Poly(phenylene methylene)-Based Coatings for Corrosion Protection: Replacement of Additives by Use of Copolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solvents
2.2. Apparatus
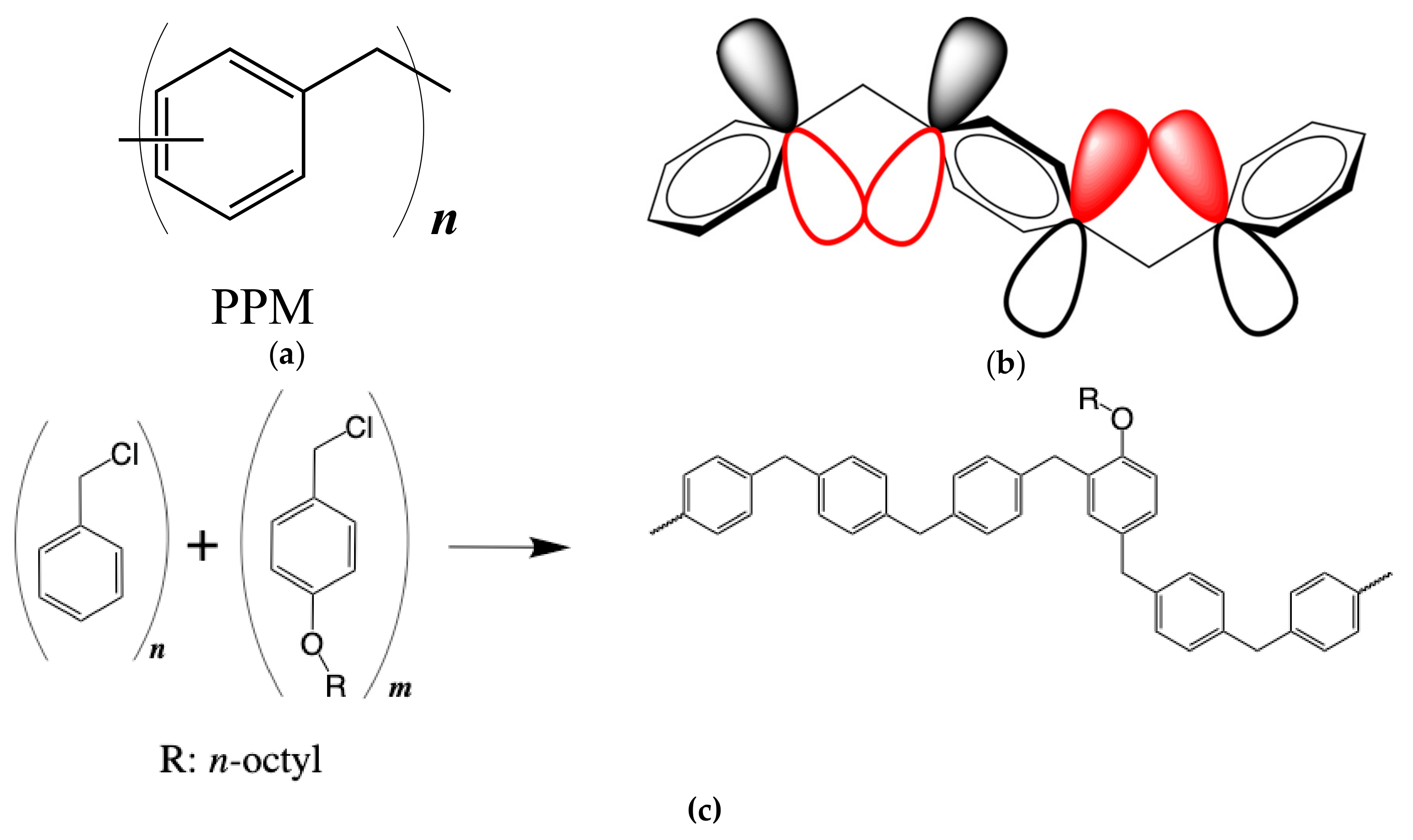
2.3. Synthesis of 4-Octyloxybenzyl Chloride
2.4. Synthesis of the Octyloxy-Containing PPM Derivatives
2.5. Preparation of Coated AA2024
2.6. Electrochemical Characterization of Coated AA2024
3. Results and Discussion
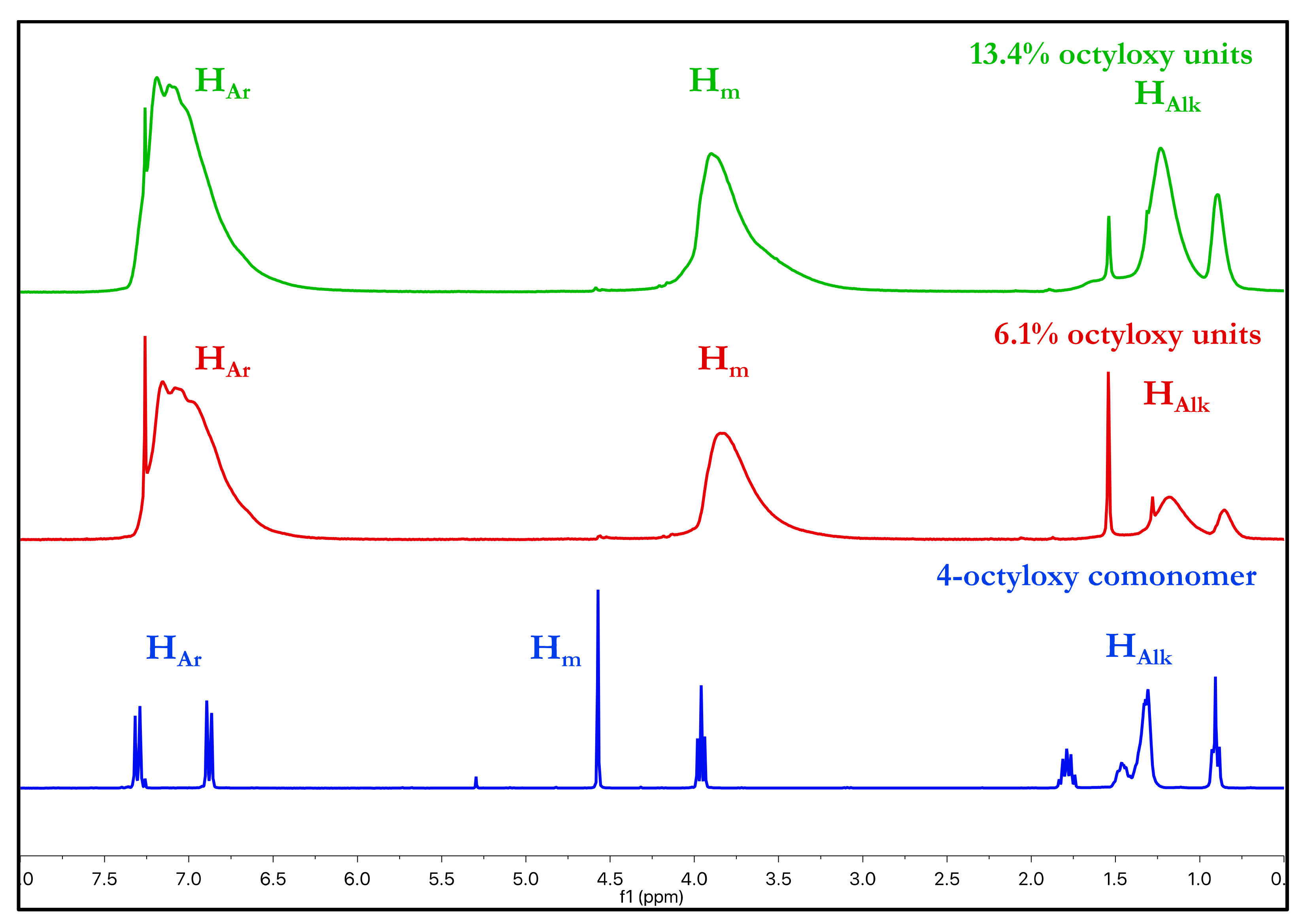
3.1. Synthesis and Structural Characterization
3.2. Thermal Analysis
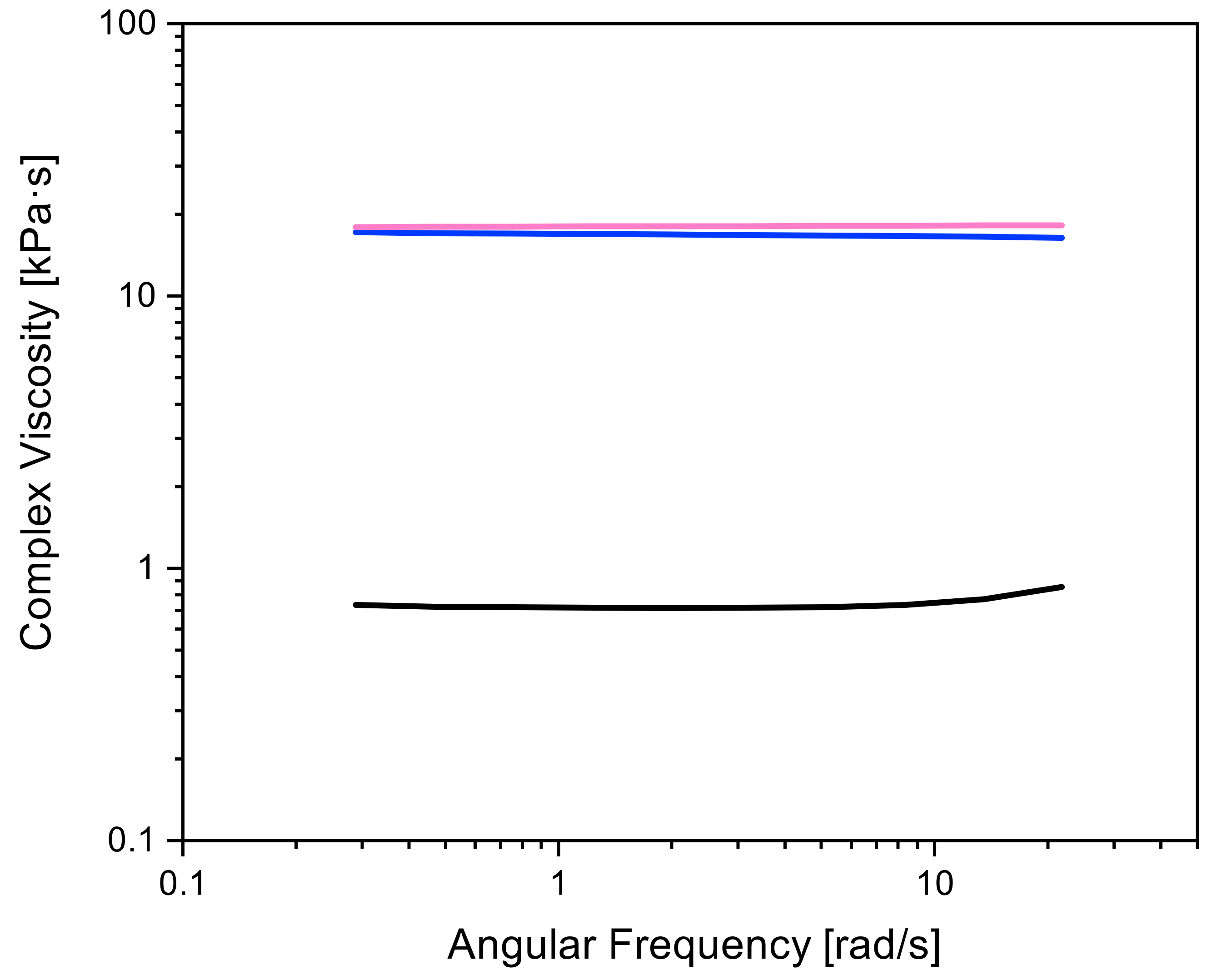
3.3. Rheology
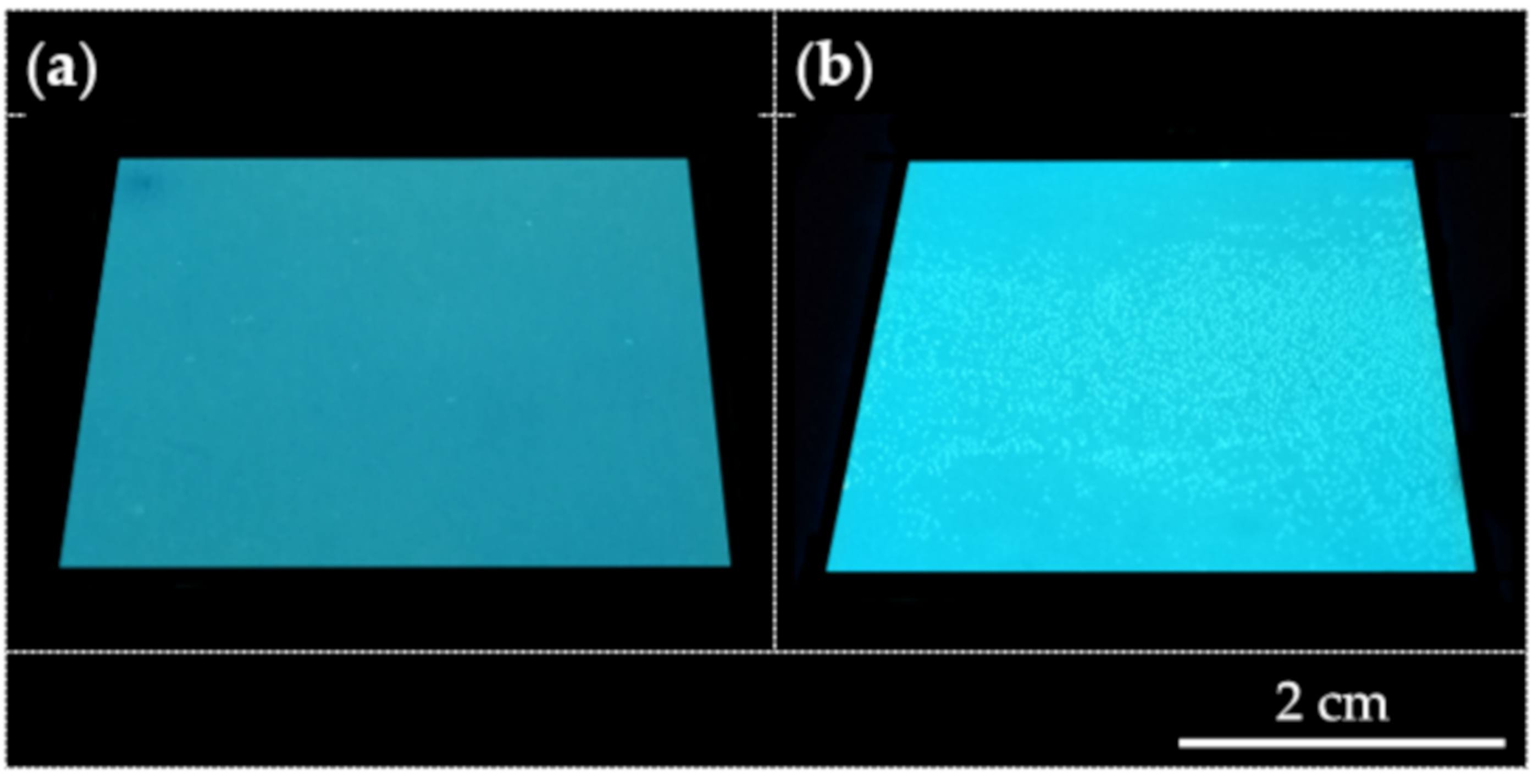
3.4. Coatings
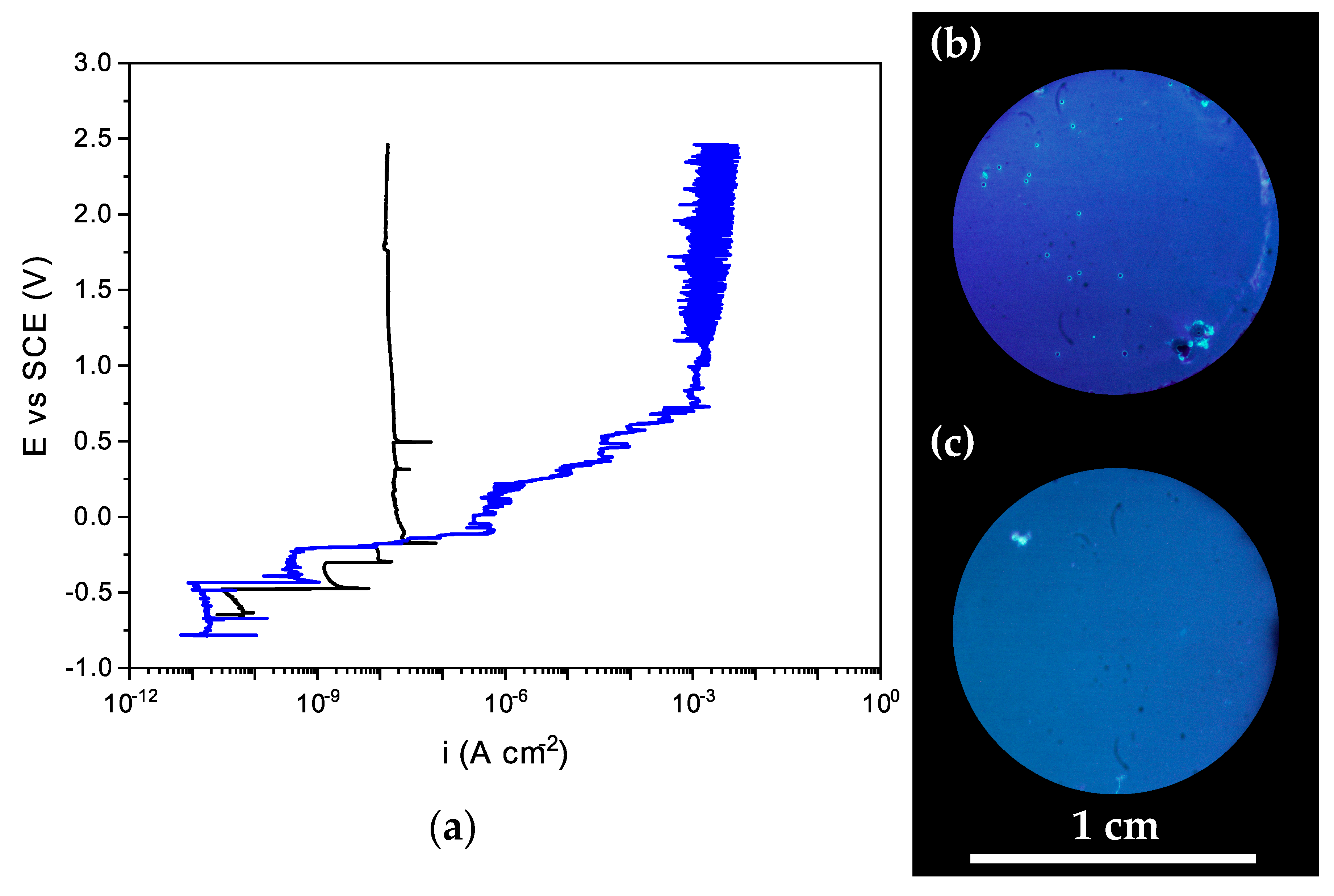
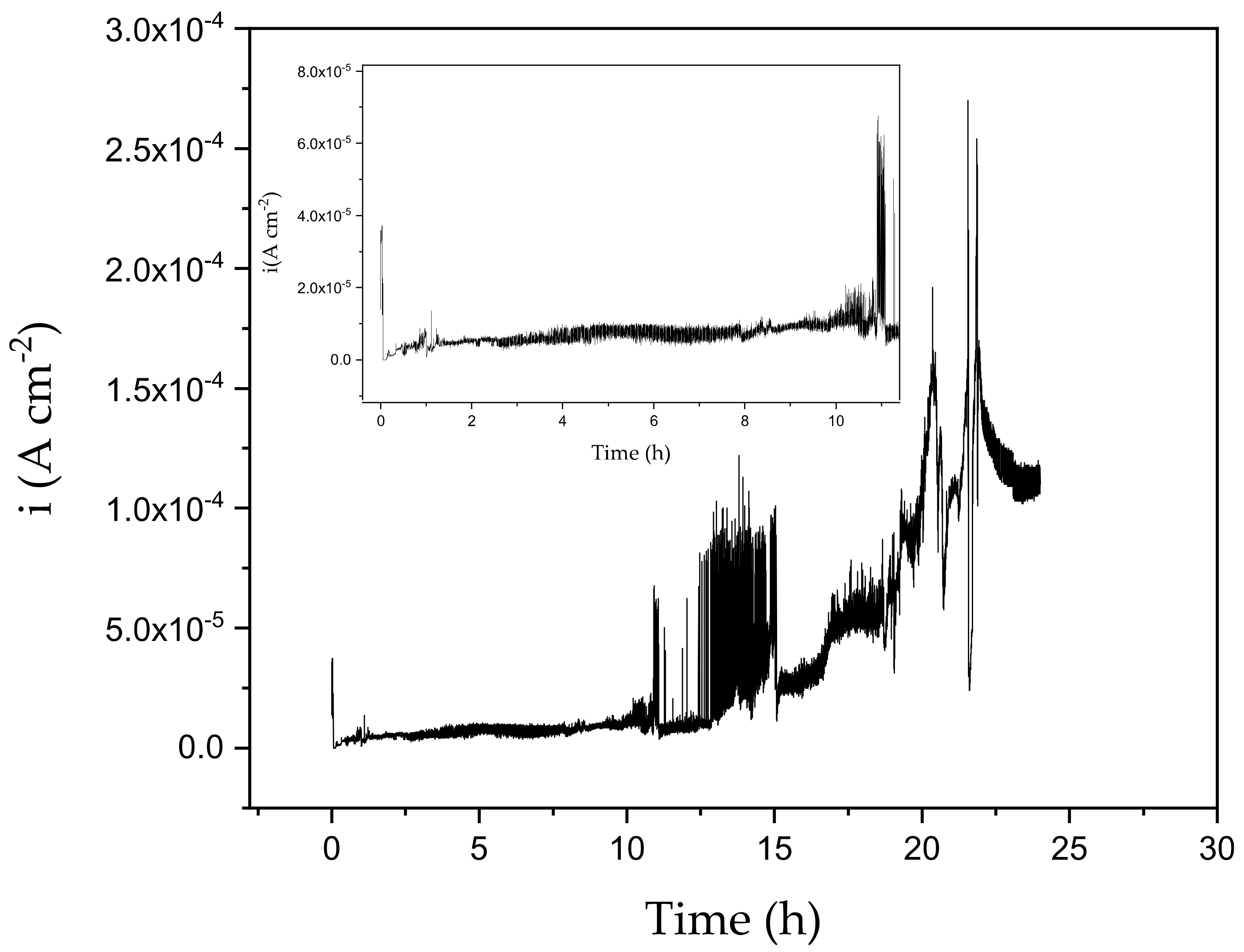
3.5. Protective Behavior of PPM-Based Coatings against Corrosion of AA2024
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- D’Elia, M.F.; Braendle, A.; Schweizer, T.; Ortenzi, M.; Trasatti, S.; Niederberger, M.; Caseri, W. Poly(Phenylene Methylene): A Multifunctional Material for Thermally Stable, Hydrophobic, Fluorescent, Corrosion-Protective Coatings. Coatings 2018, 8, 274. [Google Scholar] [CrossRef]
- Braendle, A.; Schwendimann, P.; Niederberger, M.; Caseri, W. Synthesis and fractionation of poly(phenylene methylene). J. Polym. Sci. Part A Polym. Chem. 2018, 56, 309–318. [Google Scholar] [CrossRef]
- Ellis, B.; White, P. Thermal degradation of polybenzyl. J. Polym. Sci. Polym. Chem. Ed. 1973, 11, 801–821. [Google Scholar] [CrossRef]
- Dreyer, D.; Jarvis, K.; Ferreira, P.; Bielawski, C. Graphite Oxide as a Dehydrative Polymerization Catalyst: A One-Step Synthesis of Carbon-Reinforced Poly(phenylene methylene) Composites. Macromolecules 2011, 44, 7659–7667. [Google Scholar] [CrossRef]
- Hino, M.; Arata, K. Iron oxide as an effective catalyst for the polycondensation of benzyl chloride, the formation of para-substituted polybenzyl. Chem. Lett. 1979, 8, 1141–1144. [Google Scholar] [CrossRef]
- Braendle, A.; Perevedentsev, A.; Cheetham, N.; Stavrinou, P.; Schachner, J.; Mösch-Zanetti, N.; Niederberger, M.; Caseri, W. Homoconjugation in poly(phenylene methylene)s: A case study of non-π-conjugated polymers with unexpected fluorescent properties. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 707–720. [Google Scholar] [CrossRef]
- Muller, P. Glossary of terms used in physical organic chemistry (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 306, 1077–1184. [Google Scholar] [CrossRef]
- Ferguson, L.; Nnadi, J. Electronic interactions between nonconjugated groups. J. Chem. Educ. 1965, 42, 529–535. [Google Scholar] [CrossRef]
- Rosen, S.L. Fundamental Principles of Polymeric Materials; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Rahman, M.; Brazel, C.S. The plasticizer market: An assessment of traditional plasticizers and research trends to meet new challenges. Prog. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Small, P.A. The diffusion of plasticisers from polyvinyl chloride. J. Chem. Technol. Biotechnol. 1947, 66, 17–19. [Google Scholar] [CrossRef]
- Wypych, G. 13-Plasticizers in various industrial products. In Handbook of Plasticizers, 3rd ed.; Elsevier: Toronto, ON, Canada, 2007; pp. 495–605. [Google Scholar]
- Immergut, E.H.; Mark, H.F. Principle of Plasticization. In Plasticization and Plasticizer Processes; ACS Editorial Library: Washington, DC, USA, 1965; pp. 1–26. [Google Scholar]
- Rehberg, C.E.; Fisher, C.H. Properties of Monomeric and Polymeric Alkyl Acrylates and Methacrylates. Ind. Eng. Chem. 1948, 40, 1429–1433. [Google Scholar] [CrossRef]
- Tavares, A.; Schneider, P.H.; Merlo, A.A. 3,5-Disubstituted Isoxazolines as Potential Molecular Kits for Liquid-Crystalline Materials. Eur. J. Org. Chem. 2009, 2009, 889–897. [Google Scholar] [CrossRef]
- Spreti, N.; Brinchi, L.; Germani, R.; Mancini, M.V.; Savelli, G. A New Carrier for Selective Removal of Heavy Metal Ions from Aqueous Solutions through Bulk Liquid Membranes. Eur. J. Org. Chem. 2004, 2004, 3865–3871. [Google Scholar] [CrossRef]
- Zhao, Y. Nanoparticles and Nanoparticles Compositions. U.S. Patent 2013/0101516 A1, 25 April 2013. [Google Scholar]
- Gunes, D.; Yagci, Y.; Bicak, N. Synthesis of Soluble Poly(p-phenylene methylene) from Tribenzylborate by Acid-Catalyzed Polymerization. Macromolecules 2010, 43, 7993–7997. [Google Scholar] [CrossRef]
- M’Hiri, T.; Catusse, C.; Catusse, R.; Dubry, J.L.J. Polymerization of benzyl alcohol and its derived compounds with an ion exchange resin. React. Kinet. Catal. Lett. 1983, 22, 425–428. [Google Scholar] [CrossRef]
- Banker, G.S. Film Coating Theory and Practice. J. Pharm. Sci. 1966, 55, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Trueba, M.; Trasatti, S.P. Electrochemical approach to repassivation kinetics of Al alloys: Gaining insight into environmentally assisted cracking. Corros. Rev. 2015, 33, 373–393. [Google Scholar] [CrossRef]
- Comotti, I.M.; Trueba, M.; Trasatti, S.P. The pit transition potential in the repassivation of aluminium alloys. Surf. Interface Anal. 2013, 45, 1575–1584. [Google Scholar] [CrossRef]
- Truba, M.; Trasatti, S.P. Study of Al alloy corrosion in neutral NaCl by the pitting scan technique. Mater. Chem. Phys. 2010, 121, 523–533. [Google Scholar] [CrossRef]

| Sample | Mn (g mol−1) | Mw (g mol−1) | PDI (Mw/Mn) |
|---|---|---|---|
| 6.1% octyloxy units | 2438 | 5402 | 2.21 |
| 13.4% octyloxy units | 2382 | 5496 | 2.3 |
| Sample | Tg (°C) | (T-Tg) (°C) |
|---|---|---|
| PPM | 55 | 65 |
| 6.1% octyloxy units | 48 | 71 |
| 13.4% octyloxy units | 31 | 89 |
| Sample | Advancing | Receding |
|---|---|---|
| 6.1% octyloxy units | 104° | 100° |
| 13.4% octyloxy units | 102° | 101° |
| AA 2024 (1) | 55° | 41° |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Elia, M.F.; Magni, M.; Trasatti, S.P.M.; Schweizer, T.B.; Niederberger, M.; Caseri, W. Poly(phenylene methylene)-Based Coatings for Corrosion Protection: Replacement of Additives by Use of Copolymers. Appl. Sci. 2019, 9, 3551. https://doi.org/10.3390/app9173551
D’Elia MF, Magni M, Trasatti SPM, Schweizer TB, Niederberger M, Caseri W. Poly(phenylene methylene)-Based Coatings for Corrosion Protection: Replacement of Additives by Use of Copolymers. Applied Sciences. 2019; 9(17):3551. https://doi.org/10.3390/app9173551
Chicago/Turabian StyleD’Elia, Marco F., Mirko Magni, Stefano P. M. Trasatti, Thomas B. Schweizer, Markus Niederberger, and Walter Caseri. 2019. "Poly(phenylene methylene)-Based Coatings for Corrosion Protection: Replacement of Additives by Use of Copolymers" Applied Sciences 9, no. 17: 3551. https://doi.org/10.3390/app9173551
APA StyleD’Elia, M. F., Magni, M., Trasatti, S. P. M., Schweizer, T. B., Niederberger, M., & Caseri, W. (2019). Poly(phenylene methylene)-Based Coatings for Corrosion Protection: Replacement of Additives by Use of Copolymers. Applied Sciences, 9(17), 3551. https://doi.org/10.3390/app9173551







