Aromatic Compounds and Organic Matter Behavior in Pilot Constructed Wetlands Treating Pinus Radiata and Eucalyptus Globulus Sawmill Industry Leachate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Influent Generation
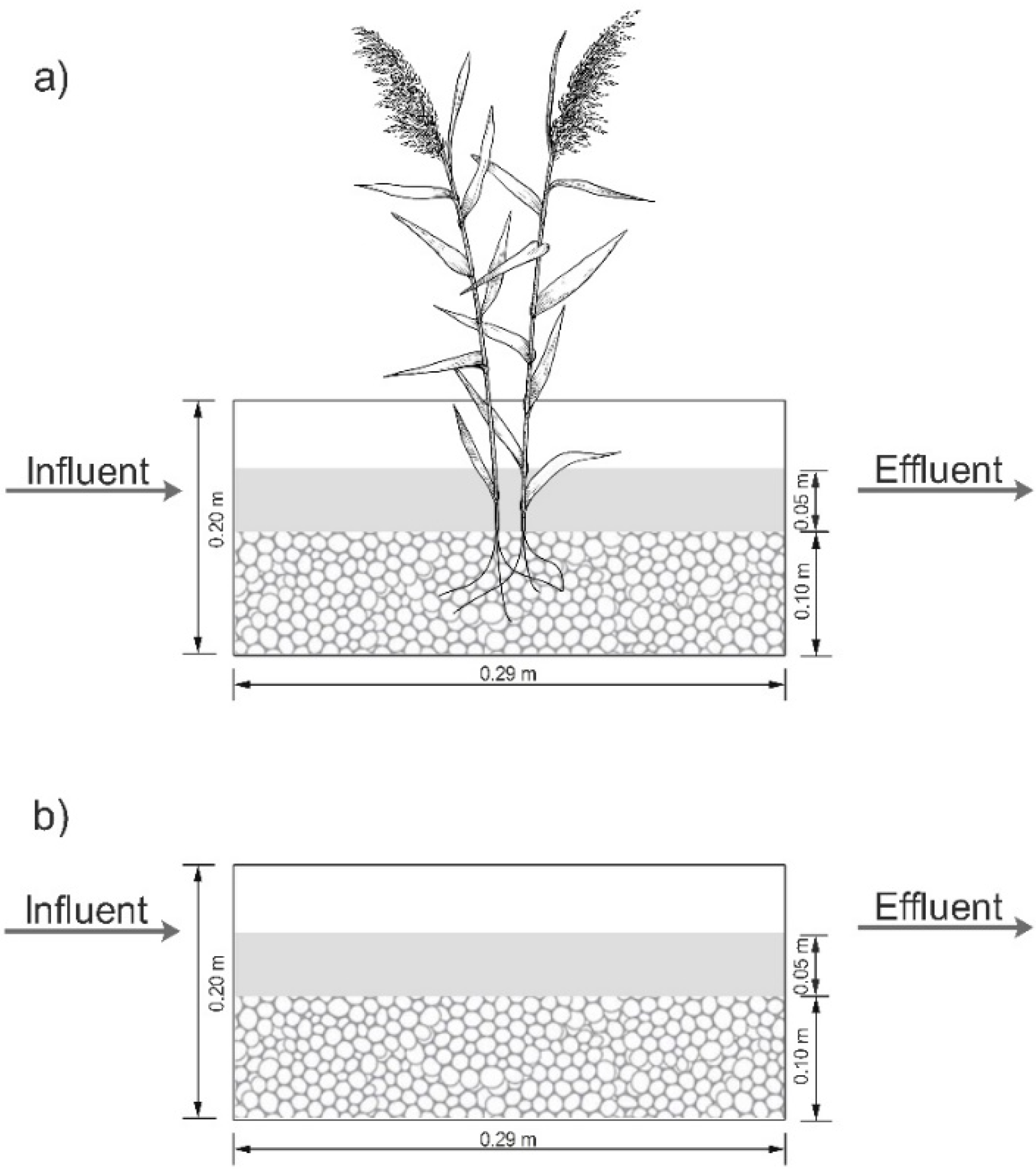
2.2. Laboratory-Scale Constructed Wetlands Description and Operation
2.3. Analytical Methods
3. Results
3.1. Physico-Chemical Characterization of the P. Radiata and E. Globulus Leachate
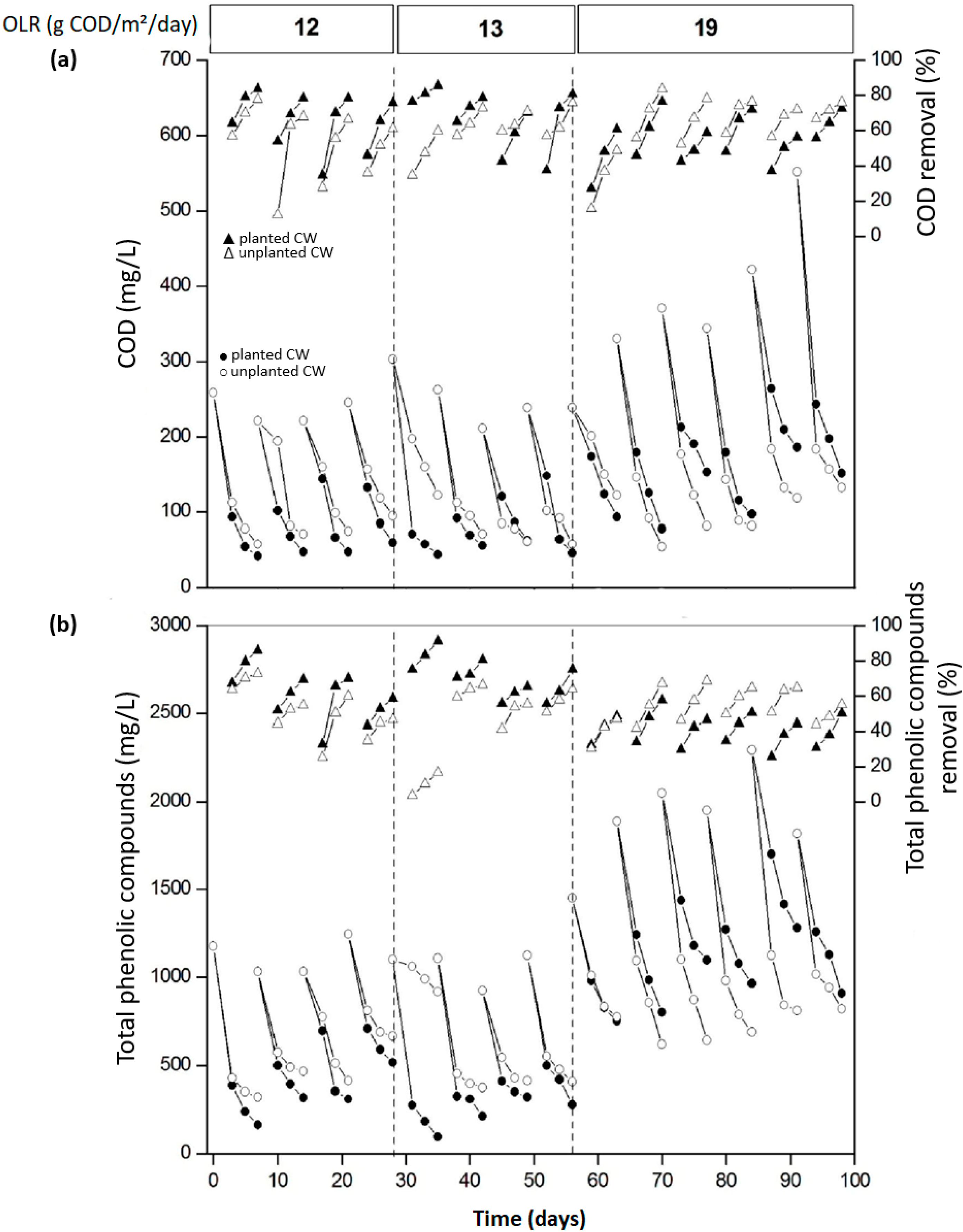
3.2. Performance of CW Treating P. Radiata Leachate
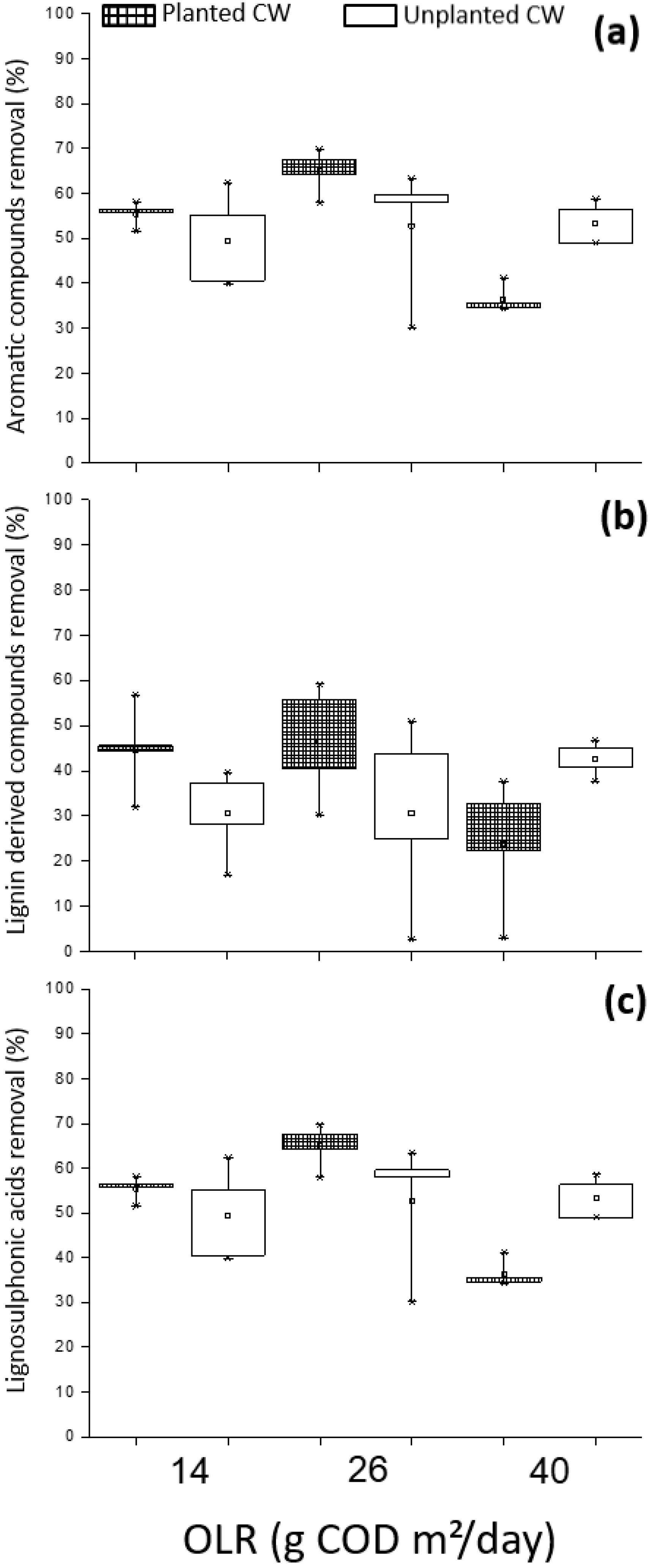
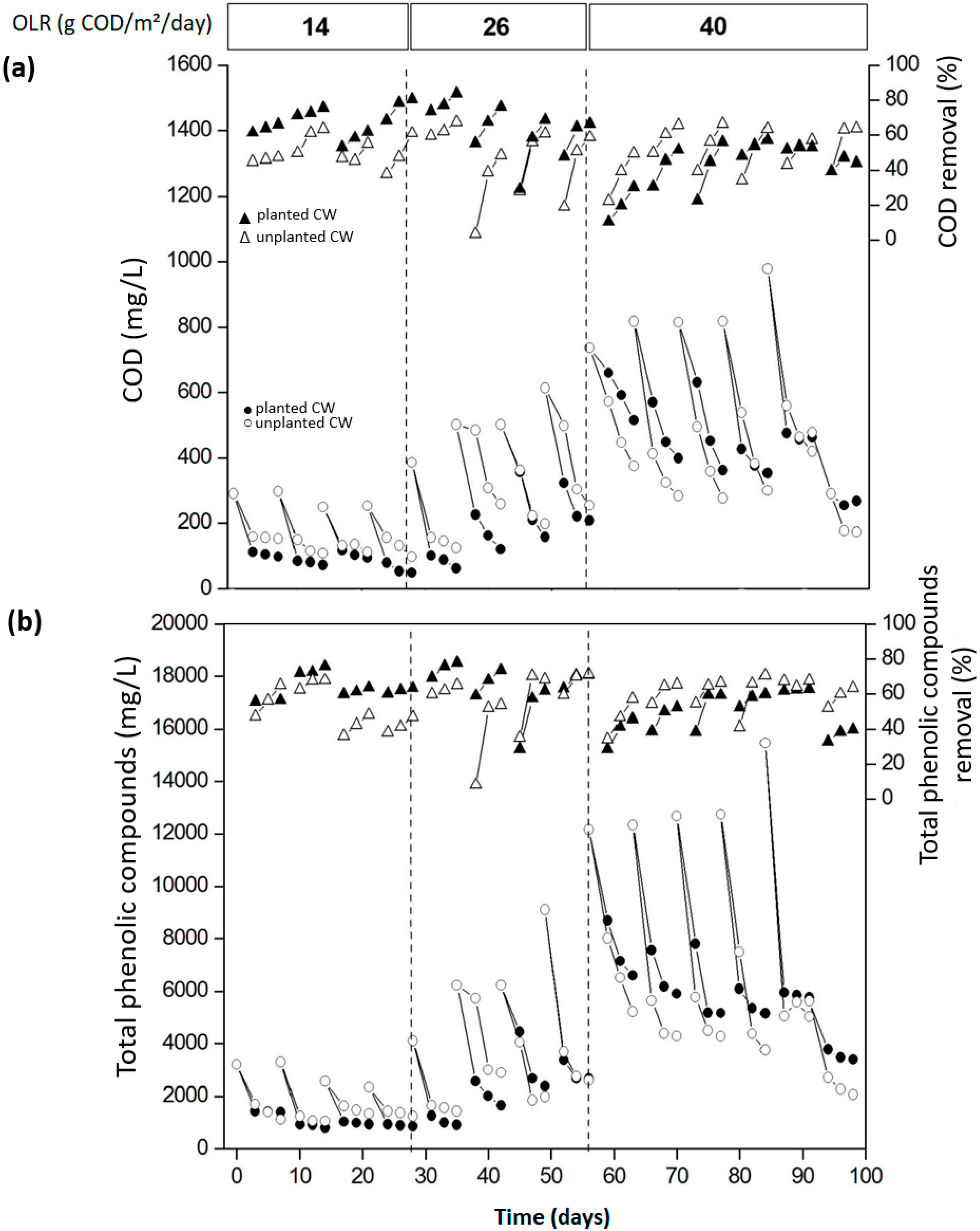
3.3. Performance of CW Treating E. Globulus Leachate
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tao, W.; Hall, K.; Masbough, A.; Frankowski, K.; Duff, S. Characterization of leachate from a woodwaste pile. Water Qual. Res. J. 2005, 40, 476–483. [Google Scholar] [CrossRef]
- Tao, W.; Hall, K.; Duff, S. Treatment of woodwaste leachate in surface flow mesocosm wetlands. Water Qual. Res. J. 2006, 41, 325–332. [Google Scholar] [CrossRef]
- Vidal, G.; Diez, M.C. Methanogenic toxicity of wood processing effluents. J. Environ. Manag. 2005, 74, 317–325. [Google Scholar] [CrossRef]
- Xavier, C.; Chamorro, S.; Vidal, G. Chronic effects of Kraft mil effluents and endocrine active chemicals on Daphnia magna. Bull. Environ. Contam. Toxicol. 2005, 75, 670–676. [Google Scholar] [CrossRef]
- Gomes, A.C.; Silva, L.; Albuquerque, A.; Simões, R.; Stefanakis, A.I. Investigation of lab-scale horizontal subsurface flow constructed wetlands treating industrial cork boiling wastewater. Chemosphere 2018, 207, 430–439. [Google Scholar] [CrossRef]
- Chamorro, S.; Pozo, G.; Jarpa, M.; Hernández, V.; Becerra, J.; Vidal, G. Monitoring endocrine activity in kraft mill effluent treated by Aerobic moving bed bioreactor system. Water Sci. Technol. 2010, 62, 157–161. [Google Scholar] [CrossRef]
- Hedmark, A.; Scholz, M. Review of environmental effects and treatment of runoff from storage and handling of wood. Bioresour. Technol. 2008, 99, 5997–6009. [Google Scholar] [CrossRef]
- Woodhouse, C.; Duff, S.J. Treatment of log yard runoff in an aerobic trickling filter. Water Qual. Res. J. 2004, 39, 230–236. [Google Scholar] [CrossRef]
- Vidal, G.; Becerra, J.; Hernández, V.; Decap, J.; Xavier, C.R. Anaerobic biodegradation of sterols contained in kraft mill effluents. J. Biosci. Bioeng. 2007, 104, 476–480. [Google Scholar] [CrossRef]
- Masbough, A.; Frankowski, K.; Hall, K.; Duff, S. The effectiveness of constructed wetland for treatment of woodwaste leachate. Ecol. Eng. 2005, 25, 552–566. [Google Scholar] [CrossRef]
- Prabu, P.C.; Udayasoorian, C. Treatment of pulp and paper mill effluent using constructed wetland. Electron. J. Environ. Agric. Food Chem. 2007, 6, 1689–1701. [Google Scholar]
- Chamorro, S.; Xavier, C.; Vidal, G. Behavior of aromatic compounds contained in the kraft mill effluents measurements by UV-VIS. Biotechnol. Progr. 2005, 21, 1567–1571. [Google Scholar] [CrossRef]
- Frankowski, K.A. The Treatment of Wood Leachate Using Constructed Wetlands. Ph.D. Thesis, The University of British Columbia, Vancouver, BC, Canada, 2000. [Google Scholar]
- Stefanakis, A.I.; Akratos, C.S.; Tsihrintzis, V.A. Vertical Flow Constructed Wetlands: Eco-Engineering Systems for Wastewater and Sludge Treatment, 1st ed.; Elsevier Publishing: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Stefanakis, A.I. Constructed Wetlands for Industrial Wastewater Treatment, 1st ed.; John Wiley & Sons Ltd.: Chichester, UK, 2018. [Google Scholar]
- Stefanakis, A.I.; Seeger, E.; Dorer, C.; Sinke, A.; Thullner, M. Performance of pilot-scale horizontal subsurface flow constructed wetlands treating groundwater contaminated with phenols and petroleum derivatives. Ecol. Eng. 2016, 95, 514–526. [Google Scholar] [CrossRef]
- Ramírez, S.; Torrealba, G.; Lameda-Cuicas, E.; Molina-Quintero, L.; Stefanakis, A.I.; Pire-Sierra, M.C. Investigation of pilot-scale Constructed Wetlands treating simulated pre-treated tannery wastewater under tropical climate. Chemosphere 2019, 234, 496–504. [Google Scholar] [CrossRef]
- Sultana, M.-Y.; Akratos, C.S.; Vayenas, D.V.; Pavlou, S. Constructed wetlands in the treatment of agro-industrial wastewater: A review. Hem. Ind. 2015, 69, 127–142. [Google Scholar] [CrossRef]
- Tatoulis, T.; Stefanakis, A.I.; Frontistis, Z.; Akratos, C.S.; Tekerlekopoulou, A.G.; Mantzavinos, D.; Vayenas, D.V. Treatment of table olive washing water using trickling filters, constructed wetlands and electrooxidation. Environ. Sci. Pollut. Res. 2017, 24, 1085–1092. [Google Scholar] [CrossRef]
- Langergraber, G.; Fleischmann, N.; Hosfstaedter, F.; Weibgartner, A. Monitoring of a paper mill wastewater treatment plant using UV/VIS spectroscopy. Water Sci. Technol. 2004, 49, 9–14. [Google Scholar] [CrossRef]
- Vidal, G.; Videla, S.; Diez, M.C. Molecular weight distribution of Pinus radiata kraft mill wastewater treated by anaerobic digestion. Bioresour. Technol. 2001, 77, 183–191. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Vidal, G.; Diez, M.C. Influence of feedstock and bleaching technologies on methanogenic toxicity of kraft mill wastewater. Water Sci. Technol. 2003, 48, 149–155. [Google Scholar] [CrossRef]
- Correa, J.; Domínguez, V.; Martínez, M.; Vidal, G. Interaction between 2,4,6-TCP increasing concentration content in ECF bleached effluent and aerobic bacteria degradative activity. Environ. Int. 2003, 29, 459–465. [Google Scholar] [CrossRef]
- Schultze-Nobre, L.; Wiessner, A.; Bartsch, C.; Paschke, H.; Stefanakis, A.I.; Aylward, L.A.; Kuschk, P. Removal of dimethylphenols and ammonium in laboratory-scale horizontal subsurface flow Constructed Wetlands. Eng. Life Sci. 2017, 17, 1224–1233. [Google Scholar] [CrossRef]
- Kurzbaum, E.; Zimmels, Y.; Kirzhner, F.; Armon, R. Removal of phenol in a constructed wetland system and the relative contribution of plant roots, microbial activity and porous bed. Water Sci. Technol. 2010, 62, 1327–1334. [Google Scholar] [CrossRef]
- Tee, H.C.; Seng, C.E.; Noor, A.M.; Lim, P.E. Performance comparison of constructed wetlands with gravel-and rice husk-based media for phenol and nitrogen removal. Sci. Total Environ. 2009, 407, 3563–3571. [Google Scholar] [CrossRef] [PubMed]
- Brix, H. Do macrophytes play a role in constructed treatment wetlands? Water Sci. Technol. 1997, 35, 11–17. [Google Scholar] [CrossRef]
- Stottmeister, U.; Wiebner, A.; Kuschk, P.; Kappelmeyer, U.; Kästner, M.; Bederski, O.; Müller, R.A.; Moormann, H. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 2003, 22, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, V.; Chazarenc, F.; Comeau, Y.; Brisson, J. Influence of macrophyte species on microbial density and activity in constructed wetlands. Water Sci. Technol. 2007, 56, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Vacca, G.; Wand, H.; Nikolausz, M.; Kuschk, P.; Kästner, M. Effect of plants and filter materials on bacteria removal in pilot-scale constructed wetlands. Water Res. 2005, 39, 1361–1373. [Google Scholar] [CrossRef]
- Sleytr, K.; Tietz, A.; Langergraber, G.; Haberl, R. Investigation of bacterial emoval during the filtration process in constructed wetlands. Sci. Total Environ. 2007, 380, 173–180. [Google Scholar] [CrossRef]
- Baptista, J.; Donnelly, T.; Rayne, D.; Davenport, R. Microbial mechanisms of carbon removal in subsurface flow wetlands. Water Sci. Technol. 2003, 48, 127–134. [Google Scholar] [CrossRef]
- Çeçen, F. The use of UV-VIS measurements in the determination of biological treatability of pulp bleaching effluents. In Proceedings of the 7th International Water Association Symposium on Forest Industry Wastewaters, Seattle, WA, USA, 1–4 June 2003. [Google Scholar]
- Vidal, G.; Navia, R.; Levet, L.; Mora, M.L.; Diez, M.C. Kraft mill anaerobic effluent color enhancement by a fixed-bed adsorption system. Biotechnol. Lett. 2001, 23, 861–865. [Google Scholar] [CrossRef]
- Milestone, C.B.; Stuthridge, T.R.; Fulthorpe, R.R. Role of high molecular mass organics in colour formation during biological treatment of pulp and paper wastewater. Water Sci. Technol. 2007, 55, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Belmont, M.; Metcalfe, C. Feasibility of using ornamental plants (Zantedeschia aethiopica) in subsurface flow treatment wetlands to remove nitrogen, chemical oxygen demand and nonylphenol ethoxylate surfactants—A laboratory-scale study. Ecol. Eng. 2003, 21, 233–247. [Google Scholar] [CrossRef]
- Ghermandi, A.; Bixio, D.; Thoeye, C. The role of free water surface constructed wetlands as polishing step in municipal wastewater reclamation and reuse. Sci. Total Environ. 2007, 380, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Lagos, C.; Urrutia, R.; Decap, J.; Martínez, M.; Vidal, G. Eichhornia crassipes used as tertiary color removal treatment for Kraft mill effluent. Desalination 2009, 246, 45–54. [Google Scholar] [CrossRef]
- Navia, R.; Levet, L.; Mora, M.L.; Vidal, G.; Diez, M.C. Allophanic soil adsorption system as a bleached kraft mill aerobic effluent post-treatment. Water Air Soil Pollut. 2003, 148, 323–333. [Google Scholar] [CrossRef]
- Vidal, G.; Nieto, J.; Mansilla, H.D.; Bornhardt, C. Combined oxidative and biological treatment of separated streams of tannery wastewater. Water Sci. Technol. 2004, 49, 287–292. [Google Scholar] [CrossRef]
- Munoz, M.; Pliego, G.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Application of intensified Fenton oxidation to the treatment of sawmill wastewater. Chemosphere 2014, 109, 34–41. [Google Scholar] [CrossRef]
- Yeber, M.C.; Soto, C.; Riveros, R.; Navarrete, J.; Vidal, G. Optimization by factorial design of copper (II) and toxicity removal using a photocatalytic process with TiO2 as semiconductor. Chem. Eng. J. 2009, 152, 14–19. [Google Scholar] [CrossRef]

| Parameter | Unit | Phase | E. Globulus Leachate | P. Radiata Leachate |
|---|---|---|---|---|
| pH | - | I | 6.58 ± 0.48 | 6.69 ± 0.63 |
| - | II | 6.32 ± 0.71 | 6.45 ± 0.55 | |
| - | III | 5.21 ± 0.48 | 5.85 ± 0.29 | |
| BOD5 | mg/L | I | n.d. | n.d. |
| II | 144.00 ± 0.00 | 78.50 ± 0.00 | ||
| III | 216.00 ± 0.00 | 104.00 ± 0.00 | ||
| COD | mg/L | I | 271.70 ± 24.79 | 236.80 ± 18.52 |
| II | 500.05 ± 93.25 | 253.80 ± 39.04 | ||
| III | 832.80 ± 88.38 | 341.40±67.44 | ||
| Aromatic compounds (UV254nm) | Absorbance | I | 3.35 ± 0.40 | 0.92 ± 0.16 |
| II | 7.74 ± 1.77 | 0.97 ± 0.17 | ||
| III | 12.26 ± 0.17 | 1.41 ± 0.71 | ||
| Lignin derived compounds (UV280nm) | Absorbance | I | 2.81 ± 0.21 | 0.80 ± 0.14 |
| II | 6.51 ± 1.42 | 0.86 ± 0.11 | ||
| III | 10.44 ± 0.37 | 1.29 ± 0.69 | ||
| Lignosulphonic acids (UV346nm) | Absorbance | I | 1.02 ± 0.17 | 0.28 ± 0.06 |
| II | 2.07 ± 0.29 | 0.35 ± 0.04 | ||
| III | 4.08 ± 0.65 | 0.56 ± 0.43 |
| Parameter | Unit | Phase | E. Globulus Leachate | P. Radiata Leachate | ||
|---|---|---|---|---|---|---|
| Planted CW | Unplanted CW | Planted CW | Unplanted CW | |||
| pH | - | I | 7.34 ± 0.06 | 7.52 ± 0.12 | 7.40 ± 0.05 | 7.40 ± 0.06 |
| - | II | 7.43 ± 0.19 | 7.30 ± 0.36 | 7.43 ± 0.14 | 7.36 ± 0.25 | |
| - | III | 7.05 ± 0.51 | 7.04 ± 0.54 | 7.32 ± 0.34 | 7.37 ± 0.46 | |
| BOD5 | mg/L | II | 78.00 ± 0.00 | 60.00 ± 0.00 | 16.10 ± 0.00 | 22.50 ± 0.00 |
| III | 15.50 ± 0.00 | 40.00 ± 0.00 | 8.25 ± 0.00 | 15.60 ± 0.00 | ||
| COD | mg/L | I | 79.28 ± 22.97 | 118.38 ± 23.98 | 49.41 ±24.10 | 74.97 ± 15.49 |
| II | 137.55 ± 61.55 | 209.20 ± 62.08 | 52.39 ± 13.04 | 78.36 ± 30.06 | ||
| III | 417.73 ± 77.44 | 330.50 ± 63.25 | 121.64 ±46.68 | 91.98 ± 28.68 | ||
| Aromatic compounds (UV254nm) | Abs | I | 1.49 ± 0.14 | 1.66 ± 0.19 | 0.26 ± 0.05 | 0.31 ± 0.06 |
| II | 2.74 ± 0.82 | 3.67 ± 1.47 | 0.32 ± 0.14 | 0.43 ± 0.15 | ||
| III | 7.80 ± 0.37 | 5.74 ± 0.67 | 0.67 ± 0.26 | 0.57 ± 0.27 | ||
| Lignin derived compounds (UV280nm) | Abs | I | 1.19 ± 0.11 | 1.38 ± 0.18 | 0.24 ± 0.04 | 0.27 ± 0.04 |
| II | 2.26 ± 0.69 | 2.94 ± 1.10 | 0.25 ± 0.12 | 0.37 ± 0.12 | ||
| III | 6.27 ± 0.39 | 4.76 ± 0.50 | 0.57 ± 0.28 | 0.49 ± 0.29 | ||
| Lignosulphonic acids (UV346nm) | Abs | I | 0.55 ± 0.04 | 0.71 ± 0.14 | 0.06 ± 0.02 | 0.08 ± 0.03 |
| II | 1.13 ± 0.37 | 1.46 ± 0.57 | 0.09 ± 0.05 | 0.11 ± 0.06 | ||
| III | 3.04 ± 0.26 | 2.33 ± 0.24 | 0.22 ± 0.16 | 0.21 ± 0.15 | ||
| OLR | Lignosulfonic Acid | Aromatic Compounds | Lignin | Lignin-Derived Compounds |
|---|---|---|---|---|
| (g COD/m2/day) | UV346/COD | UV254/COD | UV280/COD | UV254/UV280 |
| P. radiata leachate | ||||
| Planted CW | - | - | - | - |
| 12 | 0.001 | 0.005 | 0.005 | 1.083 |
| 13 | 0.002 | 0.006 | 0.005 | 1.280 |
| 19 | 0.002 | 0.006 | 0.005 | 1.175 |
| Unplanted CW | - | - | - | - |
| 12 | 0.001 | 0.004 | 0.004 | 1.148 |
| 13 | 0.001 | 0.005 | 0.005 | 1.162 |
| 19 | 0.002 | 0.006 | 0.005 | 1.163 |
| E. globulus leachate | ||||
| Planted CW | - | - | - | - |
| 14 | 0.007 | 0.019 | 0.015 | 1.252 |
| 26 | 0.008 | 0.020 | 0.016 | 1.212 |
| 40 | 0.007 | 0.019 | 0.015 | 1.258 |
| Unplanted CW | - | - | - | - |
| 14 | 0.006 | 0.010 | 0.012 | 1.203 |
| 26 | 0.007 | 0.018 | 0,014 | 1.248 |
| 40 | 0.014 | 0.017 | 0,017 | 1.206 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, C.; Gómez, G.; Stefanakis, A.I.; de los Reyes, C.P.; Vera-Puerto, I.; Vidal, G. Aromatic Compounds and Organic Matter Behavior in Pilot Constructed Wetlands Treating Pinus Radiata and Eucalyptus Globulus Sawmill Industry Leachate. Appl. Sci. 2019, 9, 5046. https://doi.org/10.3390/app9235046
Muñoz C, Gómez G, Stefanakis AI, de los Reyes CP, Vera-Puerto I, Vidal G. Aromatic Compounds and Organic Matter Behavior in Pilot Constructed Wetlands Treating Pinus Radiata and Eucalyptus Globulus Sawmill Industry Leachate. Applied Sciences. 2019; 9(23):5046. https://doi.org/10.3390/app9235046
Chicago/Turabian StyleMuñoz, C., G. Gómez, A.I. Stefanakis, C. Plaza de los Reyes, I. Vera-Puerto, and G. Vidal. 2019. "Aromatic Compounds and Organic Matter Behavior in Pilot Constructed Wetlands Treating Pinus Radiata and Eucalyptus Globulus Sawmill Industry Leachate" Applied Sciences 9, no. 23: 5046. https://doi.org/10.3390/app9235046






