Gold Nanoparticles and Nanorods in Nuclear Medicine: A Mini Review
Abstract
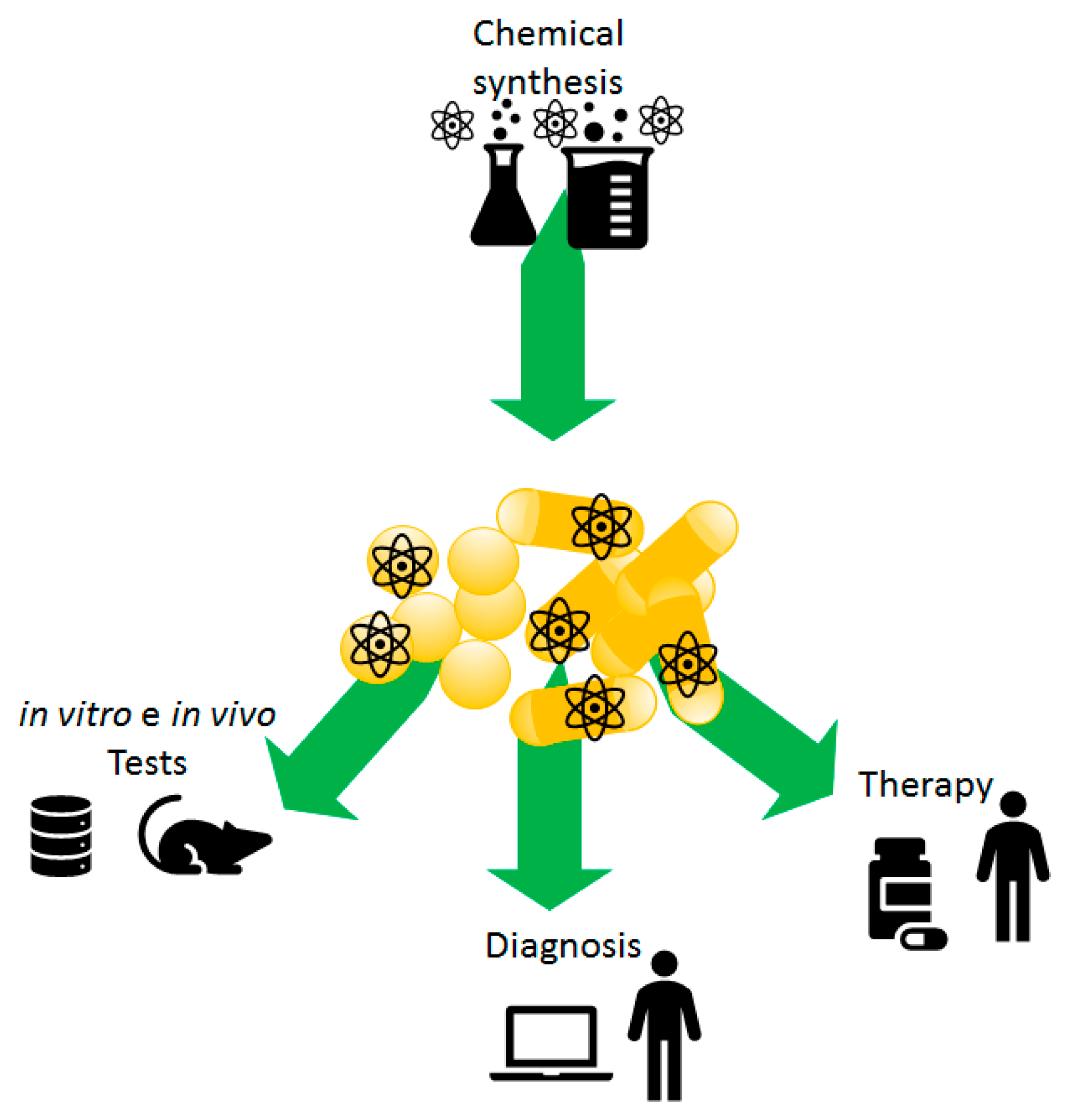
1. Introduction
2. Gold Nanoparticle and Nanorods: Preparations and Features
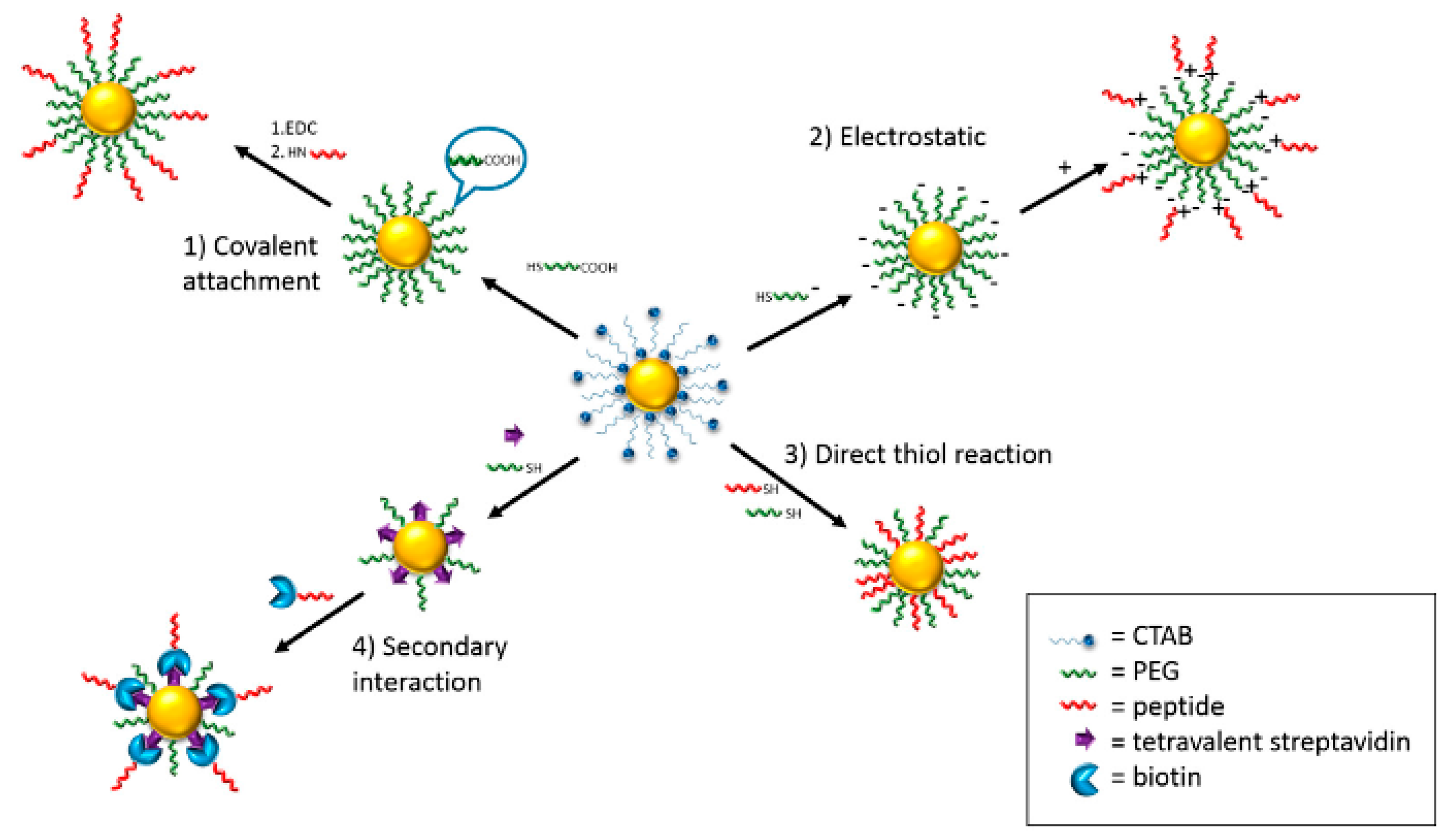
2.1. AuNPs
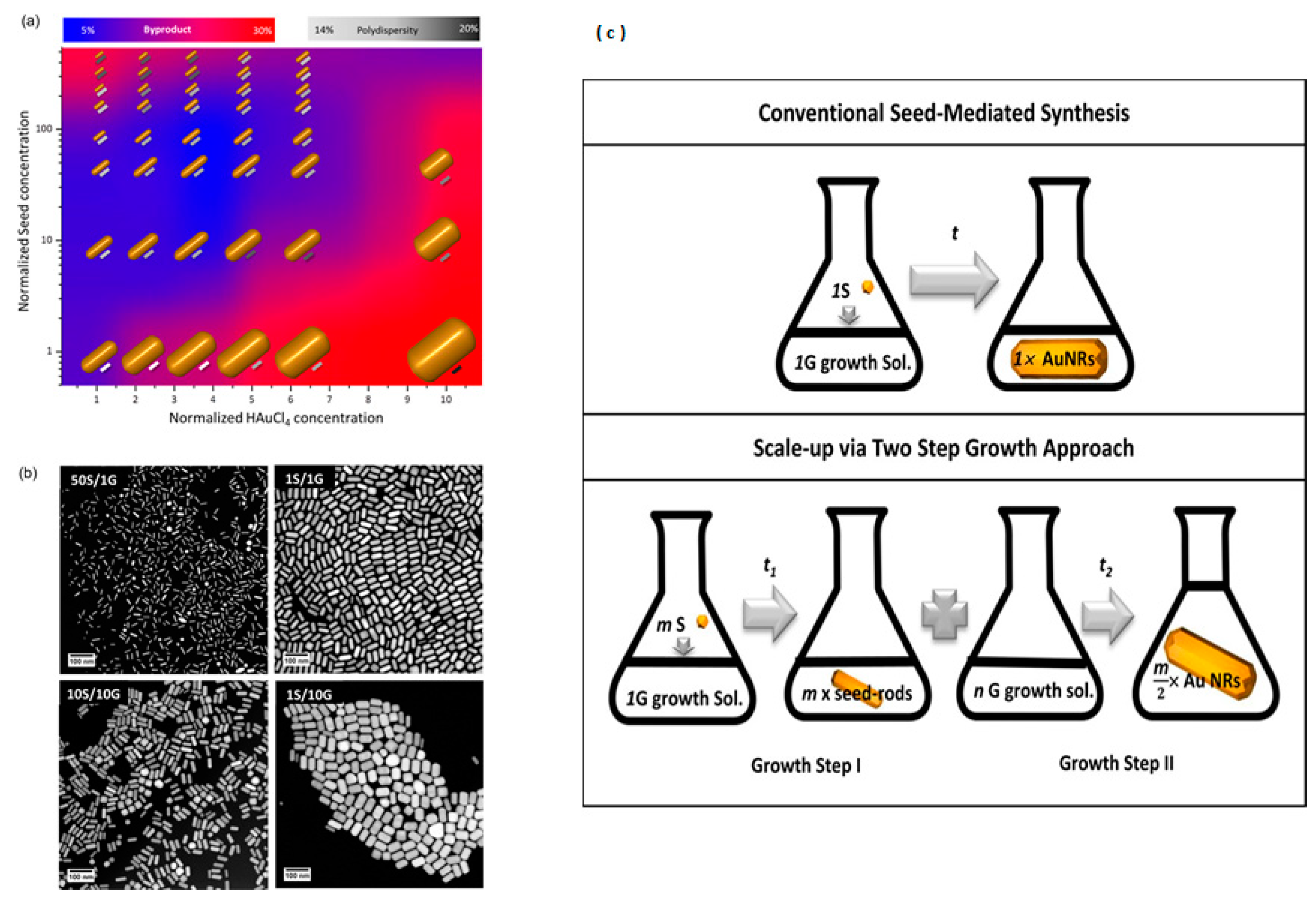
2.2. AuNRs
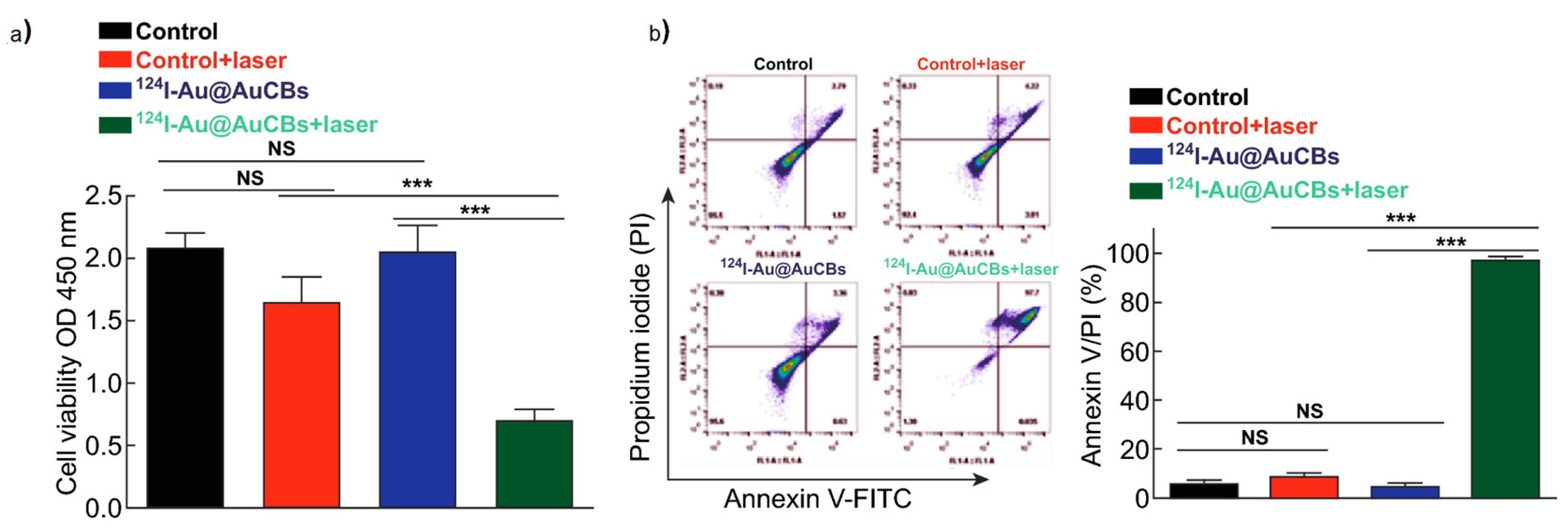
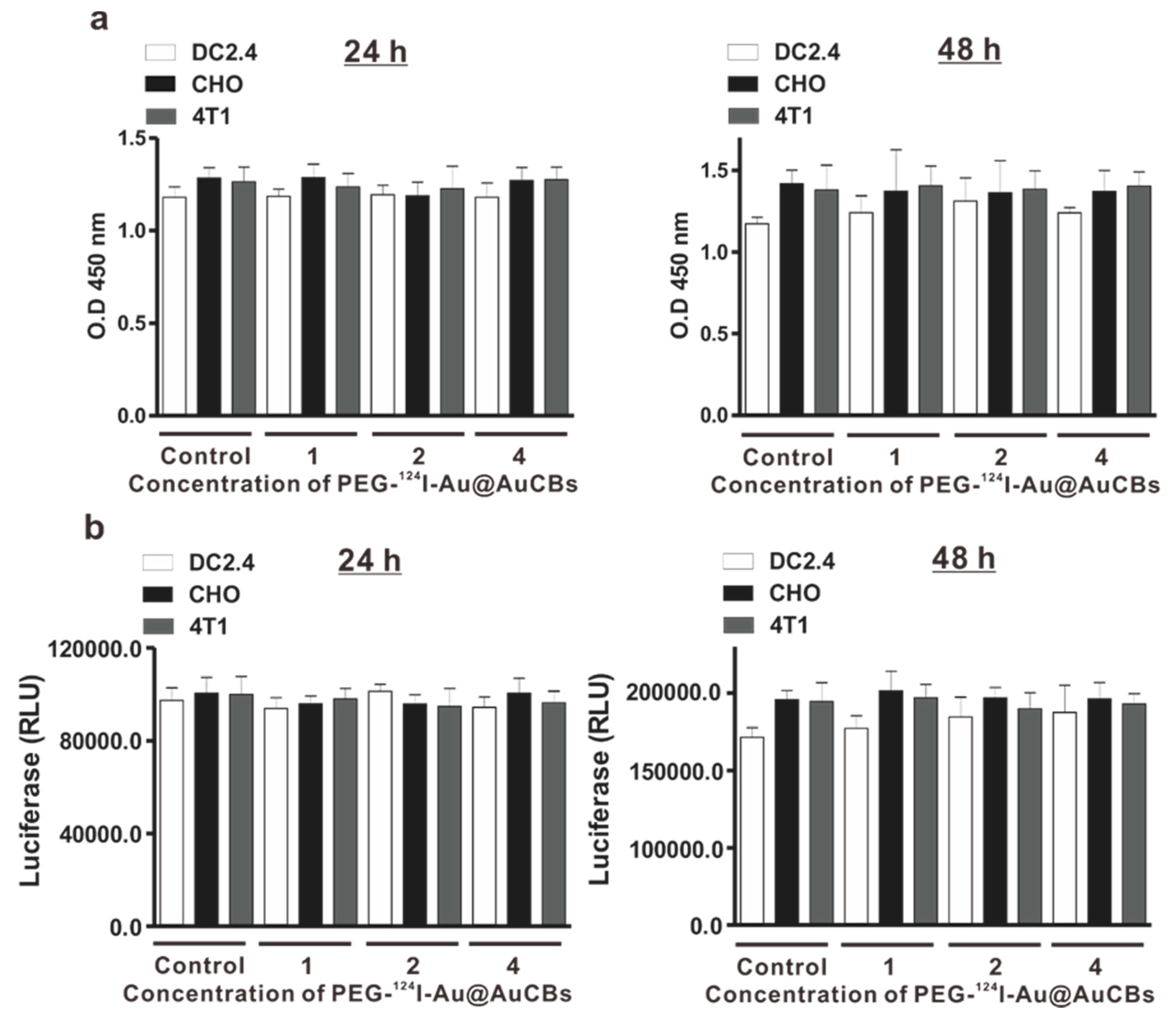
3. In Vitro Evaluation of Radionuclides Delivered by AuNPs and AuNRs
4. Gold Nanoparticle and Nanorods: Clinical Aspects
4.1. Radionuclide 198Au
4.2. Radionuclide 125I
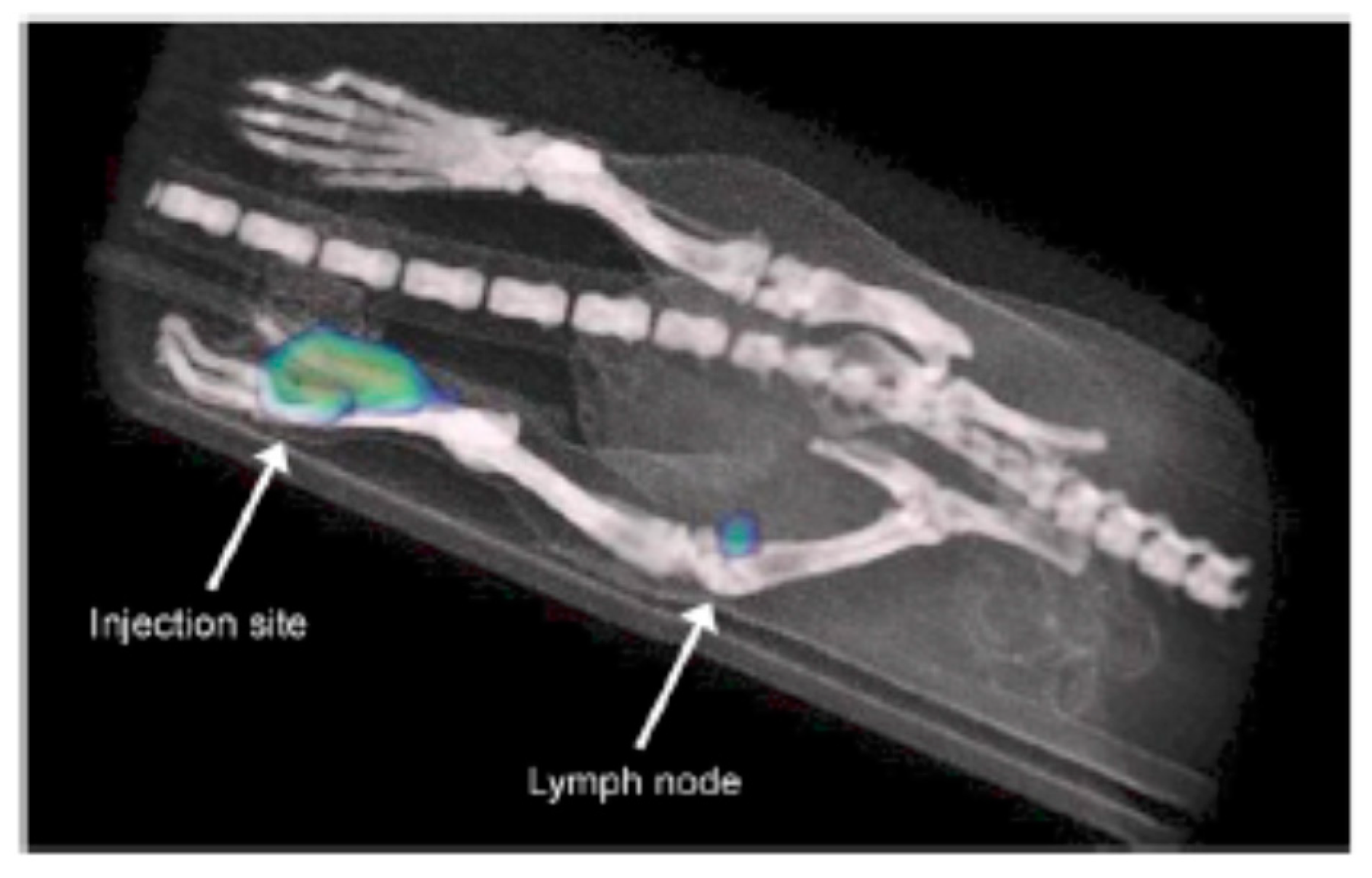
4.3. Radionuclide 99mTc
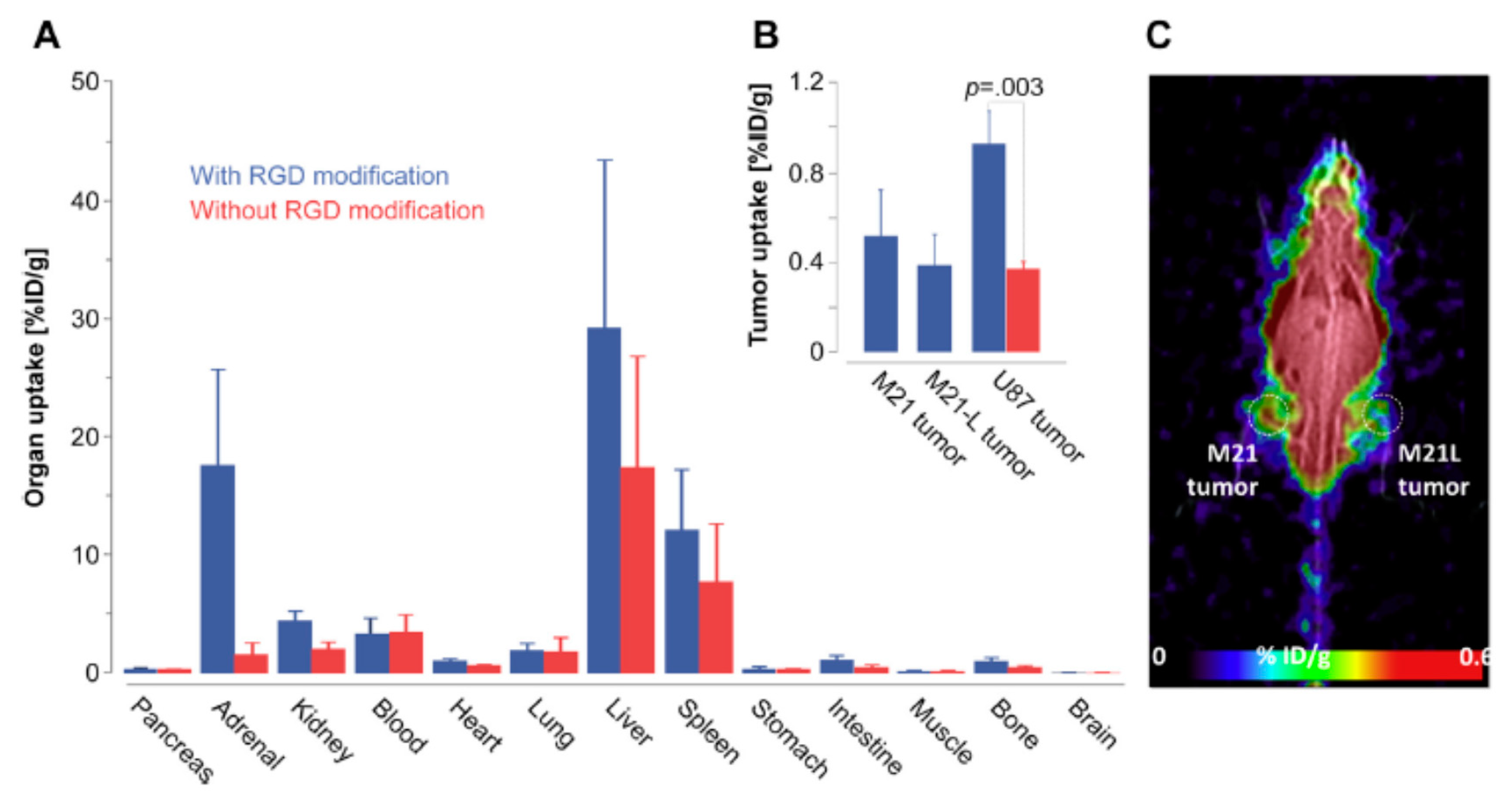
4.4. Integrin αvβ3-Based Targeting
4.4.1. Radionuclide 64Cu
4.4.2. Radionuclide 111In
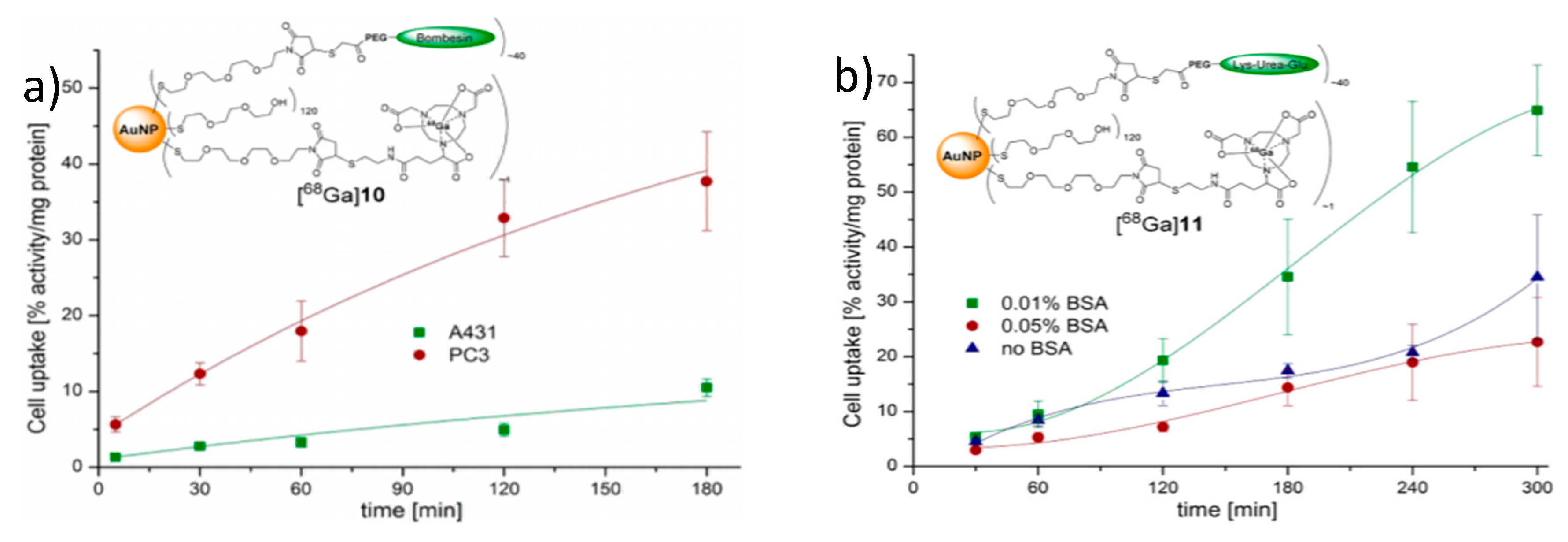
4.4.3. Radionuclide 68Ga
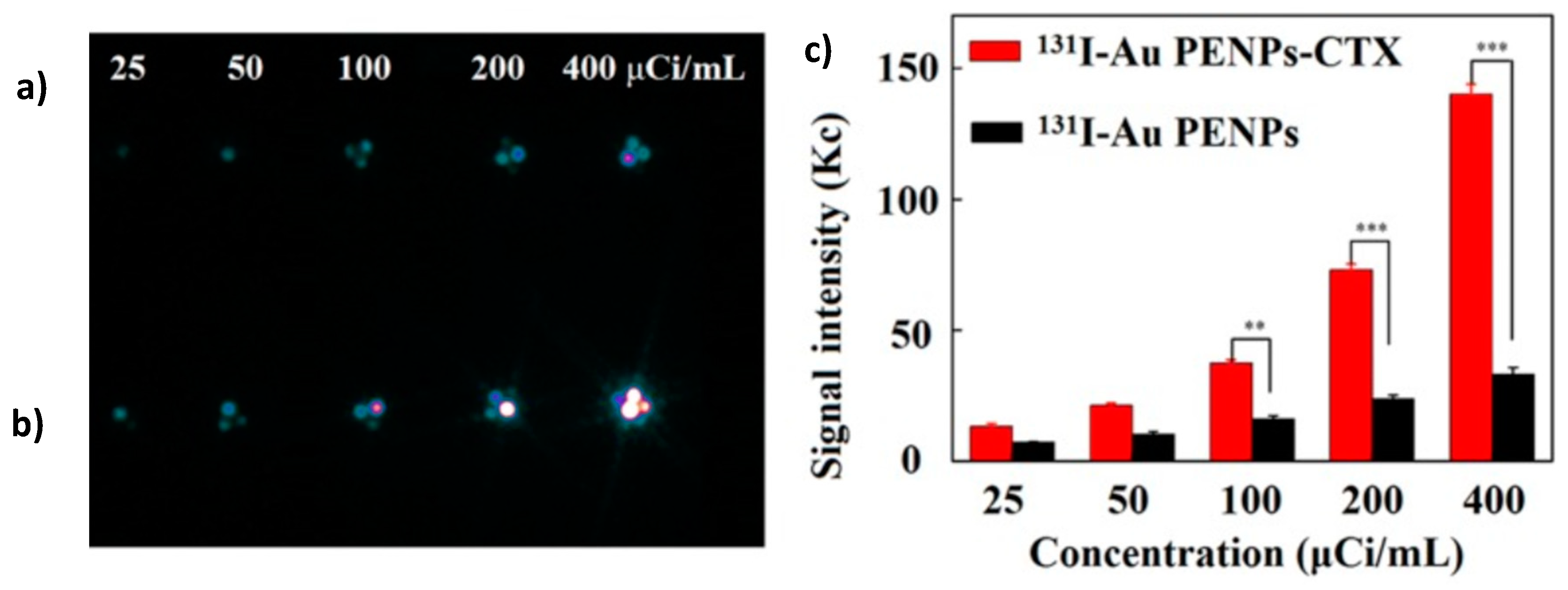
4.4.4. Radionuclide 131I
4.5. Monoclonal Antibody-Labeled AuNPs
4.5.1. Radionuclide 89Zr
4.5.2. Radionuclides 131I and 123I
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Q.X.; Liu, Y.H.; Qi, X.Z.; Liu, J.W.; Jiang, H.J.; Wang, J.L.; He, Z.; Ren, X.F.; Hou, Z.H.; Yu, S.H. Ordered Nanostructure Enhances Electrocatalytic Performance by Directional Micro-Electric Field. J. Am. Chem. Soc. 2019, 141, 10729–10735. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.K.; Erten-Ela, S.; Hassoon, K.I.; Ela, C. Plasmonic enhancement as selective scattering of gold nanoparticles based dye sensitized solar cells. Thin Solid Films 2019, 671, 127–132. [Google Scholar] [CrossRef]
- Naponiello, G.; Venditti, I.; Zardetto, V.; Saccone, D.; Di Carlo, A.; Fratoddi, I.; Barolo, C.; Dini, D. Photoelectrochemical characterization of squaraine-sensitized nickel oxide cathodes deposited via screen-printing for p-type dye-sensitized solar cells. Appl. Surf. Sci. 2015, 356, 911–920. [Google Scholar] [CrossRef]
- Song, N.J.; Ma, C. The In-Situ Synthesis of a 3D SnS/N-Doped Graphene Composite with Enhanced Electrochemical Performance as a Low-Cost Anode Material in Sodium Ion Batteries. Materials 2019, 12, 2030. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, M.; Naponiello, G.; Venditti, I.; Zardetto, V.; Di Carlo, A.; Dini, D. Electrochemical and Photoelectrochemical Properties of Screen-Printed Nickel Oxide Thin Films Obtained from Precursor Pastes with Different Compositions. J. Electrochem. Soc. 2017, 164, H137–H147. [Google Scholar] [CrossRef]
- Yao, J.; Zheng, Y.; Jia, X.; Duan, L.; Wu, Q.; Huang, C.; An, W.; Xu, Q.; Yao, W. Highly Active Pt3Sn {110} Excavated Nanocube Cocatalysts for Photocatalytic Hydrogen Production. ACS Appl. Mater. Interfaces 2019, 11, 25844–25853. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhang, J.; Deng, W.; Xie, S.; Zhang, Q.; Wang, Y. Catalytic transformation of 2,5-furandicarboxylic acid to adipic acid over niobic acid-supported Pt nanoparticles. Chem. Commun. 2019, 55, 8013–8016. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, J.; Liu, S.; Hu, C.; Li, S.; Qiu, J. Polyethyleneimine-Mediated Fabrication of Two-Dimensional Cobalt Sulfide/Graphene Hybrid Nanosheets for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 26235–26242. [Google Scholar] [CrossRef]
- D’Amato, R.; Venditti, I.; Russo, M.V.; Falconieri, M. Growth control and long-range self-assembly of poly(methyl methacrylate) nanospheres. J. Appl. Polym. Sci. 2006, 102, 4493–4499. [Google Scholar] [CrossRef]
- Halamish, H.M.; Trousil, J.; Rak, D.; Knudsen, K.D.; Pavlova, E.; Nyström, B.; Štěpánek, P.; Sosnik, A. Self-assembly and nanostructure of poly(vinyl alcohol)-graft-poly(methyl methacrylate) amphiphilic nanoparticles. J. Coll. Interface Sci. 2019, 553, 512–523. [Google Scholar] [CrossRef]
- Venditti, I. Gold Nanoparticles in Photonic Crystals Applications: A Review. Materials 2017, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Peng, W.; Yu, L.; Luo, X.; Gao, H.; Chu, S.; Masson, J.F. Hybrid Nanodisk Film for Ultra-Narrowband Filtering, Near-Perfect Absorption and Wide Range Sensing. Nanomaterials 2019, 9, 334. [Google Scholar] [CrossRef] [PubMed]
- Venditti, I.; D’Amato, R.; Russo, M.V.; Falconieri, M. Synthesis of conjugated polymeric nanobeads for photonic bandgap materials. Sens. Actuators B Chem. 2007, 126, 35–40. [Google Scholar] [CrossRef]
- Bardhan, R.; Grady, N.K.; Cole, J.R.; Joshi, A.; Halas, N.J. Fluorescence enhancement by Au nanostructures: Nanoshells and nanorods. ACS Nano 2009, 3, 744–752. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.; Medei, L.; Venditti, I.; Russo, M.V.; Falconieri, M. Chemical synthesis of polyphenylacetylene nanospheres with controlled dimensions for photonic crystals. Mater. Sci. Eng. C Biomim. Supramol. Syst. 2003, 23, 861–865. [Google Scholar] [CrossRef]
- Ayala-Orozco, C.; Liu, J.G.; Knight, M.W.; Wang, Y.; Day, J.K.; Nordlander, P.; Halas, N.J. Fluorescence enhancement of molecules inside a gold nanomatryoshka. Nano Lett. 2014, 14, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, R.; Venditti, I.; Fratoddi, I.; De Matteis, F.; Prosposito, P.; Cacciotti, I.; D’Amico, L.; Nanni, F.; Yadav, A.; Casalboni, M.; et al. From nanospheres to microribbons: Self-assembled Eosin Y doped PMMA nanoparticles as photonic crystals. J. Coll. Interface Sci. 2014, 414, 24–32. [Google Scholar] [CrossRef]
- Ganeev, R.A.; Boltaev, G.S.; Kim, V.V.; Guo, C. Effects of Laser Plasma Formation on Quasi-Phase Matching of High-Order Harmonics from Nanoparticles and Atoms. Nanomaterials 2019, 9, 572. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.; Ren, Y.; Wang, Z.; Yao, B.; Dong, Z.; Adamo, G.; Zeng, H.; Sun, H. Perovskite-Ion Beam Interactions: Toward Controllable Light Emission and Lasing. ACS Appl. Mater. Interfaces 2019, 11, 15756–15763. [Google Scholar] [CrossRef]
- Tang, T.Y.; Zhou, Y.; Arya, G. Interfacial Assembly of Tunable Anisotropic Nanoparticle Architectures. ACS Nano 2019, 13, 4111–4123. [Google Scholar] [CrossRef]
- Pantalei, S.; Zampetti, E.; Macagnano, A.; Bearzotti, A.; Venditti, I.; Russo, M.V. Enhanced sensory properties of a multichannel quartz crystal microbalance coated with polymeric nanobeads. Sensors 2007, 7, 2920–2928. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Islam, A.U.; Tariq, M.A.; Siddique, M.H.; Shah, N.S.; Khan, Z.U.H.; Amjad, M.; Din, S.U.; Shah, G.M.; Naeem, M.A.; et al. Synthesis of magnetite-based nanocomposites for effective removal of brilliant green dye from wastewater. Environ. Sci. Pollut. Res. Int. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bearzotti, A.; Macagnano, A.; Pantalei, S.; Zampetti, E.; Venditti, I.; Fratoddi, I.; Russo, M.V. Alcohol vapor sensory properties of nanostructured conjugated polymers. J. Phys. Condes. Matter 2008, 20, 6. [Google Scholar] [CrossRef]
- Santana Vega, M.; Guerrero Martínez, A.; Cucinotta, F. Facile Strategy for the Synthesis of Gold@Silica Hybrid Nanoparticles with Controlled Porosity and Janus Morphology. Nanomaterials 2019, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.F.; Lau, P.M.; Kong, S.K.; Ho, H.P. An Assay Using Localized Surface Plasmon Resonance and Gold Nanorods Functionalized with Aptamers to Sense the Cytochrome-c Released from Apoptotic Cancer Cells for Anti-Cancer Drug Effect Determination. Micromachines 2017, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Gormley, A.J.; Greish, K.; Ray, A.; Robinson, R.; Gustafson, J.A.; Ghandehari, H. Gold nanorod mediated plasmonic photothermal therapy: A tool to enhance macromolecular delivery. Int. J. Pharm. 2011, 415, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.U.; Novosad, V.; Rozhkova, E.A.; Wali, H.; Ali, A.; Fateh, A.A.; Neogi, P.B.; Neogi, A.; Wang, Z. Gold Nanoparticles-enabled Efficient Dual Delivery of Anticancer Therapeutics to HeLa Cells. Sci. Rep. 2018, 8, 2907. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, F.; Carlini, L.; Ugolini, A.; Visaggio, D.; Visca, P.; Fratoddi, I.; Venditti, I.; Meneghini, C.; Simonelli, L.; Marini, C.; et al. Synthesis and Structural Characterization of Silver Nanoparticles Stabilized with 3-Mercapto-1-Propansulfonate and 1-Thioglucose Mixed Thiols for Antibacterial Applications. Materials 2016, 9, 1028. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ishtiaq, S.; Rabia, S.; Khatoon, M.; Zeb, A.; Khan, G.M.; Ur Rehman, A.; Ud Din, F. Nanotechnology: From In Vivo Imaging System to Controlled Drug Delivery. Nanoscale Res. Lett. 2017, 12, 500. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, S.W.; Jeong, S.Y.; Yoon, G.; Cho, S.J.; Kim, S.K.; Lee, I.K.; Ahn, B.C.; Lee, J.; Jeon, Y.H. Engineering of Radioiodine-Labeled Gold Core-Shell Nanoparticles as Efficient Nuclear Medicine Imaging Agents for Trafficking of Dendritic Cells. ACS Appl. Mater. Interfaces 2017, 9, 8480–8489. [Google Scholar] [CrossRef]
- Same, S.; Aghanejad, A.; Akbari Nakhjavani, S.; Barar, J.; Omidi, Y. Radiolabeled theranostics: Magnetic and gold nanoparticles. Bioimpacts 2016, 6, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Moeendarbari, S.; Tekade, R.; Mulgaonkar, A.; Christensen, P.; Ramezani, S.; Hassan, G.; Jiang, R.; Öz, O.K.; Hao, Y.; Sun, X. Theranostic Nanoseeds for Efficacious Internal Radiation Therapy of Unresectable Solid Tumors. Sci. Rep. 2016, 6, 20614. [Google Scholar] [CrossRef] [PubMed]
- Nitipir, C.; Niculae, D.; Orlov, C.; Barbu, M.A.; Popescu, B.; Popa, A.M.; Pantea, A.M.S.; Stanciu, A.E.; Galateanu, B.; Ginghina, O.; et al. Update on radionuclide therapy in oncology. Oncol. Lett. 2017, 14, 7011–7015. [Google Scholar] [PubMed]
- Mancini-Terracciano, C.; Donnarumma, R.; Bencivenga, G.; Bocci, V.; Cartoni, A.; Collamati, F.; Fratoddi, I.; Giordano, A.; Indovina, L.; Maccora, D.; et al. Feasibility of beta-particle radioguided surgery for a variety of “nuclear medicine” radionuclides. Phys. Med. 2017, 43, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Martelli, C.; Marzano, V.; Marini, F.; Scotognella, T.; Fratoddi, I.; Venditti, I.; Rotili, D.; Solfaroli-Camillocci, E.; Collamati, F.; Mancini-Terracciano, C.; et al. Mass spectrometry characterization of DOTA-Nimotuzumab conjugate as precursor of an innovative beta(-) tracer suitable in radio-guided surgery. J. Pharm. Biomed. Anal. 2018, 156, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Collamati, F.; Bocci, V.; Castellucci, P.; De Simoni, M.; Fanti, S.; Faccini, R.; Giordano, A.; Maccora, D.; Mancini-Terracciano, C.; Marafini, M.; et al. Radioguided surgery with β radiation: A novel application with Ga. Sci. Rep. 2018, 8, 16171. [Google Scholar] [CrossRef]
- Hwang, S.; Nam, J.; Jung, S.; Song, J.; Doh, H.; Kim, S. Gold nanoparticle-mediated photothermal therapy: Current status and future perspective. Nanomedicine 2014, 9, 2003–2022. [Google Scholar] [CrossRef]
- Book Newell, B.; Wang, Y.; Irudayaraj, J. Multifunctional gold nanorod theragnostics probed by multi-photon imaging. Eur. J. Med. Chem. 2012, 48, 330–337. [Google Scholar] [CrossRef]
- Shim, M.S.; Kim, C.S.; Ahn, Y.C.; Chen, Z.; Kwon, Y.J. Combined multimodal optical imaging and targeted gene silencing using stimuli-transforming nanotheragnostics. J. Am. Chem. Soc. 2010, 132, 8316–8324. [Google Scholar] [CrossRef][Green Version]
- Venditti, I.; Cartoni, A.; Fontana, L.; Testa, G.; Scaramuzzo, F.A.; Faccini, R.; Terracciano, C.M.; Camillocci, E.S.; Morganti, S.; Giordano, A.; et al. Y3+ embedded in polymeric nanoparticles: Morphology, dimension and stability of composite colloidal system. Coll. Surf. A Physicochem. Eng. Asp. 2017, 532, 125–131. [Google Scholar] [CrossRef]
- Sun, X.; Huang, X.; Yan, X.; Wang, Y.; Guo, J.; Jacobson, O.; Liu, D.; Szajek, L.P.; Zhu, W.; Niu, G.; et al. Chelator-free (64)Cu-integrated gold nanomaterials for positron emission tomography imaging guided photothermal cancer therapy. ACS Nano 2014, 8, 8438–8446. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. PET Radiopharmaceuticals for Personalized Medicine. Curr. Drug Targets 2016, 17, 1894–1907. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.; Knapp, F.F.; Pillai, M.R. Targeted radionuclide therapy—An overview. Curr. Radiopharm. 2013, 6, 152–180. [Google Scholar] [CrossRef] [PubMed]
- Yook, S.; Cai, Z.; Lu, Y.; Winnik, M.A.; Pignol, J.P.; Reilly, R.M. Intratumorally Injected 177Lu-Labeled Gold Nanoparticles: Gold Nanoseed Brachytherapy with Application for Neoadjuvant Treatment of Locally Advanced Breast Cancer. J. Nucl. Med. 2016, 57, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Vaez-zadeh, M.; Masoudi, S.F.; Rahmani, F.; Knaup, C.; Meigooni, A.S. Gold nanoparticle-based brachytherapy enhancement in choroidal melanoma using a full Monte Carlo model of the human eye. J. Appl. Clin. Med. Phys. 2015, 16, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Minc, L.D.; Nigavekar, S.S.; Kariapper, M.S.; Nair, B.M.; Schipper, M.; Cook, A.C.; Lesniak, W.G.; Balogh, L.P. Fabrication of {198Au0} radioactive composite nanodevices and their use for nanobrachytherapy. Nanomedicine 2008, 4, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Gambhir, S.S. Molecular imaging with theranostic nanoparticles. Acc. Chem. Res. 2011, 44, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Venditti, I. Morphologies and functionalities of polymeric nanocarriers as chemical tools for drug delivery: A review. JKSUS 2019, 31, 398–411. [Google Scholar] [CrossRef]
- Venditti, I. Engineered Gold-Based Nanomaterials: Morphologies and Functionalities in Biomedical Applications. A Mini Review. Bioengineering 2019, 6, 53. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.P.; Wu, T.H.; Yang, C.H.; Lin, C.W.; Chen, C.Y. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef]
- Zong, J.; Cobb, S.L.; Cameron, N.R. Peptide-functionalized gold nanoparticles: Versatile biomaterials for diagnostic and therapeutic applications. Biomater. Sci. 2017, 5, 872–886. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Song, K.; Cha, S.H.; Cho, S.; Kim, Y.S.; Park, Y. Sesquiterpenoids from Tussilago farfara Flower Bud Extract for the Eco-Friendly Synthesis of Silver and Gold Nanoparticles Possessing Antibacterial and Anticancer Activities. Nanomaterials 2019, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Leskau, M.; Schlingmann-Molina, B.L.; Hohmeier, S.C.; Alnajjar, S.; Murua Escobar, H.; Ngezahayo, A. Functionalization of gold-nanoparticles by the Clostridium perfringens enterotoxin C-terminus for tumor cell ablation using the gold nanoparticle-mediated laser perforation technique. Sci. Rep. 2018, 8, 14963. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jeong, Y.Y.; Jon, S. A Drug-Loaded Aptamer-Gold Nanoparticle Bioconjugate for Combined CT Imaging and Therapy of Prostate Cancer. ACS Nano 2010, 4, 3689–3696. [Google Scholar] [CrossRef]
- Fratoddi, I.; Benassi, L.; Botti, E.; Vaschieri, C.; Venditti, I.; Bessar, H.; Samir, M.A.; Azzoni, P.; Magnoni, C.; Costanzo, A.; et al. Effects of topical methotrexate loaded gold nanoparticle in cutaneous inflammatory mouse model. Nanomedicine 2019, 17, 276–286. [Google Scholar] [CrossRef]
- Bae, S.H.; Yu, J.; Go, M.R.; Kim, H.J.; Hwang, Y.G.; Choi, S.J. Oral Toxicity and Intestinal Transport Mechanism of Colloidal Gold Nanoparticle-Treated Red Ginseng. Nanomaterials 2016, 6, 208. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Battocchio, C.; Carlini, L.; Amatori, S.; Porchia, M.; Tisato, F.; Bondino, F.; Magnano, E.; Pellei, M.; et al. Highly Hydrophilic Gold Nanoparticles as Carrier for Anticancer Copper(I) Complexes: Loading and Release Studies for Biomedical Applications. Nanomaterials 2019, 9, 772. [Google Scholar] [CrossRef]
- Zhou, B.; Song, J.; Wang, M.; Wang, X.; Wang, J.; Howard, E.W.; Zhou, F.; Qu, J.; Chen, W.R. BSA-bioinspired gold nanorods loaded with immunoadjuvant for the treatment of melanoma by combined photothermal therapy and immunotherapy. Nanoscale 2018, 10, 21640–21647. [Google Scholar] [CrossRef]
- Fratoddi, I.; Cartoni, A.; Venditti, I.; Catone, D.; O’Keeffe, P.; Paladini, A.; Toschi, F.; Turchini, S.; Sciubba, F.; Testa, G.; et al. Gold nanoparticles functionalized by rhodamine B isothiocyanate: A new tool to control plasmonic effects. J. Coll. Interface Sci. 2018, 513, 10–19. [Google Scholar] [CrossRef]
- Zhang, W.; Caldarola, M.; Lu, X.; Pradhan, B.; Orrit, M. Single-molecule fluorescence enhancement of a near-infrared dye by gold nanorods using DNA transient binding. Phys. Chem. Chem. Phys. 2018, 20, 20468–20475. [Google Scholar] [CrossRef]
- Venditti, I.; Testa, G.; Sciubba, F.; Carlini, L.; Porcaro, F.; Meneghini, C.; Mobilio, S.; Battocchio, C.; Fratoddi, I. Hydrophilic Metal Nanoparticles Functionalized by 2-Diethylaminoethanethiol: A Close Look at the Metal-Ligand Interaction and Interface Chemical Structure. J. Phys. Chem. C 2017, 121, 8002–8013. [Google Scholar] [CrossRef]
- Rodzik-Czalka, L.; Lewandowska-Lancucka, J.; Gatta, V.; Venditti, I.; Fratoddi, I.; Szuwarzynski, M.; Romek, M.; Nowakowska, M. Nucleobases functionalized quantum dots and gold nanoparticles bioconjugates as a fluorescence resonance energy transfer (FRET) system—Synthesis, characterization and potential applications. J. Coll. Interface Sci. 2018, 514, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Choi, C.K.K.; Wang, Q. Origin of the Plasmonic Chirality of Gold Nanorod Trimers Templated by DNA Origami. ACS Appl. Mater. Interfaces 2018, 10, 26835–26840. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Donati, S.; Fontana, L.; Porcaro, F.; Battocchio, C.; Proietti, E.; Venditti, I.; Bracci, L.; Fratoddi, I. Negatively charged gold nanoparticles as a dexamethasone carrier: Stability in biological media and bioactivity assessment in vitro. RSC Adv. 2016, 6, 99016–99022. [Google Scholar] [CrossRef]
- Venditti, I.; Hassanein, T.F.; Fratoddi, I.; Fontana, L.; Battocchio, C.; Rinaldi, F.; Carafa, M.; Marianecci, C.; Diociaiuti, M.; Agostinelli, E.; et al. Bioconjugation of gold-polymer core-shell nanoparticles with bovine serum amine oxidase for biomedical applications. Coll. Surf. B Biointerfaces 2015, 134, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. The puzzle of toxicity of gold nanoparticles. The case-study of HeLa cells. Toxicol. Res. 2015, 4, 796–800. [Google Scholar] [CrossRef]
- Singh, R.; Patil, S.; Singh, N.; Gupta, S. Dual functionality nanobioconjugates targeting intracellular bacteria in cancer cells with enhanced antimicrobial activity. Sci. Rep. 2017, 7, 5792. [Google Scholar] [CrossRef]
- Mendoza, G.; Regiel-Futyra, A.; Andreu, V.; Sebastián, V.; Kyzioł, A.; Stochel, G.; Arruebo, M. Bactericidal Effect of Gold-Chitosan Nanocomposites in Coculture Models of Pathogenic Bacteria and Human Macrophages. ACS Appl. Mater. Interfaces 2017, 9, 17693–17701. [Google Scholar] [CrossRef]
- Chowdhury, R.; Ilyas, H.; Ghosh, A.; Ali, H.; Ghorai, A.; Midya, A.; Jana, N.R.; Das, S.; Bhunia, A. Multivalent gold nanoparticle-peptide conjugates for targeting intracellular bacterial infections. Nanoscale 2017, 9, 14074–14093. [Google Scholar] [CrossRef]
- Hu, B.; Wang, N.; Han, L.; Chen, M.L.; Wang, J.H. Core-shell-shell nanorods for controlled release of silver that can serve as a nanoheater for photothermal treatment on bacteria. Acta Biomater. 2015, 11, 511–519. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wu, S.H.; Chen, C.H. A simple strategy for prompt visual sensing by gold nanoparticles: General applications of interparticle hydrogen bonds. Angew. Chem. Int. Ed. Engl. 2006, 45, 4948–4951. [Google Scholar] [CrossRef] [PubMed]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Wuithschick, M.; Birnbaum, A.; Witte, S.; Sztucki, M.; Vainio, U.; Pinna, N.; Rademann, K.; Emmerling, F.; Kraehnert, R.; Polte, J. Turkevich in New Robes: Key Questions Answered for the Most Common Gold Nanoparticle Synthesis. ACS Nano 2015, 9, 7052–7071. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.G.; Uehara, A.; Chang, S.Y.; La Fontaine, C.; Fujii, T.; Okamoto, Y.; Imai, T.; Schroeder, S.L.M.; Dryfe, R.A.W. The significance of bromide in the Brust-Schiffrin synthesis of thiol protected gold nanoparticles. Chem. Sci. 2017, 8, 7954–7962. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, B.; Aguerri, A.R.; Filipovic, N. Radiosensitization by gold nanoparticles. Clin. Transl. Oncol. 2013, 15, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Luo, T.; Li, P.; Zhou, C.; Cui, D.; Pang, B.; Ren, Q.; Fu, S. RGD-conjugated gold nanorods induce radiosensitization in melanoma cancer cells by downregulating α(v)β₃ expression. Int. J. Nanomed. 2012, 7, 915–924. [Google Scholar]
- Catone, D.; Ciavardini, A.; Di Mario, L.; Paladini, A.; Toschi, F.; Cartoni, A.; Fratoddi, I.; Venditti, I.; Alabastri, A.; Zaccaria, R.P.; et al. Plasmon Controlled Shaping of Metal Nanoparticle Aggregates by Femtosecond Laser-Induced Melting. J. Phys. Chem. Lett. 2018, 9, 5002–5008. [Google Scholar] [CrossRef]
- Chen, S.; Yang, K.; Tuguntaev, R.G.; Mozhi, A.; Zhang, J.; Wang, P.C.; Liang, X.J. Targeting tumor microenvironment with PEG-based amphiphilic nanoparticles to overcome chemoresistance. Nanomedicine 2016, 12, 269–286. [Google Scholar] [CrossRef]
- Zhao, L.; Wen, S.; Zhu, M.; Li, D.; Xing, Y.; Shen, M.; Shi, X.; Zhao, J. Tc-labelled multifunctional polyethylenimine-entrapped gold nanoparticles for dual mode SPECT and CT imaging. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 488–498. [Google Scholar] [CrossRef]
- Black, K.C.; Wang, Y.; Luehmann, H.P.; Cai, X.; Xing, W.; Pang, B.; Zhao, Y.; Cutler, C.S.; Wang, L.V.; Liu, Y.; et al. Radioactive 198Au-doped nanostructures with different shapes for in vivo analyses of their biodistribution, tumor uptake, and intratumoral distribution. ACS Nano 2014, 8, 4385–4394. [Google Scholar] [CrossRef] [PubMed]
- Dziawer, Ł.; Majkowska-Pilip, A.; Gaweł, D.; Godlewska, M.; Pruszyński, M.; Jastrzębski, J.; Wąs, B.; Bilewicz, A. Trastuzumab-Modified Gold Nanoparticles Labeled with 211At as a prospective tool for local treatment of HER2-positive breast cancer. Nanomaterials 2019, 9, 632. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, C.; Tan, H.; Cheng, L.; Liu, G.; Yang, Y.; Zhao, Y.; Zhang, Y.; Li, Y.; Zhang, C.; et al. Gold nanoparticles-based SPECT/CT imaging probe targeting for vulnerable atherosclerosis plaques. Biomaterials 2016, 108, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pang, B.; Luehmann, H.; Detering, L.; Yang, X.; Sultan, D.; Harpstrite, S.; Sharma, V.; Cutler, C.S.; Xia, Y.; et al. Gold Nanoparticles Doped with (199) Au Atoms and Their Use for Targeted Cancer Imaging by SPECT. Adv. Healthc. Mater. 2016, 5, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, R.; Chen, F.; Zhao, L.; Wang, P.; Li, X.; Bányai, I.; Ouyang, Q.; Shi, X.; Shen, M. Tc-Labeled RGD-Polyethylenimine Conjugates with Entrapped Gold Nanoparticles in the Cavities for Dual-Mode SPECT/CT Imaging of Hepatic Carcinoma. ACS Appl. Mater. Interfaces 2018, 10, 6146–6154. [Google Scholar] [CrossRef] [PubMed]
- Yook, S.; Lu, Y.; Jeong, J.J.; Cai, Z.; Tong, L.; Alwarda, R.; Pignol, J.P.; Winnik, M.A.; Reilly, R.M. Stability and Biodistribution of Thiol-Functionalized and (177)Lu-Labeled Metal Chelating Polymers Bound to Gold Nanoparticles. Biomacromolecules 2016, 17, 1292–1302. [Google Scholar] [CrossRef]
- Ocampo-García, B.E.; Ramírez, F.E.M.; Ferro-Flores, G.; De León-Rodríguez, L.M.; Santos-Cuevas, C.L.; Morales-Avila, E.; de Murphy, C.A.; Pedraza-López, M.; Medina, L.A.; Camacho-López, M.A. (99m)Tc-labelled gold nanoparticles capped with HYNIC-peptide/mannose for sentinel lymph node detection. Nucl. Med. Biol. 2011, 38, 1–11. [Google Scholar] [CrossRef]
- Karmani, L.; Labar, D.; Valembois, V.; Bouchat, V.; Nagaswaran, P.G.; Bol, A.; Gillart, J.; Levêque, P.; Bouzin, C.; Bonifazi, D.; et al. Antibody-functionalized nanoparticles for imaging cancer: Influence of conjugation to gold nanoparticles on the biodistribution of 89Zr-labeled cetuximab in mice. Contrast Media Mol. Imaging 2013, 8, 402–408. [Google Scholar] [CrossRef]
- Zhu, J.; Chin, J.; Wängler, C.; Wängler, B.; Lennox, R.B.; Schirrmacher, R. Rapid (18)F-labeling and loading of PEGylated gold nanoparticles for in vivo applications. Bioconjug Chem. 2014, 25, 1143–1150. [Google Scholar] [CrossRef]
- Khoo, A.M.; Cho, S.H.; Reynoso, F.J.; Aliru, M.; Aziz, K.; Bodd, M.; Yang, X.; Ahmed, M.F.; Yasar, S.; Manohar, N.; et al. Radiosensitization of Prostate Cancers In Vitro and In Vivo to Erbium-filtered Orthovoltage X-rays Using Actively Targeted Gold Nanoparticles. Sci. Rep. 2017, 7, 18044. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, L.; Xia, X.; Hu, F.; Liu, Q.; Qin, C.; Lan, X. Synthesis and Bioevaluation of Iodine-131 Directly Labeled Cyclic RGD-PEGylated Gold Nanorods for Tumor-Targeted Imaging. Contrast Media Mol. Imaging 2017, 2017, 6081724. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Zhang, H.; Rajian, J.R.; Chamberland, D.L.; Sherman, P.S.; Quesada, C.A.; Koch, A.E.; Kotov, N.A.; Wang, X. 125I-labeled gold nanorods for targeted imaging of inflammation. ACS Nano 2011, 5, 8967–8973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, H.; Cai, J.; Cheng, D.; Ma, Y.; Zhang, J.; Zhou, C.; Liu, S.; Shi, H.; Zhang, Y.; et al. A Multifunctional Platform for Tumor Angiogenesis-Targeted Chemo-Thermal Therapy Using Polydopamine-Coated Gold Nanorods. ACS Nano 2016, 10, 10404–10417. [Google Scholar] [CrossRef] [PubMed]
- Buckway, B.; Frazier, N.; Gormley, A.J.; Ray, A.; Ghandehari, H. Gold nanorod-mediated hyperthermia enhances the efficacy of HPMA copolymer-90Y conjugates in treatment of prostate tumors. Nucl. Med. Biol. 2014, 41, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, N.; Yavari, K.; Outokesh, M.; Sadjadi, S.; Ahmadi, S.J. Iodine-131 radiolabeling of poly ethylene glycol-coated gold nanorods for in vivo imaging. J. Label. Comp. Radiopharm. 2013, 56, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Agarwal, A.; Rajian, J.R.; Kotov, N.A.; Wang, X. Synthesis and bioevaluation of 125I-labeled gold nanorods. Nanotechnology 2011, 22, 135102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agarwal, A.; Shao, X.; Rajian, J.R.; Zhang, H.; Chamberland, D.L.; Kotov, N.A.; Wang, X. Dual-mode imaging with radiolabeled gold nanorods. J. Biomed. Opt. 2011, 16, 051307. [Google Scholar] [CrossRef] [PubMed]
- Matassa, R.; Familiari, G.; Battaglione, E.; Sibilia, C.; Leahu, G.; Belardini, A.; Venditti, I.; Fontana, L.; Fratoddi, I. Electron microscopy reveals a soluble hybrid network of individual nanocrystals self-anchored by bifunctional thiol fluorescent bridges. Nanoscale 2016, 8, 18161–18169. [Google Scholar] [CrossRef] [PubMed]
- Kluenker, M.; Mondeshki, M.; Nawaz Tahir, M.; Tremel, W. Monitoring Thiol-Ligand Exchange on Au Nanoparticle Surfaces. Langmuir 2018, 34, 1700–1710. [Google Scholar] [CrossRef]
- Balasubramanian, S.K.; Yang, L.; Yung, L.Y.; Ong, C.N.; Ong, W.Y.; Yu, L.E. Characterization, purification, and stability of gold nanoparticles. Biomaterials 2010, 31, 9023–9030. [Google Scholar] [CrossRef]
- Goldstein, A.; Soroka, Y.; Frušić-Zlotkin, M.; Popov, I.; Kohen, R. High resolution SEM imaging of gold nanoparticles in cells and tissues. J. Microsc. 2014, 256, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Grobelny, J.; DelRio, F.W.; Pradeep, N.; Kim, D.I.; Hackley, V.A.; Cook, R.F. Size measurement of nanoparticles using atomic force microscopy. Methods Mol. Biol. 2011, 697, 71–82. [Google Scholar] [PubMed]
- Porcaro, F.; Battocchio, C.; Antoccia, A.; Fratoddi, I.; Venditti, I.; Fracassi, A.; Luisetto, I.; Russo, M.V.; Polzonetti, G. Synthesis of functionalized gold nanoparticles capped with 3-mercapto-1-propansulfonate and 1-thioglucose mixed thiols and “in vitro” bioresponse. Coll. Surf. B Biointerfaces 2016, 142, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; McDonagh, B.H.; Singh, G.; Raghunathan, K.; Sandvig, A.; Sandvig, I.; Andreassen, J.P.; Glomm, W.R. Growing gold nanostructures for shape-selective cellular uptake. Nanoscale Res. Lett. 2018, 13, 254. [Google Scholar] [CrossRef] [PubMed]
- Kuo, B.H.; Hsia, C.F.; Chen, T.N.; Huang, M.H. Systematic Shape Evolution of Gold Nanocrystals Achieved through Adjustment in the Amount of HAuCl4 Solution Used. J. Phys. Chem. C 2018, 122, 25118–25126. [Google Scholar] [CrossRef]
- Carlini, L.; Fasolato, C.; Postorino, P.; Fratoddi, I.; Venditti, I.; Testa, G.; Battocchio, C. Comparison between silver and gold nanoparticles stabilized with negatively charged hydrophilic thiols: SR-XPS and SERS as probes for structural differences and similarities. Coll. Surf. A Physicochem. Eng. Asp. 2017, 532, 183–188. [Google Scholar] [CrossRef]
- Langille, M.R.; Personick, M.L.; Zhang, J.; Mirkin, C.A. Defining rules for the shape evolution of gold nanoparticles. J. Am. Chem. Soc. 2012, 134, 14542–14554. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, Y.; Zeng, J.; Zhong, X.; Jin, M.; Li, Z.Y.; Xia, Y. Seed-mediated synthesis of single-crystal gold nanospheres with controlled diameters in the range 5–30 nm and their self-assembly upon dilution. Chem. Asian J. 2013, 8, 792–799. [Google Scholar] [CrossRef]
- Prosposito, P.; Mochi, F.; Ciotta, E.; Casalboni, M.; De Matteis, F.; Venditti, I.; Fontana, L.; Testa, G.; Fratoddi, I. Hydrophilic silver nanoparticles with tunable optical properties: Application for the detection of heavy metals in water. Beilstein J. Nanotechnol. 2016, 7, 1654–1661. [Google Scholar] [CrossRef]
- Mochi, F.; Burratti, L.; Fratoddi, I.; Venditti, I.; Battocchio, C.; Carlini, L.; Iucci, G.; Casalboni, M.; de Matteis, F.; Casciardi, S.; et al. Plasmonic Sensor Based on Interaction between Silver Nanoparticles and Ni2+ or Co2+ in Water. Nanomaterials 2018, 8, 488. [Google Scholar] [CrossRef]
- Sriram, M.; Zong, K.; Vivekchand, S.R.; Gooding, J.J. Single nanoparticle plasmonic sensors. Sensors 2015, 15, 25774–25792. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.D.; Ku, S.H.; Won, Y.Y.; Kim, S.H.; Kwon, I.C. Targeted Nanotheranostics for Future Personalized Medicine: Recent Progress in Cancer Therapy. Theranostics 2016, 6, 1362–1377. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Hsiao, M.S.; Yi, Y.J.; Izor, S.; Koerner, H.; Jawaid, A.; Vaia, R.A. Highly Concentrated Seed-Mediated Synthesis of Monodispersed Gold Nanorods. ACS Appl. Mater. Interfaces 2017, 9, 26363–26371. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Wang, Z.; Sun, X.; Song, J.; Jacobson, O.; Niu, G.; Kiesewetter, D.O.; Chen, X. Size Dependent Kinetics of Gold Nanorods in EPR Mediated Tumor Delivery. Theranostics 2016, 6, 2039–2051. [Google Scholar] [CrossRef] [PubMed]
- Dahan, D.; Jude, B.A.; Lamendella, R.; Keesing, F.; Perron, G.G. Exposure to Arsenic Alters the Microbiome of Larval Zebrafish. Front. Microbiol. 2018, 9, 1323. [Google Scholar] [CrossRef] [PubMed]
- Brown, Z.B.; Takagi, J. Advances in domain and subunit localization technology for electron microscopy. Biophys. Rev. 2019, 11, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–357. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Shah, N.; Visaria, R.; Paciotti, G.F.; Bischof, J.C. Biodistribution of TNF-alpha-coated gold nanoparticles in an in vivo model system. Nanomedicine 2009, 4, 401–410. [Google Scholar] [CrossRef]
- Wan, J.; Wang, J.H.; Liu, T.; Xie, Z.; Yu, X.F.; Li, W. Surface chemistry but not aspect ratio mediates the biological toxicity of gold nanorods in vitro and in vivo. Sci. Rep. 2015, 5, 11398. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Maitra, S.S. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 2017, 7, 243–256. [Google Scholar] [CrossRef]
- Iram, F.; Iqbal, M.S.; Khan, I.U.; Rasheed, R.; Khalid, A.; Khalid, M.; Aftab, S.; Shakoori, A.R. Synthesis and Biodistribution Study of Biocompatible. Biol. Trace Elem. Res. 2019, 2019, 1–12. [Google Scholar]
- Lee, S.B.; Yoon, G.; Lee, S.W.; Jeong, S.Y.; Ahn, B.C.; Lim, D.K.; Lee, J.; Jeon, Y.H. Combined Positron Emission Tomography and Cerenkov Luminescence Imaging of Sentinel Lymph Nodes Using PEGylated Radionuclide-Embedded Gold Nanoparticles. Small 2016, 12, 4894–4901. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, A.; Krug, P.; Głowala, P.; Hungría, A.B.; Chotkowski, M.; Wiktorska, K.; Mazur, M. Nano-radiogold-decorated composite bioparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Lee, J.E.; Cho, S.J.; Chin, J.; Kim, S.K.; Lee, I.K.; Lee, S.W.; Lee, J.; Jeon, Y.H. Crushed Gold Shell Nanoparticles Labeled with Radioactive Iodine as a Theranostic Nanoplatform for Macrophage-Mediated Photothermal Therapy. Nano Micro Lett. 2019, 11, 36. [Google Scholar] [CrossRef]
- Lee, S.B.; Kumar, D.; Li, Y.; Lee, I.K.; Cho, S.J.; Kim, S.K.; Lee, S.W.; Jeong, S.Y.; Lee, J.; Jeon, Y.H. PEGylated crushed gold shell-radiolabeled core nanoballs for in vivo tumor imaging with dual positron emission tomography and Cerenkov luminescent imaging. J. Nanobiotechnol. 2018, 16, 41. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Zhu, J.; Sun, N.; Song, N.; Xing, Y.; Huang, H.; Zhao, J. Chlorotoxin peptide-functionalized polyethylenimine-entrapped gold nanoparticles for glioma SPECT/CT imaging and radionuclide therapy. J. Nanobiotechnol. 2019, 17, 30. [Google Scholar] [CrossRef]
- Xing, Y.; Zhu, J.; Zhao, L.; Xiong, Z.; Li, Y.; Wu, S.; Chand, G.; Shi, X.; Zhao, J. SPECT/CT imaging of chemotherapy-induced tumor apoptosis using. Drug Deliv. 2018, 25, 1384–1393. [Google Scholar] [CrossRef]
- Ponti, J.; Colognato, R.; Franchini, F.; Gioria, S.; Simonelli, F.; Abbas, K.; Uboldi, C.; James Kirkpatrick, C.; Holzwarth, U.; Rossi, F. A quantitative in vitro approach to study the intracellular fate of gold nanoparticles: From synthesis to cytotoxicity. Nanotoxicology 2009, 3, 296–306. [Google Scholar] [CrossRef]
- Pretze, M.; Hien, A.; Rädle, M.; Schirrmacher, R.; Wängler, C.; Wängler, B. Gastrin-Releasing Peptide Receptor- and Prostate-Specific Membrane Antigen-Specific Ultrasmall Gold Nanoparticles for Characterization and Diagnosis of Prostate Carcinoma via Fluorescence Imaging. Bioconjug Chem. 2018, 29, 1525–1533. [Google Scholar] [CrossRef]
- Zhang, L.; Su, H.; Wang, H.; Li, Q.; Li, X.; Zhou, C.; Xu, J.; Chai, Y.; Liang, X.; Xiong, L.; et al. Tumor Chemo-Radiotherapy with Rod-Shaped and Spherical Gold Nano Probes: Shape and Active Targeting Both Matter. Theranostics 2019, 9, 1893–1908. [Google Scholar] [CrossRef] [PubMed]
- Freitas de Freitas, L.; Varca, G.H.C.; Dos Santos Batista, J.G.; Benévolo Lugão, A. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef] [PubMed]
- Wiesing, U. Theranostics: Is it really a revolution? Evaluating a new term in medicine. Med. Health Care Philos. 2019, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.K.; Olariu, C.I.; Yaffee, M.; Taelman, V.F.; Marincek, N.; Krause, T.; Meier, L.; Walter, M.A. Indium-111 labeled gold nanoparticles for in-vivo molecular targeting. Biomaterials 2014, 35, 7050–7057. [Google Scholar] [CrossRef] [PubMed]
- Kao, H.W.; Lin, Y.Y.; Chen, C.C.; Chi, K.H.; Tien, D.C.; Hsia, C.C.; Lin, M.H.; Wang, H.E. Evaluation of EGFR-targeted radioimmuno-gold-nanoparticles as a theranostic agent in a tumor animal model. Bioorg. Med. Chem. Lett. 2013, 23, 3180–3185. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Chanda, N.; Zambre, A.; Upendran, A.; Katti, K.; Kulkarni, R.R.; Nune, S.K.; Casteel, S.W.; Smith, C.J.; Vimal, J.; et al. Laminin receptor specific therapeutic gold nanoparticles (198AuNP-EGCg) show efficacy in treating prostate cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 12426–12431. [Google Scholar] [CrossRef]
- Black, K.C.; Akers, W.J.; Sudlow, G.; Xu, B.; Laforest, R.; Achilefu, S. Dual-radiolabeled nanoparticle SPECT probes for bioimaging. Nanoscale 2015, 7, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Dechantsreiter, M.A.; Planker, E.; Mathä, B.; Lohof, E.; Hölzemann, G.; Jonczyk, A.; Goodman, S.L.; Kessler, H. N-Methylated cyclic RGD peptides as highly active and selective alpha(V)beta(3) integrin antagonists. J. Med. Chem. 1999, 42, 3033–3040. [Google Scholar] [CrossRef]
- Tsoukalas, C.; Laurent, G.; Jiménez Sánchez, G.; Tsotakos, T.; Bazzi, R.; Stellas, D.; Anagnostopoulos, C.; Moulopoulos, L.; Koutoulidis, V.; Paravatou-Petsotas, M.; et al. Initial in vitro and in vivo assessment of Au@DTDTPA-RGD nanoparticles for Gd-MRI and 68Ga-PET dual modality imaging. EJNMMI Phys. 2015, 2 (Suppl. 1), A89. [Google Scholar] [CrossRef]

| Morphology | Gold Surface Functionality | Size (nm) | Radionuclides | Nuclear Medicine Applications | Ref. |
|---|---|---|---|---|---|
| AuNPs | |||||
| PEI | 3 | 99Tc | SPECT/TC | [80] | |
| Tannic acid | 40 | 124I | PET | [30] | |
| PEG | 30 | 198Au | SPECT | [81] | |
| PEG | 15 | 211At | cytotoxicity | [82] | |
| PEG | 30 | 177Lu | brachytherapy | [44] | |
| Amino-PEG | 30 | 99Tc | SPECT/CT | [83] | |
| PEG | 5 | 199Au | SPECT, biodistribution | [84] | |
| PEI | 3 | 99Tc | SPECT/TC | [85] | |
| PEG | 30 | 177Lu | treatment of breast cancer | [86] | |
| thiol mannose | 20 | 99Tc | sentinel lymph node detection | [87] | |
| allylamine | 5 | 89Zr | PET, biodistribution | [88] | |
| PEG * | 10 | 18F | PET | [89] | |
| AuNRs | |||||
| s | PEG | 20–60 | 169Yb | brachytherapy | [90] |
| PEG | 25–90 | 131I | biodistribution | [91] | |
| PEG | 20–90 | 125I | bioactivity | [92] | |
| polydopamine | 15–60 | 125I | SPECT/TC | [93] | |
| PEG | 15–50; 15–75 | 64Cu | PET, biodistribution | [41] | |
| HPMA ** | --- | 90Y | treatment of prostate tumors | [94] | |
| PEG | 10–40 | 198Au | SPECT, biodistribution | [81] | |
| PEG | 10–25 | 131I | biodistribution | [95] | |
| PEG | 10–50 | 125I | biodistribution | [96] | |
| PEG | --- | 125I | SPECT, PET | [97] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maccora, D.; Dini, V.; Battocchio, C.; Fratoddi, I.; Cartoni, A.; Rotili, D.; Castagnola, M.; Faccini, R.; Bruno, I.; Scotognella, T.; et al. Gold Nanoparticles and Nanorods in Nuclear Medicine: A Mini Review. Appl. Sci. 2019, 9, 3232. https://doi.org/10.3390/app9163232
Maccora D, Dini V, Battocchio C, Fratoddi I, Cartoni A, Rotili D, Castagnola M, Faccini R, Bruno I, Scotognella T, et al. Gold Nanoparticles and Nanorods in Nuclear Medicine: A Mini Review. Applied Sciences. 2019; 9(16):3232. https://doi.org/10.3390/app9163232
Chicago/Turabian StyleMaccora, Daria, Valentina Dini, Chiara Battocchio, Ilaria Fratoddi, Antonella Cartoni, Dante Rotili, Massimo Castagnola, Riccardo Faccini, Isabella Bruno, Teresa Scotognella, and et al. 2019. "Gold Nanoparticles and Nanorods in Nuclear Medicine: A Mini Review" Applied Sciences 9, no. 16: 3232. https://doi.org/10.3390/app9163232
APA StyleMaccora, D., Dini, V., Battocchio, C., Fratoddi, I., Cartoni, A., Rotili, D., Castagnola, M., Faccini, R., Bruno, I., Scotognella, T., Giordano, A., & Venditti, I. (2019). Gold Nanoparticles and Nanorods in Nuclear Medicine: A Mini Review. Applied Sciences, 9(16), 3232. https://doi.org/10.3390/app9163232








