Development of a SnS Film Process for Energy Device Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
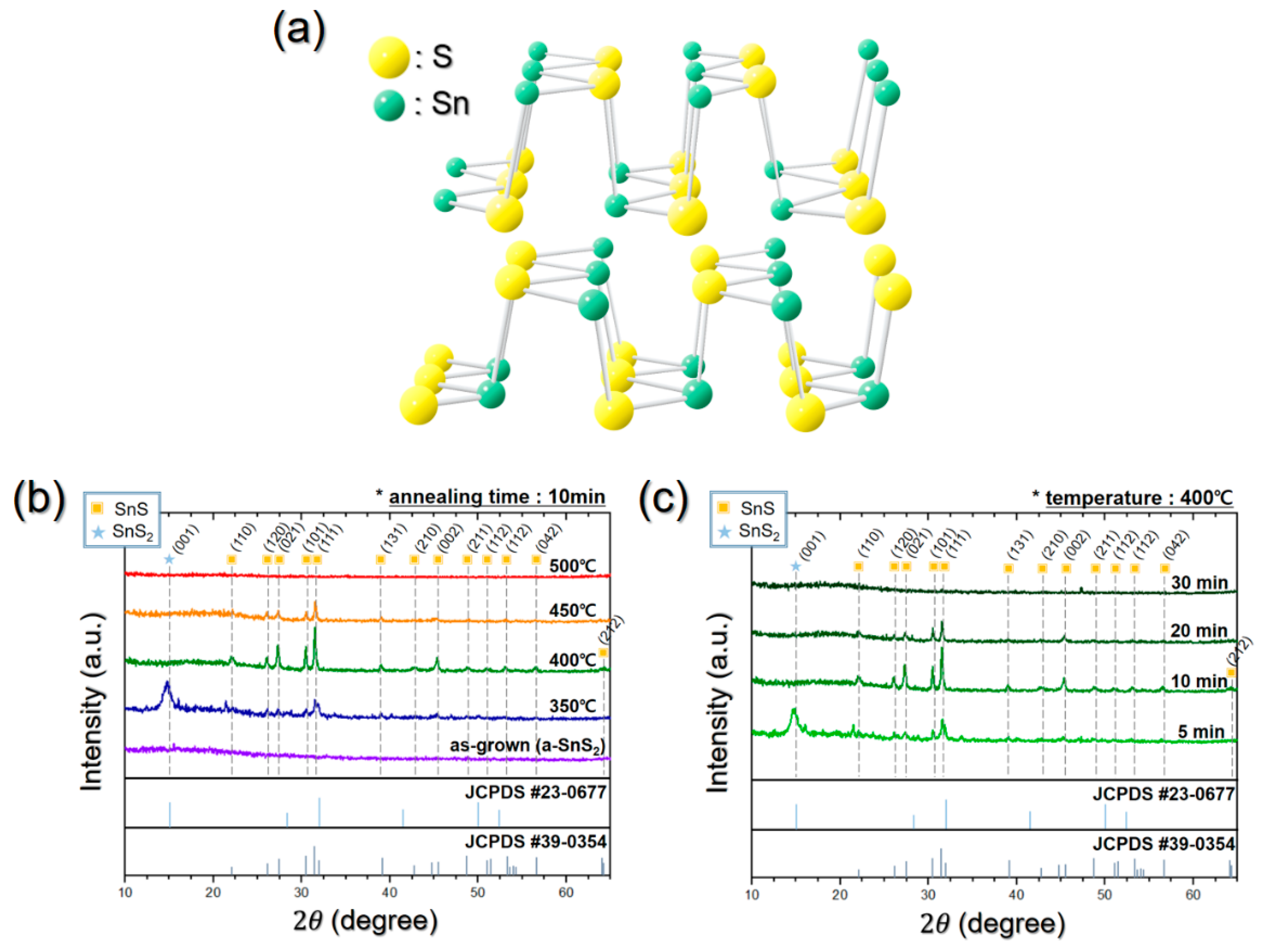
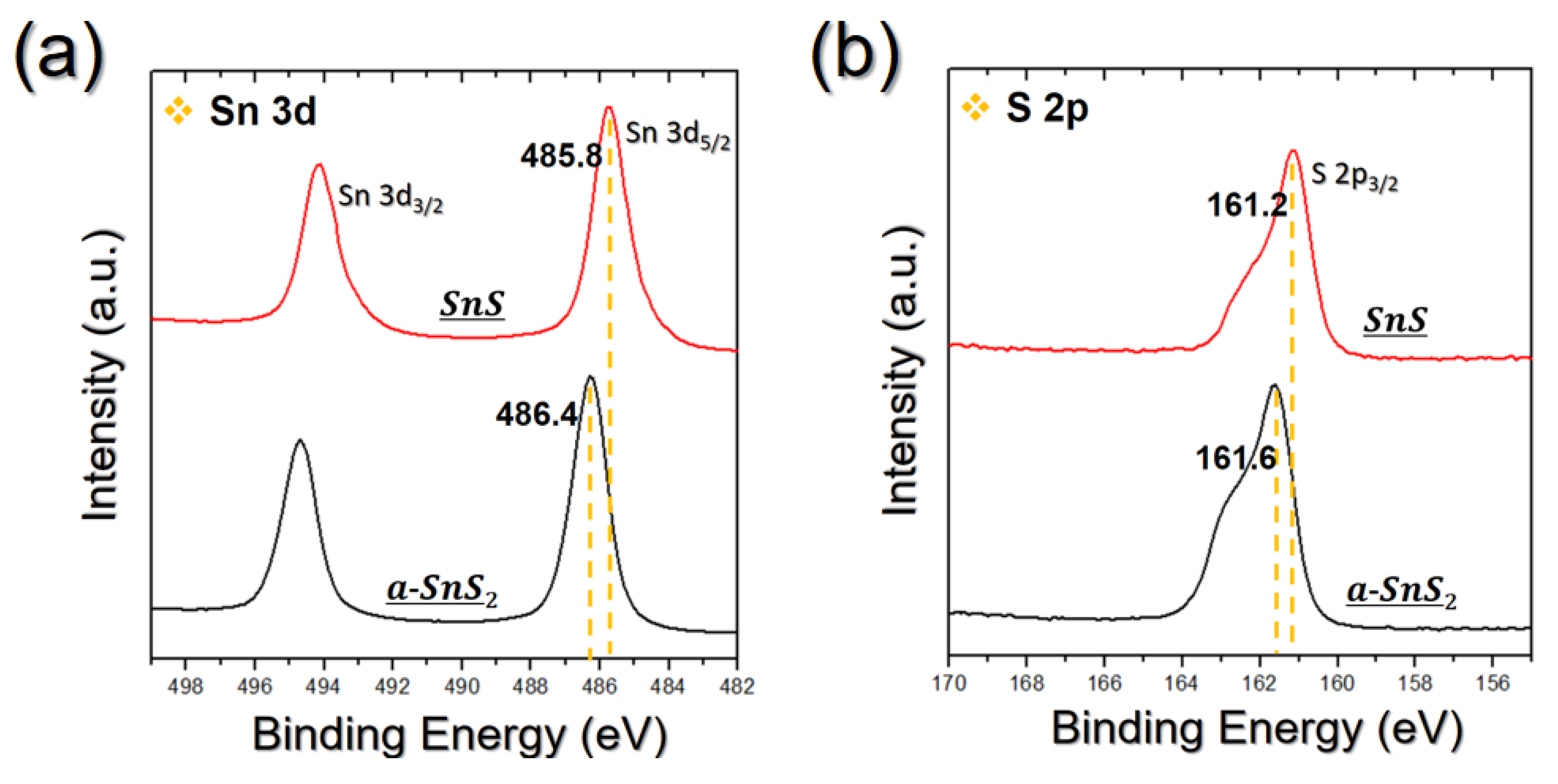
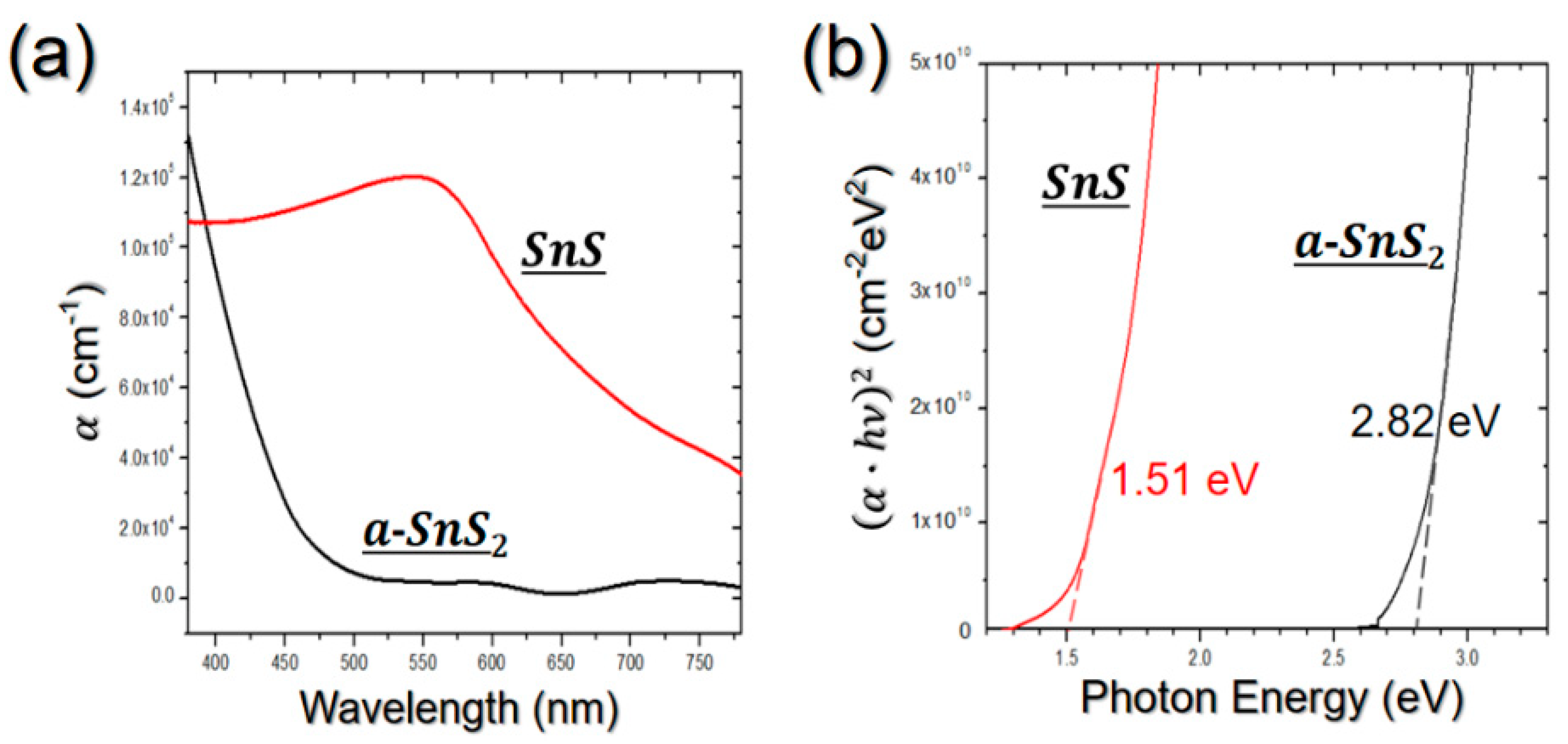
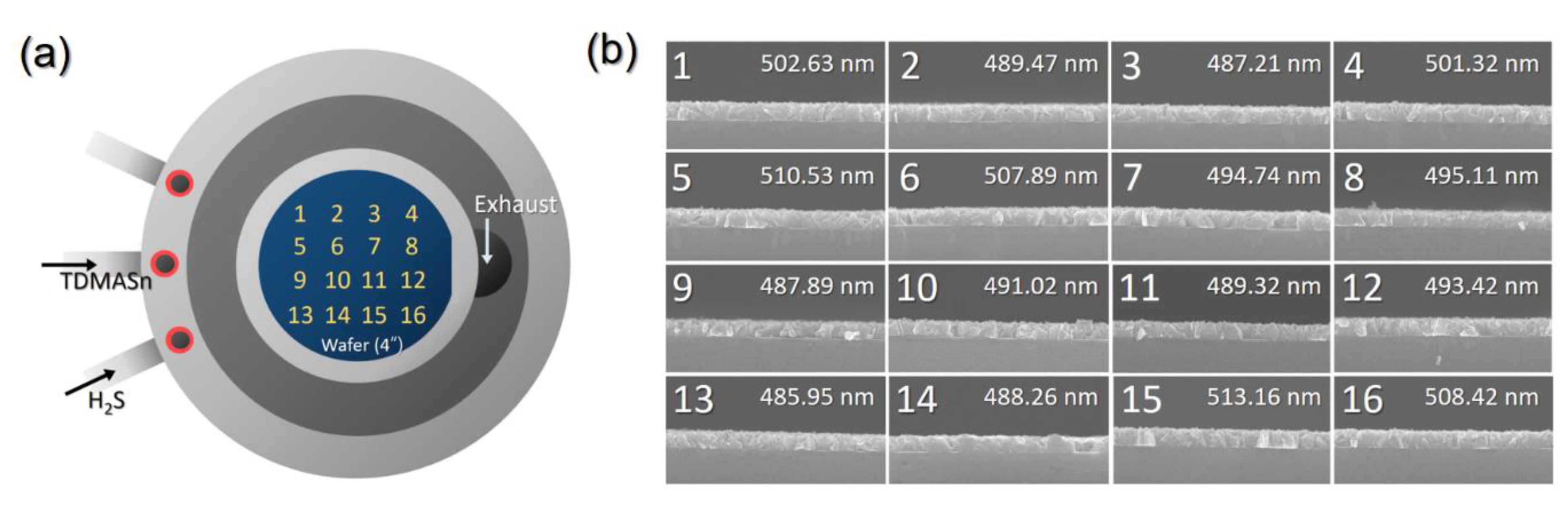
3.1. Development of a Process Proper for Obtaining a Thick SnS Film Using ALD
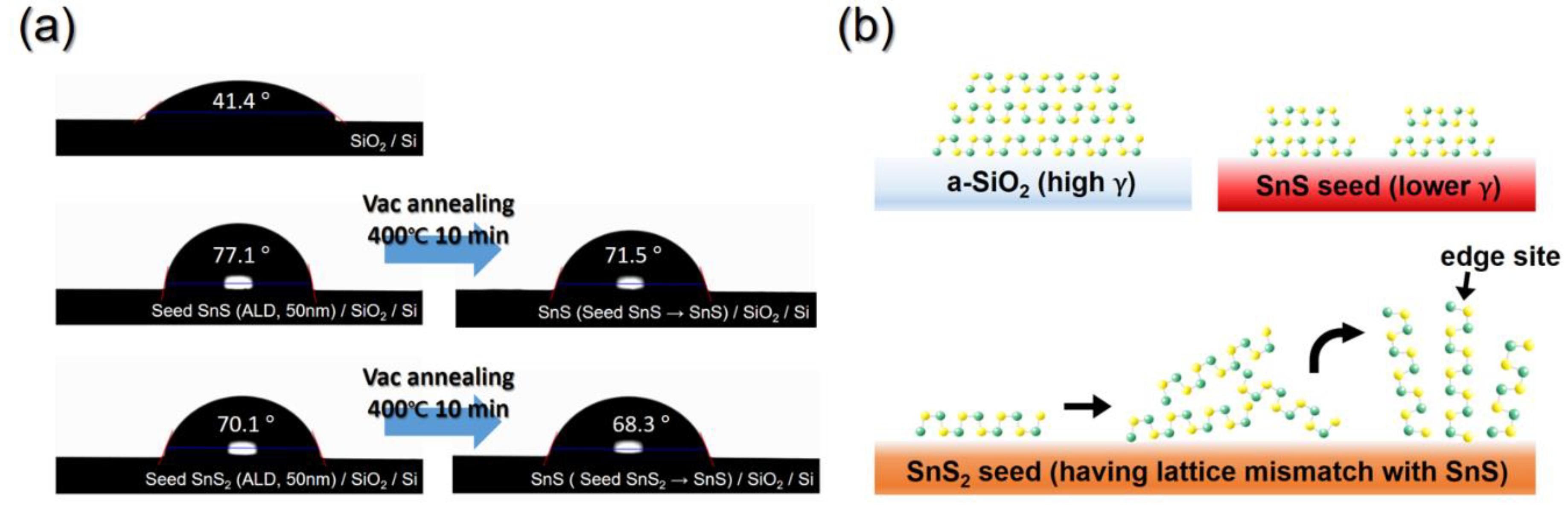
3.2. Process to Control the Film Characteristics of SnS Film Using Seed Layer
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Price, L.S.; Parkin, I.P.; Hardy, A.M.E.; Clark, R.J.H. Atmospheric Pressure Chemical Vapor Deposition of Tin Sulfides (SnS, Sn2S3, and SnS2) on Glass. Chem. Mater. 1999, 11, 1792–1799. [Google Scholar] [CrossRef]
- Sinsermsuksakul, P.; Heo, J.; Noh, W.; Hock, A.S.; Gordon, R.G. Atomic Layer Deposition of Tin Monosulfide Thin Films. Adv. Energy Mater. 2011, 1, 1116–1125. [Google Scholar] [CrossRef] [Green Version]
- Burton, L.A.; Whittles, T.J.; Hesp, D.; Linhart, W.M.; Skelton, J.M.; Hou, B.; Werster, R.F.; O’dard, G.; Reece, C.; Cherns, D.; et al. Electronic and optical properties of single crystal SnS2: An earth-abundant disulfide photocatalyst. J. Mater. Chem. A 2016, 4, 1312–1318. [Google Scholar] [CrossRef]
- Sucharitakul, S.; Kumar, U.R.; Sankar, R.; Chou, F.C.; Chen, Y.T.; Wang, C.; He, C.; Gao, X.P.A. Screening limited switching performance of multilayer 2D semiconductor FETs: The case for SnS. Nanoscale 2016, 8, 19050–19057. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, B.; Kaliannan, P. Spray pyrolytic deposition and characterization of SnS and SnS2 thin films. J. Phys. D Appl. Phys. 2000, 33, 1054–1059. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Zeyada, H.M.; Aziz, M.S.; El-Ghamaz, N.A. Optical properties of thermally evaporated SnS thin films. Opt. Mater. 2002, 20, 159–170. [Google Scholar] [CrossRef]
- Cifuentes, C.; Romero, E.; Calderon, C.; Gordillo, G. Optical and Structural Studies on SnS Films Grown by Co-Evaporation. Braz. J. Phys. 2006, 36, 1046–1049. [Google Scholar] [CrossRef]
- Burton, L.A.; Colombara, D.; Abellon, R.D.; Grozema, F.C.; Peter, L.M.; Savenije, T.J.; Dennler, G.; Walsh, A. Synthesis, Characterization, and Electronic Structure of Single-Crystal SnS, Sn2S3, and SnS2. Chem. Mater. 2013, 25, 4908–4916. [Google Scholar] [CrossRef]
- Kumagai, Y.; Burton, L.A.; Walsh, A.; Oba, F. Electronic Structure and Defect Physics of Tin Sulfides: SnS, Sn2S3, and SnS2. Phys. Rev. Appl. 2016, 6, 014009. [Google Scholar] [CrossRef]
- Burton, L.A.; Walsh, A. Phase Stability of the Earth-Abundant Tin Sulfides SnS, SnS2, and Sn2S3. J. Phys. Chem. C 2012, 116, 24262–24267. [Google Scholar] [CrossRef]
- Polizzotti, A.; Faghaninia, A.; Poindexter, J.R.; Nienhaus, L.; Steinmann, V.; Hoye, R.L.Z.; Felten, A.; Deyine, A.; Mangan, N.M.; Correa-Baena, J.P.; et al. Improving the Carrier Lifetime of Tin Sulfide via Prediction and Mitigation of Harmful Point Defects. J. Phys. Chem. Lett. 2017, 8, 3661–3667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malone, B.D.; Galibc, A.; Kaxiras, E. First principles study of point defects in SnS. Phys. Chem. Chem. Phys. 2014, 16, 26176–26183. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Heasley, R.; Sun, L.; Steinmann, V.; Jaramillo, R.; Hartman, K.; Chakraborty, R.; Sinsermsuksakul, P.; Chua, D.; Buonassisi, T.; et al. Co-optimization of SnS absorber and Zn (O, S) buffer materials for improved solar cells. Prog. Photovolt. Res. Appl. 2015, 23, 901–908. [Google Scholar] [CrossRef]
- Steinmann, V.; Jaramillo, R.; Hartman, K.; Chakraborty, R.; Brandt, R.E.; Poindexter, J.R.; Lee, Y.S.; Sun, L.; Polizzotti, A.; Park, H.H.; et al. 3.88% Efficient Tin Sulfide Solar Cells using Congruent Thermal Evaporation. Adv. Mater. 2014, 26, 7488–7492. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.T.R.; Reddy, N.K.; Miles, R.W. Photovoltaic properties of SnS based solar cells. Sol. Energy Mater. Sol. Cells 2006, 90, 3041–3046. [Google Scholar] [CrossRef]
- Dutta, R.K.; Sen, U.K.; Mitra, S. Excellent electrochemical performance of tin monosulphide (SnS) as a sodium-ion battery anode. RSC Adv. 2014, 4, 43155–43159. [Google Scholar] [CrossRef]
- Kumar, G.G.; Reddy, K.; Nahm, K.S.; Angulakshmi, N.; Stephan, A.M. Synthesis and electrochemical properties of SnS as possible anode materials for lithium batteries. J. Phys. Chem. Solids 2012, 73, 1187–1190. [Google Scholar] [CrossRef]
- Kang, J.G.; Park, J.G.; Kim, D.W. Superior rate capabilities of SnS nanosheet electrodes for Li ion batteries. Electrochem. Commun. 2010, 12, 307–310. [Google Scholar] [CrossRef]
- Zhou, M.; Lou, X.W.; Xie, Y. Two-dimensional nanosheets for photoelectrochemical water splitting: Possibilities and opportunities. Nano Today 2013, 8, 598–618. [Google Scholar] [CrossRef]
- Gao, W.; Wu, C.; Cao, M.; Huang, J.; Wang, L.; Shen, Y. Thickness tunable SnS nanosheets for photoelectrochemical water splitting. J. Alloy. Compd. 2016, 688, 668–674. [Google Scholar] [CrossRef]
- Guo, R.; Wang, X.; Kuang, Y.; Huang, B. First-principles study of anisotropic thermoelectric transport properties of IV-VI semiconductor compounds SnSe and SnS. Phys. Rev. B 2015, 92, 115202. [Google Scholar] [CrossRef]
- Sinsermsuksakul, P.; Sun, L.; Lee, S.W.; Park, H.H.; Kim, S.B.; Yang, C.; Gordon, R.G. Overcoming Efficiency Limitations of SnS-Based Solar Cells. Adv. Energy Mater. 2014, 4, 1400496. [Google Scholar] [CrossRef]
- Kim, J.Y.; George, S.M. Tin Monosulfide Thin Films Grown by Atomic Layer Deposition Using Tin 2,4-Pentanedionate and Hydrogen Sulfide. J. Phys. Chem. C 2010, 114, 17597–17603. [Google Scholar] [CrossRef]
- Bilousov, O.V.; Ren, Y.; Torndahl, T.; Donzel-Gargand, O.; Ericson, T.; Platzer-Bjorkman, C.; Edoff, M.; Hagglund, C. Atomic Layer Deposition of Cubic and Orthorhombic Phase Tin Monosulfide. Chem. Mater. 2017, 29, 2969–2978. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Lee, J.; Shin, S.; Lee, J.; Lee, S.; Park, H.; Kwon, S.; Lee, N.; Bang, K.; Lee, S.B.; et al. Fabrication of high crystalline SnS and SnS2 thin films, and their switching device characteristics. Nanotechnology 2018, 29, 215201. [Google Scholar] [CrossRef]
- Baek, I.H.; Pyeon, J.J.; Song, Y.G.; Chung, T.M.; Kim, H.R.; Baek, S.H.; Kim, J.S.; Kang, C.Y.; Choi, J.W.; Hwang, C.S.; et al. Synthesis of SnS Thin Films by Atomic Layer Deposition at Low Temperatures. Chem. Mater. 2017, 29, 8100–8110. [Google Scholar] [CrossRef]
- Steinmann, V.; Chakraborty, R.; Rekemeyer, P.H.; Hartman, K.; Brandt, R.E.; Polizzotti, A.; Yang, C.; Moriarty, T.; Gradecak, S.; Gordon, R.G.; et al. A Two-Step Absorber Deposition Approach to Overcome Shunt Losses in Thin-Film Solar Cells: Using Tin Sulfide as a Proof-of-Concept Material System. ACS Appl. Mater. 2016, 8, 22664–22670. [Google Scholar] [CrossRef]
- Kang, J.Y.; Kwon, S.M.; Yang, S.H.; Cha, J.H.; Bae, J.A.; Jeon, C.W. Control of the microstructure of SnS photovoltaic absorber using a seed layer and its impact on the solar cell performance. J. Alloy. Compd. 2017, 711, 294–299. [Google Scholar] [CrossRef]
- Jung, Y.; Shen, S.; Liu, Y.; Woods, J.M.; Sun, Y.; Cha, J.J. Metal Seed Layer Thickness-Induced Transition from Vertical to Horizontal Growth of MoS2 and WS2. Nano Lett. 2014, 14, 6842–6849. [Google Scholar] [CrossRef]
- Stern, C.; Grinvald, S.; Kirshner, M.; Sinai, O.; Oksman, M.; Alon, H.; Meiron, O.E.; Bar-Sadan, M.; Houben, L.; Naveh, D. Growth Mechanisms and Electronic Properties of Vertically Aligned MoS2. Sci. Rep. 2018, 8, 16480. [Google Scholar] [CrossRef]
- Ham, G.; Shin, S.; Park, J.; Choi, H.; Kim, J.; Lee, Y.A.; Seo, H.; Jeon, H. Tuning the Electronic Structure of Tin Sulfides Grown by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2013, 5, 8889–8896. [Google Scholar] [CrossRef] [PubMed]
- Lindwall, G.; Shang, S.; Kelly, N.R.; Anderson, T.; Liu, Z.K. Thermodynamics of the S–Sn system: Implication for synthesis of earth abundant photovoltaic absorber materials. Sol. Energy 2016, 125, 314–323. [Google Scholar] [CrossRef]
- Mandalidis, S.; Kalomiros, J.A.; Kambas, K.; Anagtopoulos, A.N. Optical investigation of SnS2 single crystals. J. Mater. Sci. 1996, 31, 5975–5978. [Google Scholar] [CrossRef]
- Powell, M.J. The effect of pressure on the optical properties of 2H and 4H SnS2. J. Phys. C Solid State Phys. 1977, 10, 2967–2977. [Google Scholar] [CrossRef]
- Sugiyama, M.; Miyauchi, K.; Minemura, T.; Nakanishi, H. Sulfurization Growth of SnS Films and Fabrication of CdS/SnS Heterojunction for Solar Cells. Jpn. J. Appl. Phys. 2008, 47, 8723. [Google Scholar] [CrossRef]
- Benabbas, S.; Rouabah, Z.; Bouarissa, N.; Chelali, N. The role of back surface field SnS layer in improvement of efficiency of CdTe thin film solar cells. Optik 2016, 127, 6210–6217. [Google Scholar] [CrossRef]
- Albers, W.; Haas, C.; Wink, H.J.; Wasscher, J.D. Investigation on SnS. J. Appl. Phys. 1961, 32, 2220–2225. [Google Scholar] [CrossRef]

| <Ref *> | (eV) | ||
|---|---|---|---|
| Sn 3d5/2 | S 2p3/2 | Spacing (Sn 3d5/2 − S 2p3/2) | |
| SnS2 | 486.5 | 161.6 | 324.9 |
| SnS | 485.7 | 161.0 | 324.7 |
| <obtained data> | |||
| Sn 3d5/2 | S 2p3/2 | Spacing (Sn 3d5/2 − S 2p3/2) | |
| SnS2 | 486.4 | 161.6 | 324.8 |
| SnS | 485.8 | 161.2 | 324.6 |
| Sample | A (No Seed) | B (Using SnS Seed) | C (Using SnS2 Seed) |
|---|---|---|---|
| 2θ (deg)/hkl | Crystallite size (nm) | ||
| 27.47/021 | 35.8 | - | 49.1 |
| 30.47/101 | 41.5 | - | 45.2 |
| 31.53/111 | 53.2 | 25.0 | 56.4 |
| 39.04/131 | - | - | 37.6 |
| 45.49/002 | 26.5 | - | 34.6 |
| Sample | A (No Seed) | B (Using SnS Seed) | C (Using SnS2 Seed) |
|---|---|---|---|
| Bulk concentration (cm−3) | 2.24 × 1019 | 1.32 × 1019 | 3.90 × 1019 |
| Resistivity (Ωm) | 4.96 × 10−2 | 6.67 × 10−2 | 5.13 × 10−2 |
| Mobility (cm2/Vs) | 32.67 | 11.50 | 8.97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Lee, N.; Park, H.; Choi, Y.; Kim, K.; Choi, Y.; Kim, J.; Song, S.; Yuk, H.; Jeon, H. Development of a SnS Film Process for Energy Device Applications. Appl. Sci. 2019, 9, 4606. https://doi.org/10.3390/app9214606
Choi H, Lee N, Park H, Choi Y, Kim K, Choi Y, Kim J, Song S, Yuk H, Jeon H. Development of a SnS Film Process for Energy Device Applications. Applied Sciences. 2019; 9(21):4606. https://doi.org/10.3390/app9214606
Chicago/Turabian StyleChoi, Hyeongsu, Namgue Lee, Hyunwoo Park, Yeonsik Choi, Keunsik Kim, Yeongtae Choi, Jongwoo Kim, Seokhwi Song, Hyunwoo Yuk, and Hyeongtag Jeon. 2019. "Development of a SnS Film Process for Energy Device Applications" Applied Sciences 9, no. 21: 4606. https://doi.org/10.3390/app9214606
APA StyleChoi, H., Lee, N., Park, H., Choi, Y., Kim, K., Choi, Y., Kim, J., Song, S., Yuk, H., & Jeon, H. (2019). Development of a SnS Film Process for Energy Device Applications. Applied Sciences, 9(21), 4606. https://doi.org/10.3390/app9214606




