Abstract
This systematic review examined available findings on spatial and temporal characteristics of cortical activity in response to unpredicted mechanical perturbations. Secondly, this review investigated associations between cortical activity and behavioral/biomechanical measures. Databases were searched from 1980–2021 and a total of 35 cross-sectional studies (31 EEG and 4 fNIRS) were included. Majority of EEG studies assessed perturbation-evoked potentials (PEPs), whereas other studies assessed changes in cortical frequencies. Further, fNIRS studies assessed hemodynamic changes. The PEP-N1, commonly identified at sensorimotor areas, was most examined and was influenced by context prediction, perturbation magnitude, motor adaptation and age. Other PEPs were identified at frontal, parietal and sensorimotor areas and were influenced by task position. Further, changes in cortical frequencies were observed at prefrontal, sensorimotor and parietal areas and were influenced by task difficulty. Lastly, hemodynamic changes were observed at prefrontal and frontal areas and were influenced by task prediction. Limited studies reported associations between cortical and behavioral outcomes. This review provided evidence regarding the involvement of cerebral cortex for sensory processing of unpredicted perturbations, error-detection of expected versus actual postural state, and planning and execution of compensatory stepping responses. There is still limited evidence examining cortical activity during reactive balance tasks in populations with high fall-risk.
1. Introduction
Maintaining upright posture and balance is critical for humans to carry out activities of daily living. Human balance control relies on anticipatory and reactive domains, respectively to maintain postural stability in response to self-induced/predicted perturbations and to restore stability after encountering unpredicted perturbations [,,,,]. Both anticipatory and reactive domains involve coordination of sensorimotor and cognitive systems for maintaining upright posture or recovering from balance losses and to avoid falling [,,,,,]. From a neural control perspective, it is well established that the cerebral cortex modulates volitional/anticipatory postural and balance adjustments prior to goal-directed movements such as reaching or obstacle crossing [,,,,,]. Previously, three reviews presented findings on cortical involvement in planning and execution of anticipatory/volitional balance and gait-related tasks among healthy young, healthy older, and clinical populations [,,]. However, there is limited evidence regarding the cortical mechanisms and/or substrates involved with initiation, execution, and modulation of reactive balance responses.
Reactive balance responses elicited upon unpredicted perturbations include feet-in-place responses (i.e., hip or ankle strategies) [] or change-in-support responses (i.e., compensatory stepping or grasping) [] and are known to be influenced by the magnitude of the perturbation (i.e., its displacement, velocity, or acceleration) []. Cumulative evidence from animal and human studies indicated that feet-in-place responses might be elicited by spinal cord and brainstem mediated short and medium latency reflexes [,,]. Conversely, change-in-support (i.e., compensatory stepping) strategies are postulated to be ‘triggered’ from brainstem control centers [,,]. Further, behavioral/neurophysiological human studies indicated the possible role of the cerebral cortex in modulating spatiotemporal characteristics of compensatory stepping based on factors such as prior experience, task demand/difficulty and current context [,,,,,,]. Studies have also indicated that physiological aging [,,,,] and neurological impairment [,,,] are associated with alterations in compensatory stepping and impaired reactive balance control, which might be related to changes in cortical substrates. Evidence from studies using neuroimaging modalities supported these postulations from behavioral studies regarding possible cortical modulation of reactive balance responses [,,,,,,,,,,]. In the past 2 decades, several neuroimaging modalities have been used by researchers to examine reactive balance control including magnetic resonance imaging (MRI) [,,], functional MRI (fMRI) [,], positron emission tomography (PET) [,], electroencephalography (EEG) [,,,,,,], and functional near-infrared spectroscopy (fNIRS) [,,,]. Studies have used fMRI to examine task-related changes in the cortico-subcortical areas for simulating unpredicted perturbations through mental imagery/observation of tasks [,]. However, with poor temporal resolution (2 s), fMRI might not provide information on true event-related activations especially for reactive balance control (e.g., at the instance a slip occurs while observing a video in the scanner) [,]. Alternatively, recently developed wireless EEG systems allow full subject mobility and can record brain activity during actual tasks. While EEG lacks spatial resolution (6–27 cm3), it offers excellent temporal resolution (up to 1 ms) and can record task-induced evoked potentials over all cortices [,]. fNIRS also allows full subject-mobility, however, the brain surfaces recorded might be often limited to prefrontal and frontal cortices []. Nevertheless, fNIRS offers greater spatial resolution (1–2 mm3) than EEG (6–27 cm3) and higher temporal resolution (up to 10 ms) than fMRI (∼3 s) over pre-frontal and frontal cortical areas [,]. Moreover, both EEG and fNIRS are portable, wireless and can be good tools to examine cortical activations in clinical and research settings [,].
The advantages of EEG have motivated researchers to use this technique for examining cortical evoked potentials associated with reactive balance control. There are two recently published reviews examining perturbation-evoked potentials (PEPs) recorded via EEG [,]. PEPs are multicomponent cortical responses comprised of positive and negative potentials recorded within the first 400 ms following an external perturbation. Payne et al. [] integrated evidence on association between error-related negative cortical potentials recorded during volitional (stimulus–response) and reactive balance tasks, whereas Varghese et al. [] focused on examining characteristics of PEPs in response to sensory (visual/auditory/proprioceptive) and mechanical perturbations. However, both Payne et al. [] and Varghese et al. [] did not provide detailed evidence on event-related changes in frequency spectrums following unpredicted perturbations. Event-related spectral perturbations (ERSPs) are one type of stimulus or task-related changes in cortical frequencies recorded via EEG and assessed via time-frequency analysis of brain activity. Further, no reviews have included findings on hemodynamic changes during unpredictable perturbations recorded via fNIRS. Lastly, there is still lack of evidence regarding the associations between unpredicted perturbation-induced cortical and behavioral/biomechanical responses. Therefore, this systematic review aimed to examine evidence on cortical activations using mobile neuroimaging techniques (EEG and/or fNIRS) during different types of reactive balance tasks among healthy young, older, and neurologically impaired populations. Additionally, this review aimed to examine the current evidence that exists regarding associations between cortical and behavioral or biomechanical measures of balance and gait.
2. Methods
2.1. Review Objectives
This systematic review examined literature on neuroimaging of reactive balance control from 1980–2021 to address the following research questions:
Research question (RQ1): What are the temporal and spatial characteristics of cortical activity recorded via EEG and fNIRS during novel unpredicted mechanical perturbations delivered across different tasks?
Research question (RQ2): What are the temporal and spatial characteristics of cortical activity recorded via EEG and fNIRS during repeated unpredicted mechanical perturbations delivered across different tasks?
Research question (RQ3): Are behavioral/biomechanical outcomes correlated with cortical activity during unpredicted mechanical perturbations delivered across different tasks?
2.2. Review Methodology
2.2.1. Search Process and Strategy
To address the proposed research questions, we used four databases: PubMed, Web of science, IEEE explore and Google scholar. The database search key terms were found in one of the following sections of a research article: Title, Abstract or Keywords. Following the initial advanced search, the results from each database were run through Mendeley to detect any duplicate studies. Once all the duplicate studies were removed, the results from the databases were uploaded to Rayyan—Intelligent systematic review software []. Each of the authors independently included and excluded research studies while being blinded to other’s decision.
We used the Preferred Items for Systematic Reviews and Meta-Analyses method (PRISMA: see Figure 1) []. The search strategy included combinations of terms related to neuroimaging studies that incorporated reactive balance paradigms. The search fields were connected with “AND” to ensure that matches included key terms from both search fields and within each search field, terms were connected with an “OR” so that at least one of the key terms was matched in the results. The advanced search terms for neuroimaging included variations of the following terms: electroencephalography, EEG, functional near-infrared spectroscopy, fNIRS, alpha, beta, gamma, delta, perturbation-evoked potentials, PEP, N1, P1, N2, and P2, cortical activity, neural activity, and brain activity. The advanced search terms for reactive balance control paradigms included variations of all the following terms: reactive balance, perturbed gait, perturbed stance, unpredicted perturbations, slips, trips, dual task slips, dual task trips, and falls. An example from the search combinations is listed below:
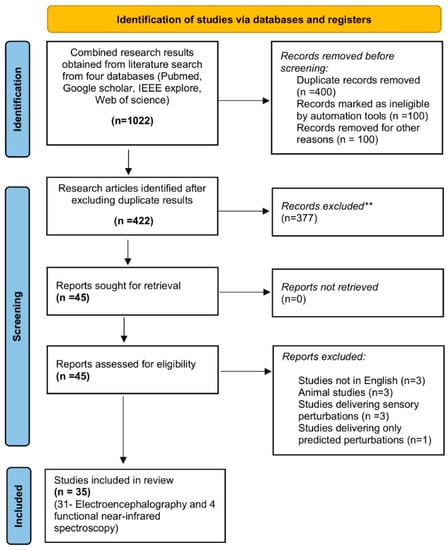
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases: Combined search results from four databases yielded 1022 research articles out of which 600 were excluded due to duplication and ineligibility. The remaining 422 were assessed for eligibility and 377 were excluded after reviewing article abstracts. Thus, 10 articles were excluded from the remaining 45 after full-text analysis and finally 35 cross-sectional studies were included. ** Records excluded by the authors due to ineligibility.
((EEG and reactive balance)) OR ((EEG and reactive balance and perturbations)) OR ((EEG and slips)) OR ((EEG and trips)) OR ((EEG and perturbed gait)) OR ((EEG and dual-task and reactive balance)) OR ((EEG and dual task and perturbations)) OR ((EEG and N1 and reactive balance)) OR ((EEG and theta and reactive balance)) OR ((EEG and beta and reactive balance)) OR ((EEG and gamma and reactive balance)) OR ((EEG and alpha and reactive balance) ((EEG and N1 and perturbations)) OR ((EEG and theta and perturbations)) OR ((EEG and beta and perturbations)) OR ((EEG and gamma and perturbations)) OR ((EEG and alpha and perturbations)).
2.2.2. Eligibility Criteria
The inclusion and exclusion criteria for this review were based on neuroimaging studies published from 1980–2021 that examined cortical changes during reactive balance tasks (via EEG and fNIRS). Studies were included based on the following criteria: (1) studies that delivered single or repeated unpredicted mechanical perturbations across one or more functional task(s) including sitting, standing, and walking, (2) studies that recorded brain activity only via EEG and fNIRS, and (3) research articles that were in English. Studies were excluded based on the following criteria: (1) studies that assessed static or dynamic volitional balance control (e.g., avoiding obstacles while walking or catching a ball), (2) studies that investigated the effects of training, exercise intervention, drug or therapy, (3) studies that did not assess cortical activity during the actual task (i.e., studies that assessed cortical activity before or after reactive balance tasks via fMRI, single-photon emission computerized tomography-SPECT, PET, or diffusion tensor imaging), (4) studies that delivered only sensory perturbations (i.e., visual, auditory or somatosensory perturbations), and (5) animal neuroimaging studies.
2.2.3. Quality Assessment
The quality of included studies was assessed using the fourteen-point National Institute of Health (NIH) Study Quality Assessment for Cross-sectional studies. Previous systematic reviews have used this tool to assess study quality []. Each of the fourteen questions was assigned a score of “2” if the criterion was met by the research paper, “1” if the criterion was partially met, “0” if the criterion was not met and N/A if the criterion was not applicable or not related to the research paper. The sum of points from all the fourteen questions divided by the total of points applicable provided a score which was then used to rate each research study (≥75% = high, 55–75% = moderate, ≤55% = low) (Table 1). Each author rated all the included studies independently.

Table 1.
Quality Assessment of Included Studies.
The majority of the studies (≈20/35) received a moderate rating from both the authors. General strengths of eligible studies included clear specification of study population(s), specification of inclusion/exclusion criteria and usage of valid and reliable tools for assessment of outcomes. Common limitations of eligible studies included lack of sample size justification, power description/analysis of variance and effect estimates. Specifically, none of the included studies provided justification for sample size for the included population(s). Secondly, a majority of studies did not incorporate key confounding factors that could impact the outcome measures into statistical analysis. Lastly, in some studies the aims/objectives/research question and the independent and dependent outcome variables were not clearly stated or defined.
2.2.4. Data Extraction
Following independent evaluation of the articles, both the authors discussed and decided whether to include or exclude the research studies under “undecided/maybe section”. Both the reviewers first worked on retrieving the data independently and then together for summarizing the results tables. Specific information was extracted from all the eligible studies including (1) first author and year of publication; (2) sample size of the study; (3) sample characteristics (e.g., age, gender and population); (4) characteristics of disease/disorders (for studies that included clinical populations); (5) neuroimaging technique(s) used; (6) information about the type, position, mode, intensity of reactive balance tasks; (7) behavioral/kinematic/biomechanical outcomes assessed; (8) key findings that specifically address RQ1–RQ3; (9) study limitations. All the above information for studies in all categories and subsets is presented in Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7.

Table 2.
Perturbation-evoked potentials (PEPs) recorded via electroencephalography (EEG) during novel unpredictable (UNPRED) mechanical perturbations (RQ1).

Table 3.
Changes in frequency spectrums recorded via electroencephalography (EEG) in response to unpredictable (UNPRED) mechanical perturbations (RQ1).

Table 4.
Changes in oxygenated and deoxygenated hemoglobin (Hb) levels recorded via functional near-infrared spectroscopy (fNIRS) in response to novel and repeated unpredictable (UNPRED) mechanical perturbations (RQ1).

Table 5.
Perturbation-evoked potentials (PEPs) recorded via electroencephalography (EEG) in response to repeated unpredictable (UNPRED) mechanical perturbations (RQ2).

Table 6.
Changes in frequency spectrums recorded via electroencephalography (EEG) in response to repeated unpredictable (UNPRED) mechanical perturbations (RQ2).

Table 7.
Changes in oxygenated and deoxygenated hemoglobin (Hb) levels recorded via functional near-infrared spectroscopy (fNIRS) in response to repeat-ed unpredictable (UNPRED) mechanical perturbations (RQ2).
2.2.5. Data Analysis
Search results obtained from four research databases yielded a total of 1022 research articles. After removing all the duplicate results via Mendeley we extracted 422 original research articles. After reviewing the abstracts of all these articles, a total of 45 articles were included. The reasons for exclusion of other 377 were (1) studies that delivered predicted mechanical or sensory perturbations for examining cortical activity, (2) studies that delivered unpredicted sensory perturbations (i.e., visual or proprioceptive perturbations, (3) intervention studies that examined the effect of treatment on changes in cortical activity to unpredicted perturbations, (4) studies that delivered unpredicted perturbations to assess changes in cortical activity in children and adolescents (below 18 years of age). The remaining 45 articles were assessed for eligibility and 10 were removed based following points: (1) studies that were not in English, (2) Animal studies, (3) Studies that delivered sensory perturbations, and (4) Studies that delivered only predicted perturbations. This was followed by full-text examination that yielded a total of 35 cross-sectional studies that assessed changes in cortical activity during novel or repeated unpredicted mechanical perturbations (Figure 1) [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].
3. Results
The results section was summarized based on the research questions (RQ1–3) and included studies from two primary domains: (1) studies using EEG to record cortical activity (n = 31) and (2) studies using fNIRS to record cortical activity (n = 4) during unpredicted mechanical perturbations. The EEG studies were sub-divided into 2 categories: studies examining PEPs (n = 20) and studies examining changes in frequency spectrums (n = 11). The fNIRS studies (n = 4) examining hemodynamic changes were summarized together. Further, each category was divided into type of reactive balance task including sitting, standing, walking and multiple tasks. Within each task, studies that assessed healthy young, heathy older and pathological populations were reported separately. Finally, for each population the results were reported based on the type of perturbations (i.e., unpredicted only, predicted versus unpredicted and dual tasks).
Although only 12 studies examined the associations between cortical activations and behavioral/biomechanical outcomes [,,,,,,,,,,,], most of the included studies assessed behavioral/biomechanical measures. Among the included studies, behavioral measures such as dynamic balance were assessed using Berg Balance Scale [,,], Mini Balance Evaluations System Test (Mini-BESTest) [] and the distance travelled on a Beam-Walking Task [,]. Other balance measures were single foot stance time, functional base of support and time-to-step using sensory organization testing []. Mobility was assessed using Short physical performance battery to stratify older adults into mobile and frail older adults []. In addition, psychological factors like pain perception, coping strategies, fear of falling, perceived balance confidence and perceived stability were assessed with clinical questionnaires [,] and autonomic responses were assessed by examining changes in electrodermal responses []. Lastly, biomechanical and kinematic outcomes including center of mass/pressure displacement [,], postural sway [] and electromyography (EMG) responses for lower limb muscles were assessed during reactive balance tasks []. Behavioral/biomechanical outcomes for only studies that examined corresponding cortical activations during reactive balance tasks are listed in the tables (Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7).
3.1. Spatial-Temporal Characteristics of Cortical Activity: Novel Perturbations (RQ1)
3.1.1. EEG Studies—PEPs
This section included 20 studies that assessed PEPs during unpredicted perturbations in sitting (n = 1) [], stance (n = 17) [,,,,,,,,,,,,,,,,], walking (n = 1) [] and multiple tasks (n = 2) [,] (Table 2). A majority of studies included young adults (n = 17), some studies compared healthy and frail older adults (n = 1) [], people with traumatic brain injury (n = 1) [] and people with low back pain (n = 1) [] with age-matched/healthy controls. Studies analyzed one or more early or late PEPs (Early PEPs: 0–150 ms post-perturbation—P1 and N1, late: 250–400 ms post-perturbation- P2 and N2) in response to unpredicted perturbations.
Task—Sitting:
Healthy young population: Unpredicted perturbations only: Ditz and colleagues [] assessed PEP characteristics during medial-lateral seated perturbations using a tilting chair. This study reported peak N1 (Amplitude-amp): −28.3 ± 14.5 µV, Latency-lat: 144 ± 9 ms post-perturbation (Mean ± SD) focused on midline central (Cz) with distribution around frontal, central and parietal areas. Peak P2 (amp: 12.2 ± 4.1 ± 4.1 µV, lat: 330 ± 30 ms) was centered at midline parietal electrode (Pz) and distributed around parietal areas. However, P1 and N2 PEPs were not identifiable using single-trial EEG analysis.
Healthy older population: None of the included studies assessed PEPs during unpredicted perturbations in sitting in healthy older adults.
Pathological populations: None of the included studies assessed PEPs during unpredicted perturbations in sitting in pathological populations.
Task—Standing:
Healthy young population: Unpredicted perturbations only: Goel et al. [] examined the effect of movable platform speed (low: 7.9 cm/s, high: 15.88 cm/s) and direction (forward/backward) on N1 response. Larger N1 amplitudes (High-backward: −10.8 ± 1.7 µV/High-forward: −10.5 ± 1.3 µV vs. Low-backward: −8.1 ± 1.1 µV/Low-forward: −8.0 ± 0.9 µV) and shorter latency (High-backward: 159.7 ± 4.4 ms/High-forward: −159.3 ± 5.4 ms vs. Low-backward: 167.3 ± 3.4 ms/Low-forward: 171.3 ± 5.1 ms) for high-speed compared to low-speed perturbations was reported. Quant and colleagues [] examined changes in late PEPs (P2 and N2) during immediate and delayed perturbation deceleration. No differences in early (P1 and N1) and late (P2 and N2) PEPs were observed between the two conditions. Further, Payne and Ting, 2020a and 2020b [,] examined the effect of backward support-surface translations delivered via movable platform on N1 characteristics over central electrodes. All participants were instructed to plan a compensatory step on half of the trials and avoid stepping on the other half [,]. In both studies, perturbations were delivered at three magnitudes (easy, moderate, and difficult: 8–22 cm distance). One of the studies found no differences in N1 amplitude based on the planning of compensatory steps []. However, this study reported significantly larger N1 amplitudes (11% or 6.3 µV higher) when compensatory steps were executed compared to those where participants resisted taking a step. The other study reported increase in N1 amplitude with increase in perturbation magnitude such that N1 amplitude increased 5–6 µV with each perturbation magnitude. This study also reported correlations between N1 amplitude and clinical balance performance. Those results have been discussed in RQ3 section.
Predicted and Unpredicted perturbations: Adkin et al. [] delivered manual unpredicted and predicted forward trunk perturbations to healthy young adults. P1, observed in less than half (3/8) of subjects, showed no differences in amplitude during predicted (Amp: ≈4.2–5.5 µV, Lat: ≈28–61 ms post-perturbation) or unpredicted (Amp: ≈5.5–6.7 µV, Lat: ≈28–61 ms post-perturbation) perturbations. Large N1 amplitudes were reported during unpredicted perturbations (≈−7.5 to −22 µV) at fronto-central and centro-parietal (FCz, Cz and CPz) electrodes. P1 and N1 during predicted perturbations were non-distinguishable from the background EEG activity. A similar study by Mochizuki et al., 2008 delivered predicted and unpredicted backward perturbations using lean-and-release of a load in standing []. During predicted perturbations, greater pre-perturbation activity was observed when compared to unpredicted perturbations. Additionally, N1 amplitude during unpredicted perturbations was significantly larger (32.0 ± 14.8 µV) than predicted perturbations (17.6 ± 7.2 µV). The N1 latency during unpredicted perturbations (156.5 ± 11.8 ms) was significantly increased when compared to predicted perturbations (140.1 ± 25.9 ms). Two studies (Adkin et al., 2008 and Sibley et al., 2010) [,] exposed young adults to predicted and unpredicted manual trunk [] and load-release [] perturbations at LOW (ground) and HIGH (1.6/3.2 m above ground) levels. In both studies, larger N1 amplitudes during HIGH (89.2 ± 17 µV) compared to LOW (70.2 ± 14 µV) were reported during unpredicted perturbations [,]. However, there were no changes in N1 latency and P2 characteristics between the two conditions []. Additionally, N1 was not generated during predicted perturbation but instead a negative cortical potential was observed before the onset of perturbation that showed no changes between HIGH and LOW ground perturbations.
Dual tasks: Four studies examined the effect of concurrent cognitive task during mechanical perturbations in young adults [,,,]. There were no task-related differences observed in P1 in any of these studies. All studies reported significant reduction in N1 amplitude with no differences in N1 latency and P2 characteristics during dual task compared to single task (i.e., cognitive task-one study or balance tasks-three studies). However, larger N1 amplitudes during dual task compared to single task even during predicted perturbations were reported []. Further, Bogost and colleagues [] identified specific regions of cortical activation during single and dual tasks. During single tasks (balance task), activations occurred primarily in the prefrontal, primary motor cortex and supplementary motor area predominantly in the left hemisphere. However, during dual task, activations was seen in prefrontal, frontal, sensorimotor and parietal cortices predominantly in the right hemisphere.
Healthy older population: Unpredicted perturbations only: Duckrow et al., 1999 compared PEPs during unpredicted stance perturbations in healthy and frail older adults with healthy young adults. Delayed P1 latency (Old: 87 ± 8 ms; Young: 60 ± 5 ms) and smaller N1 amplitudes (Old: 23 ± 12, Young: 45 ± 17 µV) were seen in older (mobile and frail) compared to young adults [].
Pathological populations: Unpredicted perturbations only: Two studies compared PEPs during stance perturbations in people with traumatic brain injury and low back pain with their younger or age-matched controls [,]. In people with chronic low back pain, no differences in N1 and larger P2 amplitudes at fronto-central areas (FCz and Cz) compared to age-matched healthy controls were reported []. On the other hand, smaller N1 amplitudes (2.1 ± 1.3 μV) with no differences in N1 latency were reported in people with chronic traumatic brain injury when compared to healthy controls (3.8 ± 1.2 μV).
Task-Walking:
Healthy young population: Unpredicted perturbations only: One study compared changes in cortical activity during unpredicted gait perturbations with stance perturbations []. This study has been mentioned in the multiple task section below.
Healthy older population: None of the included studies assessed PEPs during unpredicted perturbations in walking in healthy older adults.
Pathological populations: None of the included studies assessed PEPs during unpredicted perturbations in walking in pathological populations.
Multiple tasks:
Healthy young population: Unpredicted perturbations only: Two studies compared PEPs during multiple tasks [,]. Dietz et al. [] compared changes in cortical activity during unpredicted gait perturbations with stance perturbations delivered via motorized treadmill. P1 during gait perturbations exhibited longer latency (80 ± 11 ms) compared to stance perturbations (43 ± 6 ms) at midline central area (Cz). In addition, N1 observed during gait perturbations exhibited longer latency (131 ± 7 ms) compared to stance perturbations (95 ± 8 ms). In addition, Mochizuki et al. [] compared changes in PEPs characteristics during seated and stance perturbations. Larger P2 amplitude with no changes in latency during stance (37.87 ± 6.14 μV) compared to sitting tasks (23.66 ± 6.21 μV) was observed at centro-parietal areas (CPz) [].
Healthy older population: None of the included studies assessed PEPs during unpredicted perturbations in one or more tasks in healthy older adults.
Pathological populations: None of the included studies assessed PEPs during unpredicted perturbations in one or more tasks in pathological populations.
3.1.2. EEG Studies—Changes in Frequency Spectrums
This section included 8 studies that assessed changes in low-to-high frequency bands in response to unpredicted perturbations in stance (n = 5) [,,,,,,], walking (n = 2) [,] and multiple tasks (n = 1) [] (stance and walking) (Table 3). While a majority of these studies included young adults (n= 5), one study examined cortical activations in healthy older adults [] and two studies compared cortical activations in people with chronic cortical stroke [] and chronic traumatic brain injury [] with healthy counterparts. Studies analyzed changes in cortical frequencies including alpha (7.5–12.5 Hz), beta (13–30 Hz), gamma (40–100 Hz) and theta (4–8 Hz). For simplicity, we would like to clarify the meaning of terms related to frequency analysis: synchronization or enhancement refers to increase in frequency band(s) compared to baseline whereas desynchronization or suppression refers to decrease in frequency band(s) compared to baseline []. Event-related spectral perturbations (ERSPs) measure the average changes in spectral power over varied periods of time [] and spectral power density is measured in terms of absolute spectral power density or average spectral power density.
Task—sitting: None of the included studies assessed changes in cortical frequencies during seated perturbations in healthy young, healthy older or pathological populations.
Task—Standing:
Heathy young population: Unpredicted perturbations only: Preliminary studies examined the relationship between frequency characteristics and PEPs in young adults during unpredicted forward perturbations via lean-and-release method [,]. They reported corresponding increases in alpha, beta and theta frequencies in fronto-central and parietal areas during the time of N1 occurrence []. Further, Solis-Escalante et al. [] exposed young adults to unpredicted backward perturbations with random visual cues to either execute in-place or compensatory step. Reduction in theta and alpha bands was observed during observation of cues followed by enhancement during response preparation phase (5.6–7 s after cue). However, the reduction in alpha bands for feet-in-place responses during cue observation was maintained throughout the response preparation phase. On the other hand, both feet-in-place and compensatory stepping shared some common frequency characteristics including initial alpha band suppression in midline occipital areas during cue observation followed by beta I and II reduction in midline central and parietal area, respectively during response preparation phase (5.6–7 s after cue observation). Additionally, Ghosn et al. [] exposed young adults to unpredicted backward perturbations at different intensities (i.e., acceleration and displacement) via a movable platform. This study reported increase in beta power in central areas 50 ms post-perturbation especially during perturbations with higher intensity.
Healthy older population: None of the included studies assessed changes in frequency spectrums during unpredicted perturbations in standing in healthy older adults.
Pathological populations: Unpredicted perturbations only: Solis-Escalante et al. [] exposed people with chronic stroke and young adults to unpredicted stance perturbations via movable platform. This study reported perturbation direction-specific pictographic presentations of theta band activation patterns similar in both groups. Such direction-specific cortical changes in theta bands were accompanied by corresponding scalp topographies with frontal, central and parietal areas. Shenoy et al., 2021 exposed people with chronic traumatic brain injury and age-matched healthy adults to unpredicted forward/backward variable magnitude perturbations and compared network connectivity strength in alpha, beta and theta bands []. Network strength analysis showed task-related reductions in theta and alpha with no differences in beta network strength in people with traumatic brain injury compared to healthy counterparts. Lastly, Palmer et al., 2021 delivered multi-directional perturbations via movable platform to healthy older adults and reported correlations between beta connectivity and balance performance measures []. The study results are explained in RQ3 section.
Task—Walking:
Healthy young population: Unpredicted perturbations only: Two studies assessed band power [] and rate of variation of spectral power density [] in frequency bands in young adults during forward [] and backward perturbations [] in walking using a split-belt treadmill. During post-perturbation recovery period [], reduction in alpha power was observed in sensorimotor and posterior-parietal areas compared to unperturbed periods. Additionally, theta power increased during walking in posterior parietal cortex and in sensorimotor and posterior parietal cortices during recovery compared to unperturbed periods. On the other hand, post-perturbation theta and beta frequency increased with highest difference in beta band over fronto-central and parietal electrodes [].
Healthy older population: None of the included studies assessed changes in frequency spectrums during unpredicted perturbations in walking in healthy older adults.
Pathological populations: None of the studies included assessed changes in frequency spectrums during unpredicted perturbations in walking in pathological populations.
Multiple tasks:
Healthy young population: Unpredicted perturbations only: A study conducted by Peterson and Ferris [] examined the differences in frequency characteristics during medio-lateral stance and gait perturbations via manual waist pulls. Increased alpha power and beta power in sensorimotor and increased alpha power in supplementary motor and posterior parietal cortices were observed during stance compared to walk perturbations. Theta synchronization in supplementary motor area and anterior cingulate areas were observed during perturbation onset in both conditions. This was followed by alpha-beta desynchronization in bilateral sensorimotor areas during stance and walk perturbations.
Healthy older population: None of the included studies assessed changes in frequency spectrums during unpredicted perturbations in more than one task in healthy older adults.
Pathological populations: None of the included studies assessed changes in frequency spectrums during unpredicted perturbations in more than one task in pathological populations.
3.1.3. fNIRS Studies—Changes in Hemodynamic Responses
This section included 4 fNIRS studies that assessed changes in hemodynamic responses during stance (n = 2) [,] and gait perturbations (n = 2) [,] (Table 4). Three studies assessed healthy young populations whereas 1 study included adults with subcortical stroke [].
Task—Sitting: None of the included studies assessed changes in hemodynamic responses during seated perturbations in healthy young, healthy older, or pathological populations.
Task—Standing:
Healthy young population: Unpredicted perturbations only: None of the included studies assessed changes in oxygenated hemoglobin only during unpredicted stance perturbations in healthy young adults.
Predicted and unpredicted perturbations: A study in healthy young adults [] reported changes in oxygenated hemoglobin based on the perturbation type. While both predicted (with auditory signals) and unpredicted (without auditory signals) perturbations resulted in increase in oxygenated hemoglobin in frontal and parietal areas, predicted perturbations resulted in greater increase in oxygenated hemoglobin in right superior parietal lobes and left supplementary motor area as well as activation of left middle frontal gyrus and precentral gyrus. Although majority of temporal characteristics were similar between the two conditions, the right middle frontal gyrus displayed early increase in oxygenated hemoglobin in predicted compared to unpredicted perturbations.
Healthy older population: None of the included studies assessed changes in hemodynamic responses during unpredicted perturbations in standing in healthy older adults.
Pathological populations: Unpredicted perturbations only: A study conducted in people with subcortical stroke delivered anterior-posterior stance perturbations via a movable platform. This study reported increased oxygenated hemoglobin in bilateral prefrontal cortices and premotor cortex and parietal association area of the unaffected hemisphere [].
Task—Walking:
Healthy young population: Unpredicted perturbations only: Koren et al., 2019 exposed healthy young adults to unpredicted overground perturbations during walking via customized shoe-like mechanical system []. Increased oxygenated hemoglobin was reported in bilateral prefrontal cortices during perturbed compared to unperturbed walking/standing periods.
Healthy older population: None of the included studies assessed changes in hemodynamic responses during unpredicted perturbations in walking in healthy older adults.
Pathological populations: None of the included studies assessed changes in hemodynamic responses during unpredicted perturbations in walking in pathological populations.
3.2. Spatial-Temporal Characteristics of Cortical Activity: Repeated Perturbations (RQ2)
3.2.1. EEG Studies: PEPs
Two EEG studies assessed changes in PEPs during repeated stance perturbations in healthy young population [,] (Table 5).
Task—Sitting: None of the included studies assessed changes in PEPs during repeated seated perturbations in healthy young, healthy older and pathological populations.
Task—Standing:
Healthy young population: Unpredicted perturbations only: Studies by a group of researchers [] tested the effect of repeated backward platform perturbations by delivering easy (displacement 8 cm), moderate (13–15 cm) and difficult (18–22 cm) perturbations with variable acceleration levels. Young adults were instructed to plan a stepping response in half of the trials and to resist stepping in the other half []. The results showed an increase in N1 amplitude and reduction in N1 latency with increasing perturbation difficulty (difficult > moderate > easy) []. Additionally, repeated perturbations resulted in reduction in N1 amplitude []. Another study by Mierau et al., 2015 [] tested the effect of repeated perturbations on N1 amplitude while balancing on their dominant leg. The results showed no differences in P1 characteristics between the trials. However, the study reported reduction in N1 amplitude between novel and consecutive perturbation as well as between the second and last perturbation.
Healthy older populations: None of the included studies assessed changes in PEPs during repeated stance perturbations in healthy older adults.
Pathological populations: None of the included studies assessed changes in PEPs during repeated stance perturbations in pathological populations.
Task—Walking: None of the included studies assessed changes in PEPs during repeated gait perturbations in healthy young, healthy older and pathological populations.
Multiple tasks: None of the included studies assessed changes in PEPs during repeated perturbations across one or more different tasks in healthy young, healthy older and pathological populations.
3.2.2. EEG Studies: Changes in Frequency Spectrums
One study in healthy young adults examined changes in frequency spectrums during repeated perturbations delivered in sitting [] (Table 6).
Task—Sitting:
Healthy young population: Unpredicted perturbations only: A study by Shirazi and Huang [] delivered repeated perturbations during mid-extension versus full-extension of leg while seated stepping via a motorized stepper. Changes in theta band synchronization and desynchronization were analyzed between early (first 33%) and late (last 33%) perturbed trials. The study findings showed greater theta band synchronizations in anterior cingulate cortex and supplementary motor area during full-leg extension as compared to perturbations delivered during mid-extension. This was followed by task-specific theta desynchronization in supplementary motor area during post-perturbation recovery (i.e., desynchronization occurred in left supplementary motor area for mid-extension type and right supplementary motor area for perturbations during full-extension. While novel perturbations observed greatest theta band synchronizations in anterior cingulate cortex and supplementary motor area, subsequent perturbations resulted in reduced theta band synchronizations across anterior cingulate cortex and supplementary motor area.
Healthy older population: None of the included studies assessed changes in cortical frequencies during repeated seated perturbations in healthy older adults.
Pathological populations: None of the included studies assessed changes in cortical frequencies during repeated seated perturbations in pathological populations.
Task—Standing: None of the included studies assessed changes in cortical frequencies during repeated stance perturbations in healthy young, healthy older and pathological populations.
Task—Walking: None of the included studies assessed changes in cortical frequencies during repeated gait perturbations in healthy young, healthy older and pathological populations.
Multiple tasks: None of the included studies assessed changes in cortical frequencies during repeated perturbations across one or more tasks in healthy young, healthy older and pathological populations.
3.2.3. fNIRS Studies: Changes in Hemodynamic Responses
One study in healthy young adults examined changes in frequency spectrums during repeated perturbations delivered in walking [] (Table 7).
Task—Sitting: None of the included studies assessed changes in oxygenated hemoglobin during repeated seated perturbations in healthy young, healthy older and pathological populations.
Task—Standing: None of the included studies assessed changes in oxygenated hemoglobin during repeated stance perturbations in healthy young, healthy older and pathological populations.
Task—Walking: A study reported perturbation phase-dependent changes in bilateral hemispheres with perturbations delivered via split-belt treadmill during walking [] (Table 7). More specifically, pre-perturbation walking phase showed increased activation in bilateral dorsolateral prefrontal cortex whereas post-perturbation recovery period showed greater activations in ventrolateral and frontopolar prefrontal cortex. Repeated perturbations resulted in minimal changes in all prefrontal regions during early adaptation; however, late adaptation trials resulted in switching of activity from dorsolateral and frontopolar prefrontal to orbitofrontal cortex.
Multiple tasks: None of the included studies assessed changes in oxygenated hemoglobin during repeated perturbations across one or more tasks in healthy young, healthy older and pathological populations.
3.3. Behavioral and Kinematic Correlates of Cortical Activity (RQ3)
Eight included studies that examined PEPs, three included studies that assessed changes in cortical frequency spectrums and one included study (total: twelve) that examined hemodynamic changes during unpredicted perturbations also investigated corresponding behavioral or biomechanical outcome measures.
3.3.1. EEG Studies: PEPs
Healthy young population: Unpredicted perturbations only: Adkin et al. [] reported that N1 amplitudes recorded at midline central areas were negatively correlated with reduced perceived balance confidence, reduced perceived postural stability and positively correlated fear of falling during high-ground (1.6 m above ground) unpredicted perturbations compared to ground level. However, there were no associations between N1 characteristics and perceived anxiety. On the other hand, Sibley and colleagues [] reported no associations between changes in N1 amplitude at midline central areas and autonomic responses (i.e., electrodermal responses) during high-ground (3.2 m above ground) versus ground level unpredicted perturbations. Payne and Ting [] tested the associations between N1 amplitude during varying magnitude unpredicted perturbations with balance performance assessed by the distance walked on a narrow beam. The study reported negative correlations between N1 amplitude and the distance travelled on the beam []. Another study by Payne and colleagues [] examined associations between N1 characteristics and lower limb EMG activity (early and late postural responses) during repeated perturbations. During repeated perturbations, a reduction in N1 amplitude was positively correlated with reduction in early postural responses but not with late postural responses []. Finally, a study by Mierau et al. [] delivered repeated stance perturbations and reported positive correlations between changes in N1 amplitude with changes in postural sway and ankle plantarflexor EMG activity.
Healthy older population: Unpredicted perturbations only: Duckrow and colleagues [] stratified older adults into mobile and frail older adults based on short term physical performance battery-SPPB (tasks: standing balance, walking speed and sit-to-stand). In addition, this study examined behavioral measures like gait velocity, single foot stance time, functional base of support and standing balance performance during conflicting sensory inputs (sensory-organization testing). This study reported that older adults with reduced mobility and shorter time-to-step during sensory-organization testing exhibited delayed P1 and prolonged intervals between N1 and N2.
Pathological populations: Unpredicted perturbations only: Jacobs and colleagues [] reported a negative correlation between P2 amplitude and kinematic and psychometric measures in people with and without chronic low back pain. Kinematic measures included center of mass displacement and psychological measures like pain perception (brief pain inventory short form), fear avoidance beliefs questionnaire and coping strategies questionnaire. On the other hand, Allexandre et al. [] delivered unpredicted forward and backward perturbations via movable platforms and reported positive correlations between N1 amplitude and clinical balance performance (Berg balance scores). In addition, this study found negative correlations between N1 latency and center of pressure displacement.
3.3.2. EEG Studies: Changes in Frequency Spectrums
Healthy young population: Unpredicted perturbations only: Two studies have examined associations between beta frequency bands and clinical balance measures (Mini Balance Evaluation Systems Test and the distance travelled on the balance beam) [,]. Beta frequencies recorded after unpredicted perturbations especially during late phases (150–250 ms after perturbation onset) were negatively correlated with balance performance (Mini Balance Evaluation Systems Test scores and the distance travelled on the balance beam) [,].
Healthy older population: None of the included studies examined associations between cortical activity and behavioral/biomechanical outcomes in healthy older adults.
Pathological populations: One study reported negative correlation between theta frequency band with clinical balance performance (Berg balance scores) in individuals with traumatic brain injury and their healthy counterparts [].
3.3.3. EEG Studies: Changes in Hemodynamic Responses
Healthy young population: None of the fNIRS included studies examined the associations between cortical activations and behavioral/kinematic outcomes in healthy young adults.
Healthy older population: None of the fNIRS included studies examined the associations between cortical activations and behavioral/kinematic outcomes in healthy older adults.
Pathological populations: One fNIRS study reported positive correlation between changes in oxygenated hemoglobin in the supplementary motor area and prefrontal cortex after perturbation onset with clinical balance performance (Berg balance scale) in individuals with chronic subcortical stroke [].
4. Discussion
We examined 35 articles that used mobile neuroimaging (EEG and fNIRS) to investigate mechanical perturbation-induced changes in cortical activity among healthy and clinical populations. Further, we examined 12 articles that investigated associations between cortical activity during reactive balance tasks and behavioral/biomechanical measures. Reactive balance control was most examined in healthy young during upright stance with cortical activity commonly assessed using PEPs and behavioral outcomes were commonly assessed using clinical balance measures (e.g., Berg balance scale, Beam Walking task). Comparatively lesser studies examined changes in cortical frequencies and hemodynamic responses during reactive balance tasks. In general, changes in cortical activity during reactive balance tasks were based on several internal (e.g., age or pathology) and external (e.g., task type: seated/stance/gait perturbations, task predictability, perturbation magnitude, concurrent cognitive task, task repetition).
4.1. Spatial-Temporal Characteristics of Cortical Activity: Novel Perturbations (RQ1)
Cortical involvement in feedback reception and sensorimotor processing: Studies assessing modulations in PEPs and alpha and beta frequencies suggest the involvement of sensorimotor cortex in reception of peripheral sensory feedback and processing of perturbation characteristics. Moreover, age and pathology-related temporal changes in PEP P1, which is the first cortical event postulated to reflect afferent feedback reception, could be explained in two ways. First, delayed P1 in healthy older adults [] could be attributed to slow conduction of peripheral afferents compared to young adults []. Second, delayed P1 in adults with traumatic brain injury [] compared to young adults could arise from structural/functional alterations in the cortex itself []. Such delays in cortical perception of perturbation could have further resulted in alterations in sensorimotor processing [,], which is postulated to be reflected as the second cortical potential, PEP N1. Modulations in N1 were also seen in young adults. Specifically, changes in sensorimotor and parietal N1 responses were based on perturbation magnitude/postural threat [,,], which could be explained in two ways. First, it is possible that with increasing perturbation magnitude/higher postural threat, young adults required greater cortical resources for sensorimotor processing. Alternatively, with higher perturbation magnitudes/greater postural threat there could be a greater mismatch between the expected and the ongoing postural state, thus resulting in higher cortical error-detection. On the other hand, there was reduction in N1 amplitudes with addition of a concurrent cognitive task [,,,] and during predicted perturbations [,,]. This could suggest diversion of cognitive resources from ongoing processing in case of dual tasking or smaller mismatch between expected and ongoing state when there is prior knowledge/time to plan balance recovery in case of predicted perturbations. Remarkably, feet-in-place and compensatory stepping responses showed some similarities in sensorimotor frequency modulations [], suggesting that the cortex might also have a role in feet-in-place responses. Specifically, both feet-in-place and compensatory stepping showed alpha reductions in occipital and supplementary motor areas during cue observation [], suggesting alpha involvement in visual information processing or cortico-cortical information transfer. Further increased sensorimotor beta frequencies during perturbations with higher magnitudes [] suggested their involvement in processing of perturbation characteristics or detecting changes in ongoing postural state.
Cortical involvement in planning and execution of reactive balance responses: Changes in PEPs, beta and theta frequencies, and hemodynamic responses seen in prefrontal, frontal and sensorimotor areas suggested that the cortex is highly involved in planning and execution of reactive balance responses. Specifically, compensatory stepping responses elicited larger N1 amplitudes and increased beta frequencies in sensorimotor areas in young adults [,], especially during higher perturbation intensities [], which could be explained in several ways. It is well-established that an effective compensatory step involves multiple components including timely initiation of step (i.e., foot liftoff, simultaneous shifting of body weight to the supporting stance limb and effective foot placement for balance recovery) [,]. In addition, executing a compensatory step might also depend on environmental conditions (e.g., floor height, space for stepping) and thus require greater attentional and visuospatial resources to plan appropriate responses. Thus, it can be postulated that compensatory stepping responses demand higher sensorimotor involvement for successful step execution. Contrastingly, when young adults were asked to execute feet-in-place responses at higher perturbation intensities, there were increased prefrontal theta frequencies compared to when asked to voluntarily take compensatory steps []. Hence, it is possible that cortical contribution to response execution is also based on task difficulty and not merely the type of balance response. This postulation can further be supported when reductions in frontal and sensorimotor P2 amplitudes were seen during seated compared to stance perturbations in young adults []. It is possible that small magnitude seated perturbations that also allow larger base of support might not be perceived with higher postural threat by the cerebral cortex. Secondly, it is possible that seated perturbations might require lesser cortical resources as they are comprised of trunk or single-joint movements for balance recovery compared to multi-segmented postural responses during stance perturbations. In older adults, increased prefrontal and motor beta frequencies were observed with lower compensatory stepping threshold [], which suggested that aging adults might have heightened reliance on prefrontal cortex for step execution. Lastly, fNIRS studies also supported the involvement of prefrontal [,] and frontal [] cortices in planning and execution of compensatory steps. Specifically, predicted perturbations induced greater hemodynamic changes in prefrontal, supplementary motor, and posterior parietal areas compared to unpredicted perturbations in young adults []. This suggested that prior knowledge of perturbations might involve greater recruitment of cognitive resources for pre-planning/decision making for the upcoming perturbation. In people with chronic stroke, cortical activations during balance recovery were more localized to the prefrontal cortex of the unaffected hemisphere [], suggesting the pre-dominant role of unaffected hemisphere in planning of reactive balance responses.
4.2. Spatial-Temporal Characteristics of Cortical Activity: Repeated Perturbations (RQ2)
Included studies suggested reductions in the perturbation-evoked potential (PEP) N1 [,] and theta frequencies [] with repeated perturbations in young adults. Further, there was switching of hemodynamic responses in prefrontal sub-regions with repeated perturbations []. It can be postulated that with repeated practice, there might be reduction in cortical error-detection and enhanced sensorimotor processing of perturbations resulting from recalibration of internal central nervous system (CNS) models and/or increased habituation of postural responses. Consequently, with repeated perturbations, there might be reduced reliance on the cerebral cortex resulting from more automated subcortical control based on stored motor memory or ongoing learning []. As all the included studies examined repeated perturbations in young adults, it remains unclear whether individuals aging and those with neurological impairments can demonstrate similar changes in cortical activity with repeated perturbations.
4.3. Behavioral and Biomechanical/Kinematic Correlates of Cortical Activity (RQ3)
A few included studies indicated direct associations between changes in cortical and biomechanical/kinematic correlates of reactive balance in young adults. Specifically, N1 reductions were associated with reductions in electromyographic responses [,] and postural sway during repeated perturbations []. It can be postulated that with repeated practice, there is enhanced sensorimotor processing resulting in smaller mismatch between the expected and ongoing postural state. This can result in smaller muscular activations required for balance recovery or improvement in balance performance.
Other studies indicated indirect associations between cortical activity changes during reactive balance tasks and behavioral measures assessed before/after the tasks. Specifically, changes in PEP N1 were associated with fear of falling and balance confidence [,], which suggested that perceived threat and psychological measures can interfere with cortical sensorimotor processing. Further, clinical dynamic balance performance was associated with changes in PEPs and cortical frequencies, as well as hemodynamic changes during reactive balance tasks [,,,,]. This could suggest existence of shared pathways or common cortical areas of activation between volitional and reactive balance control. For example, previous neurophysiological literature indicated similar muscle synergy patterns between volitional and reactive stepping in healthy older adults [] and associations between dynamic balance (Timed-up and go-test) and slip-related falls during walking in people with chronic stroke [].
To summarize, even though the exact role of PEPs is not well established, their spatial-temporal changes provide a broad picture regarding the cortical control of reactive balance in healthy populations. Specifically, the role of later potentials P2 and N2 remains unknown possibly due to their undistinguishable nature from background EEG or from lack of advanced artifact removal techniques. Secondly, multicomponent PEPs provide an instantaneous snapshot of cortical changes related to reactive balance tasks; however, a majority of studies have used average trial analysis to quantify them. Previous neurophysiological studies have indicated that perturbation-induced motor adaptation can occur in as little as a single trial in healthy young and older adults [,,]. Hence, future studies should test the feasibility and robustness of using single-trial PEP analysis. Additionally, assessing PEPs alone may not provide a clear picture of cortical modulation of reactive balance. Future studies could supplement PEP assessment with changes in alpha, beta or theta frequencies to provide a more accurate interpretation of changes in cortical activity during reactive balance tasks, especially for examining training (e.g., repeated perturbations) induced changes. Thirdly, there is still limited evidence regarding alterations in PEPs and cortical frequencies in aging and neurologically impaired populations (e.g., chronic stroke, multiple sclerosis). Further, majority of fNIRS studies used the prefrontal cortex as the area of interest. Hence, there is still lack of information regarding perturbation-induced whole-brain hemodynamic changes in aging and neurologically impaired populations. Lastly, it is worth highlighting that even though majority of included studies assessed behavioral/biomechanical measures, less than 35% of them examined their associations with cortical changes during reactive balance tasks. Further, none of the included studies established a true brain-behavior relationship for reactive balance control. Hence, it remains unclear whether changes in behavioral/biomechanical substrates are independent of or are driven by cortical modulation during reactive balance tasks among healthy and pathological populations.
5. Limitations of the Review
The current review was limited by several factors. Firstly, this review only included studies in adult populations (age > 18 years). Hence, this review lacked evidence from studies that examined physiological growth-related cortical changes in children and adolescents. Secondly, the keywords chosen for the search of research articles were based on current literature and individual preferences. Hence, the final research articles might have excluded studies with different terminology and emphasis that incorporated similar research paradigms. Thirdly, this study did not focus on providing evidence on movement and perturbation-related artifact removal techniques used in studies. Movement-related artifacts can restrict single-trial analyses of EEG signal data and limit evaluation of trial-to-trial changes in cortical activations during repeated perturbations. Lastly, while perturbation-related changes in PEPs, frequency spectrums, and hemodynamic changes provide information about cortical involvement in reactive balance control in healthy young, little is known about age and pathology-related alterations in brain activations during unpredicted perturbations. Thus, this review and existing literature lacks information regarding involvement of cerebral cortex in reactive balance control in high fall-risk populations (e.g., neurologically impaired populations). Lastly, the current review included research articles that were published only in English. It is possible that this review missed research articles in other languages that have published relevant findings related to cortical activations during reactive balance tasks using EEG and/or fNIRS. The authors would like to credit all the global centers that have conducted and/or published research regarding mobile neuroimaging of reactive balance control out of English science.
6. Conclusions
This systematic review demonstrated that, in general, unpredicted mechanical perturbations elicit cortical activations in prefrontal, sensorimotor, supplementary motor and posterior parietal areas. Specifically, the cerebral cortex might be involved for sensory processing of perturbations, error-detection of expected versus actual postural state, and planning and execution of compensatory stepping responses. Further, a handful of studies indicated that repeated exposure to perturbations could be associated with reduction in cortical error-detection and corresponding improvements in reactive balance performance. Lastly, task-related changes in cortical activity might be associated with biomechanical and clinical dynamic balance measures. As most of the current studies examined healthy young adults; future studies should focus on task-related cortical activations during novel and repeated perturbations and their behavioral correlates in aging and other populations with higher fall-risk. Future studies assessing PEPs could use single-trial analysis and supplement them with assessment of changes in frequency spectrums for examining cortical dynamics during reactive balance tasks.
Author Contributions
Conceptualization, R.P. and T.B.; methodology, R.P. and T.B.; software, R.P. and T.B.; validation, R.P. and T.B.; formal analysis, R.P. and T.B.; resources, R.P. and T.B.; writing—original draft preparation, R.P.; writing—review and editing, T.B.; visualization, R.P. and T.B.; supervision, T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Institutes of Health/NIA (R01AG050672) and National Institutes of Health/NICHD (R01HD088543) awarded to Tanvi Bhatt.
Acknowledgments
The authors would like to thank Jessica Pitts and Meaghan Rubsam for assistance with editing the manuscript.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35 (Suppl. 2), ii7–ii11. [Google Scholar] [CrossRef] [PubMed]
- Deliagina, T.G.; Orlovsky, G.N.; Zelenin, P.V.; Beloozerova, I.N. Neural bases of postural control. Physiology 2006, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B. Adaptation of automatic postural responses. In The Acquisition of Motor Behavior in Vertebrates; The MIT Press: Cambridge, MA, USA, 1996; pp. 57–85. [Google Scholar]
- Massion, J. Postural control system. Curr. Opin. Neurobiol. 1994, 4, 877–887. [Google Scholar] [CrossRef]
- Sousa, A.S.; Silva, A.; Tavares, J.M. Biomechanical and neurophysiological mechanisms related to postural control and efficiency of movement: A review. Somatosens. Mot. Res. 2012, 29, 131–143. [Google Scholar] [CrossRef]
- Forbes, P.A.; Chen, A.; Blouin, J.-S. Sensorimotor control of standing balance. Handb. Clin. Neurol. 2018, 159, 61–83. [Google Scholar]
- MacKinnon, C.D. Sensorimotor anatomy of gait, balance, and falls. Handb. Clin. Neurol. 2018, 159, 3–26. [Google Scholar]
- Page, P. Sensorimotor training: A “global” approach for balance training. J. Bodyw. Mov. Ther. 2006, 10, 77–84. [Google Scholar] [CrossRef]
- Stelmach, E.G.; Worringham, C.J. Sensorimotor deficits related to postural stability: Implications for falling in the elderly. Clin. Geriatr. Med. 1985, 1, 679–694. [Google Scholar] [CrossRef]
- Li, K.Z.; Bherer, L.; Mirelman, A.; Maidan, I.; Hausdorff, J.M. Cognitive involvement in balance, gait and dual-tasking in aging: A focused review from a neuroscience of aging perspective. Front. Neurol. 2018, 9, 913. [Google Scholar] [CrossRef]
- Maki, B.E.; McIlroy, W.E. Cognitive demands and cortical control of human balance-recovery reactions. J. Neural Transm. 2007, 114, 1279–1296. [Google Scholar] [CrossRef]
- Ng, T.H.; Sowman, P.F.; Brock, J.; Johnson, B.W. Neuromagnetic brain activity associated with anticipatory postural adjustments for bimanual load lifting. Neuroimage 2013, 66, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Horak, F.B.; Fujiwara, K.; Tomita, H.; Furune, N.; Kunita, K. Changes in activity at the cerebral cortex associate with the optimization of responses to external postural perturbations when given prior warning. Gait Posture Suppl. 2007. [Google Scholar]
- Takakusaki, K. Functional neuroanatomy for posture and gait control. J. Mov. Disord. 2017, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Van Boxtel, G.; Brunia, C.H. Motor and non-motor aspects of slow brain potentials. Biol. Psychol. 1994, 38, 37–51. [Google Scholar] [CrossRef]
- Saitou, K.; Washimi, Y.; Koike, Y.; Takahashi, A.; Kaneoke, Y. Slow negative cortical potential preceding the onset of postural adjustment. Electroencephalogr. Clin. Neurophysiol. 1996, 98, 449–455. [Google Scholar] [CrossRef]
- Slobounov, S.; Hallett, M.; Stanhope, S.; Shibasaki, H. Role of cerebral cortex in human postural control: An EEG study. Clin. Neurophysiol. 2005, 116, 315–323. [Google Scholar] [CrossRef]
- Bolton, D.A. The role of the cerebral cortex in postural responses to externally induced perturbations. Neurosci. Biobehav. Rev. 2015, 57, 142–155. [Google Scholar] [CrossRef]
- Kahya, M.; Moon, S.; Ranchet, M.; Vukas, R.R.; Lyons, K.E.; Pahwa, R.; Akinwuntan, A.; Devos, H. Brain activity during dual task gait and balance in aging and age-related neurodegenerative conditions: A systematic review. Exp. Gerontol. 2019, 128, 110756. [Google Scholar] [CrossRef]
- Wittenberg, E.; Thompson, J.; Nam, C.S.; Franz, J.R. Neuroimaging of Human Balance Control: A Systematic Review. Front. Hum. Neurosci. 2017, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Nashner, L.M. Central programming of postural movements: Adaptation to altered support-surface configurations. J. Neurophysiol. 1986, 55, 1369–1381. [Google Scholar] [CrossRef]
- Maki, B.E.; Mcilroy, W.E.; Fernie, G.R. Change-in-support reactions for balance recovery. IEEE Eng. Med. Biol. Mag. 2003, 22, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E.; McIlroy, W.E. The role of limb movements in maintaining upright stance: The “change-in-support” strategy. Phys. Ther. 1997, 77, 488–507. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Horak, F.B. Cortical control of postural responses. J. Neural. Transm. 2007, 114, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E.; McIlroy, W.E. Postural control in the older adult. Clin. Geriatr. Med. 2007, 12, 635–658. [Google Scholar] [CrossRef]
- Chan, C.W.; Jones, G.M.; Kearney, R.E.; Watt, D.G.D. The ‘late’ electromyographic response to limb displacement in man. I. Evidence for supraspinal contribution. Electroencephalogr. Clin. Neurophysiol. 1979, 46, 173–181. [Google Scholar] [CrossRef]
- Taube, W.; Schubert, M.; Gruber, M.; Beck, S.; Faist, M.; Gollhofer, A. Direct corticospinal pathways contribute to neuromuscular control of perturbed stance. J. Appl. Physiol. 2006, 101, 420–429. [Google Scholar] [CrossRef]
- Beloozerova, I.N.; Sirota, M.G.; Swadlow, H.A.; Orlovsky, G.N.; Popova, L.B.; Deliagina, T.G. Activity of different classes of neurons of the motor cortex during postural corrections. J. Neurosci. 2003, 23, 7844–7853. [Google Scholar] [CrossRef][Green Version]
- Brauer, S.; Woollacott, M.; Shumway-Cook, A. The influence of a concurrent cognitive task on the compensatory stepping response to a perturbation in balance-impaired and healthy elders. Gait Posture 2002, 15, 83–93. [Google Scholar] [CrossRef]
- Maki, B.E.; Zecevic, A.; Bateni, H.; Kirshenbaum, N.; McIlroy, W.E. Cognitive demands of executing postural reactions: Does aging impede attention switching? Neuroreport 2001, 12, 3583–3587. [Google Scholar] [CrossRef]
- McIlroy, W.E.; Norrie, R.G.; Brooke, J.D.; Bishop, D.C.; Nelson, A.J.; Maki, B.E. Temporal properties of attention sharing consequent to disturbed balance. Neuroreport 1999, 10, 2895–2899. [Google Scholar] [CrossRef]
- Kim, H.; Nnodim, J.O.; Richardson, J.K.; Ashton-Miller, J.A. Effect of age on the ability to recover from a single unexpected underfoot perturbation during gait: Kinematic responses. Gait Posture 2013, 38, 853–857. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pavol, M.J.; Owings, T.M.; Foley, K.T.; Grabiner, M.D. Mechanisms leading to a fall from an induced trip in healthy older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M428–M437. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.-F.; Woollacott, M.H.; Chong, R.K. Control of reactive balance adjustments in perturbed human walking: Roles of proximal and distal postural muscle activity. Exp. Brain Res. 1998, 119, 141–152. [Google Scholar] [CrossRef]
- Tseng, S.-C.; Stanhope, S.J.; Morton, S.M. Impaired reactive stepping adjustments in older adults. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2009, 64, 807–815. [Google Scholar] [CrossRef]
- Woollacott, M.H.; Tang, P.-F. Balance control during walking in the older adult: Research and its implications. Phys. Ther. 1997, 77, 646–660. [Google Scholar] [CrossRef]
- Kajrolkar, T.; Bhatt, T. Falls-risk post-stroke: Examining contributions from paretic versus non paretic limbs to unexpected forward gait slips. J. Biomech. 2016, 49, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.; Wong, J.S.; McIlroy, W.E.; Biasin, L.; Brunton, K.; Bayley, M.; Inness, E.L. Do measures of reactive balance control predict falls in people with stroke returning to the community? Physiotherapy 2015, 101, 373–380. [Google Scholar] [CrossRef]
- Patel, P.J.; Bhatt, T. Does aging with a cortical lesion increase fall-risk: Examining effect of age versus stroke on intensity modulation of reactive balance responses from slip-like perturbations. Neuroscience 2016, 333, 252–263. [Google Scholar] [CrossRef]
- Salot, P.; Patel, P.; Bhatt, T. Reactive Balance in Individuals with Chronic Stroke: Biomechanical Factors Related to Perturbation-Induced Backward Falling. Phys. Ther. 2016, 96, 338–347. [Google Scholar] [CrossRef]
- Adkin, A.L.; Campbell, A.D.; Chua, R.; Carpenter, M.G. The influence of postural threat on the cortical response to unpredictable and predictable postural perturbations. Neurosci. Lett. 2008, 435, 120–125. [Google Scholar] [CrossRef]
- Mochizuki, G.; Sibley, K.M.; Cheung, H.J.; Camilleri, J.M.; McIlroy, W.E. Generalizability of perturbation-evoked cortical potentials: Independence from sensory, motor and overall postural state. Neurosci. Lett. 2009, 451, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.M.; Ting, L.H. Balance perturbation-evoked cortical N1 responses are larger when stepping and not influenced by motor planning. J. Neurophysiol. 2020, 124, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.M.; Ting, L.H. Worse balance is associated with larger perturbation-evoked cortical responses in healthy young adults. Gait Posture 2020, 80, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Quant, S.; Adkin, A.L.; Staines, W.R.; McIlroy, W.E. Cortical activation following a balance disturbance. Exp. Brain Res. 2004, 155, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Marlin, A.; Mochizuki, G.; Staines, W.R.; McIlroy, W.E. Localizing evoked cortical activity associated with balance reactions: Does the anterior cingulate play a role? J. Neurophysiol. 2014, 111, 2634–2643. [Google Scholar] [CrossRef]
- Bolton, D.A.; Williams, L.; Staines, W.R.; McIlroy, W.E. Contribution of primary motor cortex to compensatory balance reactions. BMC Neurosci. 2012, 13, 102. [Google Scholar] [CrossRef]
- Peterson, S.M.; Ferris, D.P. Differentiation in Theta and Beta Electrocortical Activity between Visual and Physical Perturbations to Walking and Standing Balance. eNeuro 2018, 5. [Google Scholar] [CrossRef]
- Solis-Escalante, T.; de Kam, D.; Weerdesteyn, V. Classification of Rhythmic Cortical Activity Elicited by Whole-Body Balance Perturbations Suggests the Cortical Representation of Direction-Specific Changes in Postural Stability. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2566–2574. [Google Scholar] [CrossRef]
- Koren, Y.; Parmet, Y.; Bar-Haim, S. Treading on the unknown increases prefrontal activity: A pilot fNIRS study. Gait Posture 2019, 69, 96–100. [Google Scholar] [CrossRef]
- Lee, B.C.; Choi, J.; Martin, B.J. Roles of the prefrontal cortex in learning to time the onset of pre-existing motor programs. PLoS ONE 2020, 15, e0241562. [Google Scholar] [CrossRef]
- Bultmann, U.; Pierscianek, D.; Gizewski, E.R.; Schoch, B.; Fritsche, N.; Timmann, D.; Maschke, M.; Frings, M. Functional recovery and rehabilitation of postural impairment and gait ataxia in patients with acute cerebellar stroke. Gait Posture 2014, 39, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, L.; Petsas, N.; Raz, E.; Sbardella, E.; Tona, F.; Mancinelli, C.R.; Pozzilli, C.; Pantano, P. Balance deficit with opened or closed eyes reveals involvement of different structures of the central nervous system in multiple sclerosis. Mult. Scler. J. 2014, 20, 81–90. [Google Scholar] [CrossRef]
- Prosperini, L.; Sbardella, E.; Raz, E.; Cercignani, M.; Tona, F.; Bozzali, M.; Petsas, N.; Pozzilli, C.; Pantano, P. Multiple sclerosis: White and gray matter damage associated with balance deficit detected at static posturography. Radiology 2013, 268, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, T.; Patel, P.; Dusane, S.; DelDonno, S.R.; Langenecker, S.A. Neural mechanisms involved in mental imagery of slip-perturbation while walking: A preliminary fMRI study. Front. Behav. Neurosci. 2018, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.J.; Bhatt, T.; DelDonno, S.R.; Langenecker, S.A.; Dusane, S. Examining neural plasticity for slip-perturbation training: An fMRI study. Front. Neurol. 2019, 9, 1181. [Google Scholar]
- Ouchi, Y.; Okada, H.; Yoshikawa, E.; Nobezawa, S.; Futatsubashi, M. Brain activation during maintenance of standing postures in humans. Brain 1999, 122, 329–338. [Google Scholar] [CrossRef]
- Ouchi, Y.; Okada, H.; Yoshikawa, E.; Futatsubashi, M.; Nobezawa, S. Absolute changes in regional cerebral blood flow in association with upright posture in humans: An orthostatic PET study. J. Nucl. Med. 2001, 42, 707–712. [Google Scholar]
- Adkin, A.L.; Quant, S.; Maki, B.E.; McIlroy, W.E. Cortical responses associated with predictable and unpredictable compensatory balance reactions. Exp. Brain Res. 2006, 172, 85–93. [Google Scholar] [CrossRef]
- Dietz, V.; Quintern, J.; Berger, W. Cerebral evoked potentials associated with the compensatory reactions following stance and gait perturbation. Neurosci. Lett. 1984, 50, 181–186. [Google Scholar] [CrossRef]
- Dietz, V.; Quintern, J.; Berger, W. Afferent control of human stance and gait: Evidence for blocking of group I afferents during gait. Exp. Brain Res. 1985, 61, 153–163. [Google Scholar] [CrossRef]
- Dietz, V.; Quintern, J.; Berger, W.; Schenck, E. Cerebral potentials and leg muscle e.m.g. responses associated with stance perturbation. Exp. Brain Res. 1985, 57, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Duckrow, R.B.; Abu-Hasaballah, K.; Whipple, R.; Wolfson, L. Stance perturbation-evoked potentials in old people with poor gait and balance. Clin. Neurophysiol. 1999, 110, 2026–2032. [Google Scholar] [CrossRef]
- Mihara, M.; Miyai, I.; Hatakenaka, M.; Kubota, K.; Sakoda, S. Role of the prefrontal cortex in human balance control. Neuroimage 2008, 43, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Miyai, I.; Hattori, N.; Hatakenaka, M.; Yagura, H.; Kawano, T.; Kubota, K. Cortical control of postural balance in patients with hemiplegic stroke. Neuroreport 2012, 23, 314–319. [Google Scholar] [CrossRef]
- Glover, G.H. Overview of functional magnetic resonance imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139. [Google Scholar] [CrossRef]
- Goense, J.; Bohraus, Y.; Logothetis, N.K. fMRI at high spatial resolution: Implications for BOLD-models. Front. Comput. Neurosci. 2016, 10, 66. [Google Scholar] [CrossRef]
- Dijkstra, B.W.; Bekkers, E.M.; Gilat, M.; de Rond, V.; Hardwick, R.M.; Nieuwboer, A. Functional neuroimaging of human postural control: A systematic review with meta-analysis. Neurosci. Biobehav. Rev. 2020, 115, 351–362. [Google Scholar] [CrossRef]
- Varghese, J.P.; McIlroy, R.E.; Barnett-Cowan, M. Perturbation-evoked potentials: Significance and application in balance control research. Neurosci. Biobehav. Rev. 2017, 83, 267–280. [Google Scholar] [CrossRef]
- Scarapicchia, V.; Brown, C.; Mayo, C.; Gawryluk, J.R. Functional Magnetic Resonance Imaging and Functional Near-Infrared Spectroscopy: Insights from Combined Recording Studies. Front. Hum. Neurosci. 2017, 11, 419. [Google Scholar] [CrossRef]
- Wilcox, T.; Biondi, M. fNIRS in the developmental sciences. Wiley Interdiscip. Rev. Cogn. Sci. 2015, 6, 263–283. [Google Scholar] [CrossRef]
- Payne, A.M.; Ting, L.H.; Hajcak, G. Do sensorimotor perturbations to standing balance elicit an error-related negativity? Psychophysiology 2019, 56, e13359. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Ditz, J.C.; Schwarz, A.; Muller-Putz, G.R. Perturbation-evoked potentials can be classified from single-trial EEG. J. Neural Eng. 2020, 17, 036008. [Google Scholar] [CrossRef]
- Varghese, J.P.; Marlin, A.; Beyer, K.B.; Staines, W.R.; Mochizuki, G.; McIlroy, W.E. Frequency characteristics of cortical activity associated with perturbations to upright stability. Neurosci. Lett. 2014, 578, 33–38. [Google Scholar] [CrossRef]
- Goel, R.; Ozdemir, R.A.; Nakagome, S.; Contreras-Vidal, J.L.; Paloski, W.H.; Parikh, P.J. Effects of speed and direction of perturbation on electroencephalographic and balance responses. Exp. Brain Res. 2018, 236, 2073–2083. [Google Scholar] [CrossRef]
- Varghese, J.P.; Staines, W.R.; McIlroy, W.E. Activity in Functional Cortical Networks Temporally Associated with Postural Instability. Neuroscience 2019, 401, 43–58. [Google Scholar] [CrossRef]
- Quant, S.; Maki, B.E.; McIlroy, W.E. The association between later cortical potentials and later phases of postural reactions evoked by perturbations to upright stance. Neurosci. Lett. 2005, 381, 269–274. [Google Scholar] [CrossRef]
- Solis-Escalante, T.; Van Der Cruijsen, J.; De Kam, D.; Van Kordelaar, J.; Weerdesteyn, V.; Schouten, A.C. Cortical dynamics during preparation and execution of reactive balance responses with distinct postural demands. Neuroimage 2019, 188, 557–571. [Google Scholar] [CrossRef]
- Ghosn, N.J.; Palmer, J.A.; Borich, M.R.; Ting, L.H.; Payne, A.M. Cortical Beta Oscillatory Activity Evoked during Reactive Balance Recovery Scales with Perturbation Difficulty and Individual Balance Ability. Brain Sci. 2020, 10, 860. [Google Scholar] [CrossRef] [PubMed]
- Shenoy Handiru, V.; Alivar, A.; Hoxha, A.; Saleh, S.; Suviseshamuthu, E.S.; Yue, G.H.; Allexandre, D. Graph-theoretical analysis of EEG functional connectivity during balance perturbation in traumatic brain injury: A pilot study. Hum. Brain Mapp. 2021, 42, 4427–4447. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, G.; Sibley, K.M.; Esposito, J.G.; Camilleri, J.M.; McIlroy, W.E. Cortical responses associated with the preparation and reaction to full-body perturbations to upright stability. Clin. Neurophysiol. 2008, 119, 1626–1637. [Google Scholar] [CrossRef]
- Palmer, J.A.; Payne, A.M.; Ting, L.H.; Borich, M.R. Cortical Engagement Metrics during Reactive Balance Are Associated with Distinct Aspects of Balance Behavior in Older Adults. Front. Aging Neurosci. 2021, 13, 684743. [Google Scholar] [CrossRef]
- An, J.; Yoo, D.; Lee, B.C. Electrocortical activity changes in response to unpredictable trip perturbations induced by a split-belt treadmill. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 110–113. [Google Scholar] [PubMed]
- Sibley, K.M.; Mochizuki, G.; Frank, J.S.; McIlroy, W.E. The relationship between physiological arousal and cortical and autonomic responses to postural instability. Exp. Brain Res. 2010, 203, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Mezzina, G.; Aprigliano, F.; Micera, S.; Monaco, V.; De Venuto, D. Cortical reactive balance responses to unexpected slippages while walking: A pilot study. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 6868–6871. [Google Scholar]
- Bogost, M.D.; Burgos, P.I.; Little, C.E.; Woollacott, M.H.; Dalton, B.H. Electrocortical Sources Related to Whole-Body Surface Translations during a Single- and Dual-Task Paradigm. Front. Hum. Neurosci. 2016, 10, 524. [Google Scholar] [CrossRef]
- Little, C.E.; Woollacott, M. EEG measures reveal dual-task interference in postural performance in young adults. Exp. Brain Res. 2015, 233, 27–37. [Google Scholar] [CrossRef]
- Mochizuki, G.; Boe, S.G.; Marlin, A.; McIlroy, W.E. Performance of a concurrent cognitive task modifies pre- and post-perturbation-evoked cortical activity. Neuroscience 2017, 348, 143–152. [Google Scholar] [CrossRef]
- Quant, S.; Adkin, A.L.; Staines, W.R.; Maki, B.E.; McIlroy, W.E. The effect of a concurrent cognitive task on cortical potentials evoked by unpredictable balance perturbations. BMC Neurosci. 2004, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.V.; Roy, C.L.; Hitt, J.R.; Popov, R.E.; Henry, S.M. Neural mechanisms and functional correlates of altered postural responses to perturbed standing balance with chronic low back pain. Neuroscience 2016, 339, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Mierau, A.; Hulsdunker, T.; Struder, H.K. Changes in cortical activity associated with adaptive behavior during repeated balance perturbation of unpredictable timing. Front. Behav. Neurosci. 2015, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Allexandre, D.; Hoxha, A.; Handiru, V.S.; Saleh, S.; Selvan, S.E.; Yue, G.H. Altered Cortical and Postural Response to Balance Perturbation in Traumatic Brain Injury—An EEG Pilot Study. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 1543–1546. [Google Scholar]
- Payne, A.M.; Hajcak, G.; Ting, L.H. Dissociation of muscle and cortical response scaling to balance perturbation acceleration. J. Neurophysiol. 2019, 121, 867–880. [Google Scholar] [CrossRef]
- Shirazi, S.Y.; Huang, H.J. Differential Theta-Band Signatures of the Anterior Cingulate and Motor Cortices during Seated Locomotor Perturbations. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 468–477. [Google Scholar] [CrossRef]
- Fujimoto, H.; Mihara, M.; Hattori, N.; Hatakenaka, M.; Kawano, T.; Yagura, H.; Miyai, I.; Mochizuki, H. Cortical changes underlying balance recovery in patients with hemiplegic stroke. Neuroimage 2014, 85, 547–554. [Google Scholar] [CrossRef]
- Palve, S.S.; Palve, S.B. Impact of Aging on Nerve Conduction Velocities and Late Responses in Healthy Individuals. J. Neurosci. Rural Pract. 2018, 9, 112–116. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, Y.; Xi, J.H.; Chen, Z.Q. Structural and functional connectivity in traumatic brain injury. Neural Regen. Res. 2015, 10, 2062–2071. [Google Scholar]
- Pai, Y.C.; Bhatt, T.S. Repeated-slip training: An emerging paradigm for prevention of slip-related falls among older adults. Phys. Ther. 2007, 87, 1478–1491. [Google Scholar] [CrossRef]
- Wang, S.; Varas-Diaz, G.; Bhatt, T. Muscle synergy differences between voluntary and reactive backward stepping. Sci. Rep. 2021, 11, 15462. [Google Scholar] [CrossRef]
- Gangwani, R.; Dusane, S.; Wang, S.; Kannan, L.; Wang, E.; Fung, J.; Bhatt, T. Slip-Fall Predictors in Community-Dwelling, Ambulatory Stroke Survivors: A Cross-sectional Study. J. Neurol. Phys. Ther. 2020, 44, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bhatt, T.; Wang, S.; Yang, F.; Pai, Y.C.C. Retention of the “first-trial effect” in gait-slip among community-living older adults. Geroscience 2017, 39, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Reschechtko, S.; Wang, S.; Pai, Y.C.C. The recovery response to a novel unannounced laboratory-induced slip: The “first trial effect” in older adults. Clin. Biomech. 2017, 48, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Pavol, M.J.; Runtz, E.F.; Edwards, B.J.; Pai, Y.C. Age influences the outcome of a slipping perturbation during initial but not repeated exposures. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M496–M503. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).