The Protection Role of Cysteine for Cu-5Zn-5Al-1Sn Alloy Corrosion in 3.5 wt.% NaCl Solution
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Potentiodynamic Polarization
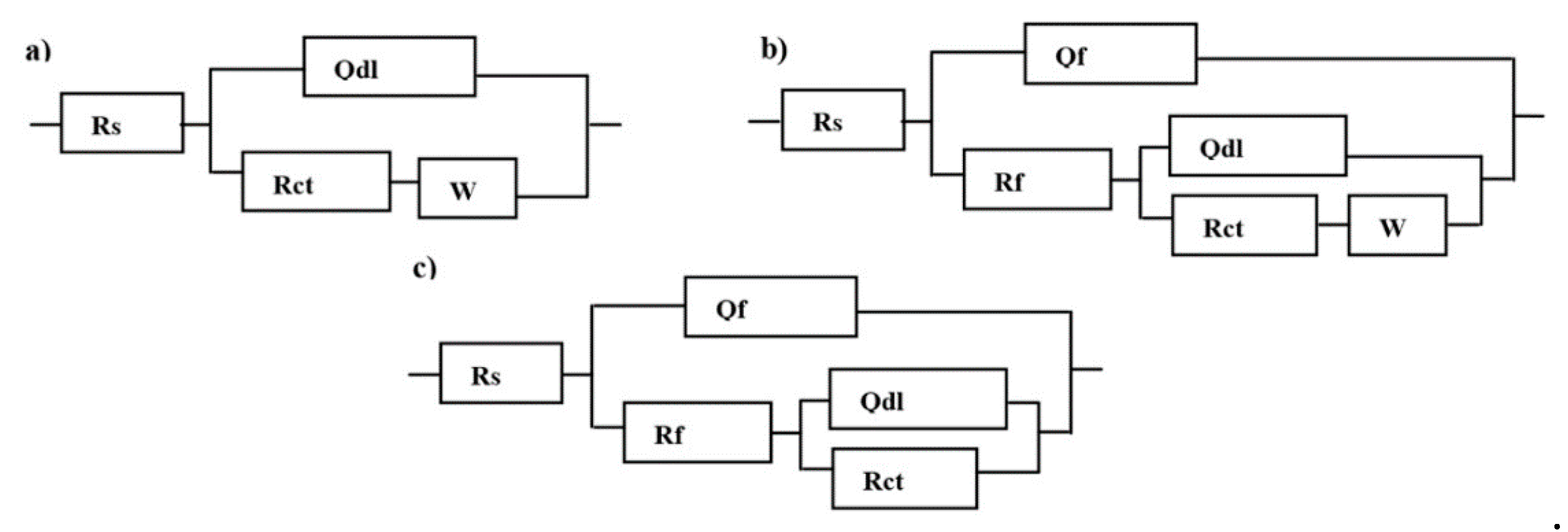
3.2. Electrochemical Impedance Spectroscopy
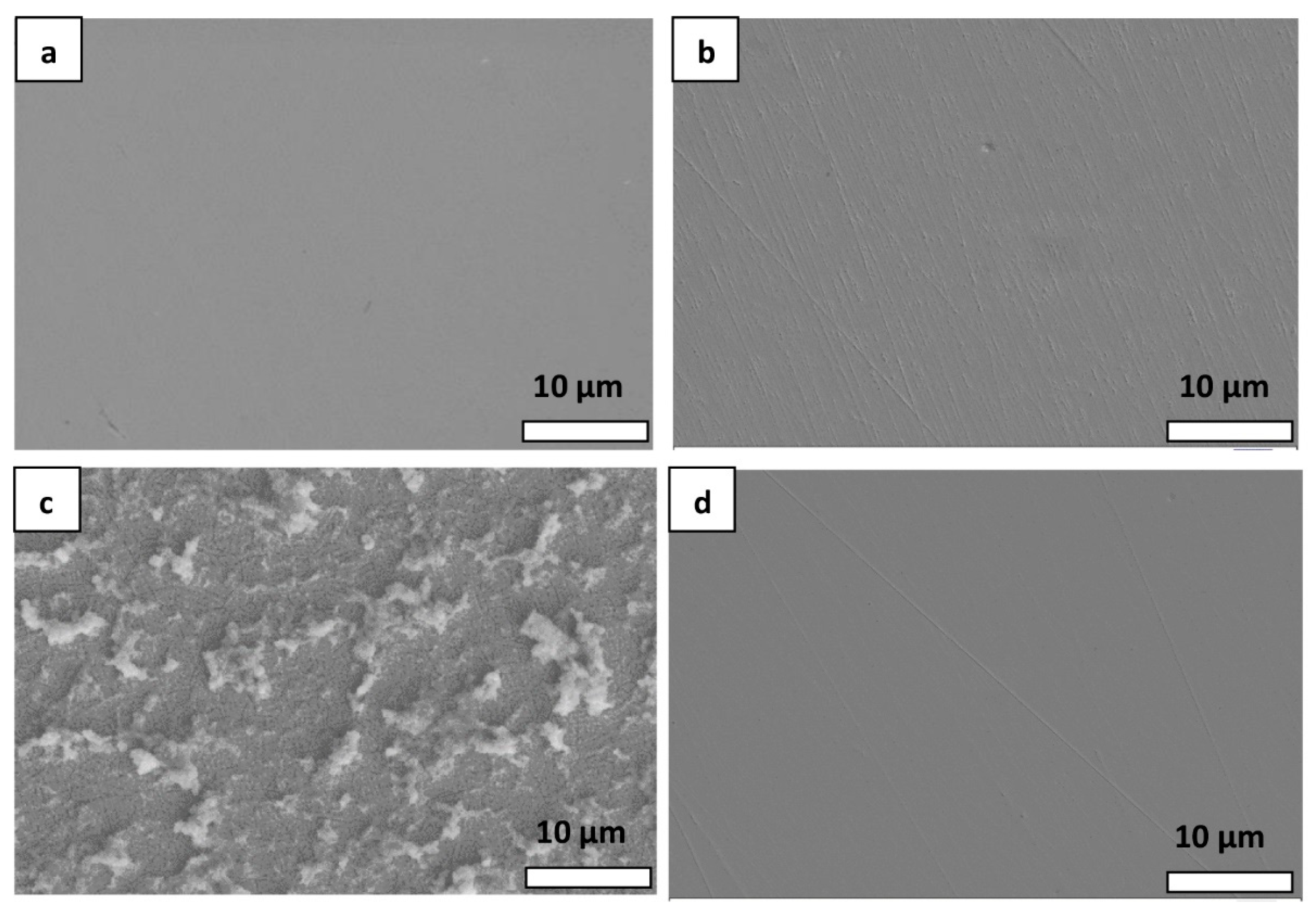
3.3. SEM Analysis
3.4. XPS and Auger Results
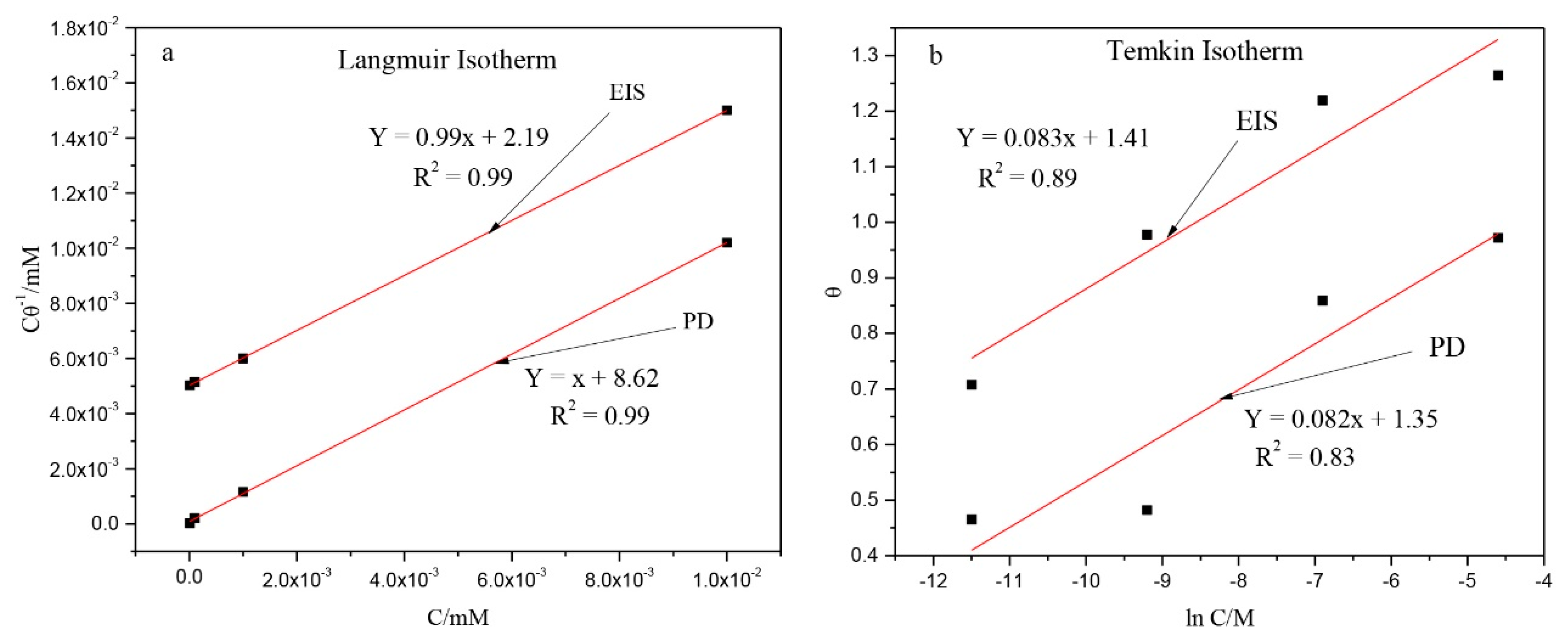
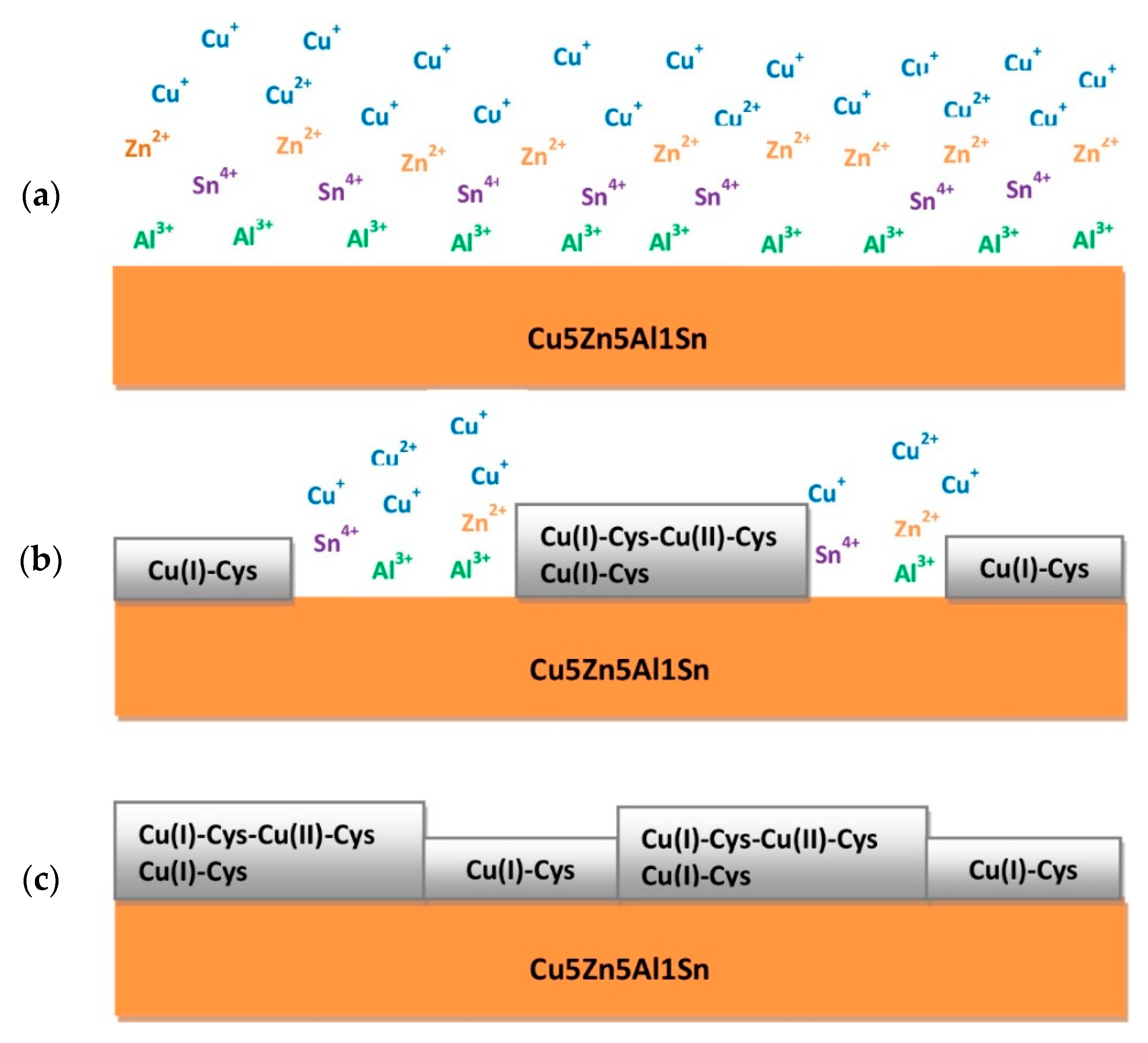
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, Y.; Yang, D.; Zhang, D.; Wang, Y.; Gao, L. Anti-corrosion film formed on HAl77-2 copper alloy surface by aliphatic polyamine in 3 wt.% NaCl solution. Appl. Surf. Sci. 2017, 392, 768–776. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, S.; Qiang, Y.; Guo, L.; Feng, L.; Liao, C. A combined experimental and theoretical study of the inhibition effect of three disulfide-based flavouring agents for copper corrosion in 0.5M sulfuric acid. J. Colloid Interface Sci. 2018, 526, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Tasić, Ž.Z.; Petrović, M.M.B.; Radovanović, M.B.; Simonović, A.T.; Antonijević, M.M. Cephradine as corrosion inhibitor for copper in 0.9% NaCl solution. J. Mol. Struct. 2018, 1159, 46–54. [Google Scholar] [CrossRef]
- Sayed, G.H.; Azab, M.E.; Anwer, K.E.; Raouf, M.A.; Negm, N.A. Pyrazole, pyrazolone and enaminonitrile pyrazole derivatives: Synthesis, characterization and potential in corrosion inhibition and antimicrobial applications. J. Mol. Liq. 2018, 252, 329–338. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Zhang, J.; Gao, L.; Feng, L.; Zhang, D. Click-assembling triazole membrane on copper surface via one-step or two-steps and their corrosion inhibition performance. Appl. Surf. Sci. 2018, 427, 1120–1128. [Google Scholar] [CrossRef]
- Zhang, X.; Odnevall, W.I.; Leygraf, C. Mechanistic studies of corrosion product flaking on copper and copper-based alloys in marine environments. Corros. Sci. 2014, 85, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Horton, D.J.; Ha, H.; Foster, L.L.; Bindig, H.J.; Scully, J.R. Tarnishing and Cu Ion release in Selected Copper-Base Alloys: Implications towards Antimicrobial Functionality. Electrochim. Acta 2015, 169, 351–366. [Google Scholar] [CrossRef]
- Chang, T.; Odnevall, W.I.; Jin, Y.; Leygraf, C. The golden alloy Cu-5Zn-5Al-1Sn: A multi-analytical surface characterization. Corros. Sci. 2018, 131, 94–103. [Google Scholar] [CrossRef]
- Lapeire, L.; Lombardia, E.M.; Verbeken, K.; De Graeve, I.; Terryn, H.; Kestens, L. Structural dependence of gold deposition by nanoplating in polycrystalline copper. J. Mater. Sci. 2014, 49, 3909–3916. [Google Scholar] [CrossRef]
- Martinez-Lombardia, E.; Maurice, V.; Lapeire, L.; De Graeve, I.; Verbeken, K.; Kestens, L.; Marcus, P.; Terryn, H. In situ scanning tunneling microscopy study of grain-dependent corrosion on microcrystalline copper. J. Phys. Chem. 2014, 118, 25421–25428. [Google Scholar] [CrossRef]
- Chang, T.; Herting, G.; Jin, Y.; Leygraf, C.; Odnevall, W.I. The golden alloy Cu5Zn5Al1Sn: Patina evolution in chloride-containing atmospheres. Corros. Sci. 2018, 133, 190–203. [Google Scholar] [CrossRef]
- Foster, L.L.; Scully, J.R. Corrosion of Cu-5Zn-5Al-1Sn (89% Cu, 5% Zn, 5% Al, 1% Sn) Compared to Copper in Synthetic Perspiration During Cyclic Wetting and Drying: The Fate of Copper. Corrosion 2016, 72, 1095–1106. [Google Scholar] [CrossRef]
- Goyal, M.; Kumar, S.; Bahadur, I.; Verma, C.; Ebenso, E.E. Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review. J. Mol. Liq. 2018, 256, 565–573. [Google Scholar] [CrossRef]
- Liu, G.; Huang, Y.; Qu, X.; Xiao, J.; Yang, X.; Xu, Z. Understanding the hydrophobic mechanism of 3-hexyl-4-amino-1, 2,4-triazole-5-thione to malachite by ToF-SIMS, XPS, FTIR, contact angle, zeta potential and micro-flotation. Colloids Surf. A Physicochem. Eng. Asp. 2016, 503, 34–42. [Google Scholar] [CrossRef]
- El-Deab, M.S. Interaction of cysteine and copper ions on the surface of iron: EIS, polarization and XPS study. Mater. Chem. Phys. 2011, 129, 223–227. [Google Scholar] [CrossRef]
- Nazeer, A.A.; Allam, N.K.; Fouda, A.S.; Ashour, E.A. Effect of cysteine on the electrochemical behavior of Cu10Ni alloy in sulfide polluted environments: Experimental and theoretical aspects. Mater. Chem. Phys. 2012, 136, 1–9. [Google Scholar] [CrossRef]
- Radovanovic, M.B.; Petrovic, M.B.; Simonovic, A.T.; Milic, S.M.; Antonijevic, M.M. Cysteine as a green corrosion inhibitor for Cu37Zn brass in neutral and weakly alkaline sulphate solutions. Environ. Sci. Pollut. Res. Int. 2013, 20, 4370–4381. [Google Scholar] [CrossRef]
- Kazansky, L.P.; Selyaninov, I.A.; Kuznetsov, Y.I. Adsorption of 2-mercaptobenzothiazole on copper surface from phosphate solutions. Appl. Surf. Sci. 2012, 258, 6807–6813. [Google Scholar] [CrossRef]
- Ghelichkhah, Z.; Sharifi-Asl, S.; Farhadi, K.; Banisaied, S.; Ahmadi, S.; Macdonald, D.D. L-cysteine/polydopamine nanoparticle-coatings for copper corrosion protection. Corros. Sci. 2015, 91, 129–139. [Google Scholar] [CrossRef]
- Milosev, I.; Pavlinac, J.; Hodoscek, M.; Lesar, A. Amino acids as corrosion inhibitors for copper in acidic medium: Experimental and theoretical study. J. Serb. Chem. Soc. 2013, 78, 2069–2086. [Google Scholar] [CrossRef]
- El-Hafez, G.M.A.; Badawy, W.A. The use of cysteine, N-acetyl cysteine and methionine as environmentally friendly corrosion inhibitors for Cu–10Al–5Ni alloy in neutral chloride solutions. Electrochim. Acta 2013, 108, 860–866. [Google Scholar] [CrossRef]
- Sabet, B.K.; Dehghanian, C. Adsorption behavior of 1H-benzotriazole corrosion inhibitor on aluminum alloy 1050, mild steel and copper in artificial seawater. J. Environ. Chem. Eng. 2018, 6, 1613–1624. [Google Scholar] [CrossRef]
- Tasić, Ž.Z.; Mihajlović, M.B.P.; Radovanović, M.B.; Antonijević, M.M. Electrochemical investigations of copper corrosion inhibition by azithromycin in 0.9% NaCl. J. Mol. Liq. 2018, 265, 687–692. [Google Scholar] [CrossRef]
- Karthik, G.; Sundaravadivelu, M. Investigations of the inhibition of copper corrosion in nitric acid solutions by levetiracetam drug. Egypt. J. Pet. 2016, 25, 481–493. [Google Scholar] [CrossRef] [Green Version]
- Khadom, A.A.; Yaro, A.S. Mass transfer effect on corrosion inhibition process of copper–nickel alloy in hydrochloric acid by Benzotriazole. J. Saudi Chem. Soc. 2014, 18, 214–219. [Google Scholar] [CrossRef]
- Vastag, G.; Shaban, A.; Vraneš, M.; Tot, A.; Belić, S.; Gadžurić, S. Influence of the N-3 alkyl chain length on improving inhibition properties of imidazolium-based ionic liquids on copper corrosion. J. Mol. Liq. 2018, 264, 526–533. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; El Basiony, N.M.; Sadeek, S.A.; Migahed, M.A. Scale and corrosion inhibition performance of the newly synthesized anionic surfactant in desalination plants: Experimental, and theoretical investigations. Desalination 2018, 437, 45–58. [Google Scholar] [CrossRef]
- Sabet, B.K.; Dehghanian, C.; Yari, S. Corrosion inhibition of copper, mild steel and galvanically coupled copper-mild steel in artificial sea water in presence of 1H-benzotriazole, sodium molybdate and sodium phosphate. Corros. Sci. 2017, 126, 272–285. [Google Scholar] [CrossRef]
- Srivastava, M.; Tiwari, P.; Srivastava, S.K.; Kumar, A.; Ji, G.; Prakash, R. Low cost aqueous extract of Pisum sativum peels for inhibition of mild steel corrosion. J. Mol. Liq. 2018, 254, 357–368. [Google Scholar] [CrossRef]
- Kong, D.; Dong, C.; Ni, X.; Man, C.; Xiao, K.; Li, X. Insight into the mechanism of alloying elements (Sn, Be) effect on copper corrosion during long-term degradation in harsh marine environment. Appl. Surf. Sci. 2018, 455, 543–553. [Google Scholar] [CrossRef]
- Prakashaiah, B.G.; Vinaya, K.D.; Anup, P.A.; Nityananda, S.A.; Amitha, R.B.E. Corrosion inhibition of 2024-T3 aluminum alloy in 3.5% NaCl by thiosemicarbazone derivatives. Corros. Sci. 2018, 136, 326–338. [Google Scholar] [CrossRef]
- Jiang, L.; Qiang, Y.; Lei, Z.; Wang, J.; Qin, Z.; Xiang, B. Excellent corrosion inhibition performance of novel quinoline derivatives on mild steel in HCl media: Experimental and computational investigations. J. Mol. Liq. 2018, 255, 53–63. [Google Scholar] [CrossRef]
- Boyapati, V.A.R.; Kanukula, C.K. Corrosion Inhibition of Cu-Ni (90/10) Alloy in Seawater and Sulphide-Polluted Seawater Environments by 1,2,3-Benzotriazole. ISRN Corros. 2013, 2013, 703929. [Google Scholar] [CrossRef]
- Deyab, M.A. Corrosion inhibition of heat exchanger tubing material (titanium) in MSF desalination plants in acid cleaning solution using aromatic nitro compounds. Desalination 2018, 439, 73–79. [Google Scholar] [CrossRef]
- Negm, N.A.; Migahed, M.A.; Farag, R.K.; Fadda, A.A.; Awad, M.K.; Shaban, M.M. High performance corrosion inhibition of novel tricationic surfactants on carbon steel in formation water: Electrochemical and computational evaluations. J. Mol. Liq. 2018, 262, 363–375. [Google Scholar] [CrossRef]
- Chaubey, N.; Singh, V.K.; Quraishi, M.A. Corrosion inhibition performance of different bark extracts on aluminium in alkaline solution. J. Assoc. Arab Univ. Basic Appl. Sci. 2018, 22, 38–44. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Pugazhendhi, A.; Lee, Y.R.; Sethuraman, M.G. Corrosion inhibition performance of spermidine on mild steel in acid media. J. Mol. Liq. 2018, 264, 483–489. [Google Scholar] [CrossRef]
- Raman, K.; Hansung, K.; Reddicherla, U.; Ompal, S.Y.; Gurmeet, S. Comprehensive adsorption characteristics of a newly synthesized and sustainable anti-corrosion catalyst onmild steel surface exposed to a highly corrosive electrolytic solution. J. Mol. Liq. 2018, 268, 37–48. [Google Scholar]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. (Eds.) Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MN, USA, 1979; pp. 41–127. [Google Scholar]
- Wang, T.; Wang, J.; Wu, Y. The inhibition effect and mechanism of l-cysteine on the corrosion of bronze covered with a CuCl patina. Corros. Sci. 2015, 97, 89–99. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, Y.; Jing, C.; Huang, H.; Li, H.; Zhang, S. Synthesis of dibenzotriazole derivatives bearing alkylene linkers as corrosion inhibitors for copper in sodium chloride solution: A new thought for the design of organic inhibitors. Corros. Sci. 2016, 113, 64–77. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Gong, Y.; Gao, F.; Luo, Z.; Zhang, S. Water soluble corrosion inhibitors for copper in 3.5 wt% sodium chloride solution. Corros. Sci. 2017, 123, 339–350. [Google Scholar] [CrossRef]
- Milošev, I.; Kovačević, N.; Kovač, J.; Kokalj, A. The roles of mercapto, benzene and methyl groups in the corrosion inhibition of imidazoles on copper: I. Experimental characterization. Corros. Sci. 2015, 98, 107–118. [Google Scholar] [CrossRef]
- Finšgar, M. EQCM and XPS analysis of 1,2,4-triazole and 3-amino-1,2,4-triazole as copper corrosion inhibitors in chloride solution. Corros. Sci. 2013, 77, 350–359. [Google Scholar] [CrossRef]
- Finšgar, M. 2-Mercaptobenzimidazole as a copper corrosion inhibitor: Part II. Surface analysis using X-ray photoelectron spectroscopy. Corros. Sci. 2013, 72, 90–98. [Google Scholar] [CrossRef]
- Finšgar, M.; Merl, D.K. An electrochemical, long-term immersion, and XPS study of 2-mercaptobenzothiazole as a copper corrosion inhibitor in chloride solution. Corros. Sci. 2014, 83, 164–175. [Google Scholar] [CrossRef]
- Finšgar, M. X-ray excited Auger Cu L₃L₄,₅M₄,₅ spectra measured at low take-off angles as a fingerprint for a Cu-organics connection. J. Electron Spectrosc. Relat. Phenom. 2018, 222, 10–14. [Google Scholar] [CrossRef]
- Qiang, Y.; Fu, S.; Zhang, S.; Chen, S.; Zou, X. Designing and fabricating of single and double alkyl-chain indazole derivatives self-assembled monolayer for corrosion inhibition of copper. Corros. Sci. 2018, 140, 111–121. [Google Scholar] [CrossRef]
- Koitaya, T.; Shiozawa, Y.; Yoshikura, Y.; Mukai, K.; Yoshimoto, S.; Torii, S. Electronic states and growth modes of Zn atoms deposited on Cu(111) studied by XPS, UPS and DFT. Surf. Sci. 2017, 663, 1–10. [Google Scholar] [CrossRef]
- Morozov, I.G.; Belousova, O.V.; Ortega, D.; Mafina, M.K.; Kuznetcov, M.V. Structural, optical, XPS and magnetic properties of Zn particles capped by ZnO nanoparticles. J. Alloy. Compd. 2015, 633, 237–245. [Google Scholar] [CrossRef]
- Sun, J.; Yarmolenko, M.A.; Rogachev, A.A.; Rogachev, A.V.; Jiang, X.; Gorbachev, D.L. Investigation of structural properties of electron-beam deposition of zinc oxide coatings doped with copper. Surf. Interfaces 2017, 6, 24–32. [Google Scholar] [CrossRef]
- Babu, B.; Neelakanta, R.I.; Yoo, K.; Kim, D.; Shim, J. Bandgap tuning and XPS study of SnO₂ quantum dots. Mater. Lett. 2018, 221, 211–215. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, L.; An, Y.; Wang, X.; Xu, G.; Chen, Y. Promotional synergistic effect of Sn doping into a novel bimetallic Sn-W oxides/graphene catalyst for selective oxidation of alcohols using aqueous H₂O₂ without additives. Appl. Catal. A Gen. 2018, 558, 26–33. [Google Scholar] [CrossRef]
- Hutchison, M.J.; Zhou, P.; Ogle, K.; Scully, J.R. Enhanced Electrochemical Cu Release from Commercial Cu-Sn Alloys: Fate of the Alloying Elements in Artificial Perspiration. Electrochim. Acta 2017, 241, 73–88. [Google Scholar] [CrossRef] [Green Version]
- Zatsepin, D.A.; Zatsepin, A.F.; Boukhvalov, D.W.; Kurmaev, E.Z.; Gavrilov, N.V. Sn-loss effect in a Sn-implanted α-SiO₂ host-matrix after thermal annealing: A combined XPS, PL, and DFT study. Appl. Surf. Sci. 2016, 367, 320–326. [Google Scholar] [CrossRef]
- Sangaiya, P.; Jayaprakash, R. Tuning effect of Sn doping on structural, morphological, optical, electrical and photocatalytic properties of iron oxide nanoparticles. Mater. Sci. Semicond. Process. 2018, 85, 40–51. [Google Scholar] [CrossRef]
- Moscu, A.; Theodoridi, C.; Cardenas, L.; Thieuleux, C.; Motta-Meira, D.; Agostini, G. CO dissociation on Pt-Sn nanoparticles triggers Sn oxidation and alloy segregation. J. Catal. 2018, 359, 76–81. [Google Scholar] [CrossRef]
- Finšgar, M.; Merl, D.K. 2-Mercaptobenzoxazole as a copper corrosion inhibitor in chloride solution: Electrochemistry, 3D-profilometry, and XPS surface analysis. Corros. Sci. 2014, 80, 82–95. [Google Scholar] [CrossRef]
- Appa, R.B.V.; Narsihma, R.M. Formation, characterization and corrosion protection efficiency of self-assembled 1-octadecyl-1H-imidazole films on copper for corrosion protection. Arab. J. Chem. 2017, 10, S3270–S3283. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Gong, Y.; Zhang, L.; Jing, C.; Gao, F.; Zhang, S. Self-assembly of new dendrimers basing on strong π-π intermolecular interaction for application to protect copper. Chem. Eng. J. 2018, 342, 238–250. [Google Scholar] [CrossRef]
- Wagner, A.J.; Wolfe, G.M.; Fairbrother, D.H. Reactivity of vapor-deposited metal atoms with nitrogen-containing polymers and organic surfaces studied by in situ XPS. Appl. Surf. Sci. 2003, 219, 317–328. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Gao, L.X.; Zhou, G.D. Synergistic effect of 2-mercapto benzimidazole and KI on copper corrosion inhibition in aerated sulfuric acid solution. J. Appl. Electrochem. 2003, 33, 361–366. [Google Scholar] [CrossRef]
- Krzysztof, S.; Roman, D.; Zofia, P.; Stefan, W. transformation of nitrogen structures in carbonization of model compounds determined by XPS. Carbon 1995, 33, 1383–1392. [Google Scholar]
- Wang, C.; Luo, X.; Jia, Z. Linkage, charge state and layer of L-Cysteine on copper surfaces. Colloids Surf. B Biointerfaces 2017, 160, 33–39. [Google Scholar] [CrossRef]
- Ismail, K.M. Evaluation of cysteine as environmentally friendly corrosion inhibitor for copper in neutral and acidic chloride solutions. Electrochim. Acta 2007, 52, 7811–7819. [Google Scholar] [CrossRef]
- Vinothkumar, K.; Sethuraman, M.G. Corrosion inhibition ability of electropolymerised composite film of 2-amino-5-mercapto-1,3,4-thiadiazole/TiO₂ deposited over the copper electrode in neutral medium. Mater. Today Commun. 2018, 14, 27–39. [Google Scholar] [CrossRef]
- Mahdavian, M.; Tehrani-Bagha, A.R.; Alibakhshi, E.; Ashhari, S.; Palimi, M.J.; Farashi, S.; Javadiane, S.; Ektefae, F. Corrosion of mild steel in hydrochloric acid solution in the presence of two cationic gemini surfactants with and without hydroxyl substituted spacers. Corros. Sci. 2018, 137, 62–75. [Google Scholar] [CrossRef]
- Roland, T.L.; Olukeye, T. Corrosion inhibition properties of the synergistic effect of 4-hydroxy-3-methoxybenzaldehyde and hexadecyltrimethylammoniumbromide on mild steel in dilute acid solutions. J. King Saud Univ. Eng. Sci. 2018, 30, 384–390. [Google Scholar] [Green Version]
- Hany, M.A.; Ahmed, M.A.; Bahaa, E.M. Investigation of adsorption and inhibition effects of some novel anil compounds towards mild steel in H₂SO₄ solution: Electrochemical and theoretical quantum studies. J. Electroanal Chem. 2015, 758, 135–147. [Google Scholar]
- Yang, W.; Li, T.; Zhou, H.; Huang, Z.; Fu, C.; Chen, L.; Li, M.; Kuang, Y. Electrochemical and anti-corrosion properties of octadecanethiol and benzotriazole binary self-assembled monolayers on copper. Electrochim. Acta. 2016, 220, 245–251. [Google Scholar] [CrossRef]
- Chen, W.; Hong, S.; Li, H.B.; Li, B.; Luo, H.Q.; Li, M.; Li, N.B. Protection of copper corrosion in 0.5 M NaCl solution by modification of 5-mercapto-3-phenyl-1,3,4-thiadiazole-2-thione potassium self-assembled monolayer. Corros. Sci. 2012, 61, 53–62. [Google Scholar] [CrossRef]
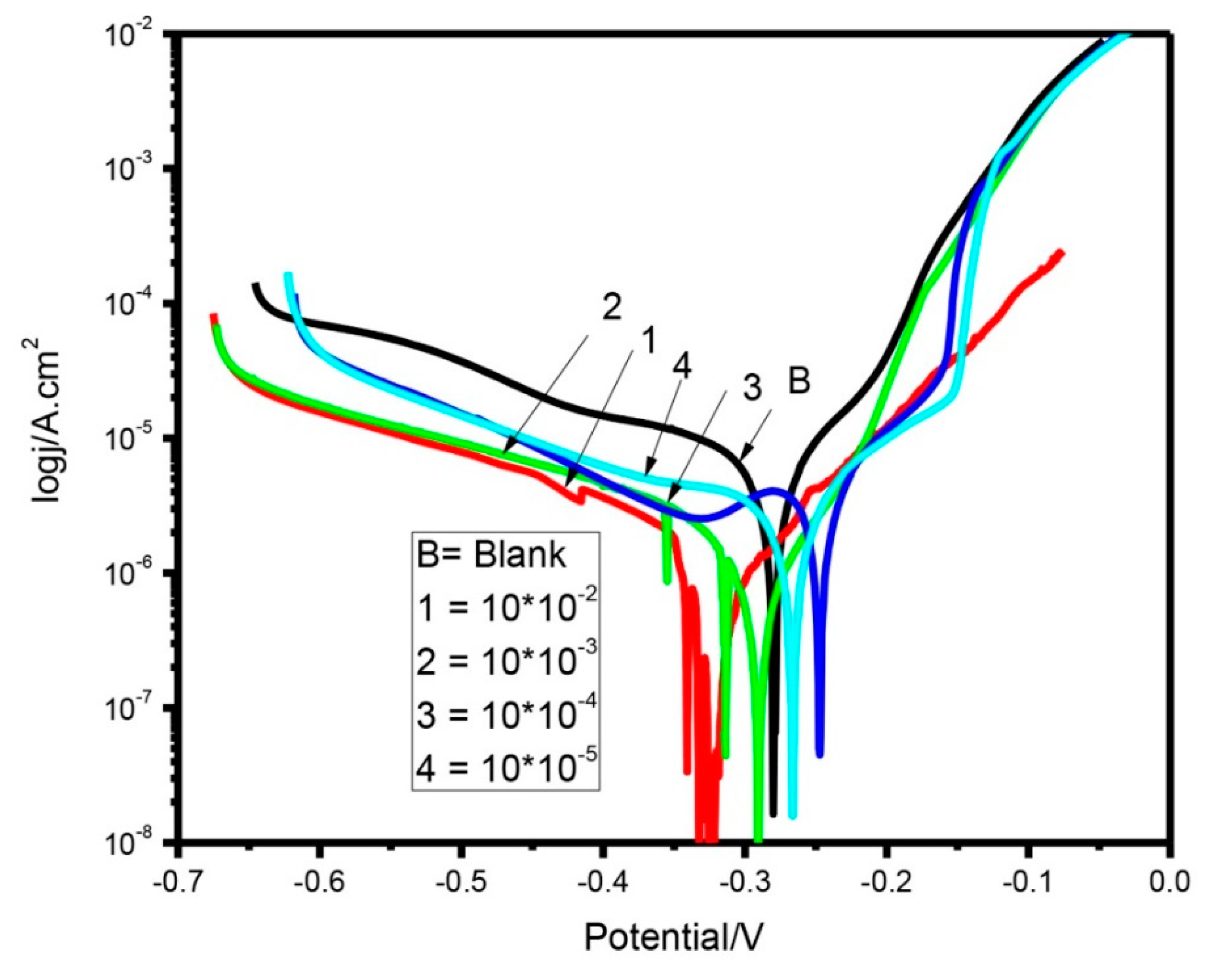


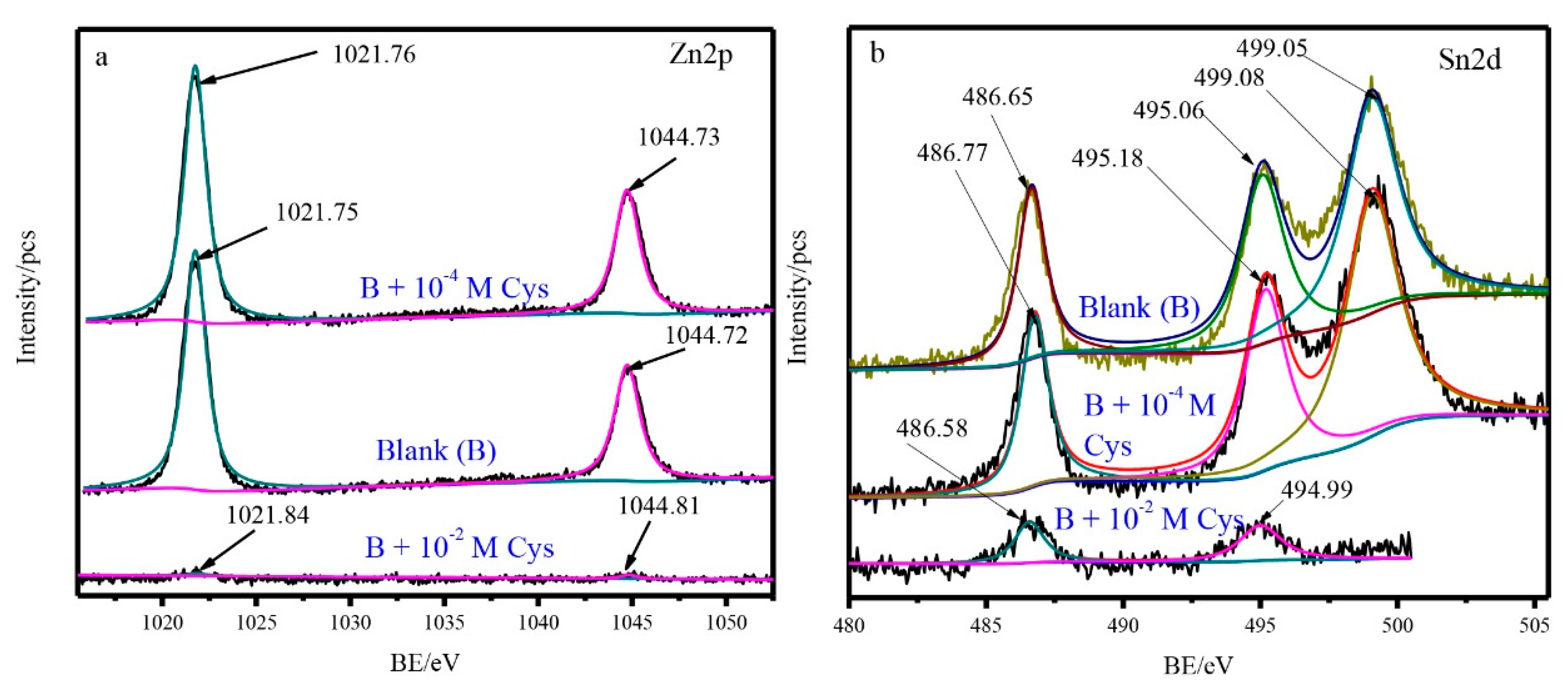

| Cysteine Concentration | Ecorr (V versus Ag/AgCl) | βa (mV/Decade) | βc (mV/Decade) | Icorr (µAcm−2) | µ% |
|---|---|---|---|---|---|
| Blank (0) | −0.280 | 69.18 | 102.3 | 3.44 | - |
| 10−5 M | −0.226 | 77.77 | 127.2 | 1.84 | 46.5 |
| 10−4 M | −0.247 | 48.5 | 63.86 | 1.78 | 48.2 |
| 10−3 M | −0.292 | 61.23 | 56.96 | 0.483 | 85.9 |
| 10−2 M | −0.326 | 23.71 | 19.49 | 0.094 | 97.2 |
| Cys Conc. (M) | Rs (Ω) | Qf | Rf (Ω) | Qct | Rct (KΩ) | W | Rp (KΩ) | µ% |
|---|---|---|---|---|---|---|---|---|
| Blank | 2.668 | 1.55 × 10−4 | 692.7 | 0.0032 | 0.695 | - | ||
| 10−5 | 3.224 | 8.09 × 10−5 | 588.1 | 1.64 × 10−4 | 1169 | 0.0053 | 1.197 | 40.76 |
| 10−4 | 2.894 | 1.05 × 10−4 | 212.7 | 1.61 × 10−4 | 1.986 | 2.201 | 67.78 | |
| 10−3 | 3.345 | 8.24 × 10−5 | 2382 | 1.10 × 10−4 | 6.399 | 8.784 | 91.92 | |
| 10−2 | 3.067 | 5.28 × 10−5 | 12.02 | 4.41 × 10−6 | 19.940 | 19.955 | 96.44 |
| Element | Atomic % | ||
|---|---|---|---|
| Blank | Blank + 10−4 M Cys | Blank + 10−2 M Cys | |
| C1s | 37.9 | 33.54 | 49.4 |
| N1s | 2.77 | 4.52 | 7.78 |
| O1s | 36.99 | 37.07 | 29.38 |
| S2p | 0 | 2.1 | 3.29 |
| Cu2p | 13.22 | 14.42 | 9.36 |
| Zn2p | 7.98 | 7.26 | 0.63 |
| Sn3d | 1.14 | 1.08 | 0.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinato, K.W.; Huang, F.; Xue, Y.; Wen, L.; Jin, Y. The Protection Role of Cysteine for Cu-5Zn-5Al-1Sn Alloy Corrosion in 3.5 wt.% NaCl Solution. Appl. Sci. 2019, 9, 3896. https://doi.org/10.3390/app9183896
Shinato KW, Huang F, Xue Y, Wen L, Jin Y. The Protection Role of Cysteine for Cu-5Zn-5Al-1Sn Alloy Corrosion in 3.5 wt.% NaCl Solution. Applied Sciences. 2019; 9(18):3896. https://doi.org/10.3390/app9183896
Chicago/Turabian StyleShinato, Kebede W., Feifei Huang, Yanpeng Xue, Lei Wen, and Ying Jin. 2019. "The Protection Role of Cysteine for Cu-5Zn-5Al-1Sn Alloy Corrosion in 3.5 wt.% NaCl Solution" Applied Sciences 9, no. 18: 3896. https://doi.org/10.3390/app9183896
APA StyleShinato, K. W., Huang, F., Xue, Y., Wen, L., & Jin, Y. (2019). The Protection Role of Cysteine for Cu-5Zn-5Al-1Sn Alloy Corrosion in 3.5 wt.% NaCl Solution. Applied Sciences, 9(18), 3896. https://doi.org/10.3390/app9183896






