Effects of Pore Structures of Different Maceral Compositions on Methane Adsorption and Diffusion in Anthracite
Abstract
:- (1)
- The pore structure data of MIP and LP-NA can be connected at a pore diameter of 100 nm.
- (2)
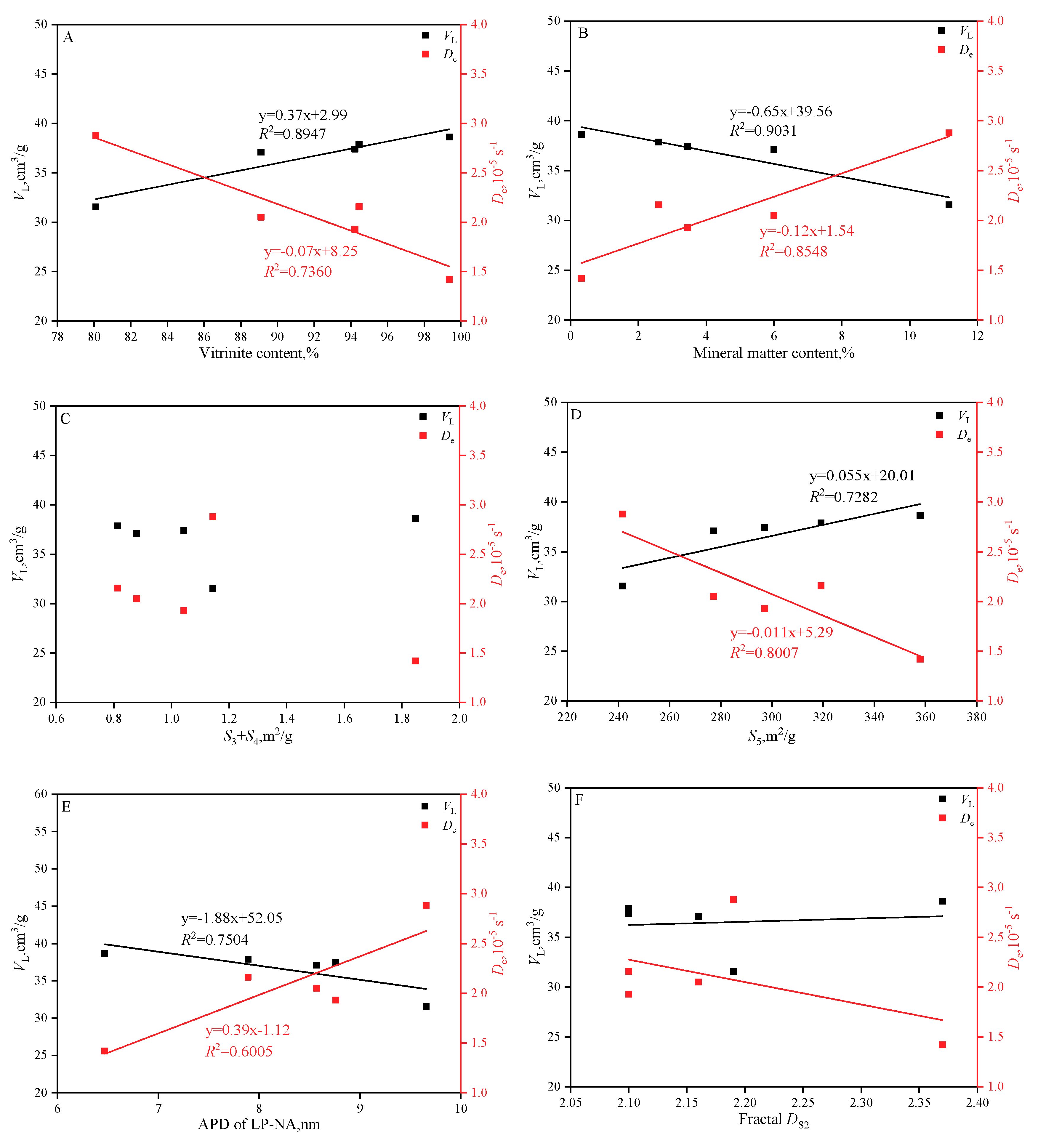
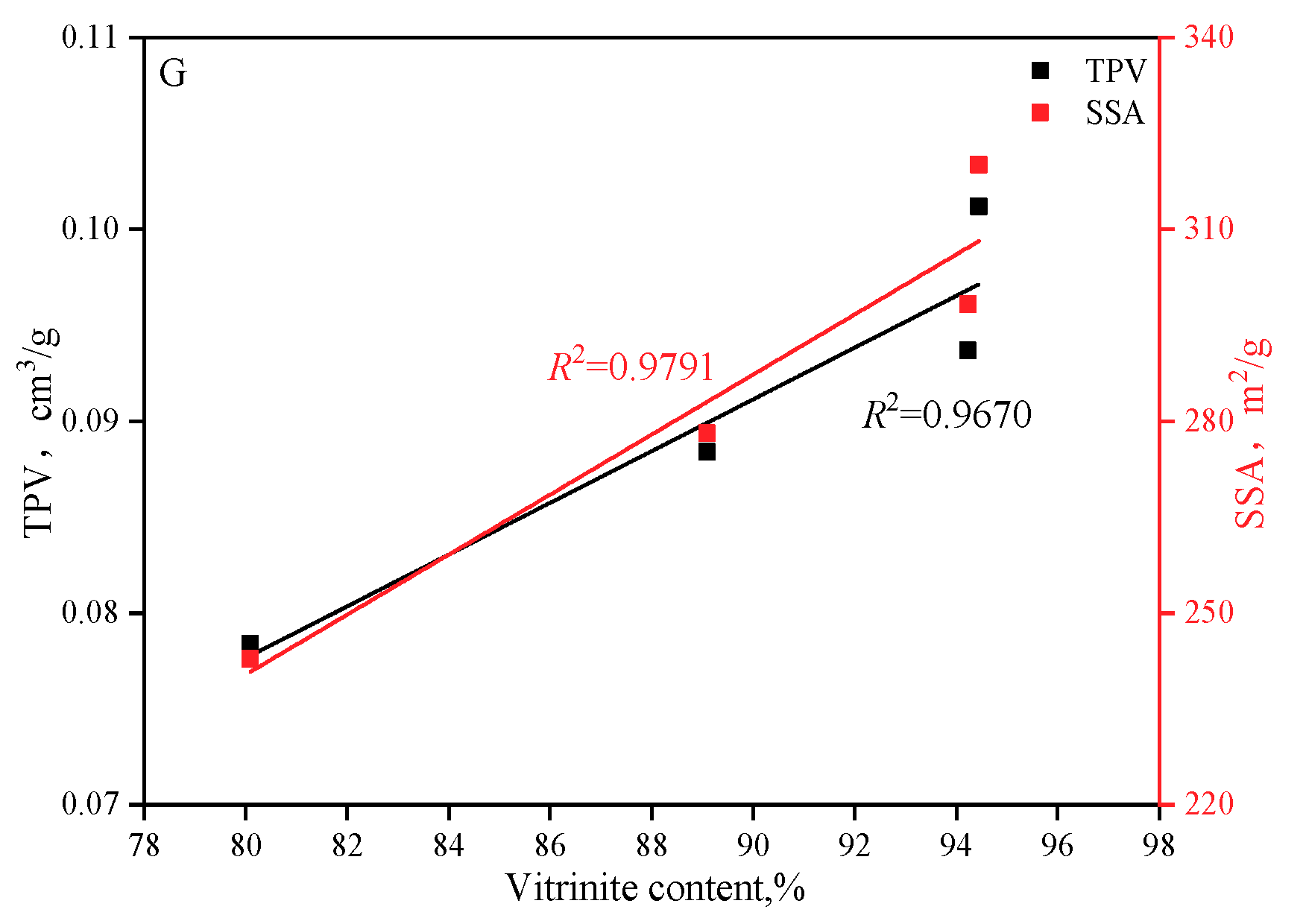
- The larger the vitrinite and the SSA, the larger the adsorption capacity but the weaker the diffusion capacity.
- (3)
- The larger the APD of micro-transitional pores, the stronger the diffusion capacity.
1. Introduction
2. Materials and Methods
2.1. Materials Collection and Preparation
2.2. Experimental Methods
2.3. Fractal Theory
2.4. Adsorption Kinetics
3. Results and Discussions
3.1. Sample Characterization
3.2. Pore Structures
3.2.1. MIP Test Results
3.2.2. LP-NA and LP-CA Tests Results
3.2.3. Comprehensive Characterization of Pore Fractals and Structures
Comprehensive Characterization of Pore Fractals
Comprehensive Characterization of Pore Structures
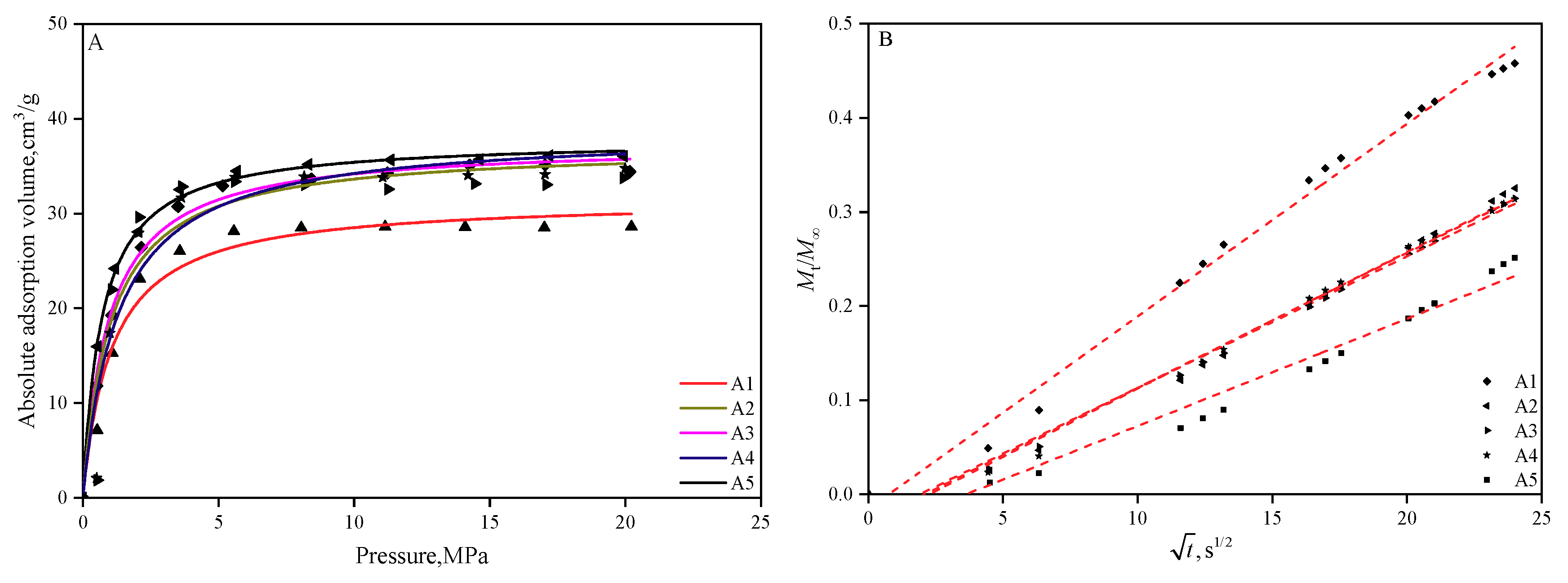
3.3. Isothermal Adsorption and Its Kinetics
3.4. Effects of PSD and Macerals on Adsorption Capacity and Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tutak, M.; Brodny, J. Analysing the Utilisation Effectiveness of Mining Machines Using Independent Data Acquisition Systems: A Case Study. Energies 2019, 12, 2505. [Google Scholar]
- Tao, S.; Pan, Z.; Chen, S.; Tang, S. Coal seam porosity and fracture heterogeneity of marcolithotypes in the Fanzhuang Block, southern Qinshui Basin, China. J. Nat. Gas Sci. Eng. 2019, 66, 148–158. [Google Scholar] [CrossRef]
- Qin, Y.; Moore, T.A.; Shen, J.; Yang, Z.B.; Shen, Y.L.; Wang, G. Resources and geology of coalbed methane in China: A review. Int. Geol. Rev. 2018, 60, 777–812. [Google Scholar] [CrossRef]
- Tutak, M.; Brodny, J. Forecasting Methane Emissions from Hard Coal Mines Including the Methane Drainage Process. Energies 2019, 12, 3840. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, J.; Zhao, X.; Gao, N. Dynamic assessment of coalbed methane resources and availability in China. J. China Coal Soc. 2018, 43, 1598–1604. [Google Scholar]
- Tao, S.; Chen, S.; Pan, Z.J. Current status, challenges, and policy suggestions for coalbed methane industry development in China: A review. Energy Sci. Eng. 2019, 1–16. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Chen, L.; Shen, J.; Chao, W.; Shao, G. Production controlling mechanism and mode of solid-fluid coupling of high rank coal reservoirs. Acta Pet. Sin. 2015, 43, 332–339. [Google Scholar]
- Giffin, S.; Littke, R.; Klaver, J.; Urai, J.L. Application of BIB–SEM technology to characterize macropore morphology in coal. Int. J. Coal Geol. 2013, 114, 85–95. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D. Comparison of low-field NMR and mercury intrusion porosimetry in characterizing pore size distributions of coals. Fuel 2012, 95, 152–158. [Google Scholar] [CrossRef]
- Okolo, G.N.; Everson, R.C.; Neomagus, H.W.J.P.; Roberts, M.J.; Sakurovs, R. Comparing the porosity and surface areas of coal as measured by gas adsorption, mercury intrusion and SAXS techniques. Fuel 2015, 141, 293–304. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Freeman, M.; He, L.; Agamalian, M.; Melnichenko, Y.B.; Mastalerz, M.; Bustin, R.M.; Radlinski, A.P.; Blach, T.P. Characterization of Tight Gas Reservoir Pore Structure Using USANS/SANS and Gas Adsorption Analysis. Fuel 2012, 95, 371–385. [Google Scholar] [CrossRef]
- Kuila, U.; Prasad, M. Surface area and pore-size distribution in clays and shales. Geophys. Prospect. 2013, 61, 341–362. [Google Scholar] [CrossRef]
- Li, G.; Qin, Y.; Wu, M.; Zhang, B.; Wu, X.; Tong, G.; Liu, J. The pore structure of the transitional shale in the Taiyuan formation, Linxing area, Ordos Basin. J. Pet. Sci. Eng. 2019, 181, 1–15. [Google Scholar] [CrossRef]
- Zhao, J.; Hao, X.; Tang, D.; Mathews, J.P.; Song, L.; Shu, T. A comparative evaluation of coal specific surface area by CO2 and N2 adsorption and its influence on CH4 adsorption capacity at different pore sizes. Fuel 2016, 183, 420–431. [Google Scholar] [CrossRef]
- Schmitt, M.; Fernandes, C.P.; da Cunha Neto, J.A.; Wolf, F.G.; dos Santos, V.S. Characterization of pore systems in seal rocks using Nitrogen Gas Adsorption combined with Mercury Injection Capillary Pressure techniques. Mar. Pet. Geol. 2013, 39, 138–149. [Google Scholar] [CrossRef]
- Wang, B.Y.; Qin, Y.; Shen, J.; Zhang, Q.S.; Wang, G. Pore structure characteristics of low- and medium-rank coals and their differential adsorption and desorption effects. J. Pet. Sci. Eng. 2018, 165, 1–12. [Google Scholar] [CrossRef]
- Jian, K.; Fu, X.H.; Ding, Y.M.; Wang, H.D.; Li, T. Characteristics of pores and methane adsorption of low-rank coal in China. J. Nat. Gas Sci. Eng. 2015, 27, 207–218. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, X. Characterization of pore structure and its impact on methane adsorption capacity for semi-anthracite in Shizhuangnan Block, Qinshui Basin. J. Nat. Gas Sci. Eng. 2018, 60, 49–62. [Google Scholar] [CrossRef]
- Chen, Y.L.; Qin, Y.; Wei, C.T.; Huang, L.L.; Shi, Q.M.; Wu, C.F.; Zhang, X.Y. Porosity changes in progressively pulverized anthracite subsamples: Implications for the study of closed pore distribution in coals. Fuel 2018, 225, 612–622. [Google Scholar] [CrossRef]
- Chen, Y.L.; Qin, Y.; Li, Z.P.; Shi, Q.M.; Wei, C.T.; Wu, C.F.; Cao, C.H.; Qu, Z.H. Differences in desorption rate and composition of desorbed gases between undeformed and mylonitic coals in the Zhina Coalfield, Southwest China. Fuel 2019, 239, 905–916. [Google Scholar] [CrossRef]
- Chen, S.D.; Tao, S.; Tang, D.Z.; Xu, H.; Li, S.; Zhao, J.L.; Jiang, Q.; Yang, H.X. Pore Structure Characterization of Different Rank Coals Using N-2 and CO2 Adsorption and Its Effect on CH4 Adsorption Capacity: A Case in Panguan Syncline, Western Guizhou, China. Energy Fuels 2017, 31, 6034–6044. [Google Scholar] [CrossRef]
- Ju, Y.; Huang, C.; Sun, Y.; Wan, Q.; Lu, X.; Lu, S.; He, H.; Wang, X.; Zou, C.; Wu, J. Nanogeosciences: Research history, current status, and development trends. J. Nanosci. Nanotechnol. 2017, 17, 5930–5965. [Google Scholar] [CrossRef]
- Singh, A.; Singh, M.; Singh, P. Microstructural relation of macerals with mineral matter in Eocene coal. Energy Sources Part A Recovery Util. Environ. Eff. 2015, 37, 1089–1097. [Google Scholar] [CrossRef]
- Moore, T.A. Coalbed methane: A review. Int. J. Coal Geol. 2012, 101, 36–81. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Bustin, R.M. The effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modeling study. 1. Isotherms and pore volume distributions. Fuel 1999, 78, 1333–1344. [Google Scholar] [CrossRef]
- Mastalerz, M.; Drobniak, A.; Strapoc, D.; Acosta, W.S.; Rupp, J. Variations in pore characteristics in high volatile bituminous coals: Implications for coal bed gas content. Int. J. Coal Geol. 2008, 76, 205–216. [Google Scholar] [CrossRef]
- Gürdal, G.; Yalçın, M.N. Pore volume and surface area of the Carboniferous coals from the Zonguldak basin (NW Turkey) and their variations with rank and maceral composition. Int. J. Coal Geol. 2001, 48, 133–144. [Google Scholar] [CrossRef]
- Crosdale, P.J.; Beamish, B.B.; Valix, M.; Flores, R.M. Coalbed methane sorption related to coal composition. Int. J. Coal Geol. 1998, 35, 147–158. [Google Scholar] [CrossRef]
- Lefebvre, Y.; Jolicoeur, C. Gas phase adsorption studies of selected probe molecules on powdered silica polymorphs. Colloids Surf. 1992, 63, 67–76. [Google Scholar] [CrossRef]
- Posnansky, O.; Guo, J.; Hirsch, S.; Papazoglou, S.; Braun, J.; Sack, I. Fractal network dimension and viscoelastic powerlaw behavior: I. A modeling approach based on a coarse-graining procedure combined with shear oscillatory rheometry. Phys. Med. Biol. 2012, 57, 4023–4040. [Google Scholar] [CrossRef]
- Wang, Y.; Baiyu, D.U.; Dou, X.; Liu, J.; Shi, B.; Wang, D.; Tang, H. Study on the pore surface fractal dimension and surface acid-base properties of natural particles around Guanting reservoir. Colloids Surf. A Physicochem. Eng. Asp. 2007, 307, 16–27. [Google Scholar] [CrossRef]
- Jiang, X.P.; Qian, M.L.; Qian, C. Morphological Characterization of Super Fine Pulverized Coal Particle. Part 4. Nitrogen adsorption and Small Angle X-ray Scattering Study. Energy Fuels 2010, 24, 084302–084604. [Google Scholar]
- Yao, Y.; Liu, D.; Tang, D.; Tang, S.; Huang, W. Fractal characterization of adsorption-pores of coals from North China: An investigation on CH adsorption capacity of coals. Int. J. Coal Geol. 2008, 73, 27–42. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Liu, S.; Zhang, R. Pore characterization and its impact on methane adsorption capacity for organic-rich marine shales. Fuel 2016, 181, 227–237. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, D.; Cai, Y.; Yao, Y. Gas sorption and flow capabilities of lignite, subbituminous and high-volatile bituminous coals in the Southern Junggar Basin, NW China. J. Nat. Gas Sci. Eng. 2016, 34, 6–21. [Google Scholar] [CrossRef]
- Shen, J.; Qin, Y.; Zhao, J. Maceral contribution to pore size distribution in anthracite in the south Qinshui Basin. Energy Fuels 2019, 33, 7234–7243. [Google Scholar] [CrossRef]
- Li, T.; Wu, C. Research on the Abnormal Isothermal Adsorption of Shale. Energy Fuels 2015, 29, 634–640. [Google Scholar] [CrossRef]
- Zhou, S.D.; Liu, D.M.; Cai, Y.D.; Karpyn, Z.; Yao, Y.B. Comparative analysis of nanopore structure and its effect on methane adsorption capacity of Southern Junggar coalfield coals by gas adsorption and FIB-SEM tomography. Micropor. Mesopor. Mater. 2018, 272, 117–128. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, S.; Tang, D.; Huang, W.; Pan, Z. Determining fractal dimensions of coal pores by FHH model: Problems and effects. J. Nat. Gas Sci. Eng. 2014, 21, 929–939. [Google Scholar] [CrossRef]
- Friesen, W.I.; Mikula, R.J. Mercury porosimetry of coalsPore volume distribution and compressibility. Fuel 1988, 67, 1516–1520. [Google Scholar] [CrossRef]
- Charrière, D.; Pokryszka, Z.; Behra, P. Effect of pressure and temperature on diffusion of CO and CH into coal from the Lorraine basin (France). Int. J. Coal Geol. 2010, 81, 373–380. [Google Scholar] [CrossRef]
- Shen, J.; Qin, Y.; Fu, X.; Wang, G.; Chen, R.; Zhao, L. Study of high-pressure sorption of methane on Chinese coals of different rank. Arab. J. Geosci. 2015, 8, 3451–3460. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Bustin, R.M. The effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modeling study. 2. Adsorption rate modeling. Fuel 1999, 78, 1345–1362. [Google Scholar] [CrossRef]
- Tao, S.; Chen, S.; Tang, D.; Zhao, X.; Xu, H.; Li, S. Material composition, pore structure and adsorption capacity of low-rank coals around the first coalification jump: A case of eastern Junggar Basin, China. Fuel 2018, 211, 804–815. [Google Scholar] [CrossRef]
- Gregg, S.; Sing, K.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982; pp. 195–197. [Google Scholar]
- Li, Z.; Liu, D.; Cai, Y.; Yao, Y.; Wang, H. Pore structure and compressibility of coal matrix with elevated temperatures by mercury intrusion porosimetry. Energy Explor. Exploit. 2015, 33, 809–826. [Google Scholar] [CrossRef] [Green Version]
- Shao, P.; Wang, X.; Song, Y.; Li, Y. Study on the characteristics of matrix compressibility and its influence factors for different rank coals. J. Nat. Gas Sci. Eng. 2018, 56, 93–106. [Google Scholar] [CrossRef]
- Zhou, F.; Hussain, F.; Guo, Z.; Yanici, S.; Cinar, Y. Adsorption/desorption characteristics for methane, nitrogen and carbon dioxide of coal samples from Southeast Qinshui Basin, China. Energy Explor. Exploit. 2013, 31, 645–665. [Google Scholar] [CrossRef] [Green Version]
- Laxminarayana, C.; Crosdale, P.J. Role of coal type and rank on methane sorption characteristics of Bowen Basin, Australia coals. Int. J. Coal Geol. 1999, 40, 309–325. [Google Scholar] [CrossRef]
- Guo, D.Y.; Guo, X.J. The influence factors for gas adsorption with different ranks of coals. Adsorpt. Sci. Technol. 2018, 36, 904–918. [Google Scholar] [CrossRef] [Green Version]
- Lamberson, M.N.; Bustin, R.M.J.A.b. Coalbed methane characteristics of Gates Formation coals, northeastern British Columbia: Effect of maceral composition. AAPG Bull. 1993, 77, 2062–2076. [Google Scholar]
- Gan, H.; Nandi, S.; Walker, P., Jr. Nature of the porosity in American coals. Fuel 1972, 51, 272–277. [Google Scholar] [CrossRef]
- Walker, R.; Glikson, M.; Mastalerz, M. Relations between coal petrology and gas content in the Upper Newlands Seam, central Queensland, Australia. Int. J. Coal Geol. 2001, 46, 83–92. [Google Scholar] [CrossRef]
- Yao, Y.B.; Liu, D.M.; Cai, Y.D.; Li, J.Q. Advanced characterization of pores and fractures in coals by nuclear magnetic resonance and X-ray computed tomography. Sci. China 2010, 53, 854–862. [Google Scholar] [CrossRef]
- Wang, F.; Cheng, Y.; Lu, S.; Jin, K.; Zhao, W. Influence of coalification on the pore characteristics of middle–high rank coal. Energy Fuels 2014, 28, 5729–5736. [Google Scholar] [CrossRef]
- Zhang, X.D.; Du, Z.G.; Li, P.P. Physical characteristics of high-rank coal reservoirs in different coal-body structures and the mechanism of coalbed methane production. Sci. China Earth Sci. 2017, 60, 246–255. [Google Scholar] [CrossRef]
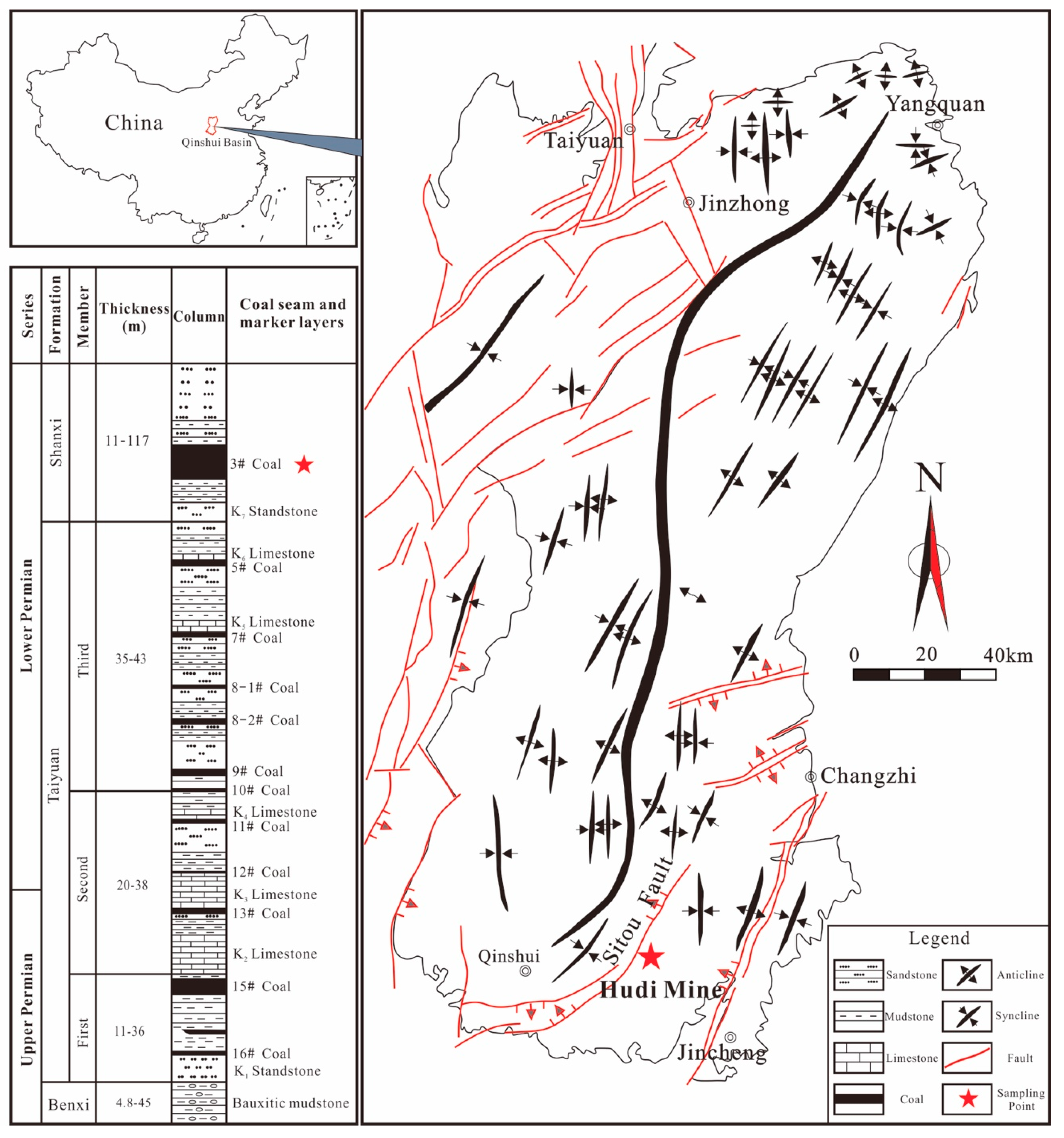


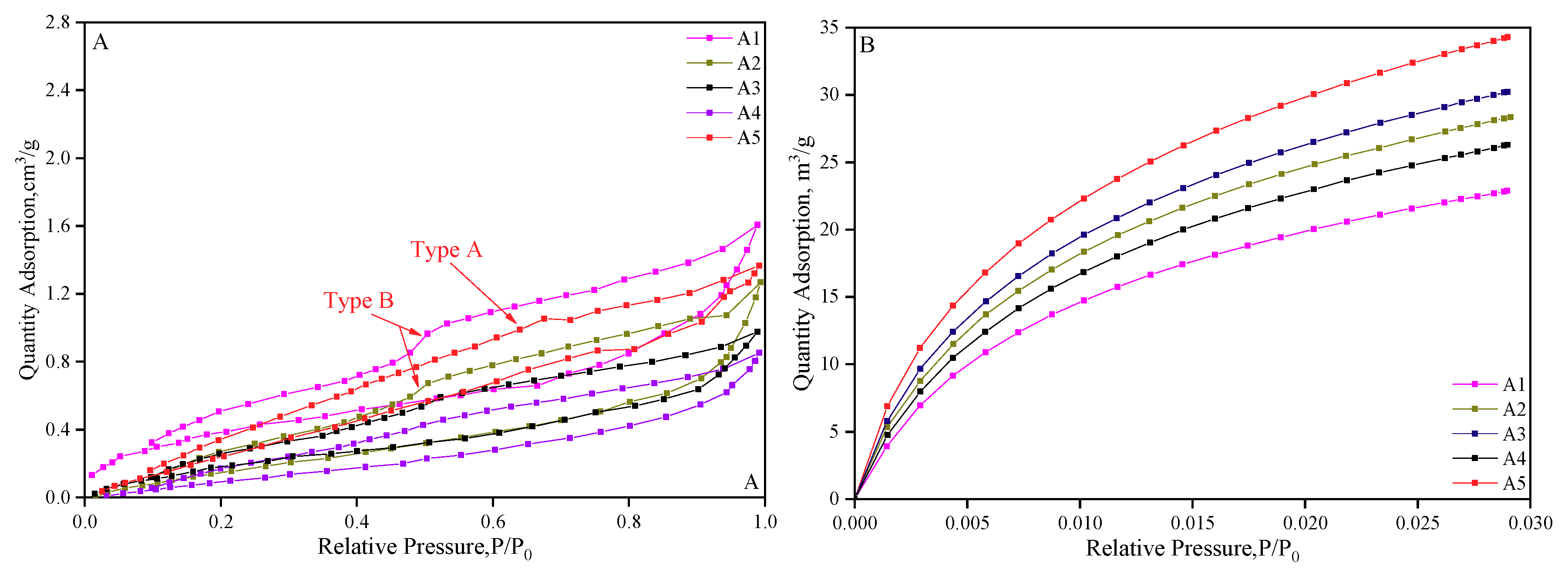
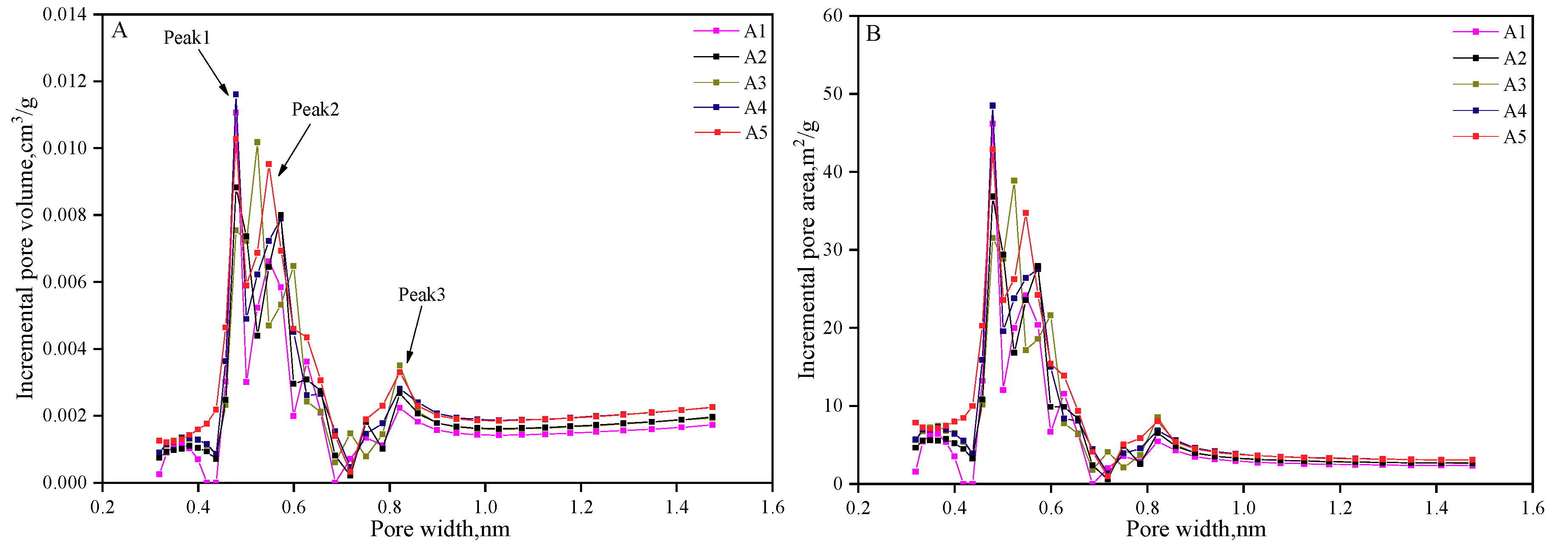

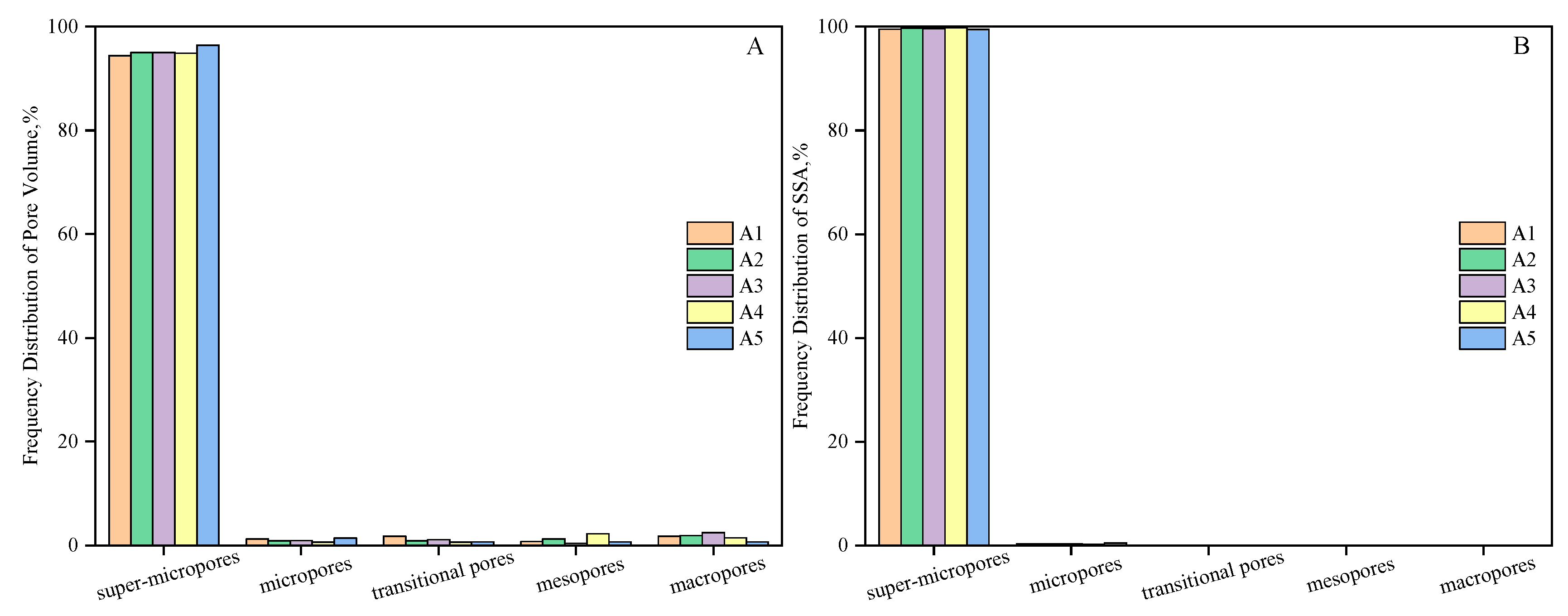

| Samples | Coal Particle Size(mesh) | Ro,max (%) | Macerals Composition (%) | Proximate Analysis (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| V | I | M | Mad | Ad | Vdaf | FCad | |||
| A1 | 40–60 | 2.94 | 80.10 | 8.67 | 11.23 | 0.92 | 21.89 | 7.56 | 71.55 |
| A2 | 40–60 | 2.94 | 89.10 | 4.90 | 4.62 | 1.02 | 15.33 | 6.71 | 78.19 |
| A3 | 40–60 | 2.95 | 94.23 | 2.37 | 3.51 | 1.13 | 12.05 | 6.14 | 81.61 |
| A4 | 40–60 | 2.94 | 94.55 | 2.95 | 3.18 | 1.10 | 8.54 | 5.86 | 85.16 |
| A5 | 40–60 | 2.95 | 99.36 | 0.32 | 0.32 | 1.50 | 1.81 | 5.16 | 91.72 |
| Average | 40–60 | 2.94 | 91.47 | 3.84 | 4.57 | 1.13 | 11.92 | 6.29 | 81.65 |
| Samples | MIP | LP-NA | LP-CA | CH4 Adsorption | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VM (cm3/g) | SM (m2/g) | VBJH (cm3/g) | SBET (m2/g) | APD (nm) | VCO2 (cm3/g) | VDFT (cm3/g) | SDFT (m2/g) | VL, cm3/g | PL, MPa | |
| A1 | 0.0277 | 7.5 | 0.0023 | 1.496 | 9.656 | 22.88 | 0.074 | 241.67 | 31.55 | 1.07 |
| A2 | 0.0301 | 11.55 | 0.0016 | 0.922 | 8.571 | 26.31 | 0.084 | 277.16 | 37.08 | 1.03 |
| A3 | 0.0275 | 10.62 | 0.0021 | 0.915 | 8.761 | 28.36 | 0.089 | 297.20 | 37.41 | 0.96 |
| A4 | 0.0338 | 12.16 | 0.0015 | 0.913 | 7.891 | 30.24 | 0.096 | 319.26 | 37.87 | 0.7 |
| A5 | 0.0298 | 12.67 | 0.0025 | 1.559 | 6.470 | 34.29 | 0.106 | 357.96 | 38.63 | 1.29 |
| Average | 0.0298 | 10.90 | 0.0020 | 1.161 | 8.270 | 28.42 | 0.090 | 298.65 | 36.51 | 1.01 |
| Samples | k | D | A1 | δ1 | Ds1 | A2 | δ2 | Ds2 | De, 10−5 s−1 |
|---|---|---|---|---|---|---|---|---|---|
| A1 | −1.45 | 2.55 | −0.57 | −0.71 | 2.43 | −0.27 | 0.19 | 2.19 | 2.88 |
| A2 | −1.50 | 2.5 | −0.92 | −1.76 | 2.08 | −0.28 | 0.16 | 2.16 | 2.05 |
| A3 | −1.64 | 2.36 | −1.15 | −2.45 | 1.85 | −0.30 | 0.10 | 2.10 | 1.93 |
| A4 | −1.62 | 2.38 | −1.31 | −2.93 | 1.69 | −0.30 | 0.10 | 2.10 | 2.16 |
| A5 | −1.40 | 2.60 | −1.27 | −2.81 | 1.73 | −0.21 | 0.37 | 2.37 | 1.42 |
| Average | −1.52 | 2.48 | −1.04 | −2.13 | 1.96 | −0.27 | 0.18 | 2.18 | 2.09 |
| Samples | Vt (cm3/g) | St (m2/g) | Pore Volume Ratio (%) | Specific Surface Area Ratio (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V1/Vt | V2/Vt | V3/Vt | V4/Vt | V5/Vt | S1/St | S2/St | S3/St | S4/St | S5/St | |||
| A1 | 0.078 | 242.823 | 1.786 | 0.765 | 1.786 | 1.276 | 94.388 | 0.001 | 0.003 | 0.085 | 0.386 | 99.525 |
| A2 | 0.088 | 278.051 | 1.923 | 1.244 | 0.905 | 0.905 | 95.023 | 0.001 | 0.004 | 0.039 | 0.278 | 99.68 |
| A3 | 0.094 | 298.250 | 2.455 | 0.427 | 1.174 | 0.961 | 94.984 | 0 | 0.002 | 0.046 | 0.304 | 99.648 |
| A4 | 0.101 | 320.121 | 1.482 | 2.273 | 0.692 | 0.692 | 94.862 | 0.001 | 0.014 | 0.032 | 0.222 | 99.731 |
| A5 | 0.110 | 359.818 | 0.727 | 0.727 | 0.727 | 1.455 | 96.364 | 0 | 0.003 | 0.04 | 0.474 | 99.484 |
| Average | 0.094 | 299.81 | 1.675 | 1.087 | 1.057 | 1.058 | 95.124 | 0.001 | 0.005 | 0.048 | 0.333 | 99.614 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Qin, Y.; Shen, J.; Zhou, B.; Li, C.; Li, G. Effects of Pore Structures of Different Maceral Compositions on Methane Adsorption and Diffusion in Anthracite. Appl. Sci. 2019, 9, 5130. https://doi.org/10.3390/app9235130
Zhao J, Qin Y, Shen J, Zhou B, Li C, Li G. Effects of Pore Structures of Different Maceral Compositions on Methane Adsorption and Diffusion in Anthracite. Applied Sciences. 2019; 9(23):5130. https://doi.org/10.3390/app9235130
Chicago/Turabian StyleZhao, Jincheng, Yong Qin, Jian Shen, Binyang Zhou, Chao Li, and Geng Li. 2019. "Effects of Pore Structures of Different Maceral Compositions on Methane Adsorption and Diffusion in Anthracite" Applied Sciences 9, no. 23: 5130. https://doi.org/10.3390/app9235130






