Is There a Relation between 677C>T Polymorphism in the MTHFR Gene and the Susceptibility to Epilepsy in Young Patients? A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Data Extraction and Methodological Quality
2.3. Statistical Analyses
3. Results
3.1. Characteristics of the Studies Included
3.2. Association between 677C>T Polymorphism in MTHFR Gene and Epilepsy in Total Groups
3.3. Sensitivity Analysis for Comparisons in Total Groups
3.4. Publication Bias in Total Groups
3.5. Subgroup Analyses
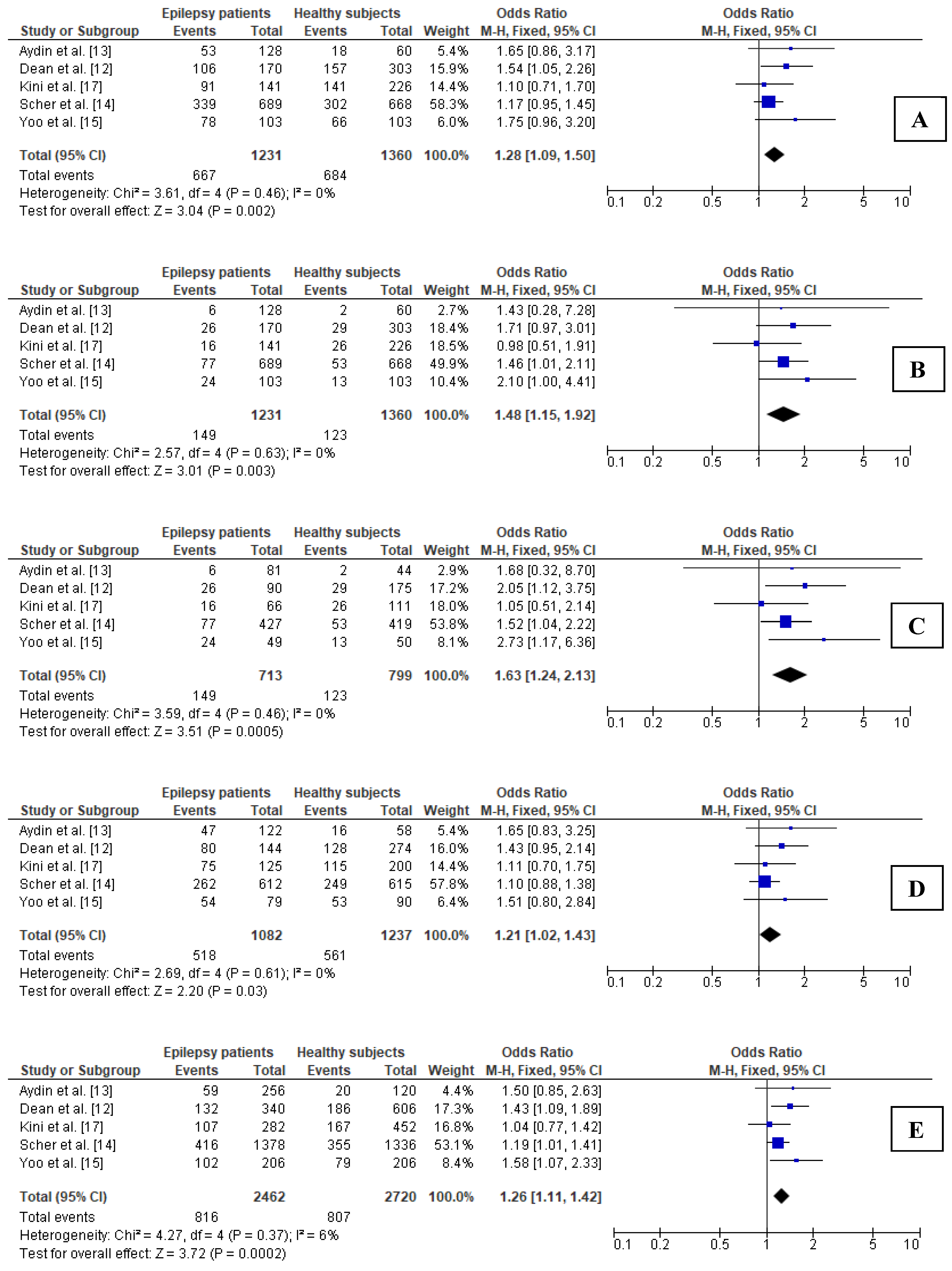
3.5.1. Association between 677C>T Polymorphism in MTHFR Gene and Epilepsy in Young Adults
3.5.2. Sensitivity Analysis for Comparisons in Subgroup of Young Adults
3.5.3. Publication Bias in Subgroup of Young Adults
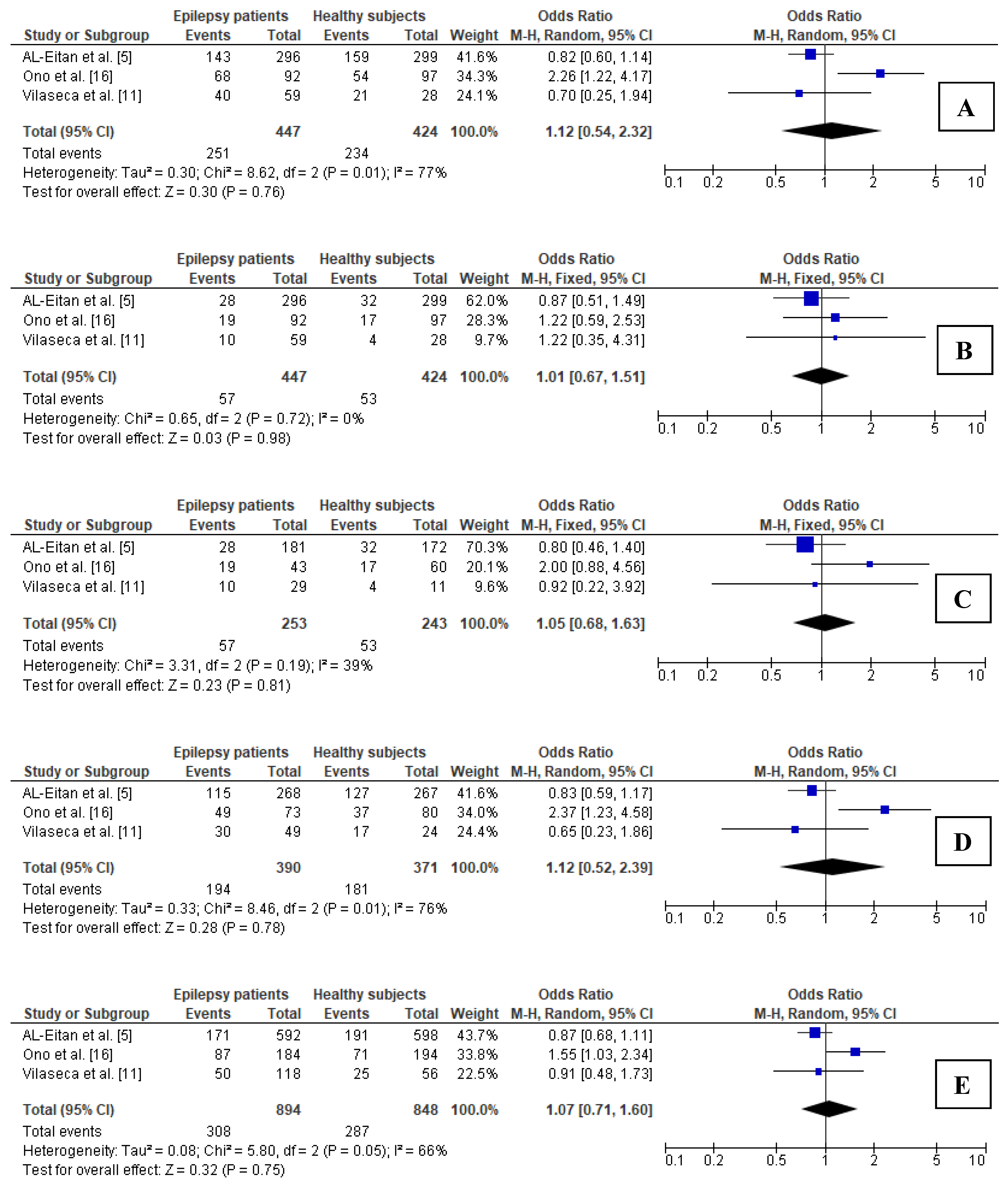
3.5.4. Association between 677C>T Polymorphism in MTHFR Gene and Epilepsy in Children
3.5.5. Sensitivity Analysis for Comparisons in Subgroup of Children
3.5.6. Publication Bias in Subgroup of Children
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitkänen, A.; Lukasiuk, K.; Dudek, F.E.; Staley, K.J. Epileptogenesis. Cold Spring Harb. Perspect. Med. 2015, 5, a022822. [Google Scholar] [CrossRef] [Green Version]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [Green Version]
- Magiorkinis, E.; Sidiropoulou, K.; Diamantis, A. Hallmarks in the History of Epilepsy: From Antiquity till the Twentieth Century. In Novel Aspects on Epilepsy; Foyaca-Sibat, H., Ed.; InTech: Rijeka, Croatia, 2011; pp. 132–133. [Google Scholar]
- Vadlamudi, L.; Milne, R.L.; Lawrence, K.; Heron, S.E.; Eckhaus, J.; Keay, D.; Connellan, M.; Torn-Broers, Y.; Howell, R.A.; Mulley, J.C.; et al. Genetics of epilepsy: The testimony of twins in the molecular era. Neurology 2014, 83, 1042–1048. [Google Scholar] [CrossRef] [Green Version]
- Al-Eitan, L.N.; Al-Dalalah, I.M.; Mustafa, M.M.; Alghamdi, M.A.; Elshammari, A.K.; Khreisat, W.H.; Aljamal, H.A. Effects of MTHFR and ABCC2 gene polymorphisms on antiepileptic drug responsiveness in Jordanian epileptic patients. Pharmgenom. Pers. Med. 2019, 12, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Dębski, K.J.; Pitkanen, A.; Puhakka, N.; Bot, A.M.; Khurana, I.; Harikrishnan, K.N.; Ziemann, M.; Kaspi, A.; El-Osta, A.; Lukasiuk, K.; et al. Etiology matters—Genomic DNA Methylation Patterns in Three Rat Models of Acquired Epilepsy. Sci. Rep. 2016, 6, 25668. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ou, S.; Xu, T.; Liu, S.; Yuan, J.; Huang, H.; Qin, L.; Yang, H.; Chen, L.; Tan, X.; et al. New differentially expressed genes and differential DNA methylation underlying refractory epilepsy. Oncotarget 2016, 7, 87402–87416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Fu, X.; Peng, X.; Xiao, Z.; Li, Z.; Chen, G.; Wang, X. DNA Methylation Profiling Reveals Correlation of Differential Methylation Patterns with Gene Expression in Human Epilepsy. J. Mol. Neurosci. 2016, 59, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Rivera, I.; Ravasco, P.; Camilo, M.E.; Jakobs, C.; Blom, H.J.; de Almeida, I.T. 5,10-methylenetetrahydrofolate reductase (MTHFR) 677C-->T and 1298A-->C mutations are associated with DNA hypomethylation. J. Med. Genet. 2004, 41, 454–458. [Google Scholar] [CrossRef] [Green Version]
- Schwahn, B.; Rozen, R. Polymorphisms in the methylenetetrahydrofolate reductase gene: Clinical consequences. Am. J. Pharm. 2001, 1, 189–201. [Google Scholar] [CrossRef]
- Vilaseca, M.A.; Monrós, E.; Artuch, R.; Colomé, C.; Farré, C.; Valls, C.; Cardo, E.; Pineda, M. Anti-epileptic drug treatment in children: Hyperhomocysteinaemia, B-vitamins and the 677C-->T mutation of the methylenetetrahydrofolate reductase gene. Eur. J. Paediatr. Neurol. 2000, 4, 269–277. [Google Scholar] [CrossRef]
- Dean, J.C.; Robertson, Z.; Reid, V.; Wang, Q.; Hailey, H.; Moore, S.; Rasalam, A.D.; Turnpenny, P.; Lloyd, D.; Shaw, D.; et al. A high frequency of the MTHFR 677C>T polymorphism in Scottish women with epilepsy: Possible role in pathogenesis. Seizure 2008, 17, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydin, A.; Varoglu, A.O. Methylenetetrahydrofolate reductase gene polymorphism and clinical importance in epilepsy patients using valproic acid, carbamazepine and levetiracetam. J. Lab. Med. 2017, 41, 147–151. [Google Scholar] [CrossRef]
- Scher, A.I.; Wu, H.; Tsao, J.W.; Blom, H.J.; Feit, P.; Nevin, R.L.; Schwab, K.A. MTHFR C677T genotype as a risk factor for epilepsy including post-traumatic epilepsy in a representative military cohort. J. Neurotrauma 2011, 28, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Hong, S.B. A common mutation in the methylenetetrahydrofolate reductase gene is a determinant of hyperhomocysteinemia in epileptic patients receiving anticonvulsants. Metabolism 1999, 48, 1047–1051. [Google Scholar] [CrossRef]
- Ono, H.; Sakamoto, A.; Mizoguchi, N.; Sakura, N. Methylenetetrahydrofolate reductase 677C > T mutation and epilepsy. J. Inherit. Metab. Dis. 2000, 23, 525–526. [Google Scholar] [CrossRef]
- Kini, U.; Lee, R.; Jones, A.; Smith, S.; Ramsden, S.; Fryer, A.; Clayton-Smith, J.; Liverpool Manchester Neurodevelopmental Study Group. Influence of the MTHFR genotype on the rate of malformations following exposure to antiepileptic drugs in utero. Eur. J. Med. Genet. 2007, 50, 411–420. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 12 August 2021).
- Minelli, C.; Thompson, J.R.; Abrams, K.R.; Thakkinstian, A.; Attia, J. How should we use information about HWE in the meta-analyses of genetic association studies? Int. J. Epidemiol. 2008, 37, 136–146. [Google Scholar] [CrossRef]
- Wu, Y.L.; Yang, H.Y.; Ding, X.X.; Zhao, X.; Chen, J.; Bi, P.; Sun, Y.H. Association between methylenetetrahydrofolate reductase C677T polymorphism and epilepsy susceptibility: A meta-analysis. Seizure 2014, 23, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Rai, V.; Kumar, P. Methylenetetrahydrofolate reductase C677T polymorphism and susceptibility to epilepsy. Neurol. Sci. 2018, 39, 2033–2041. [Google Scholar] [CrossRef]
- Balamuralikrishnan, B.; Balachandar, V.; Devi, S.M.; Kumar, A.; Sasikala, K. Cytogenetic evaluation in epilepsy patients correlated with MTHFR C677T gene mutation and frequency of homocysteine levels. J. Neurol. Sci. 2013, 333, e44–e45. [Google Scholar] [CrossRef]
- Munisamy, M.; Al-Gahtany, M.; Tripathi, M.; Subbiah, V. Impact of MTHFR (C677T) gene polymorphism on antiepileptic drug monotherapy in North Indian epileptic population. Ann. Saudi Med. 2015, 35, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Caccamo, D.; Condello, S.; Gorgone, G.; Crisafulli, G.; Belcastro, V.; Gennaro, S.; Striano, P.; Pisani, F.; Ientile, R. Screening for C677T and A1298C MTHFR polymorphisms in patients with epilepsy and risk of hyperhomocysteinemia. Neuromol. Med. 2004, 6, 117–126. [Google Scholar] [CrossRef]
- Belcastro, V.; Striano, P.; Gorgone, G.; Costa, C.; Ciampa, C.; Caccamo, D.; Pisani, L.R.; Oteri, G.; Marciani, M.G.; Aguglia, U.; et al. Hyperhomocysteinemia in epileptic patients on new antiepileptic drugs. Epilepsia 2010, 51, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Gorgone, G.; Caccamo, D.; Pisani, L.R.; Currò, M.; Parisi, G.; Oteri, G.; Ientile, R.; Rossini, P.M.; Pisani, F. Hyperhomocysteinemia in patients with epilepsy: Does it play a role in the pathogenesis of brain atrophy? A preliminary report. Epilepsia 2009, 50, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Sniezawska, A.; Dorszewska, J.; Rozycka, A.; Przedpelska-Ober, E.; Lianeri, M.; Jagodzinski, P.P.; Kozubski, W. MTHFR, MTR, and MTHFD1 gene polymorphisms compared to homocysteine and asymmetric dimethylarginine concentrations and their metabolites in epileptic patients treated with antiepileptic drugs. Seizure 2011, 20, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Rasić-Marković, A.; Stanojlović, O.; Hrncić, D.; Krstić, D.; Colović, M.; Susić, V.; Radosavljević, T.; Djuric, D. The activity of erythrocyte and brain Na+/K+ and Mg2+-ATPases in rats subjected to acute homocysteine and homocysteine thiolactone administration. Mol. Cell Biochem. 2009, 327, 39–45. [Google Scholar] [CrossRef]
- Ullah, S.; Ali, N.; Khan, A.; Ali, S.; Nazish, H.R.; Uddin, Z. Epilepsy control with carbamazepine monotherapy from a genetic perspective. BMC Pharmacol. Toxicol. 2018, 19, 73. [Google Scholar] [CrossRef]
- Coppola, G.; Ingrosso, D.; Operto, F.F.; Signoriello, G.; Lattanzio, F.; Barone, E.; Matera, S.; Verrotti, A. Role of folic acid depletion on homocysteine serum level in children and adolescents with epilepsy and different MTHFR C677T genotypes. Seizure 2012, 21, 340–343. [Google Scholar] [CrossRef] [Green Version]
- Vurucu, S.; Demirkaya, E.; Kul, M.; Unay, B.; Gul, D.; Akin, R.; Gokçay, E. Evaluation of the relationship between C677T variants of methylenetetrahydrofolate reductase gene and hyperhomocysteinemia in children receiving antiepileptic drug therapy. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 844–848. [Google Scholar] [CrossRef]
- Zhu, S.; Ni, G.; Sui, L.; Zhao, Y.; Zhang, X.; Dai, Q.; Chen, A.; Lin, W.; Li, Y.; Huang, M.; et al. Genetic Polymorphisms in Enzymes Involved in One-Carbon Metabolism and Anti-epileptic Drug Monotherapy on Homocysteine Metabolism in Patients with Epilepsy. Front. Neurol. 2021, 12, 683275. [Google Scholar] [CrossRef] [PubMed]


| Study | Year of Publication | Population | Epilepsy Group n | Age (Years) | Control Groupn | Age (Years) | Established Relationship |
|---|---|---|---|---|---|---|---|
| AL-Eitan et al. [5] | 2019 | Jordan | 269 | Under 15 | 299 | 5.9 ± 3.8 | No (in the total group) Yes (in generalized epilepsy subgroup) |
| Vilaseca et al. [11] | 2000 | Spain | 59 | Range: 1–18 | 28 | Age-matched to cases | No |
| Dean et a. [12] | 2008 | Scotland | 170 | NA (but both groups were age-matched) | 303 | 29.0 ± 11.3 | Yes |
| Aydin et al. [13] | 2017 | Turkey | 128 | 27.6 ± 16.0 | 60 | Age-matched to cases | No |
| Scher et al. [14] | 2011 | USA | 689 | 32.0 ± 8.5 | 668 | 32.0 ± 8.5 | Yes |
| Yoo et al. [15] | 1999 | South Korea | 103 | 27.5 ± 8.5 | 103 | 28.1 ± 9.8 | Yes |
| Ono et al. [16] | 2000 | Japan | 92 | Mean: 14.8 (range: 1–40) | 97 | Mean: 25 (range: 20–30) | Yes (in symptomatic or cryptogenic epilepsy) |
| Kini et al. [17] | 2007 | UK | 141 | NA | 226 | Age-matched to cases | NA |
| Total | 1678 | 1784 | |||||
| Study | Epilepsy Group | Control Group | HWE (for Controls) (χ2; p) | NOS | ||
|---|---|---|---|---|---|---|
| Genotypes of MTHFR 677C>T Polymorphism n (%) | Alleles of MTHFR 677C>T Polymorphism n (%) | Genotypes of MTHFR 677C>T Polymorphism n (%) | Alleles of MTHFR 677C>T Polymorphism n (%) | |||
| AL-Eitan et al. [5] | CC: 153 (51.7) CT: 115 (38.8) TT: 28 (9.5) | C: 421 (71.1) T: 171 (28.9) | CC: 140 (46.8) CT: 127 (42.5) TT: 32 (10.7) | C: 407 (68.1) T: 191 (31.9) | 0.051; 0.82 | 8 |
| Vilaseca et al. [11] | CC: 19 (32.2) CT: 30 (50.8) TT: 10 (17.0) | C: 68 (57.6) T: 50 (42.4) | CC: 7 (25.0) CT: 17 (60.7) TT: 4 (14.3) | C: 31 (55.4) T: 25 (44.6) | 5.022; 0.02 | 6 |
| Dean et a. [12] | CC: 64 (37.6) CT: 80 (47.1) TT: 26 (15.3) | C: 208 (61.2) T: 132 (38.8) | CC: 146 (48.2) CT: 128 (42.2) TT: 29 (9.6) | C: 420 (69.3) T: 186 (30.7) | 0.007; 0.93 | 7 |
| Aydin et al. [13] | CC: 75 (58.6) CT: 47 (36.7) TT: 6 (4.7) | C: 197 (76.9) T: 59 (23.1) | CC: 42 (70.0) CT: 16 (26.7) TT: 2 (3.3) | C: 100 (83.3) T: 20 (16.7) | 0.145; 0.70 | 7 |
| Scher et al. [14] | CC: 350 (50.8) CT: 262 (38.0) TT: 77 (11.2) | C: 962 (69.8) T: 416 (30.2) | CC: 366 (54.8) CT: 249 (37.3) TT: 53 (7.9) | C: 981 (73.4) T: 355 (26.6) | 0.190; 0.66 | 7 |
| Yoo et al. [15] | CC: 25 (24.3) CT: 54 (52.4) TT: 24 (23.3) | C: 104 (50.5) T: 102 (49.5) | CC: 37 (35.9) CT: 53 (51.5) TT: 13 (12.6) | C: 127 (61.6) T: 79 (38.4) | 0.794; 0.37 | 6 |
| Ono et al. [16] | CC: 24 (26.1) CT: 49 (53.3) TT: 19 (20.6) | C: 97 (52.7) T: 87 (47.3) | CC: 43 (44.4) CT: 37 (38.1) TT: 17 (17.5) | C: 123 (63.4) T: 71 (36.6) | 0.007; 0.93 | 5 |
| Kini et al. [17] | CC: 50 (35.5) CT: 75 (53.2) TT: 16 (11.3) | C: 175 (62.1) T: 107 (37.9) | CC: 85 (37.6) CT: 115 (50.9) TT: 26 (11.5) | C: 285 (63.1) T: 167 (36.9) | 0.854; 0.36 | 7 |
| Total | CC: 760 (45.3) CT: 712 (42.4) TT: 206 (12.3) | C: 2232 (66.5) T: 1124 (33.5) | CC: 866 (48.5) CT: 742 (41.6) TT: 176 (9.9) | C: 2474 (69.3) T: 1094 (30.7) | ||
| Genetic Model | Egger’s Test | Begg’s Test | |||
|---|---|---|---|---|---|
| Intercept | 95% CI | p | Kendall’s Tau | p | |
| Dominant | 1.023 | −1.956 to 4.002 | 0.433 | 0.071 | 0.805 |
| Recessive | −0.075 | −2.304 to 2.153 | 0.937 | 0.214 | 0.458 |
| Additive | 0.216 | −2.522 to 2.955 | 0.853 | 0.143 | 0.621 |
| Heterozygous | 1.069 | −1.660 to 3.797 | 0.375 | 0.214 | 0.458 |
| Allelic | 0.784 | −2.467 to 4.036 | 0.577 | 0.071 | 0.805 |
| Genetic Model | Egger’s Test | Begg’s Test | |||
|---|---|---|---|---|---|
| Intercept | 95% CI | p | Kendall’s Tau | p | |
| Dominant | 1.485 | −1.202 to 4.171 | 0.177 | 0.400 | 0.327 |
| Recessive | 0.074 | −3.269 to 3.418 | 0.948 | 0.200 | 0.624 |
| Additive | 0.475 | −3.252 to 4.203 | 0.712 | 0.200 | 0.624 |
| Heterozygous | 1.498 | −0.440 to 3.436 | 0.091 | 0.600 | 0.142 |
| Allelic | 1.256 | −2.549 to 5.062 | 0.371 | 0.200 | 0.624 |
| Genetic Model | Egger’s Test | Begg’s Test | |||
|---|---|---|---|---|---|
| Intercept | 95% CI | p | Kendall’s Tau | p | |
| Dominant | 1.464 | −46.030 to 48.958 | 0.762 | 0.333 | 0.602 |
| Recessive | 1.100 | −11.880 to 14.081 | 0.476 | 0.333 | 0.602 |
| Additive | 1.083 | −35.202 to 37.368 | 0.769 | 0.333 | 0.602 |
| Heterozygous | 1.324 | −46.659 to 49.307 | 0.785 | 0.333 | 0.602 |
| Allelic | 1.713 | −42.493 to 45.919 | 0.709 | 0.333 | 0.602 |
| Genetic Model | Heterogeneity I2 (%), p | Effect Model | Pooled OR, 95% CI, p | Stability of Results (Yes/No) |
|---|---|---|---|---|
| Total Groups (8 Studies) | ||||
| Dominant (CT + TT vs. CC) | 52.19, 0.041 | random | 1.264, 1.005–1.589, 0.045 | No |
| Recessive (TT vs. CC + CT) | 0.00, 0.572 | fixed | 1.327, 1.069–1.648, 0.010 | No |
| Additive (TT vs. CC) | 27.35, 0.210 | fixed | 1.440, 1.144–1.812, 0.002 | Yes |
| Heterozygous (CT vs. CC) | 43.39, 0.089 | fixed | 1.153, 0.997–1.333, 0.055 | Yes |
| Allelic (T vs. C) | 48.84, 0.057 | fixed | 1.183, 1.067–1.311, 0.001 | Yes |
| Young adults (5 studies) | ||||
| Dominant (CT + TT vs. CC) | 0.00, 0.462 | fixed | 1.281, 1.092–1.503, 0.002 | Yes |
| Recessive (TT vs. CC + CT) | 0.00, 0.632 | fixed | 1.483, 1.148–1.918, 0.003 | Yes |
| Additive (TT vs. CC) | 0.00, 0.464 | fixed | 1.627, 1.240–2.135, <0.001 | Yes |
| Heterozygous (CT vs. CC) | 0.00, 0.611 | fixed | 1.209, 1.021–1.431, 0.028 | Yes |
| Allelic (T vs. C) | 6.42, 0.370 | fixed | 1.256, 1.114–1.417, <0.001 | Yes |
| Children (3 studies) | ||||
| Dominant (CT + TT vs. CC) | 76.80, 0.013 | random | 1.119, 0.540–2.318, 0.762 | Yes |
| Recessive (TT vs. CC + CT) | 0.00, 0.722 | fixed | 1.006, 0.670–1.510, 0.978 | Yes |
| Additive (TT vs. CC) | 39.50, 0.192 | fixed | 1.054, 0.682–1.628, 0.814 | Yes |
| Heterozygous (CT vs. CC) | 76.36, 0.015 | random | 1.117, 0.521–2.394, 0.777 | Yes |
| Allelic (T vs. C) | 65.53, 0.055 | random | 1.070, 0.710–1.600, 0.750 | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarecka-Hujar, B. Is There a Relation between 677C>T Polymorphism in the MTHFR Gene and the Susceptibility to Epilepsy in Young Patients? A Meta-Analysis. Brain Sci. 2021, 11, 1327. https://doi.org/10.3390/brainsci11101327
Sarecka-Hujar B. Is There a Relation between 677C>T Polymorphism in the MTHFR Gene and the Susceptibility to Epilepsy in Young Patients? A Meta-Analysis. Brain Sciences. 2021; 11(10):1327. https://doi.org/10.3390/brainsci11101327
Chicago/Turabian StyleSarecka-Hujar, Beata. 2021. "Is There a Relation between 677C>T Polymorphism in the MTHFR Gene and the Susceptibility to Epilepsy in Young Patients? A Meta-Analysis" Brain Sciences 11, no. 10: 1327. https://doi.org/10.3390/brainsci11101327






