Saccharomyces Cerevisiae Var. Boulardii: Valuable Probiotic Starter for Craft Beer Production
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Wort Preparation and Beer Fermentation Procedure
2.3. Polyphenols and Antioxidant Activity
2.3.1. Total Phenolic Content
2.3.2. DPPH Scavenging Activity
2.3.3. Polyphenol Profiles
2.4. Bioreactor Culture
2.5. Growth Analysis and Modelling
2.6. Biovolume Estimation
2.7. Yeast Viability
2.8. Specific Gravity and Alcohol Concentration
2.9. Sensory Evaluation
2.10. Software
3. Results
3.1. Physicochemical Parameters During Fermentation
3.2. Antioxidant Activity and Total Phenolic Content After Fermentation
3.3. HPLC/DAD Analysis After Fermentation
3.4. Yeast Growth Analysis in Bioreactor Culture
3.5. Physicochemical Parameters During Bioreactor Culture
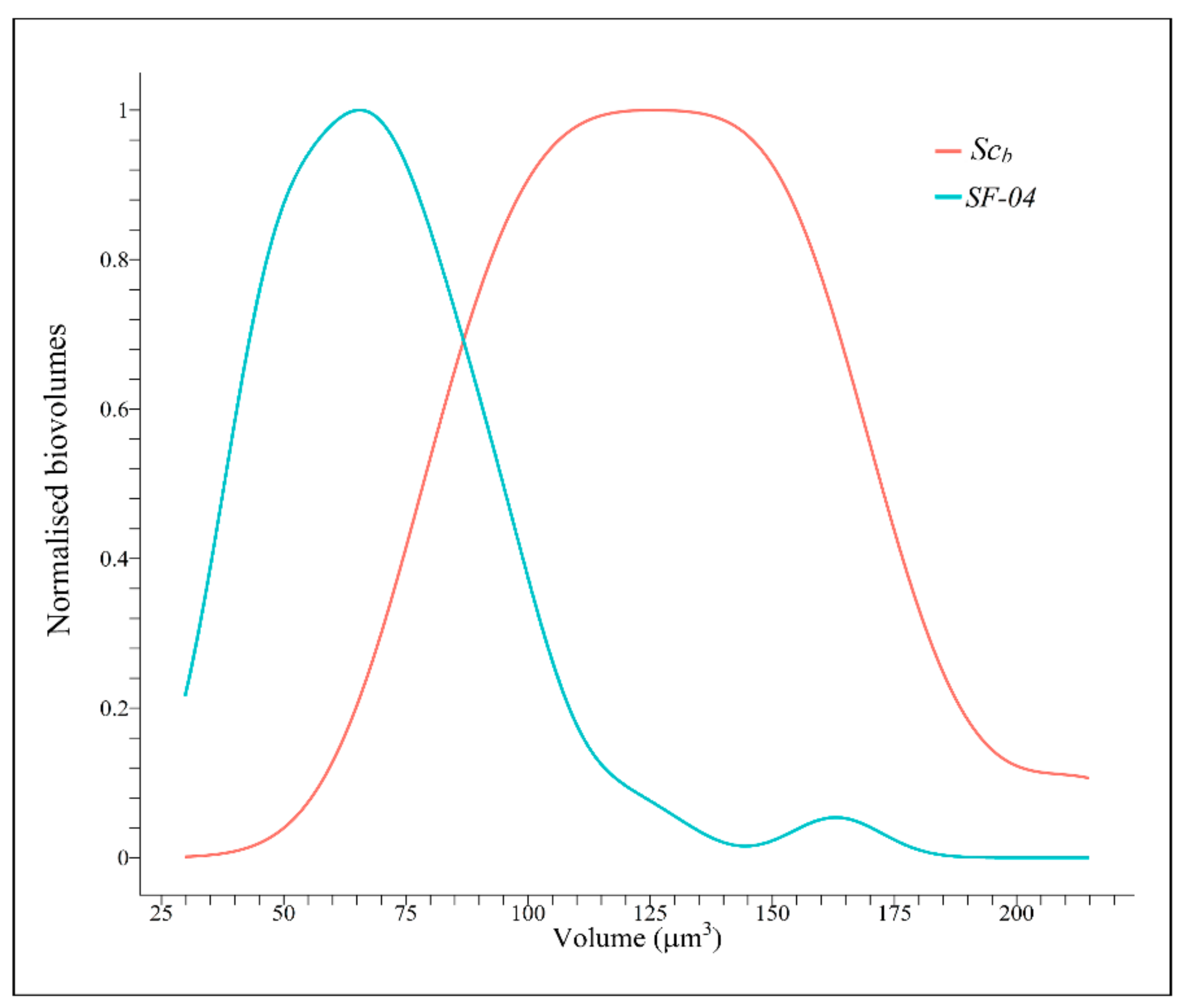
3.6. Yeast Biovolumes
3.7. Yeast Viability in the Craft Beers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T. The Yeasts: A Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780080931272. [Google Scholar]
- Ding, J.; Huang, X.; Zhang, L.; Zhao, N.; Yang, D.; Zhang, K. Tolerance and stress response to ethanol in the yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2009, 85, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Lodolo, E.J.; Kock, J.L.F.; Axcell, B.C.; Brooks, M. The yeast Saccharomyces cerevisiae—The main character in beer brewing. In FEMS Yeast Research; Wiley/Blackwell: Hoboken, NJ, USA, 2008; Volume 8, pp. 1018–1036. [Google Scholar]
- McCullough, M.J.; Clemons, K.V.; Mccusker, J.H.; Stevens, D.A. Species identification and virulence attributes of Saccharomyces boulardii. J. Clin. Microbiol. 1998, 36, 2613–2617. [Google Scholar] [PubMed]
- Hancox, L.R.; Le Bon, M.; Richards, P.J.; Guillou, D.; Dodd, C.E.R.; Mellits, K.H. Effect of a single dose of Saccharomyces cerevisiae var. boulardii on the occurrence of porcine neonatal diarrhoea. Animal 2015, 9, 1756–1759. [Google Scholar] [CrossRef] [PubMed]
- Leggat, P.A.; Goldsmid, J.M. Others the returned traveller with diarrhoea. Aust. Fam. Physician 2007, 36, 322. [Google Scholar]
- McFarland, L.V. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med. Infect. Dis. 2007, 5, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Moslehi-Jenabian, S.; Pedersen, L.L.; Jespersen, L. Beneficial effects of probiotic and food borne yeasts on human health. Nutrients 2010, 2, 449–473. [Google Scholar] [CrossRef] [PubMed]
- Czerucka, D.; Piche, T.; Rampal, P. Review article: Yeast as probiotics—Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, T.A.; Pallav, K.; Dowd, S.E.; Villafuerte-Galvez, J.; Vanga, R.R.; Castillo, N.E.; Hansen, J.; Dennis, M.; Leffler, D.A.; Kelly, C.P. Prospective randomized controlled study on the effects of Saccharomyces boulardii CNCM I-745 and amoxicillin-clavulanate or the combination on the gut microbiota of healthy volunteers. Gut Microbes 2017, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M. Treatment of recurrent Clostridium difficile-associated disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2004, 1, 32–38. [Google Scholar] [CrossRef]
- Greenhill, C. Probiotic helps to eradicate H. pylori. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 362. [Google Scholar] [CrossRef]
- Edwards-Ingram, L.; Gitsham, P.; Burton, N.; Warhurst, G.; Clarke, I.; Hoyle, D.; Oliver, S.G.; Stateva, L. Genotypic and physiological characterization of Saccharomyces boulardii, the probiotic strain of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2007, 73, 2458–2467. [Google Scholar] [CrossRef] [PubMed]
- Khatri, I.; Tomar, R.; Ganesan, K.; Prasad, G.S.; Subramanian, S. Complete genome sequence and comparative genomics of the probiotic yeast Saccharomyces boulardii. Sci. Rep. 2017, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Nourani, A.; Wesolowski-Louvel, M.; Delaveau, T.; Jacq, C.; Delahodde, A. Multiple-drug-resistance phenomenon in the yeast Saccharomyces cerevisiae: Involvement of two hexose transporters. Mol. Cell. Biol. 1997, 17, 5453–5460. [Google Scholar] [CrossRef] [PubMed]
- League, G.P.; Slot, J.C.; Rokas, A. The ASP3 locus in Saccharomyces cerevisiae originated by horizontal gene transfer from Wickerhamomyces. FEMS Yeast Res. 2012, 12, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Verstrepen, K.J.; Klis, F.M. Flocculation, adhesion and biofilm formation in yeasts. Mol. Microbiol. 2006, 60, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Stratford, M. Genetic aspects of yeast flocculation: In particular, the role of FLO genes in the flocculation of Saccharomyces cerevisiae. Colloids Sur. B Biointerfaces 1994, 2, 151–158. [Google Scholar] [CrossRef]
- Karen, M.; Yuksel, O.; Akyürek, N.; Ofluoǧlu, E.; Çaǧlar, K.; Şahin, T.T.; Paşaoǧlu, H.; Memiş, L.; Akyürek, N.; Bostanci, H. Probiotic Agent Saccharomyces boulardii Reduces the Incidenceof Lung Injury in Acute Necrotizing Pancreatitis Induced Rats. J. Surg. Res. 2010, 160, 139–144. [Google Scholar] [CrossRef]
- Surawicz, C.M.; McFarland, L.V.; Greenberg, R.N.; Rubin, M.; Fekety, R.; Mulligan, M.E.; Garcia, R.J.; Brandmarker, S.; Bowen, K.; Borjal, D.; et al. The Search for a Better Treatment for Recurrent Clostridium difficile Disease: Use of High-Dose Vancomycin Combined with Saccharomyces boulardii. Clin. Infect. Dis. 2000, 31, 1012–1017. [Google Scholar] [CrossRef]
- Czerucka, D.; Dahan, S.; Mograbi, B.; Rossi, B.; Rampal, P. Saccharomyces boulardii preserves the barrier function and modulates the signal transduction pathway induced in enteropathogenic Escherichia coli-infected T84 cells. Infect. Immun. 2000, 68, 5998–6004. [Google Scholar] [CrossRef]
- Berg, R.; Bernasconi, P.; Fowler, D.; Gautreaux, M. Inhibition of Candida albicans Translocation from the Gastrointestinal Tract of Mice by Oral Administration of Saccharomyces boulardii. J. Infect. Dis. 1993, 168, 1314–1318. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Dominance and influence of selected Saccharomyces cerevisiae strains on the analytical profile of craft beer refermentation. J. Inst. Brew. 2014, 120, 262–267. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; de Faria, A.J.; Cruz, A.G. Characterization of Brazilian lager and brown ale beers based on color, phenolic compounds, and antioxidant activity using chemometrics. J. Sci. Food Agric. 2011, 91, 563–571. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, G.; Cerletti, C.; Alkerwi, A.; Iacoviello, L.; Badimon, L.; Costanzo, S.; Pounis, G.; Trevisan, M.; Panico, S.; Stranges, S.; et al. Effects of moderate beer consumption on health and disease: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esslinger, H.M. Handbook of Brewing: Processes, Technology, Markets; Wiley-VCH: Hoboken, NJ, USA, 2009; ISBN 3527623493. [Google Scholar]
- Stewart, G. Saccharomyces species in the Production of Beer. Beverages 2016, 2, 34. [Google Scholar] [CrossRef]
- Callemien, D.; Collin, S. Structure, organoleptic properties, quantification methods, and stability of phenolic compounds in beer-A review. Food Rev. Int. 2010, 26, 1–84. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisiae var. boulardii in co-fermentations with S. cerevisiae for the production of craft beers with potential healthy value-added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Senkarcinova, B.; Graça Dias, I.A.; Nespor, J.; Branyik, T. Probiotic alcohol-free beer made with Saccharomyces cerevisiae var. boulardii. LWT 2019, 100, 362–367. [Google Scholar] [CrossRef]
- Sanekata, A.; Tanigawa, A.; Takoi, K.; Nakayama, Y.; Tsuchiya, Y. Identification and Characterization of Geranic Acid as a Unique Flavor Compound of Hops (Humulus lupulus L.) Variety Sorachi Ace. J. Agric. Food Chem. 2018, 66, 12285–12295. [Google Scholar] [CrossRef]
- Bishop, L.R. The Nitrogen Compounds of Wort And Beer. J. Inst. Brew. 1943, 49, 173–178. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Chang, W.-H.; Chen, C.-S.; Liao, J.-W.; Huang, C.-J.; Lu, F.-J.; Chia, Y.-C.; Hsu, H.-K.; Wu, J.-J.; Yang, H.-L. Antioxidant activities of Toona Sinensis leaves extracts using different antioxidant models. Food Chem. Toxicol. 2008, 46, 105–114. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Phenolic Constituents in the Leaves of Northern Willows: Methods for the Analysis of Certain Phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Ditrych, M.; Kordialik-Bogacka, E.; Czyżowska, A. Antiradical and Reducing Potential of Commercial Beers. Czech. J. Food Sci. 2015, 33. [Google Scholar] [CrossRef]
- Quifer-Rada, P.; Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R. A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS). Food Chem. 2015, 169, 336–343. [Google Scholar] [CrossRef]
- Bovee, T.F.H.; Helsdingen, R.J.R.; Hamers, A.R.M.; van Duursen, M.B.M.; Nielen, M.W.F.; Hoogenboom, R.L.A.P. A new highly specific and robust yeast androgen bioassay for the detection of agonists and antagonists. Anal. Bioanal. Chem. 2007, 389, 1549–1558. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, R.L.; Whiting, R.C.; Damert, W.C. When is simple good enough: A comparison of the Gompertz, Baranyi, and three-phase linear models for fitting bacterial growth curves. Food Microbiol. 1997, 14, 313–326. [Google Scholar] [CrossRef]
- Kacena, M.A.; Merrell, G.A.; Manfredi, B.; Smith, E.E.; Klaus, D.M.; Todd, P. Bacterial growth in space flight: Logistic growth curve parameters for Escherichia coli and Bacillus subtilis. Appl. Microbiol. Biotechnol. 1999, 51, 229–234. [Google Scholar] [CrossRef]
- R Core Team. R Language Definition; R Foundation for Statistical Computing: Vienna, Austria, 2000. [Google Scholar]
- Nonlinear Regression Analysis and Its Applications; Bates, D.M.; Watts, D.G. (Eds.) Wiley Series in Probability and Statistics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1988. [Google Scholar]
- Saccà, A. Methods for the estimation of the biovolume of microorganisms: A critical review. Limnol. Oceanogr. Methods 2017, 15, 337–348. [Google Scholar] [CrossRef]
- Saccà, A. A simple yet accurate method for the estimation of the biovolume of planktonic microorganisms. PLoS ONE 2016, 11, e0151955. [Google Scholar] [CrossRef]
- Lenz, M.; Roumans, N.J.T.; Vink, R.G.; Van Baak, M.A.; Mariman, E.C.M.; Arts, I.C.W.; De Kok, T.M.; Ertaylan, G. Estimating real cell size distribution from cross-section microscopy imaging. Bioinformatics 2016, 32, 396–404. [Google Scholar] [CrossRef]
- Pau, G.; Fuchs, F.; Sklyar, O.; Boutros, M.; Huber, W. EBImage--an R package for image processing with applications to cellular phenotypes. Bioinformatics 2010, 26, 979–981. [Google Scholar] [CrossRef]
- Jekel, J.F.; Katz, D.L.; Elmore, J.G.; Wild, D. Epidemiology, Biostatistics and Preventive Medicine; Elsevier Health Sciences: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Massey, F.J. The Kolmogorov-Smirnov Test for Goodness of Fit. J. Am. Stat. Assoc. 1951, 46, 68–78. [Google Scholar] [CrossRef]
- Martí, M.; Frígols, B.; Serrano-Aroca, Á. Antimicrobial Characterization of Advanced Materials for Bioengineering Applications. J. Vis. Exp. 2018, e57710. [Google Scholar] [CrossRef] [Green Version]
- Spedding, G. Alcohol and Its Measurement. In Brewing Materials and Processes: A Practical Approach to Beer Excellence; Academic Press: Cambridge, MA, USA, 2016; pp. 123–149. [Google Scholar]
- Erkan, H.; Çelik, S.; Bilgi, B.; Köksel, H. A new approach for the utilization of barley in food products: Barley tarhana. Food Chem. 2006, 97, 12–18. [Google Scholar] [CrossRef]
- Agu, R.C.; Palmer, G.H. A reassessment of sorghum for lager-beer brewing. Bioresour. Technol. 1998, 66, 253–261. [Google Scholar] [CrossRef]
- Verbelen, P.J.; De Schutter, D.P.; Delvaux, F.; Verstrepen, K.J.; Delvaux, F.R. Immobilized yeast cell systems for continuous fermentation applications. Biotechnol. Lett. 2006, 28, 1515–1525. [Google Scholar] [CrossRef]
- Strezov, V.; Evans, T.J. Biomass Processing Technologies; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Lowe, D.P.; Ulmer, H.M.; Barta, R.C.; Goode, D.L.; Arendt, E.K. Biological acidification of a mash containing 20% barley using Lactobacillus amylovorus FST 1.1: Its effects on Wort and beer quality. J. Am. Soc. Brew. Chem. 2005, 63, 96–106. [Google Scholar] [CrossRef]
- Araki, S.; Kimura, T.; Shimizu, C.; Furusho, S.; Takashio, M.; Shinotsuka, K. Estimation of Antioxidative Activity and its Relationship to Beer Flavor Stability. J. Am. Soc. Brew. Chem. 1999, 57, 34–37. [Google Scholar] [CrossRef]
- Montanari, L.; Perretti, G.; Natella, F.; Guidi, A.; Fantozzi, P. Organic and phenolic acids in beer. LWT-Food Sci. Technol. 1999, 32, 535–539. [Google Scholar] [CrossRef]
- Pothoulakis, C.; Kelly, C.P.; Joshi, M.A.; Gao, N.; O’Keane, C.J.; Castagliuolo, I.; Lamont, J.T. Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastroenterology 1993, 104, 1108–1115. [Google Scholar] [CrossRef]
- Castagliuolo, I.; Riegler, M.F.; Valenick, L.; LaMont, J.T.; Pothoulakis, C. Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect. Immun. 1999, 67, 302–307. [Google Scholar] [CrossRef]
- Czerucka, D.; Roux, I.; Rampal, P. Saccharomyces boulardii inhibits secretagogue-mediated adenosine 3′, 5′-cyclic monophosphate induction in intestinal cells. Gastroenterology 1994, 106, 65–72. [Google Scholar] [CrossRef]
- Castagliuolo, I.; Thomas Lamont, J.; Nikulasson, S.T.; Pothoulakis, C. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect. Immun. 1996, 64, 5225–5232. [Google Scholar]
- Chen, X.; Kokkotou, E.G.; Mustafa, N.; Bhaskar, K.R.; Sougioultzis, S.; O’Brien, M.; Pothoulakis, C.; Kelly, C.P. Saccharomyces boulardii inhibits ERK1/2 mitogen-activated protein kinase activation both in vitro and in vivo and protects against Clostridium difficile toxin A-induced enteritis. J. Biol. Chem. 2006, 281, 24449–24454. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Kogan, G.; Pajtinka, M.; Babincova, M.; Miadokova, E.; Rauko, P.; Slamenova, D.; Korolenko, T.A. Yeast cell wall polysaccharides as antioxidants and antimutagens: Can they fight cancer. In Neoplasma; In Tech: Rijeka, Croatia, 2008; Volume 55, pp. 387–393. [Google Scholar]
- Pearl, R.; Slobodkin, L. The Growth of Populations. Q. Rev. Biol. 1976, 51, 6–24. [Google Scholar] [CrossRef]
- Chowdhury, B.R.; Chakraborty, R.; Chaudhuri, U.R. Validity of modified Gompertz and Logistic models in predicting cell growth of Pediococcus acidilactici H during the production of bacteriocin pediocin AcH. J. Food Eng. 2007, 80, 1171–1175. [Google Scholar] [CrossRef]
- Chirivella-Martorell, J.; Briz-Redón, Á.; Serrano-Aroca, Á. Modelling of Biomass Concentration, Multi-Wavelength Absorption and Discrimination Method for Seven Important Marine Microalgae Species. Energies 2018, 11, 1089. [Google Scholar] [CrossRef]
- Fietto, J.L.; Araújo, R.S.; Valadão, F.N.; Fietto, L.G.; Brandão, R.L.; Neves, M.J.; Gomes, F.C.; Nicoli, J.R.; Castro, I.M. Molecular and physiological comparisons between Saccharomyces cerevisiae and Saccharomyces boulardii. Can. J. Microbiol. 2004, 50, 615–621. [Google Scholar] [CrossRef]
- Sun, J.; Yang, J.; Gao, Y.; Xu, D.; Li, D. Reference channel-based microfluidic resistance sensing for single yeast cell volume growth measurement. Microfluid. Nanofluidics 2017, 21, 33. [Google Scholar] [CrossRef]
- Wang, J.; Mao, J.; Yang, G.; Zheng, F.; Niu, C.; Li, Y.; Liu, C.; Li, Q. The FKS family genes cause changes in cell wall morphology resulted in regulation of anti-autolytic ability in Saccharomyces cerevisiae. Bioresour. Technol. 2018, 249, 49–56. [Google Scholar] [CrossRef]





| Polyphenol | Retention Time (min) | Wavelength (nm) |
|---|---|---|
| Phloroglucinol | 4.01 | 270 |
| Catequin | 12.66 | |
| Vanillic acid | 14.29 | |
| Epicatechin | 15.14 | |
| Protocatechuic acid | 18.49 | |
| Rutin | 19.20 | |
| Gentisic acid | 11.86 | 324 |
| Chlorogenic acid | 13.81 | |
| Caffeic acid | 14.63 | |
| p-coumaric acid | 16.84 | |
| Ferulic acid | 17.41 | |
| Myricetin | 20.48 | 373 |
| Quercetin | 24.18 |
| Yeast Strain | TPC (mg GA/L) | RSA (%) | ρwort (kg/m3) | ρbeer (kg/m3) | ABWt (%) | ABV (%) |
|---|---|---|---|---|---|---|
| SF-04 | 0.1597 ± 0.0373 | 11.51 ± 0.36 | 1028 | 1008 | 1.87 | 2.39 |
| Scb | 0.1545 ± 0.0192 | 16.80 ± 0.31 * | 1028 | 1014 | 1.29 | 1.65 |
| Yeast Strain | K | Error | a | Error | r | Error | Model |
|---|---|---|---|---|---|---|---|
| SF-04 | 1.832 | 0.066 | 35.858 | 0.799 | 0.113 | 0.005 | Logistic |
| Scb | 1.644 | 0.008 | 23.092 | 0.093 | 0.302 | 0.007 | |
| SF-04 | 2.719 | 0.143 | 5.960 | 0.179 | 0.047 | 0.002 | Gompertz |
| Scb | 1.671 | 0.016 | 69.873 | 14.079 | 0.203 | 0.009 |
| Yeast Strain | Appearance | Aroma | Flavor | Bitterness |
|---|---|---|---|---|
| SF-04 | 6.25 ± 1.03 | 6.12 ± 0.99 | 5.87 ± 1.13 | 4.75 ± 1.04 |
| Scb | 6.50 ± 1.41 | 5.62 ± 1.51 | 5.37 ± 1.30 | 5.25 ± 1.58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulero-Cerezo, J.; Briz-Redón, Á.; Serrano-Aroca, Á. Saccharomyces Cerevisiae Var. Boulardii: Valuable Probiotic Starter for Craft Beer Production. Appl. Sci. 2019, 9, 3250. https://doi.org/10.3390/app9163250
Mulero-Cerezo J, Briz-Redón Á, Serrano-Aroca Á. Saccharomyces Cerevisiae Var. Boulardii: Valuable Probiotic Starter for Craft Beer Production. Applied Sciences. 2019; 9(16):3250. https://doi.org/10.3390/app9163250
Chicago/Turabian StyleMulero-Cerezo, Joaquín, Álvaro Briz-Redón, and Ángel Serrano-Aroca. 2019. "Saccharomyces Cerevisiae Var. Boulardii: Valuable Probiotic Starter for Craft Beer Production" Applied Sciences 9, no. 16: 3250. https://doi.org/10.3390/app9163250
APA StyleMulero-Cerezo, J., Briz-Redón, Á., & Serrano-Aroca, Á. (2019). Saccharomyces Cerevisiae Var. Boulardii: Valuable Probiotic Starter for Craft Beer Production. Applied Sciences, 9(16), 3250. https://doi.org/10.3390/app9163250







