Tablet Scoring: Current Practice, Fundamentals, and Knowledge Gaps
Abstract
1. Introduction
2. Overview of Drug Administration Routes
2.1. Intranasal Route
2.2. Oral Route
2.3. Ocular Route
2.4. Otic Route
2.5. Parenteral Route
2.6. Rectal Route
2.7. Sublingual Route
2.8. Transdermal Route
2.9. Vaginal Route
3. Oral Solid Dosages: Tablets
4. Tablet Scoring
4.1. Tablet Splitting: Current Practice
- (1)
- (2)
- Reduce cost and adjust dose. When a higher therapy dose is no longer needed, patients split tablets to save cost. The price variation among different tablet strengths is typically minimal. Requesting a higher dosage tablet strength in order to partition the tablets can be more economical to some patients. Recent studies suggest that the practice of splitting tablets has become more common because of economic hardship [90].
- (3)
- Facilitate dose alteration. Dose alteration involves changing the marketed dose to achieve a target dose, hence, dose tapering or dose titrating [91]. Dose tapering refers to starting a medication at a high dose and slowly decrease the dose to wean the patient out of the medication. Dose tapering is typically done to prevent the effect of medication withdrawal [92]. Medical withdrawal often is associated to clinical adverse reactions. Dose titration refers to starting a medication at a low dose and slowly increasing the dose to the target level [93]. Tablet splitting provides proper dosage in cases where slow dose titration and dose tapering are necessary, particularly with medicines that control the central nervous system [94]. Recently, it has become more of a practice for pharmaceutical companies to manufacture tablets of multiple dose strengths. In some cases, a lower desired dose strength may not be available, thus the need to partition a larger dose tablets into smaller doses.
- (4)
- Overcome changes in insurance policies. This practice aligns with cost savings; the down turn of the economy has mas made it a more common practice. Some insurance companies have denied payments for lower-strength tablets, which requires patients to obtain a larger dosage and then split the tablets [90].
- Out of specification tablets. Controlled release tablets have been designed to release the medication in a predictable manner over time [102]. To accomplish this, a variety of methods have been employed. Some methods, such as the use of coated granules, may be suitable for tablet splitting [103]. Other dosage forms, however, would have their designed features impaired by splitting. The difficulty in assessing the suitability of each controlled dosage form and the potential effect on their function makes it not favorable to partition these tablets.
- Non-robust tablets. Tablets with inadequate physical properties (e.g., low hardness, high friability) can crumble or shatter because of the brittleness property and/or low hardness values during splitting/partitioning [104]. This can compromise the desired dose and may lead to product fragmentation and wastage [104,105,106].
- Inadequate dose. This can present serious clinical adverse reaction, particularly in the case in which a drug of narrower therapeutic index is used [107]. Uneven split tablets may lead to administration of incorrect dose. Certain products, particularly potent compounds, are available commercially at doses of less than 1 mg. Splitting such smaller dose tablet can lead to dose inaccuracy, hence can pose serious clinical risks [108].
4.2. Tablet Splitting: Regulatory Guidance
- (a)
- Drug underdose. This is a case of a patient who is continuously being exposed to a low (inefficacious) amount of a drug, hence under dosing. This can potentially create resistance to that drug, which can render the therapy ineffective. That may cause serious side effects, may prevent the drug from working properly, and/or may slow down the efficacy of the therapy [140].
- (b)
- Drug overdose. This can be very harmful to patients, especially for high potency drugs [138].
- All scored tablets should be stable at: 5 °C, 25 °C/60%RH, 40 °C/75%RH for up to 90 days. Stability studies should be performed in appropriate container closures.
- Scored tablets should be stable in pharmacy dispensing containers for up to 90 days 25 °C/60%RH.
- The label should encompass the therapeutic dose.
- Enteric coated tablets should not be scored.
- The physical characteristic criteria for scored tablets are similar to that of whole tablets.
- Scored tablet Content Uniformity and Uniformity of Dosage unit as specified in USP 37 chapter 905.
- Scored tablet Water Content as specified in in USP 37 chapter 921 [65].
- Scored tablet Dissolution as specified in USP 37 chapter 711.
- Scored tablet Microbial Examination as specified in USP 61 and 62.
5. Overview of Tablet Preparation
5.1. Tablet Manufacturing
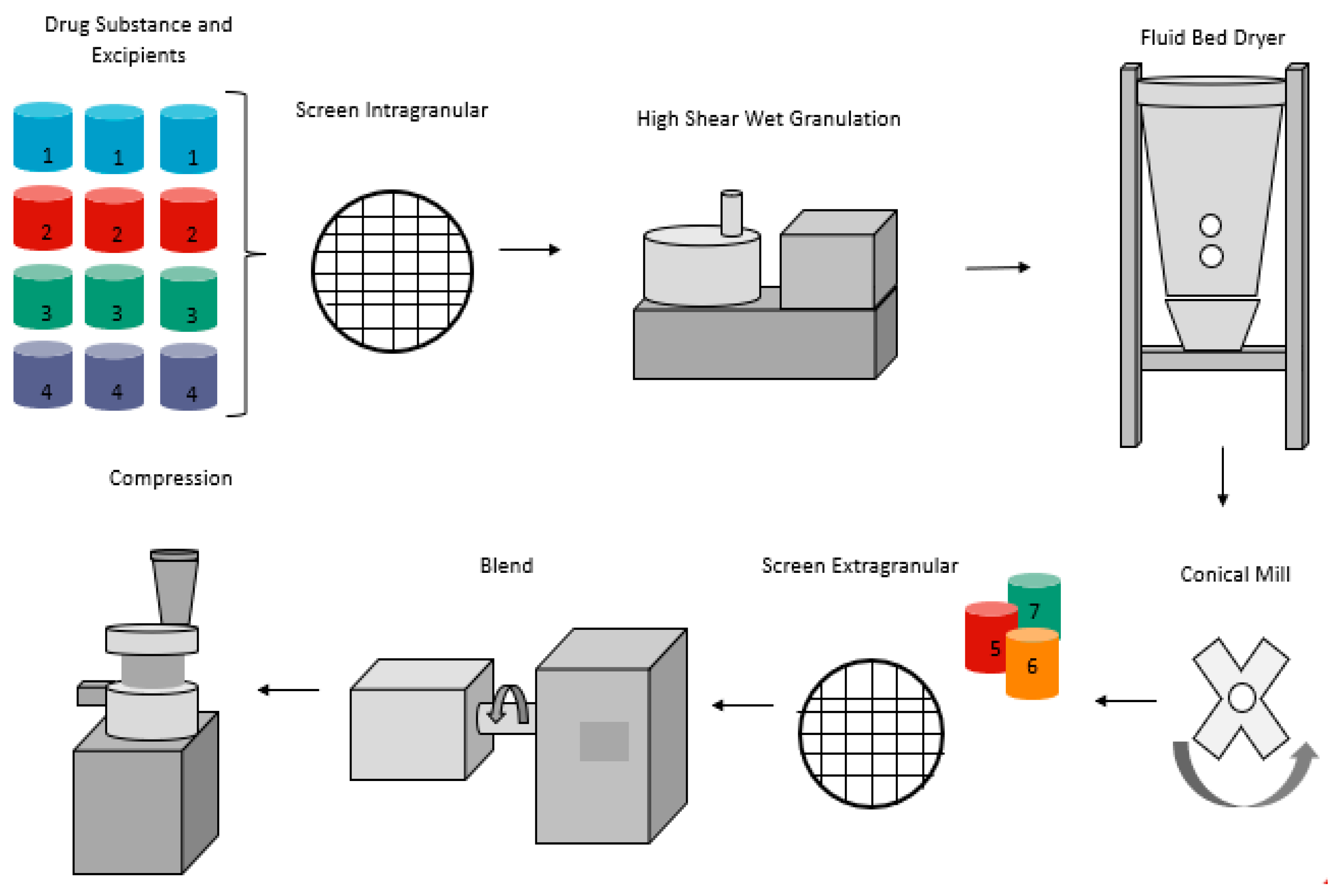
5.1.1. Dry Granulation (Direct Compression Method)
5.1.2. High Shear Wet Granulation
5.1.3. Fluidized Bed Spray Granulation
5.1.4. Dry Granulation (Roller Compaction)
5.1.5. Tablet Compression
- (1)
- Capping. This typically occurs when the top part of the tablet separates from the body of the tablet. The most common reason for this issue is air entrapment. To circumvent this effect, a pre-compression force is applied to the compressed tablet, prior to applying the final compression force [161].
- (2)
- Lamination. Lamination is often misconstrued for capping. Lamination occurs when bands or cracks are observed anywhere on the tablet rather than at the top of the tablet. This is typically process-related, especially when a large portion of fine and/or coarse granules is generated. This can also be formulation related, especially when not sufficient amount of binder is used to compress a robust tablet [162].
- (3)
- Sticking. Sticking is caused by granulation adhesion to the punches. Several factors can influence tablet sticking: formulation (drug substance, excipient, and other components), granulation properties (granule particle size, high amount of fines and/or coarse granules), tablet design (tablet shape and sizes). Tablet-press conditions and tablet-tool properties can also influence sticking [162].
5.2. Excipients Commonly Used in Tablet Manufacturing
5.2.1. Diluents
5.2.2. Binders
5.2.3. Lubricants
5.2.4. Glidants
5.2.5. Disintegrants
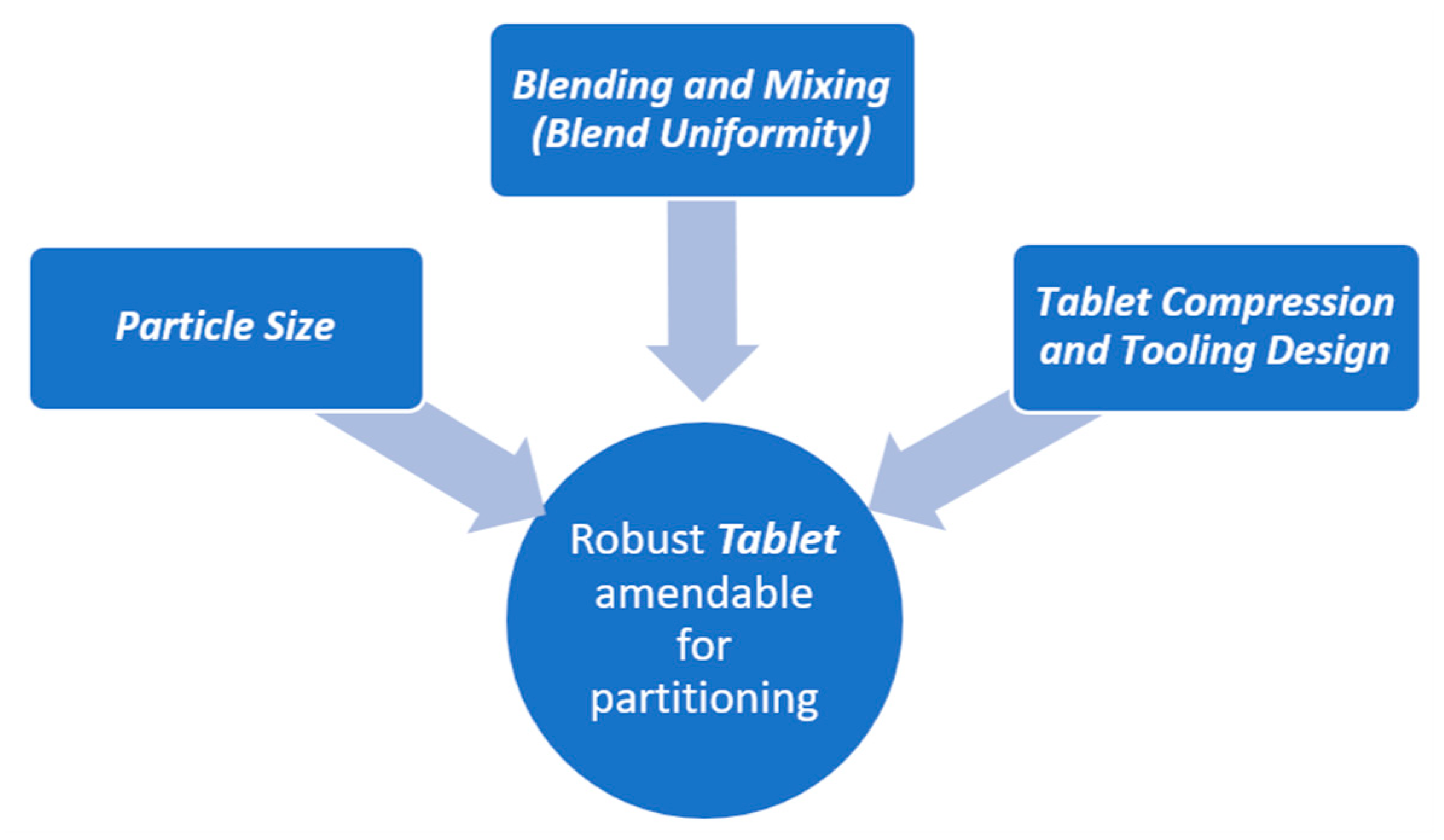
6. Tablet Scoring: Challenges and Opportunities
- (a)
- Lack of drug substance uniformity with a single tablet. Although a tablet may fall within specified specification of dosage strength, the active ingredient may not be equally distributed within that tablet. Research has revealed that halves of partitioned tablets may contain different concentration of actives [190]. This was typically observed when a drug substance of wide particle size distribution was used. Researchers have studied the effect of drug content uniformity only as variation in half tablet weights [190]. However, there are not enough data to support the in-vitro effect of the drug content of half tablets [191,192].
- (b)
- Non-conventional shaped tablets. Certain tablet shapes may be difficult to partition, since the geometry of the tablet plays a crucial role in positioning the embossing scored line in the tablet [168].
- (c)
- Fragments or crumble tablets. This issue is related to the lack of robust tablet physical property. The right selection of excipients can circumvent the fragmentation issue [193].
- Hardness (good breakability will not crumble during splitting)
- Thickness (bulkier tablets can be difficult to split)
- Good friability (tablets are not brittle and do not loose mass during splitting)
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Unit Operation | Process Parameter | Physical Properties |
|---|---|---|
| Mixing |
| Blend uniformity Particle size distribution Bulk/tapped density Moisture content Flow properties |
| Milling |
| Particle size Particle size distribution Particle shape Bulk/tapped density Flow properties Polymorphic form |
| High Shear Wet Granulation |
| Power consumption (process control) Blend uniformity Flow Moisture content Particle size and distribution Granule strength and uniformity Solid form |
| Fluid Bed Granulation |
| Granule size and distribution Granule strength, and uniformity Particle size Flow Bulk/tapped density Moisture content Residual solvents |
| Fluid Bed Drying |
| Moisture content Particle size and distribution Granule strength and uniformity Solid form |
| Unit Operation | Process Parameter | Physical Properties |
|---|---|---|
| Tray Drying |
| Moisture content Residual solvents |
| Roller Compaction |
| Appearance Ribbon/particle size and shape Ribbon density, strength, and thickness Solid form |
| Tablet Compression |
| Target weight Weight uniformity Content uniformity Hardness Thickness Tablet porosity Friability Visual attributes Moisture content Weight of core tablets |
References
- Davidson, L.; Greblov, G. The Pharmaceutical Industry in the Global Economy; Indiana University Kelley School of Business: Bloomington, IN, USA, 2005. [Google Scholar]
- Wening, K.; Breitkreutz, J. Oral drug delivery in personalized medicine: Unmet needs and novel approaches. Int. J. Pharm. 2011, 404, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Truffer, C.J.; Keehan, S.; Smith, S.; Cylus, J.; Sisko, A.; Poisal, J.A.; Lizonitz, J.; Clemens, M.K. Health spending projections through 2019: The recession’s impact continues. Health Aff. 2010, 29, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Benet, L.Z. Predicting drug disposition via application of BCS: Transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm. Res. 2005, 22, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Ku, M.S. Use of the biopharmaceutical classification system in early drug development. AAPS J. 2008, 10, 208–212. [Google Scholar] [CrossRef]
- Dahan, A.; Miller, J.M.; Amidon, G.L. Prediction of solubility and permeability class membership: Provisional BCS classification of the world’s top oral drugs. AAPS J. 2009, 11, 740–746. [Google Scholar] [CrossRef]
- Dressman, J.; Butler, J.; Hempenstall, J.; Reppas, C. The BCS: Where do we go from here? Pharm. Technol. Eur. 2001, 25, 68–77. [Google Scholar]
- Hancock, B.C.; Carlson, G.T.; Ladipo, D.D.; Langdon, B.A.; Mullarney, M.P. Comparison of the mechanical properties of the crystalline and amorphous forms of a drug substance. Int. J. Pharm. 2002, 241, 73–85. [Google Scholar] [CrossRef]
- Guinot, S.; Leveiller, F. The use of MTDSC to assess the amorphous phase content of a micronised drug substance. Int. J. Pharm. 1999, 192, 63–75. [Google Scholar] [CrossRef]
- Pudipeddi, M.; Serajuddin, A.T. Trends in solubility of polymorphs. J. Pharm. Sci. 2005, 94, 929–939. [Google Scholar] [CrossRef]
- Popović, M.R.; Popović, G.V.; Agbaba, D.D. The effects of anionic, cationic, and nonionic surfactants on acid–base equilibria of ACE inhibitors. J. Chem. Eng. Data 2013, 58, 2567–2573. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S. Molecular understanding and design of zwitterionic materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Hari, A.C.; Paruchuri, R.A.; Sabatini, D.A.; Kibbey, T.C. Effects of pH and cationic and nonionic surfactants on the adsorption of pharmaceuticals to a natural aquifer material. Environ. Sci. Technol. 2005, 39, 2592–2598. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.P.; Dugar, R. Application of surfactants in solid dispersion technology for improving solubility of poorly water soluble drugs. J. Drug Deliv. Sci. Technol. 2017, 41, 68–77. [Google Scholar] [CrossRef]
- Chiappetta, D.A.; Carcaboso, Á.M.; Bregni, C.; Rubio, M.; Bramuglia, G.; Sosnik, A. Indinavir-loaded pH-sensitive microparticles for taste masking: Toward extemporaneous pediatric anti-HIV/AIDS liquid formulations with improved patient compliance. AAPS PharmSciTech 2009, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nunn, T.; Williams, J. Formulation of medicines for children. Br. J. Clin. Pharmacol. 2005, 59, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Meyboom, R.H.; Lindquist, M.; Flygare, A.-K.; Biriell, C.; Edwards, I.R. The value of reporting therapeutic ineffectiveness as an adverse drug reaction. Drug Saf. 2000, 23, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, X.Y. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2008, 25, 781–791. [Google Scholar]
- Monforte, A.D.A.; Lepri, A.C.; Rezza, G.; Pezzotti, P.; Antinori, A.; Phillips, A.N.; Angarano, G.; Colangeli, V.; De Luca, A.; Ippolito, G. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. AIDS J. Acquir. Immune Defic. Syndr. 2000, 14, 499–507. [Google Scholar] [CrossRef]
- Howard, R.; Avery, A.; Howard, P.; Partridge, M. Investigation into the reasons for preventable drug related admissions to a medical admissions unit: Observational study. BMJ Qual. Saf. 2003, 12, 280–285. [Google Scholar] [CrossRef]
- Hänsel, A.; Bucher, H.C.; Nüesch, R.; Battegay, M. Reasons for discontinuation of first highly active antiretroviral therapy in a cohort of proteinase inhibitor-naive HIV-infected patients. AIDS J. Acquir. Immune Defic. Syndr. 2001, 26, 191–193. [Google Scholar] [CrossRef]
- Peyriere, H.; Guillemin, V.; Lotthe, A.; Baillat, V.; Fabre, J.; Favier, C.; Atoui, N.; Hansel, S.; Hillaire-Buys, D.; Reynes, J. Reasons for early abacavir discontinuation in HIV-infected patients. Ann. Pharmacother. 2003, 37, 1392–1397. [Google Scholar] [CrossRef]
- Verma, P.; Thakur, A.; Deshmukh, K.; Jha, A.; Verma, S. Routes of drug administration. Int. J. Pharm. Stud. Res. 2010, 1, 54–59. [Google Scholar]
- Vila, A.; Sanchez, A.; Tobıo, M.; Calvo, P.; Alonso, M. Design of biodegradable particles for protein delivery. J. Controll. Release 2002, 78, 15–24. [Google Scholar] [CrossRef]
- Olsson, P.; Bende, M.; Ohlin, P. The laser Doppler flowmeter for measuring microcirculation in human nasal mucosa. Acta Oto-Laryngol. 1985, 99, 133–139. [Google Scholar] [CrossRef]
- Thorne, R.; Pronk, G.; Padmanabhan, V.; Frey Ii, W. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef]
- Staniforth, J.N.; Sherwood, B.E.; Hunter, E.A. Pharmaceutical Formulations Having Improved Disintegration and/or Absorptivity. U.S. Patent 5,948,438, 7 September 1999. [Google Scholar]
- Bodratti, A.M.; Alexandridis, P. Amphiphilic block copolymers in drug delivery: Advances in formulation structure and performance. Expert Opin. Drug Deliv. 2018, 15, 1085–1104. [Google Scholar] [CrossRef]
- Bodratti, A.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Yang, L.; Alexandridis, P. Physicochemical aspects of drug delivery and release from polymer-based colloids. Curr. Opin. Colloid Interface Sci. 2000, 5, 132–143. [Google Scholar] [CrossRef]
- Agyilirah, G.A.; Banker, G.S. Polymers for enteric coating applications. Polym. Controll. Drug Deliv. 1991, 3, 39–66. [Google Scholar]
- Klein, C.E.; Chiu, Y.-L.; Awni, W.; Zhu, T.; Heuser, R.S.; Doan, T.; Breitenbach, J.; Morris, J.B.; Brun, S.C.; Hanna, G.J. The tablet formulation of lopinavir/ritonavir provides similar bioavailability to the soft-gelatin capsule formulation with less pharmacokinetic variability and diminished food effect. J. Acquir. Immune Defic. Syndr. 2007, 44, 401–410. [Google Scholar] [CrossRef]
- Wong, P.S.-L.; Edgren, D.E. Gastric Retaining Oral Liquid Dosage Form. U.S. Patent 6,635,281, 21 October 2003. [Google Scholar]
- Lang, J.C. Ocular drug delivery conventional ocular formulations. Adv. Drug Deliv. Rev. 1995, 16, 39–43. [Google Scholar] [CrossRef]
- Calvo, P.; Alonso, M.J.; Vila-Jato, J.L.; Robinson, J.R. Improved ocular bioavailability of indomethacin by novel ocular drug carriers. J. Pharm. Pharmacol. 1996, 48, 1147–1152. [Google Scholar] [CrossRef]
- Sankar, V.; Durga, S.; Prasanth, K.; Nilani, P.; Geetha, G.; Ravich, V.; Vijayakumar, A.; Raghuraman, S. Formulation and stability evaluation of diclofenac sodium ophthalmic gels. Indian J. Pharm. Sci. 2005, 67, 473. [Google Scholar]
- Mohamed, M.I. Optimization of chlorphenesin emulgel formulation. AAPS J. 2004, 6, 81–87. [Google Scholar] [CrossRef]
- Wang, X.; Dellamary, L.; Fernandez, R.; Ye, Q.; LeBel, C.; Piu, F. Principles of inner ear sustained release following intratympanic administration. Laryngoscope 2011, 121, 385–391. [Google Scholar] [CrossRef]
- Bergamini, M.V.; Mas, J.A.V.; Cabello, G.T.; Cabrera, A.L. Diclofenac and Tobramycin Formulations for Ophthalmic and Otic Topical Use. U.S. Patent 5,597,560, 28 January 1997. [Google Scholar]
- Sweetana, S.; Akers, M.J. Solubility principles and practices for parenteral drug dosage form development. PDA J. Pharm. Sci. Technol. 1996, 50, 330–342. [Google Scholar]
- Jain, J.; Fernandes, C.; Patravale, V.J.A.P. Formulation development of parenteral phospholipid-based microemulsion of etoposide. AAPS PharmSciTech 2010, 11, 826–831. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Zocharski, P.D.; Samas, B. An intravenous formulation decision tree for discovery compound formulation development. Int. J. Pharm. 2003, 253, 111–119. [Google Scholar] [CrossRef]
- Dobrozsi, D.J.; Jerry, W.H.I.; Lindman, B.O.; Ivanova, R.H.; Alexandridis, P. Pourable Liquid Vehicles. U.S. Patent 6,503,955, 7 January 2003. [Google Scholar]
- Miyake, M.; Kamada, N.; Oka, Y.; Mukai, T.; Minami, T.; Toguchi, H.; Odomi, M.; Ogawara, K.-i.; Higaki, K.; Kimura, T. Development of suppository formulation safely improving rectal absorption of rebamipide, a poorly absorbable drug, by utilizing sodium laurate and taurine. J. Controll. Release 2004, 99, 63–71. [Google Scholar] [CrossRef]
- Setnikar, I.; Fantelli, S. Liquefaction time of rectal suppositories. J. Pharm. Sci. 1962, 51, 566–571. [Google Scholar] [CrossRef]
- Harrison, T.S.; Cokeley, B.J. Suppository Applicator. U.S. Patent 5,788,664, 4 August 1998. [Google Scholar]
- Sperti, G.S. Suppository. U.S. Patent 3,415,249, 10 December 1968. [Google Scholar]
- Gowthamarajan, K.; Kulkarni, T.G.; Venkateswaran, G.; Samanta, M.; Suresh, B. Formulation and dissolution properties of meloxicam solid dispersion incorporated suppositories. Indian J. Pharm. Sci. 2002, 64, 525. [Google Scholar]
- Ghodake, P.P.; Karande, K.M.; Osmani, R.A.; Bhosale, R.R.; Harkare, B.R.; Kale, B.B. Mouth dissolving films: Innovative vehicle for oral drug delivery. Int. J. Pharma Res. Rev. 2013, 2, 41–47. [Google Scholar]
- Richey, R.H.; Hughes, C.; Craig, J.V.; Shah, U.U.; Ford, J.L.; Barker, C.E.; Peak, M.; Nunn, A.J.; Turner, M.A. A systematic review of the use of dosage form manipulation to obtain required doses to inform use of manipulation in paediatric practice. Int. J. Pharm. 2017, 518, 155–166. [Google Scholar] [CrossRef]
- Cox, L.S.; Linnemann, D.L.; Nolte, H.; Weldon, D.; Finegold, I.; Nelson, H.S. Sublingual immunotherapy: A comprehensive review. J. Allergy Clin. Immunol. 2006, 117, 1021–1035. [Google Scholar] [CrossRef]
- Singh, S.K.; Sameer, A.A. Development and characterization of sublingual tablet of Lisinopril. Asian Pac. J. Trop. Biomed. 2012, 2, S1711–S1719. [Google Scholar] [CrossRef]
- Bhyan, B.; Jangra, S.; Kaur, M.; Singh, H. Orally fast dissolving films: Innovations in formulation and technology. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 9–15. [Google Scholar]
- Wehling, F.; Schuehle, S.; Madamala, N. Pediatric Effervescent Dosage Form. U.S. Patent 5,223,264, 29 June 1993. [Google Scholar]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261. [Google Scholar] [CrossRef]
- Benson, H.A. Transdermal drug delivery: Penetration enhancement techniques. Curr. Drug Deliv. 2005, 2, 23–33. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Donnelly, L.; Curran, R.M.; Tregoning, J.S.; McKay, P.F.; Cole, T.; Morrow, R.J.; Kett, V.L.; Andrews, G.P.; Woolfson, A.D.; Malcolm, R.K. Intravaginal immunization using the recombinant HIV-1 clade-C trimeric envelope glycoprotein CN54gp140 formulated within lyophilized solid dosage forms. Vaccine 2011, 29, 4512–4520. [Google Scholar] [CrossRef][Green Version]
- Sastry, S.V.; Nyshadham, J.R.; Fix, J.A. Recent technological advances in oral drug delivery—A review. Pharm. Sci. Technol. Today 2000, 3, 138–145. [Google Scholar] [CrossRef]
- Charman, W.N.; Porter, C.J.; Mithani, S.; Dressman, J.B. Physicochemical and physiological mechanisms for the effects of food on drug absorption: The role of lipids and pH. J. Pharm. Sci. 1997, 86, 269–282. [Google Scholar] [CrossRef]
- Levy, G. Comparison of dissolution and absorption rates of different commercial aspirin tablets. J. Pharm. Sci. 1961, 50, 388–392. [Google Scholar] [CrossRef]
- Greenblatt, D.J.; Smith, T.W.; Koch-Weser, J. Bioavailability of drugs: The digoxin dilemma. Clin. Pharmacokinet. 1976, 1, 36–51. [Google Scholar] [CrossRef]
- Ford, J.L.; Rubinstein, M.H.; McCaul, F.; Hogan, J.E.; Edgar, P.J. Importance of drug type, tablet shape and added diluents on drug release kinetics from hydroxypropylmethylcellulose matrix tablets. Int. J. Pharm. 1987, 40, 223–234. [Google Scholar] [CrossRef]
- Wan, X.; Woods, A.T.; Salgado-Montejo, A.; Velasco, C.; Spence, C. Assessing the expectations associated with pharmaceutical pill colour and shape. Food Qual. Prefer. 2015, 45, 171–182. [Google Scholar] [CrossRef]
- Rähse, W.; Hoffmann, S. Product Design–The Interaction between Chemistry, Technology and Marketing to Meet Customer Needs. Chem. Eng. Technol. 2003, 26, 931–940. [Google Scholar] [CrossRef]
- Aagaard, L.; Rossi, J.J. RNAi therapeutics: Principles, prospects and challenges. Adv. Drug Deliv. Rev. 2007, 59, 75–86. [Google Scholar] [CrossRef]
- Ramkissoon-Ganorkar, C.; Liu, F.; Baudyš, M.; Kim, S.W. Modulating insulin-release profile from pH/thermosensitive polymeric beads through polymer molecular weight. J. Controll. Release 1999, 59, 287–298. [Google Scholar] [CrossRef]
- Thanoo, B.; Sunny, M.; Jayakrishnan, A. Oral Sustained-release Drug Delivery Systems using Polycarbonate Microspheres Capable of Floating on the Gastric Fluid. J. Pharm. Pharmacol. 1993, 45, 21–24. [Google Scholar] [CrossRef]
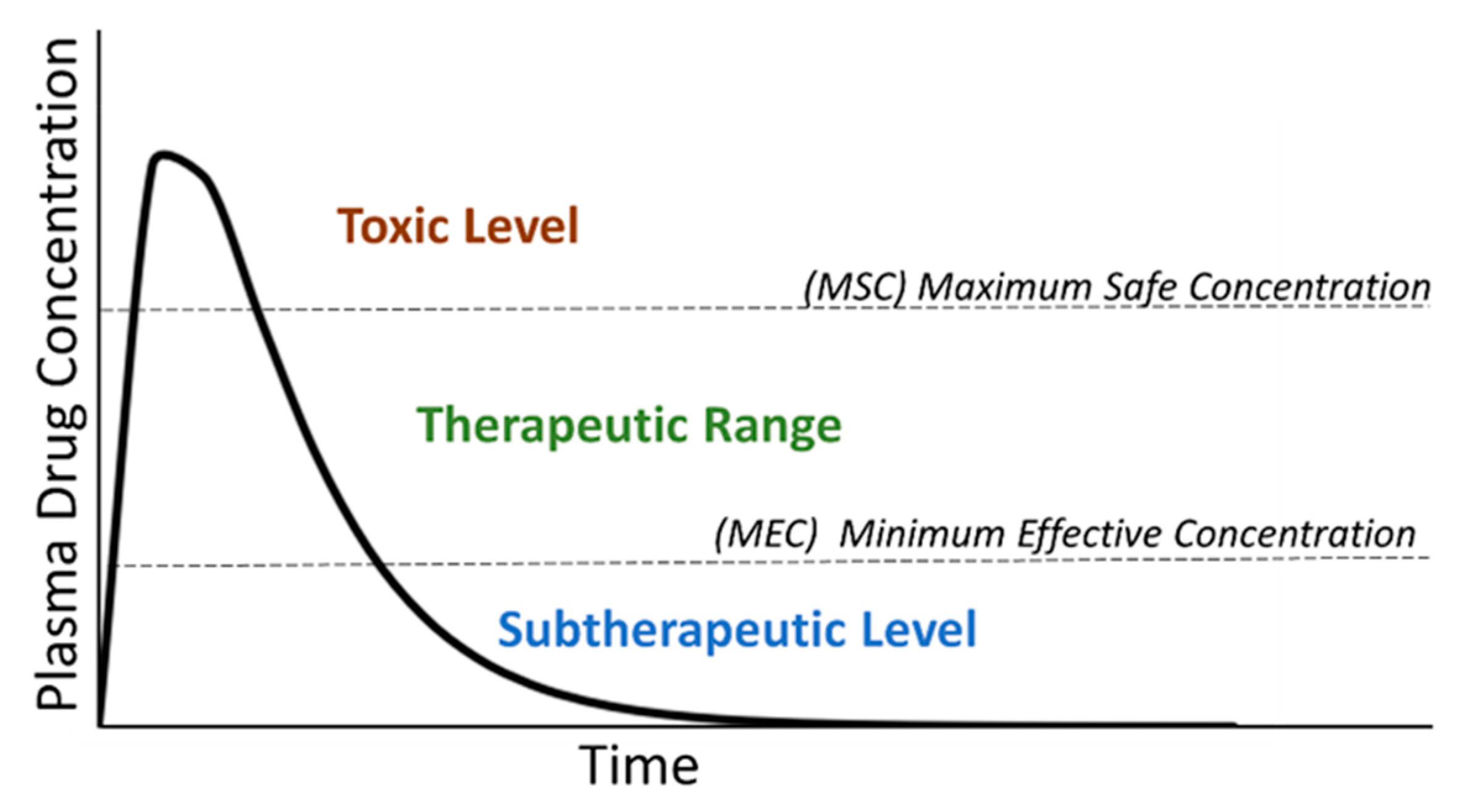
- Lehmann, K.; Gerhard, A.; Horrichs-Haermeyer, G.; Grond, S.; Zech, D. Postoperative patient-controlled analgesia with sufentanil: Analgesic efficacy and minimum effective concentrations. Acta Anaesthesiol. Scand. 1991, 35, 221–226. [Google Scholar] [CrossRef]
- Goldstein, A.; Aronow, L.; Kalman, S.M. Principles of Drug Action; the Basis of Pharmacology; Hoeber Medical Division, Harper & Row: New York, NY, USA, 1968. [Google Scholar]
- Huang, L.-F.; Tong, W.-Q.T. Impact of solid state properties on developability assessment of drug candidates. Adv. Drug Deliv. Rev. 2004, 56, 321–334. [Google Scholar] [CrossRef]
- Zhang, G.G.; Law, D.; Schmitt, E.A.; Qiu, Y. Phase transformation considerations during process development and manufacture of solid oral dosage forms. Adv. Drug Deliv. Rev. 2004, 56, 371–390. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Bernstein, J. Disappearing polymorphs. Acc. Chem. Res. 1995, 28, 193–200. [Google Scholar] [CrossRef]
- Warne, N.W. Development of high concentration protein biopharmaceuticals: The use of platform approaches in formulation development. Eur. J. Pharm. Biopharm. 2011, 78, 208–212. [Google Scholar] [CrossRef]
- Davis, J.M. Comparative doses and costs of antipsychotic medication. Arch. Gen. Psychiatry 1976, 33, 858–861. [Google Scholar] [CrossRef]
- Van Santen, E.; Barends, D.; Frijlink, H. Breaking of scored tablets: A review. Eur. J. Pharm. Biopharm. 2002, 53, 139–145. [Google Scholar] [CrossRef]
- Rodenhuis, N.; De Smet, P.A.; Barends, D.M. The rationale of scored tablets as dosage form. Eur. J. Pharm. Sci. 2004, 21, 305–308. [Google Scholar] [CrossRef]
- Verrue, C.; Mehuys, E.; Boussery, K.; Remon, J.P.; Petrovic, M. Tablet-splitting: A common yet not so innocent practice. J. Adv. Nurs. 2011, 67, 26–32. [Google Scholar] [CrossRef]
- Habib, W.A.; Alanizi, A.S.; Abdelhamid, M.M.; Alanizi, F.K. Accuracy of tablet splitting: Comparison study between hand splitting and tablet cutter. Saudi Pharm. J. 2014, 22, 454–459. [Google Scholar] [CrossRef]
- Quinzler, R.; Szecsenyi, J.; Haefeli, W. Tablet splitting: Patients and physicians need better support. Eur. J. Clin. Pharmacol. 2007, 63, 1203–1204. [Google Scholar] [CrossRef]
- Teng, J.; Song, C.K.; Williams, R.L.; Polli, J.E. Lack of medication dose uniformity in commonly split tablets. J. Am. Pharm. Assoc. 2002, 42, 195–199. [Google Scholar] [CrossRef]
- Mc Gillicuddy, A.; Kelly, M.; Crean, A.M.; Sahm, L.J. The knowledge, attitudes and beliefs of patients and their healthcare professionals around oral dosage form modification: A systematic review of the qualitative literature. Res. Soc. Adm. Pharm. 2017, 13, 717–726. [Google Scholar] [CrossRef]
- Freeman, M.K.; White, W.; Iranikhah, M. Tablet Splitting: A Review of Weight and Content Uniformity Part 1 of a 2-Part Series. Consult. Pharm. J. Am. Soc. Consult. Pharm. 2012, 27, 341–352. [Google Scholar] [CrossRef]
- Debotton, N.; Dahan, A. Applications of polymers as pharmaceutical excipients in solid oral dosage forms. Med. Res. Rev. 2017, 37, 52–97. [Google Scholar] [CrossRef]
- Leslie, S.T. Slow Release Pharmaceutical Compositions. U.S. Patent 3,965,256, 22 June 1976. [Google Scholar]
- Krause, G.M.; Ninger, F.C. Tablet Structure. U.S. Patent 3,336,200, 15 August 1967. [Google Scholar]
- Lau, E.T.; Steadman, K.J.; Cichero, J.A.; Nissen, L.M. Dosage form modification and oral drug delivery in older people. Adv. Drug Deliv. Rev. 2018, 135, 75–84. [Google Scholar] [CrossRef]
- Forough, A.S.; Lau, E.T.; Steadman, K.J.; Cichero, J.A.; Kyle, G.J.; Santos, J.M.S.; Nissen, L.M. A spoonful of sugar helps the medicine go down? A review of strategies for making pills easier to swallow. Patient Prefer. Adherence 2018, 12, 1337–1346. [Google Scholar] [CrossRef]
- Elliott, I.; Mayxay, M.; Yeuichaixong, S.; Lee, S.J.; Newton, P.N. The practice and clinical implications of tablet splitting in international health. Trop. Med. Int. Health 2014, 19, 754–760. [Google Scholar] [CrossRef]
- Helmy, S.A. Tablet splitting: Is it worthwhile? Analysis of drug content and weight uniformity for half tablets of 16 commonly used medications in the outpatient setting. J. Manag. Care Spec. Pharm. 2015, 21, 76–88. [Google Scholar] [CrossRef]
- Harrison, M.; Busto, U.; Naranjo, C.; Kaplan, H.; Sellers, E. Diazepam tapering in detoxification for high-dose benzodiazepine abuse. Clin. Pharmacol. Ther. 1984, 36, 527–533. [Google Scholar] [CrossRef]
- Cathcart, R.F. Vitamin C, titrating to bowel tolerance, anascorbemia, and acute induced scurvy. Med. Hypotheses 1981, 7, 1359–1376. [Google Scholar] [CrossRef]
- Breitkreutz, J.; Boos, J. Paediatric and geriatric drug delivery. Expert Opin. Drug Deliv. 2007, 4, 37–45. [Google Scholar] [CrossRef]
- Yusuf, S.; Peto, R.; Lewis, J.; Collins, R.; Sleight, P. Beta blockade during and after myocardial infarction: An overview of the randomized trials. Prog. Cardiovasc. Dis 1985, 27, 335–371. [Google Scholar] [CrossRef]
- Yeager, R.A.; Moneta, G.L.; Edwards, J.M.; Taylor, L.M.; McConnell, D.B.; Porter, J.M. Reducing Perioperative Myocardial Infarction Following Vascular Surgery: The Potential Role of β-Blockade. Arch. Surg. 1995, 130, 869–873. [Google Scholar] [CrossRef]
- Rydén, L.; Standl, E.; Bartnik, M.; Van den Berghe, G.; Betteridge, J.; De Boer, M.-J.; Cosentino, F.; Jönsson, B.; Laakso, M.; Malmberg, K. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: Executive summary: The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2007, 28, 88–136. [Google Scholar]
- Stafford, R.S.; Radley, D.C. The potential of pill splitting to achieve cost savings. Am. J. Manag. Care 2002, 8, 706–712. [Google Scholar]
- Cobby, J.; Mayersohn, M.; Walker, G.C. Influence of shape factors on kinetics of drug release from matrix tablets I: Theoretical. J. Pharm. Sci. 1974, 63, 725–732. [Google Scholar] [CrossRef]
- Gupta, A.; Hunt, R.L.; Khan, M.A. Influence of tablet characteristics on weight variability and weight loss in split tablets. Am. J. Health-Syst. Pharm. 2008, 65, 2326–2328. [Google Scholar] [CrossRef]
- Didier, A.; Malling, H.-J.; Worm, M.; Horak, F.; Jäger, S.; Montagut, A.; André, C.; de Beaumont, O.; Melac, M. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5–grass pollen tablet for seasonal allergic rhinitis. J. Allergy Clin. Immunol. 2007, 120, 1338–1345. [Google Scholar] [CrossRef]
- Katikaneni, P.R.; Upadrashta, S.M.; Neau, S.H.; Mitra, A.K. Ethylcellulose matrix controlled release tablets of a water-soluble drug. Int. J. Pharm. 1995, 123, 119–125. [Google Scholar] [CrossRef]
- Bodmeier, R. Tableting of coated pellets. Eur. J. Pharm. Biopharm. 1997, 43, 1–8. [Google Scholar] [CrossRef]
- Seitz, J.A.; Flessland, G.M. Evaluation of the physical properties of compressed tablets I: Tablet hardness and friability. J. Pharm. Sci. 1965, 54, 1353–1357. [Google Scholar] [CrossRef]
- Muti, H.; Othman, S. Effects of binders and moisture content on the disintegration, hardness and friability of paracetamol and orphenadrine citrate tablets. Drug Dev. Ind. Pharm. 1989, 15, 2017–2035. [Google Scholar] [CrossRef]
- Lakshman, J.P.; Kowalski, J.; Vasanthavada, M.; Tong, W.Q.; Joshi, Y.M.; Serajuddin, A.T. Application of melt granulation technology to enhance tabletting properties of poorly compactible high-dose drugs. J. Pharm. Sci. 2011, 100, 1553–1565. [Google Scholar] [CrossRef]
- Cook, T.J.; Edwards, S.; Gyemah, C.; Shah, M.; Shah, I.; Fox, T. Variability in tablet fragment weights when splitting unscored cyclobenzaprine 10 mg tablets. J. Am. Pharm. Assoc. 2004, 44, 583–586. [Google Scholar] [CrossRef]
- Zaid, A.N.; Rowa’J, A.-R.; Ghoush, A.A.; Qaddumi, A.; Zaaror, Y.A. Weight and content uniformity of lorazepam half-tablets: A study of correlation of a low drug content product. Saudi Pharm. J. 2013, 21, 71–75. [Google Scholar] [CrossRef]
- Bachynsky, J.; Wiens, C.; Melnychuk, K. The practice of splitting tablets. Pharmacoeconomics 2002, 20, 339–346. [Google Scholar] [CrossRef]
- Kolodny, A.; Courtwright, D.T.; Hwang, C.S.; Kreiner, P.; Eadie, J.L.; Clark, T.W.; Alexander, G.C. The prescription opioid and heroin crisis: A public health approach to an epidemic of addiction. Ann. Rev. Public Health 2015, 36, 559–574. [Google Scholar] [CrossRef]
- Yarborough, B.J.H.; Stumbo, S.P.; Janoff, S.L.; Yarborough, M.T.; McCarty, D.; Chilcoat, H.D.; Coplan, P.M.; Green, C.A. Understanding opioid overdose characteristics involving prescription and illicit opioids: A mixed methods analysis. Drug Alcohol Depend. 2016, 167, 49–56. [Google Scholar] [CrossRef]
- Alexander, L.; Mannion, R.O.; Weingarten, B.; Fanelli, R.J.; Stiles, G.L. Development and impact of prescription opioid abuse deterrent formulation technologies. Drug Alcohol Depend. 2014, 138, 1–6. [Google Scholar] [CrossRef]
- Koob, G.F.; Pettit, H.O.; Ettenberg, A.; Bloom, F.E. Effects of opiate antagonists and their quaternary derivatives on heroin self-administration in the rat. J. Pharmacol. Exp. Ther. 1984, 229, 481–486. [Google Scholar]
- Vosburg, S.K.; Jones, J.D.; Manubay, J.M.; Ashworth, J.B.; Benedek, I.H.; Comer, S.D. Assessment of a formulation designed to be crush-resistant in prescription opioid abusers. Drug Alcohol Depend. 2012, 126, 206–215. [Google Scholar] [CrossRef][Green Version]
- Schneider, J.P.; Matthews, M.; Jamison, R.N. Abuse-deterrent and tamper-resistant opioid formulations. CNS Drugs 2010, 24, 805–810. [Google Scholar] [CrossRef]
- Stegemann, S.; Ecker, F.; Maio, M.; Kraahs, P.; Wohlfart, R.; Breitkreutz, J.; Zimmer, A.; Bar-Shalom, D.; Hettrich, P.; Broegmann, B. Geriatric drug therapy: Neglecting the inevitable majority. Ageing Res. Rev. 2010, 9, 384–398. [Google Scholar] [CrossRef]
- Rodenhuis, N.; Barends, D.; de Smet, P. Patient experiences with the performance of tablet score lines needed for dosing. Pharm. World Sci. 2003, 25, 173–176. [Google Scholar] [CrossRef]
- Van der Steen, K.C.; Frijlink, H.W.; Schipper, C.M.A.; Barends, D.M. Prediction of the ease of subdivision of scored tablets from their physical parameters. AAPS Pharmscitech 2010, 11, 126–132. [Google Scholar] [CrossRef][Green Version]
- Van Riet-Nales, D.A.; Doeve, M.E.; Nicia, A.E.; Teerenstra, S.; Notenboom, K.; Hekster, Y.A.; van den Bemt, B.J. The accuracy, precision and sustainability of different techniques for tablet subdivision: Breaking by hand and the use of tablet splitters or a kitchen knife. Int. J. Pharm. 2014, 466, 44–51. [Google Scholar] [CrossRef]
- Somogyi, O.; Meskó, A.; Csorba, L.; Szabó, P.; Zelkó, R. Pharmaceutical counselling about different types of tablet-splitting methods based on the results of weighing tests and mechanical development of splitting devices. Eur. J. Pharm. Sci. 2017, 106, 262–273. [Google Scholar] [CrossRef]
- Fahelelbom, K.; Al-Tabakha, M.; Eissa, N.; Javadi, J. Evaluation of Certain Pharmaceutical Quality Attributes of Lisinopril Split Tablets. Sci. Pharm. 2016, 84, 646–653. [Google Scholar] [CrossRef]
- Van Reuler, A.V.; van Diemen, J.J.; Harmsze, A.M.; Fuijkschot, W.W.; Thijs, A. Subdivision of aspirin tablets? Use your hands: A study on aspirin tablet subdivision using four different methods. J. Pharm. Pract. Res. Soc. Adm. Pharm. 2018, 48, 44–48. [Google Scholar] [CrossRef]
- Eserian, J.K.; Lombardo, M. Tablet subdivision: Far beyond the splitting technique. Int. J. Pharm. 2014, 476, 77. [Google Scholar] [CrossRef]
- Van Vooren, L.; De Spiegeleer, B.; Thonissen, T.; Joye, P.; Van Durme, J.; Slegers, G. Statistical analysis of tablet breakability methods. Int. J. Pharm. Pharm. Sci. 2002, 5, 190–198. [Google Scholar]
- Zhang, Y.; Johnson, K.C.J. Effect of drug particle size on content uniformity of low-dose solid dosage forms. Int. J. Pharm. 1997, 154, 179–183. [Google Scholar] [CrossRef]
- Abu-Geras, D.; Hadziomerovic, D.; Leau, A.; Khan, R.N.; Gudka, S.; Locher, C.; Razaghikashani, M.; Lim, L.Y. Accuracy of tablet splitting and liquid measurements: An examination of who, what and how. J. Pharm. Pharmacol. 2017, 69, 603–612. [Google Scholar] [CrossRef]
- Temer, A.C.; Teixeira, M.T.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Silva, I.C.; Taveira, S.F.; Marreto, R.N.; Cunha-Filho, M. Subdivision of Tablets Containing Modified Delivery Technology: The Case of Orally Disintegrating Tablets. J. Pharm. Innov. 2018, 13, 261–269. [Google Scholar] [CrossRef]
- Chou, C.-Y.; Hsu, C.-C.; Chiang, S.-C.; Ho, C.-C.; Chou, C.-L.; Wu, M.-S.; Chang, Y.-L.; Tsai, H.-Y.; Chen, T.-J.; Chou, Y.-C. Association between physician specialty and risk of prescribing inappropriate pill splitting. PLoS ONE 2013, 8, e70113. [Google Scholar] [CrossRef]
- Thong, M.Y.; Manrique, Y.J.; Steadman, K.J. Drug loss while crushing tablets: Comparison of 24 tablet crushing devices. PLoS ONE 2018, 13, e0193683. [Google Scholar] [CrossRef]
- Trivedi, M.R.; Patel, H.H.; Dave, R.H. A Review on Tablet Scoring: Background, History and Current Regulatory Considerations. J. Pharm. Res. Int. 2017, 20, 1–7. [Google Scholar] [CrossRef]
- Harbir, K. Processing technologies for pharmaceutical tablets: A review. Int. Res. J. Pharm. 2012, 3, 20–23. [Google Scholar]
- Nayak, A.K.; Manna, K. Current developments in orally disintegrating tablet technology. J. Pharm. Educ. Res. Soc. Adm. Pharm. 2011, 2, 21–34. [Google Scholar]
- Spiegeleer, B.D.; Hoorebeke, L.V.; Spiegeleer, A.D.; Castelein, P.; Bortel, L.V. The paradox of scored tablets: A cost-saving risk. Die Pharm. Int. J. Pharm. Sci. 2009, 64, 550–552. [Google Scholar]
- Biron, C.; Licznar, P.; Hansel, S.; Schved, J. Oral anticoagulant drugs: Do not cut tablets in quarters. Thromb. Haemost. 1999, 82, 1201. [Google Scholar]
- Glickman, S.W.; Anstrom, K.J.; Lin, L.; Chandra, A.; Laskowitz, D.T.; Woods, C.W.; Freeman, D.H.; Kraft, M.; Beskow, L.M.; Weinfurt, K.P. Challenges in enrollment of minority, pediatric, and geriatric patients in emergency and acute care clinical research. Ann. Emerg. Med. 2008, 51, 775–780. [Google Scholar] [CrossRef]
- Pahwa, R.; Piplani, M.; Sharma, P.C.; Kaushik, D.; Nanda, S. Orally disintegrating tablets-Friendly to pediatrics and geriatrics. Arch. Appl. Sci. Res. 2010, 2, 35–48. [Google Scholar]
- Peek, B.T.; Al-Achi, A.; Coombs, S. Accuracy of tablet splitting by elderly patients. J. Am. Med. Assoc. 2002, 288, 451–452. [Google Scholar] [CrossRef]
- Gerber, J.E.; Cawthon, B. Overdose and death with olanzapine: Two case reports. Am. J. Forensic Med. Pathol. 2000, 21, 249–251. [Google Scholar] [CrossRef]
- Marques, M.R.; Brown, W. Updates on USP activities related to dissolution, disintegration, and drug release. Dissolution Technol. 2013, 20, 54–56. [Google Scholar] [CrossRef]
- Allemann, S.S.; Bornand, D.; Hug, B.; Hersberger, K.E.; Arnet, I. Issues around the prescription of half tablets in Northern Switzerland: The irrational case of quetiapine. BioMed Res. Int. 2015, 2015, 602021. [Google Scholar] [CrossRef]
- Strickley, R.G.; Iwata, Q.; Wu, S.; Dahl, T.C. Pediatric drugs—A review of commercially available oral formulations. J. Pharm. Sci. 2008, 97, 1731–1774. [Google Scholar] [CrossRef]
- Nagar, P.; Singh, K.; Chauhan, I.; Verma, M.; Yasir, M.; Khan, A.; Sharma, R.; Gupta, N. Orally disintegrating tablets: Formulation, preparation techniques and evaluation. J. Appl. Pharm. Sci. 2011, 1, 35–45. [Google Scholar]
- Ghebre-Sellassie, I.; Gordon, R.H.; Harris, M.R.; Nesbitt, R.U., Jr. Process for Treating Dosage Forms. U.S. Patent 4,600,645, 15 June 1986. [Google Scholar]
- Breitenbach, J.; Kleinke, A.; Kothrade, S.; Rosenberg, J. Process for Producing Solid Dosage Forms by Extrusion. U.S. Patent 6,221,368, 24 April 2001. [Google Scholar]
- Bushra, R.; Shoaib, M.H.; Aslam, N.; Hashmat, D.; Rehman, M. Formulation Development and Optimization of Ibuprofen Tablets by Direct Compression Method. J. Pharm. Sci. 2008, 21, 113–120. [Google Scholar]
- Nusheh, M.; Yoozbashizadeh, H.; Askari, M.; Kuwata, N.; Kawamura, J.; Kano, J.; Saito, F.; Kobatake, H.; Fukuyama, H. Effect of mechanical milling on carbothermic reduction of magnesia. ISIJ Int. 2010, 50, 668–672. [Google Scholar] [CrossRef][Green Version]
- Olsen, J.L.; Rippie, E.G.J. Segregation kinetics of particulate solids systems I. Influence of particle size and particle size distribution. J. Pharm. Sci. 1964, 53, 147–150. [Google Scholar] [CrossRef]
- Suresh, P.; Sreedhar, I.; Vaidhiswaran, R.; Venugopal, A. A comprehensive review on process and engineering aspects of pharmaceutical wet granulation. Chem. Eng. J. 2017, 328, 785–815. [Google Scholar] [CrossRef]
- Pereira, G.R.S.; Taveira, S.F.; Cunha-Filho, M.; Marreto, R.N. The Effects of Fillers and Binders on the Accuracy of Tablet Subdivision. AAPS PharmSciTech 2018, 19, 2929–2933. [Google Scholar] [CrossRef]
- Faure, A.; York, P.; Rowe, R. Process control and scale-up of pharmaceutical wet granulation processes: A review. Eur. J. Pharm. Biopharm. 2001, 52, 269–277. [Google Scholar] [CrossRef]
- Tan, H.; Salman, A.; Hounslow, M. Kinetics of fluidised bed melt granulation I: The effect of process variables. Chem. Eng. Sci. 2006, 61, 1585–1601. [Google Scholar] [CrossRef]
- Rajniak, P.; Mancinelli, C.; Chern, R.; Stepanek, F.; Farber, L.; Hill, B. Experimental study of wet granulation in fluidized bed: Impact of the binder properties on the granule morphology. Int. J. Pharm. 2007, 334, 92–102. [Google Scholar] [CrossRef]
- Daniher, D.; Briens, L.; Tallevi, A. End-point detection in high-shear granulation using sound and vibration signal analysis. Powder Technol. 2008, 181, 130–136. [Google Scholar] [CrossRef]
- McMillan, J.; Briens, C.; Berruti, F.; Chan, E. High velocity attrition nozzles in fluidized beds. Powder Technol. 2007, 175, 133–141. [Google Scholar] [CrossRef]
- Frake, P.; Greenhalgh, D.; Grierson, S.; Hempenstall, J.; Rudd, D. Process control and end-point determination of a fluid bed granulation by application of near infra-red spectroscopy. Int. J. Pharm. 1997, 151, 75–80. [Google Scholar] [CrossRef]
- Dixit, R.; Puthli, S. Fluidization technologies: Aerodynamic principles and process engineering. J. Pharm. Sci. 2009, 98, 3933–3960. [Google Scholar] [CrossRef]
- Osborne, J.D.; Sochon, R.P.; Cartwright, J.J.; Doughty, D.G.; Hounslow, M.J.; Salman, A.D. Binder addition methods and binder distribution in high shear and fluidised bed granulation. Chem. Eng. Res. Des. 2011, 89, 553–559. [Google Scholar] [CrossRef]
- Kleinebudde, P. Roll compaction/dry granulation: Pharmaceutical applications. Eur. J. Pharm. Biopharm. 2004, 58, 317–326. [Google Scholar] [CrossRef]
- Ito, A.; Dobashi, Y.; Sugihara, M. The relationship between dividing properties of scored tablets and dynamic characteristics of various mixed powders. Chem. Pharm. Bull. 1993, 41, 590–594. [Google Scholar] [CrossRef]
- Jivraj, M.; Martini, L.G.; Thomson, C.M. An overview of the different excipients useful for the direct compression of tablets. Pharm. Sci. Technol. Today 2000, 3, 58–63. [Google Scholar] [CrossRef]
- Sugimori, K.; Mori, S.; Kawashima, Y. Characterization of die wall pressure to predict capping of flat-or convex-faced drug tablets of various sizes. Powder Technol. 1989, 58, 259–264. [Google Scholar] [CrossRef]
- Patel, S.; Kaushal, A.M.; Bansal, A.K. Compression physics in the formulation development of tablets. Crit. Rev. Ther. Drug Carr. Syst. 2006, 23, 15–19. [Google Scholar] [CrossRef]
- De Spiegeleer, B.; Van Vooren, L.; Thonissen, T.; Joye, P. Mass uniformity: Influence of operational compression conditions on breakability of scored tablets as part of manufacturing robustness evaluation. J. Food Drug Anal. 2005, 13, 22–29. [Google Scholar]
- Katare, Y.K.; Panda, A.K. Influences of excipients on in vitro release and in vivo performance of tetanus toxoid loaded polymer particles. Eur. J. Pharm. Sci. 2006, 28, 179–188. [Google Scholar] [CrossRef]
- David, S.; Augsburger, L. Plastic flow during compression of directly compressible fillers and its effect on tablet strength. J. Pharm. Sci. 1977, 66, 155–159. [Google Scholar] [CrossRef]
- Morkhade, D.M. Comparative impact of different binder addition methods, binders and diluents on resulting granule and tablet attributes via high shear wet granulation. Powder Technol. 2017, 320, 114–124. [Google Scholar] [CrossRef]
- Castello, R.A.; Mattocks, A.M. Discoloration of tablets containing amines and lactose. J. Pharm. Sci. 1962, 51, 106–108. [Google Scholar] [CrossRef]
- Teixeira, M.T.; Sá-Barreto, L.C.; Gratieri, T.; Gelfuso, G.M.; Silva, I.C.; Cunha-Filho, M.S. Key technical aspects influencing the accuracy of tablet subdivision. AAPS PharmSciTech 2017, 18, 1393–1401. [Google Scholar] [CrossRef]
- Roberts, R.; Rowe, R. Brittle/ductile behaviour in pharmaceutical materials used in tabletting. Int. J. Pharm. 1987, 36, 205–209. [Google Scholar] [CrossRef]
- Vromans, H.; De Boer, A.; Bolhuis, G.; Lerk, C.; Kussendrager, K.; Bosch, H. Studies on tableting properties of lactose. Pharm. Weekbl. 1985, 7, 186–193. [Google Scholar] [CrossRef]
- Bolhuis, G.; Zuurman, K. Tableting properties of experimental and commercially available lactose granulations for direct compression. Drug Dev. Ind. Pharm. 1995, 21, 2057–2071. [Google Scholar] [CrossRef]
- Tu, W.-D.; Hsiau, S.-S.; Ingram, A.; Seville, J. The effect of powder size on induction behaviour and binder distribution during high shear melt agglomeration of calcium carbonate. Powder Technol. 2008, 184, 298–312. [Google Scholar] [CrossRef]
- Arndt, O.-R.; Kleinebudde, P. Towards a better understanding of dry binder functionality. Int. J. Pharm. 2018, 552, 258–264. [Google Scholar] [CrossRef]
- Zhang, Y.; Law, Y.; Chakrabarti, S. Physical properties and compact analysis of commonly used direct compression binders. AAPS PharmSciTech 2003, 4, 489–499. [Google Scholar] [CrossRef]
- Joneja, S.; Harcum, W.; Skinner, G.; Barnum, P.; Guo, J.-H. Investigating the fundamental effects of binders on pharmaceutical tablet performance. Drug Dev. Ind. Pharm. 1999, 25, 1129–1135. [Google Scholar] [CrossRef]
- Du, T. Binders in pharmaceutical granulation. In Handbook of Pharmaceutical Granulation Technology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 93–112. [Google Scholar]
- Krycer, I.; Pope, D.G.; Hersey, J.A. An evaluation of tablet binding agents part I. Solution binders. Powder Technol. 1983, 34, 39–51. [Google Scholar] [CrossRef]
- Shah, A.; Mlodozeniec, A. Mechanism of surface lubrication: Influence of duration of lubricant-excipient mixing on processing characteristics of powders properties of compressed tablets. J. Pharm. Sci. 1977, 66, 1377–1382. [Google Scholar] [CrossRef]
- Paul, S.; Sun, C.C. Systematic evaluation of common lubricants for optimal use in tablet formulation. Eur. J. Pharm. Sci. 2018, 117, 118–127. [Google Scholar] [CrossRef]
- Wang, J.; Wen, H.; Desai, D.J. Lubrication in tablet formulations. Eur. J. Pharm. Biopharm. 2010, 75, 1–15. [Google Scholar] [CrossRef]
- Rizk, S.; Guyot, J.; Duru, C.; Gaudy, D. Influence of lubricant properties on compression behaviour and drug dissolution rate of scleroglucan hydrophilic matrix. Int. J. Pharm. 1995, 126, 57–63. [Google Scholar] [CrossRef]
- Dürig, T.; Fassihi, R. Mechanistic evaluation of binary effects of magnesium stearate and talc as dissolution retardants at 85% drug loading in an experimental extended-release formulation. J. Pharm. Sci. 1997, 86, 1092–1098. [Google Scholar] [CrossRef]
- Chattoraj, S.; Sun, C.C. Crystal and particle engineering strategies for improving powder compression and flow properties to enable continuous tablet manufacturing by direct compression. J. Pharm. Sci. 2018, 107, 968–974. [Google Scholar] [CrossRef]
- Jonat, S.; Hasenzahl, S.; Gray, A.; Schmidt, P.C. Mechanism of glidants: Investigation of the effect of different colloidal silicon dioxide types on powder flow by atomic force and scanning electron microscopy. J. Pharm. Sci. 2004, 93, 2635–2644. [Google Scholar] [CrossRef]
- Wojcik-Pastuszka, D.; Juszkiewicz, K.; Özhan, G.; Musiał, W. Influence of Tablet Splitting on Dissolution of Tablets with Naproxen Sodium. Dissolution Technol. 2018, 25, 16–23. [Google Scholar] [CrossRef]
- Pandey, P.; Levins, C.; Pafiakis, S.; Zacour, B.; Bindra, D.S.; Trinh, J.; Buckley, D.; Gour, S.; Sharif, S.; Stamato, H. Enhancing tablet disintegration characteristics of a highly water-soluble high-drug-loading formulation by granulation process. Pharm. Dev. Technol. 2018, 23, 587–595. [Google Scholar] [CrossRef]
- Van Kamp, H.; Bolhuis, G.; Lerk, C. Improvement by super disintegrants of the properties of tablets containing lactose, prepared by wet granulation. Pharm. Weekbl. 1983, 5, 165–171. [Google Scholar] [CrossRef]
- Alebiowu, G.; Itiola, O.A. Effects of starches on the mechanical properties of paracetamol tablet formulations. II. Sorghum and plantain starches as disintegrants. Acta Pharm. 2003, 53, 313–320. [Google Scholar]
- Mohanachandran, P.; Sindhumol, P.; Kiran, T. Superdisintegrants: An overview. Int. J. Pharm. Sci. Rev. Res. 2011, 6, 105–109. [Google Scholar]
- Rohrs, B.R.; Amidon, G.E.; Meury, R.H.; Secreast, P.J.; King, H.M.; Skoug, C.J. Particle size limits to meet USP content uniformity criteria for tablets and capsules. J. Pharm. Sci. 2006, 95, 1049–1059. [Google Scholar] [CrossRef]
- Zhao, N.; Zidan, A.; Tawakkul, M.; Sayeed, V.A.; Khan, M. Tablet splitting: Product quality assessment of metoprolol succinate extended release tablets. Int. J. Pharm. 2010, 401, 25–31. [Google Scholar] [CrossRef]
- Duman, E.; Yuksel, N.; Olin, B.; Sakr, A. Effect of scoring design on the uniformity of extended release matrix tablet halves. Pharm. Ind. 2000, 62, 547–550. [Google Scholar]
- Saha, S.; Shahiwala, A. Multifunctional coprocessed excipients for improved tabletting performance. Expert Opin. Drug Deliv. 2009, 6, 197–208. [Google Scholar] [CrossRef]
- Navarro, R.P. Tablet splitting: Much ado about nothing? J. Manag. Care Pharm. 2009, 15, 272–274. [Google Scholar] [CrossRef]
- Shah, R.B.; Collier, J.S.; Sayeed, V.A.; Bryant, A.; Habib, M.J.; Khan, M.A. Tablet splitting of a narrow therapeutic index drug: A case with levothyroxine sodium. AAPS Pharmscitech 2010, 11, 1359–1367. [Google Scholar] [CrossRef]
- Mosena, M.S.; Van der Merwe, E. The appropriateness and risks of tablet splitting: Medifile: Clinical review. SA Pharm. J. 2009, 76, 30–36. [Google Scholar]
- Gordon, M.S. Process considerations in reducing tablet friability and their effect on in vitro dissolution. Drug Dev. Ind. Pharm. 1994, 20, 11–29. [Google Scholar] [CrossRef]
- Alkan, M.; Yuksel, A. Granulation in a fluidized bed II Effect of binder amount on the final granules. Drug Dev. Ind. Pharm. 1986, 12, 1529–1543. [Google Scholar] [CrossRef]
- Campbell, C.S.; Brennen, C.E. Computer simulation of granular shear flows. J. Fluid Mech. 1985, 151, 167–188. [Google Scholar] [CrossRef]
- Fassihi, A.; Kanfer, I. Effect of compressibility and powder flow properties on tablet weight variation. Drug Dev. Ind. Pharm. 1986, 12, 1947–1966. [Google Scholar] [CrossRef]
- Liss, E.D.; Conway, S.L.; Zega, J.A.; Glasser, B. Segregation of powders during gravity flow through vertical pipes. Pharm. Technol. Eur. 2004, 28, 78–97. [Google Scholar]
- Malliaris, A.; Turner, D. Influence of particle size on the electrical resistivity of compacted mixtures of polymeric and metallic powders. J. Appl. Phys. 1971, 42, 614–618. [Google Scholar] [CrossRef]
- Müller, M.; Albe, K. Lattice Monte Carlo simulations of FePt nanoparticles: Influence of size, composition, and surface segregation on order-disorder phenomena. Phys. Rev. B 2005, 72, 094203. [Google Scholar] [CrossRef]
- Gauthier, D.; Zerguerras, S.; Flamant, G. Influence of the particle size distribution of powders on the velocities of minimum and complete fluidization. Chem. Eng. J. 1999, 74, 181–196. [Google Scholar] [CrossRef]
- Narayan, P.; Hancock, B. The influence of particle size on the surface roughness of pharmaceutical excipient compacts. Mater. Sci. Eng. A 2005, 407, 226–233. [Google Scholar] [CrossRef]
- Bolhuis, G.K.; Anthony Armstrong, N. Excipients for direct compaction—An update. Pharm. Dev. Technol. 2006, 11, 111–124. [Google Scholar] [CrossRef]
- Reynolds, G.K. Modelling of pharmaceutical granule size reduction in a conical screen mill. Chem. Eng. J. 2010, 164, 383–392. [Google Scholar] [CrossRef]
- Chikhalia, V.; Forbes, R.; Storey, R.; Ticehurst, M. The effect of crystal morphology and mill type on milling induced crystal disorder. Eur. J. Pharm. Sci. 2006, 27, 19–26. [Google Scholar] [CrossRef]
- Yeh, A.-I.; Huang, Y.-C.; Chen, S.H. Effect of particle size on the rate of enzymatic hydrolysis of cellulose. Carbohydr. Polym. 2010, 79, 192–199. [Google Scholar] [CrossRef]
- Ghasemi, M.; Alexandridis, P.; Tsianou, M. Conversion of particle size distribution data from mass to number-based and its application to biomass processing. Biosyst. Eng. 2018, 176, 73–87. [Google Scholar] [CrossRef]
- Deveswaran, R.; Bharath, S.; Basavaraj, B.; Abraham, S.; Furtado, S.; Madhavan, V. Concepts and techniques of pharmaceutical powder mixing process: A current update. Res. J. Pharm. Technol. 2009, 2, 245–249. [Google Scholar]
- Li, W.; Bagnol, L.; Berman, M.; Chiarella, R.A.; Gerber, M. Applications of NIR in early stage formulation development. Part II. Content uniformity evaluation of low dose tablets by principal component analysis. Int. J. Pharm. 2009, 380, 49–54. [Google Scholar] [CrossRef]
- Lemieux, M.; Bertrand, F.; Chaouki, J.; Gosselin, P. Comparative study of the mixing of free-flowing particles in a V-blender and a bin-blender. Chem. Eng. Sci. 2007, 62, 1783–1802. [Google Scholar] [CrossRef]
- Gyenis, J. Assessment of mixing mechanism on the basis of concentration pattern. Chem. Eng. Process. 1999, 38, 665–674. [Google Scholar] [CrossRef]
- Sudah, O.S.; Coffin-Beach, D.; Muzzio, F.J. Quantitative characterization of mixing of free-flowing granular material in tote (bin)-blenders. Powder Technol. 2002, 126, 191–200. [Google Scholar] [CrossRef]
- Jia, X.; Williams, R.A. A packing algorithm for particles of arbitrary shapes. Powder Technol. 2001, 120, 175–186. [Google Scholar] [CrossRef]
- Portillo, P.M.; Ierapetritou, M.G.; Muzzio, F.J. Characterization of continuous convective powder mixing processes. Powder Technol. 2008, 182, 368–378. [Google Scholar] [CrossRef]
- Escudie, R.; Epstein, N.; Grace, J.; Bi, H. Effect of particle shape on liquid-fluidized beds of binary (and ternary) solids mixtures: Segregation vs. mixing. Chem. Eng. Sci. 2006, 61, 1528–1539. [Google Scholar] [CrossRef]
- Mendez, R.; Muzzio, F.; Velazquez, C. Study of the effects of feed frames on powder blend properties during the filling of tablet press dies. Powder Technol. 2010, 200, 105–116. [Google Scholar] [CrossRef]
- Savage, S.; Lun, C. Particle size segregation in inclined chute flow of dry cohesionless granular solids. J. Fluid Mech. 1988, 189, 311–335. [Google Scholar] [CrossRef]
- Rosato, A.D.; Blackmore, D.L.; Zhang, N.; Lan, Y. A perspective on vibration-induced size segregation of granular materials. Chem. Eng. Sci. 2002, 57, 265–275. [Google Scholar] [CrossRef]
- Sinka, I.; Motazedian, F.; Cocks, A.; Pitt, K. The effect of processing parameters on pharmaceutical tablet properties. Powder Technol. 2009, 189, 276–284. [Google Scholar] [CrossRef]
- Peck, G.E.; Baley, G.J.; McCurdy, V.E.; Banker, G.S. Tablet formulation and design. Pharm. Dos. Forms Tablets. Marcel Decker N. Y. 1989, 1, 75–130. [Google Scholar]
- Wilczyński, S.; Koprowski, R.; Duda, P.; Banyś, A.; Błońska-Fajfrowska, B. Microtomographic studies of subdivision of modified-release tablets. Int. J. Pharm. 2016, 511, 899–912. [Google Scholar] [CrossRef]
- Gómez, D.A.; Coello, J.; Maspoch, S. Raman spectroscopy for the analytical quality control of low-dose break-scored tablets. J. Pharm. Biomed. Anal. 2016, 124, 207–215. [Google Scholar] [CrossRef]



| Administration Route | Examples of Formulations |
|---|---|
| Intranasal | Solutions, Sprays, Ointments, Creams |
| Oral | Syrup, Elixir, Suspension, Capsules, Tablets, or Chewable Tablets |
| Ocular | Solutions and Suspensions |
| Otic | Solutions and Suspensions |
| Parenteral | Solutions and Suspensions |
| Rectal | Solutions, Ointments, Creams, Suppositories |
| Sublingual | Chewable Tablets and Lozenges |
| Transdermal | Ointments, Creams, Lotions, Transdermal Patches |
| Vaginal | Solutions, Ointments, Creams, Suppositories |
| Excipients | Function | Example |
|---|---|---|
| Diluent | Serve as bulking agent and facilitate accurate dosing. | Sugar compounds: lactose, mannitol, dextrose, sorbitol, silicate, calcium, magnesium salt, sodium chloride, potassium chloride, cellulose derivatives |
| Binder, compression aid, granulating agents | Facilitate tablet compression. Ensure tablet robustness. | Natural and synthetic polymers: starch, gelatin and sugars as sucrose, glucose, dextrose, and lactose |
| Disintegrants | Aid with tablet disintegration and dissolution by increasing the surface area of the tablets, facilitate release of drug substance. | Compounds which swell in the presence of water: Starch, cellulose derivatives, alginates and crospovidone |
| Glidants | Granulation flow enhancer, aid with tablet compression and eliminate particles agglomeration (anticaking) | Colloidal anhydrous silicon, silica compounds, talc |
| Lubricants | Tablet compression aid, reduce blend cohesiveness characteristic during compression, reduce disintegration rate | Steric acid, salts and derivatives of steric acid, talc, hydrogenated vegetable oils and PEG |
| Coating agent | Prevent tablet degradation environmental conditions (Temperature, light and moisture). Serve as taste masking agent, inhibit odor, facilitate administration and appearance enhancer | Natural and synthetic polymers, polymers that are insoluble in acid |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacques, E.R.; Alexandridis, P. Tablet Scoring: Current Practice, Fundamentals, and Knowledge Gaps. Appl. Sci. 2019, 9, 3066. https://doi.org/10.3390/app9153066
Jacques ER, Alexandridis P. Tablet Scoring: Current Practice, Fundamentals, and Knowledge Gaps. Applied Sciences. 2019; 9(15):3066. https://doi.org/10.3390/app9153066
Chicago/Turabian StyleJacques, Emmanuel Reginald, and Paschalis Alexandridis. 2019. "Tablet Scoring: Current Practice, Fundamentals, and Knowledge Gaps" Applied Sciences 9, no. 15: 3066. https://doi.org/10.3390/app9153066
APA StyleJacques, E. R., & Alexandridis, P. (2019). Tablet Scoring: Current Practice, Fundamentals, and Knowledge Gaps. Applied Sciences, 9(15), 3066. https://doi.org/10.3390/app9153066





