Hydrogen-Rich Saline—A Novel Neuroprotective Agent in a Mouse Model of Experimental Cerebral Ischemia via the ROS-NLRP3 Inflammasome Signaling Pathway In Vivo and In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and dMCAO Model
2.2. Preparation of HS and Hydrogen-Rich Medium
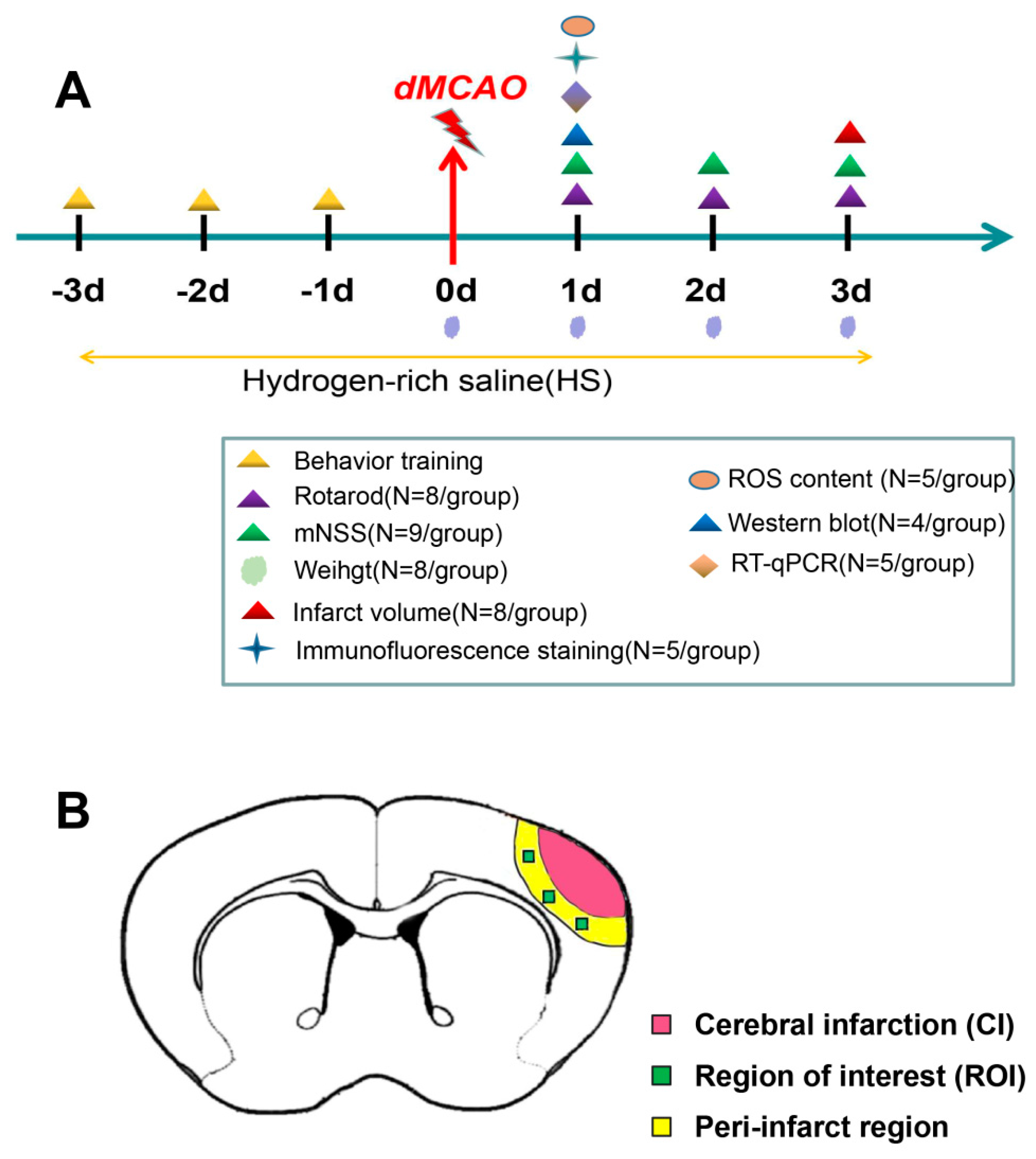
2.3. Experimental Plan and Drug Administration In Vivo
2.4. Neurobehavioral Test
2.4.1. Rotarod Test
2.4.2. Modified Neurological Severity Score (mNSS)
2.5. Brain Infarct Volume
2.6. Cerebral Blood Flow (CBF) Monitoring
2.7. Immunofluorescence Staining
2.8. Western Blotting
2.9. Reverse Transcription Quantitative Real-Time PCR (RT-qPCR)
2.10. Culture and Treatment of Microglial Cells
2.11. Cell Viability Assay
2.12. ROS Measurement
2.13. Statistical Analyses
3. Results
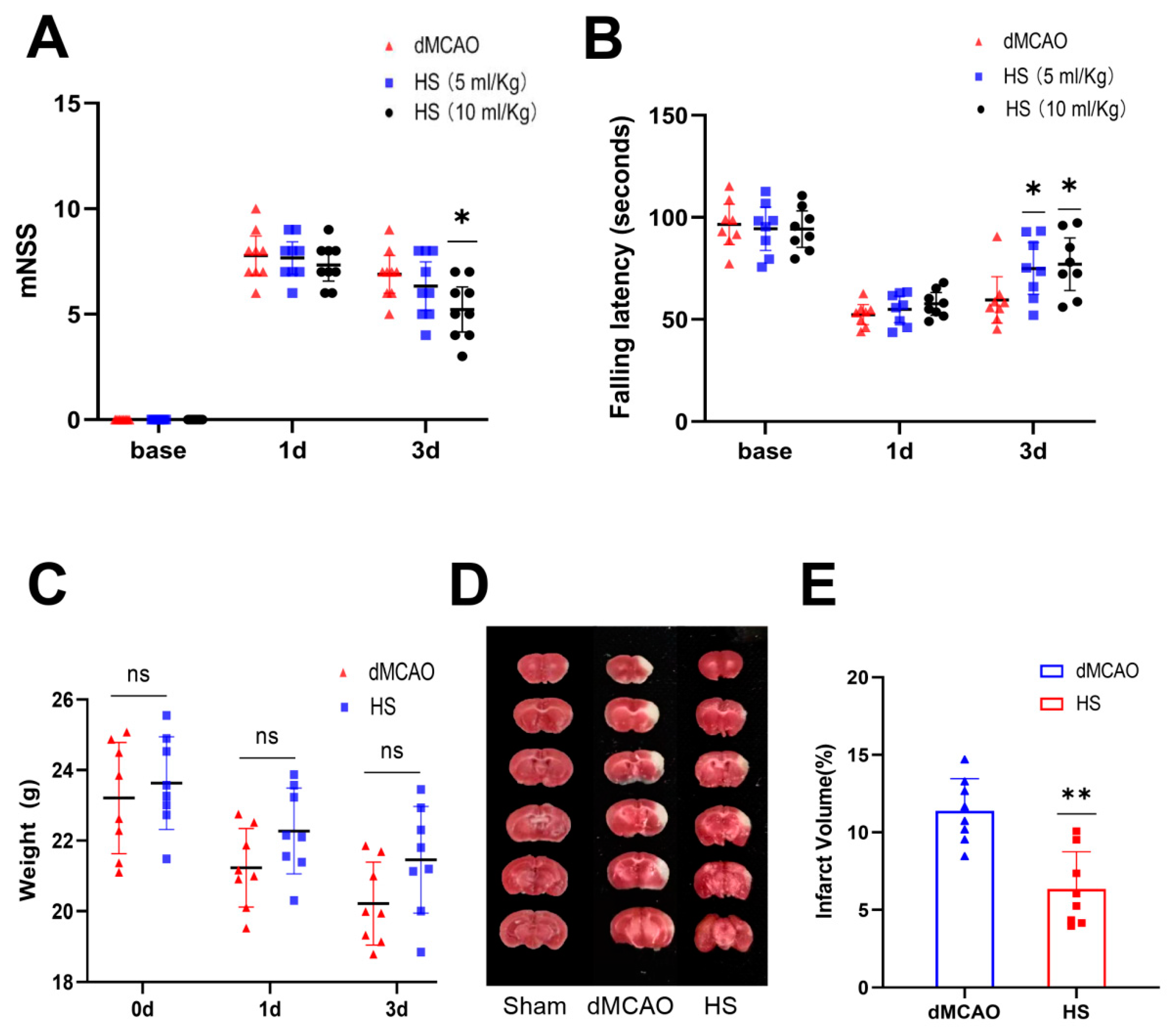
3.1. HS Reduced Neurological Deficits
3.2. HS Reduced the Infarct Volume
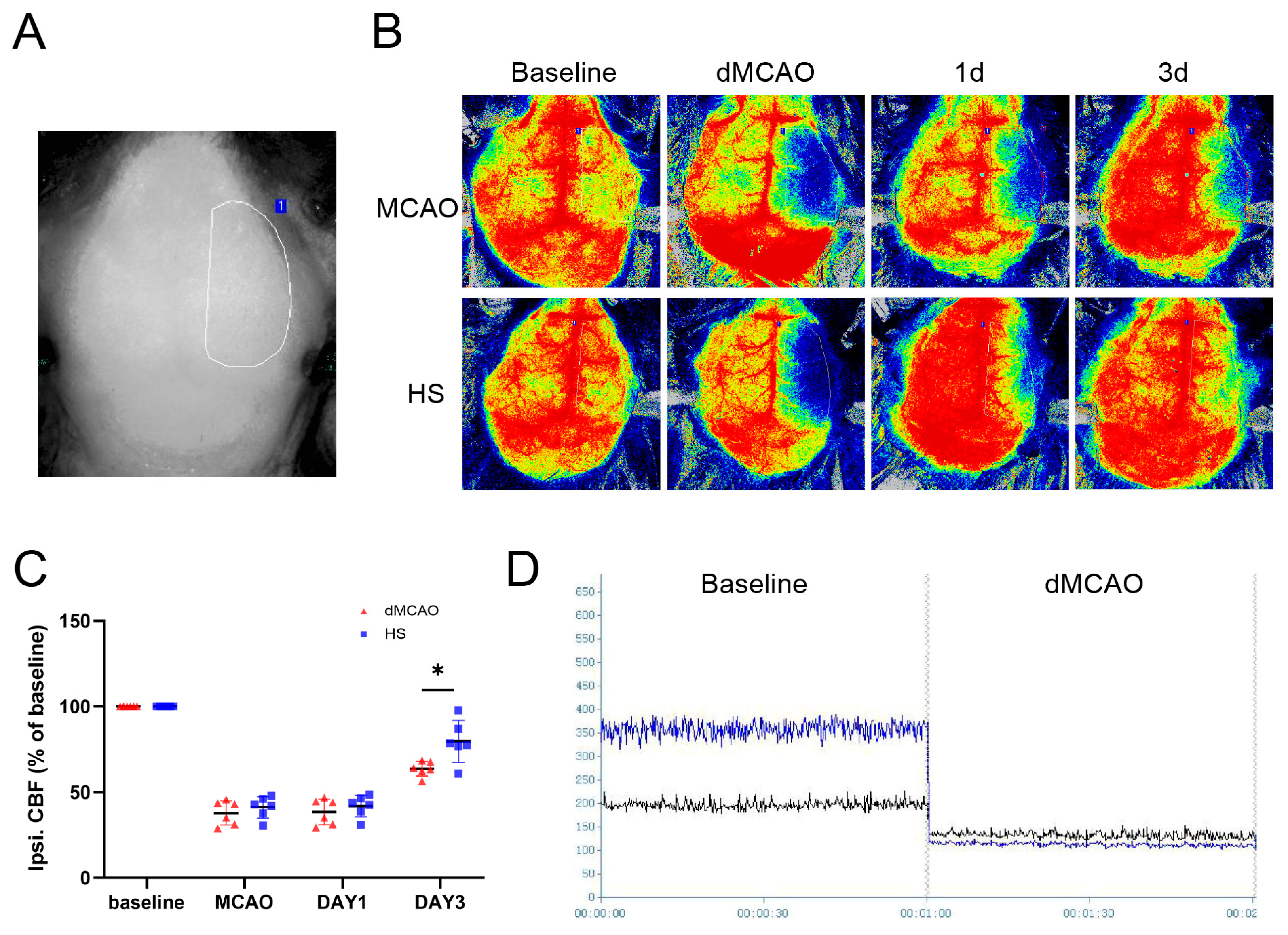
3.3. HS Increased Cerebral Blood Flow after dMCAO
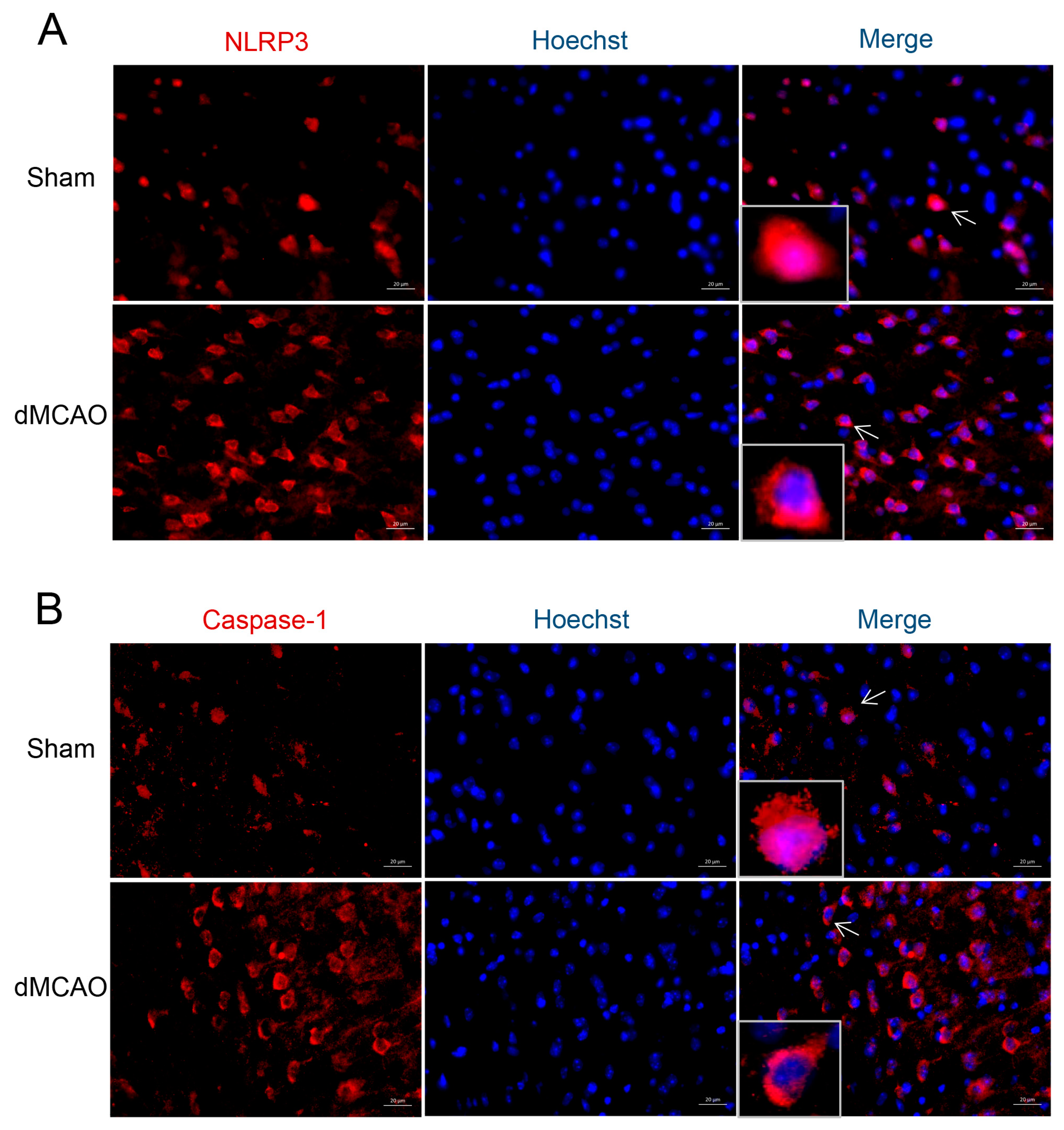
3.4. The Cellular Location of NLRP3 and Caspase-1 after dMCAO
3.5. HS Reduced the Expression of ROS, NLRP3, Caspase-1, and IL-1β
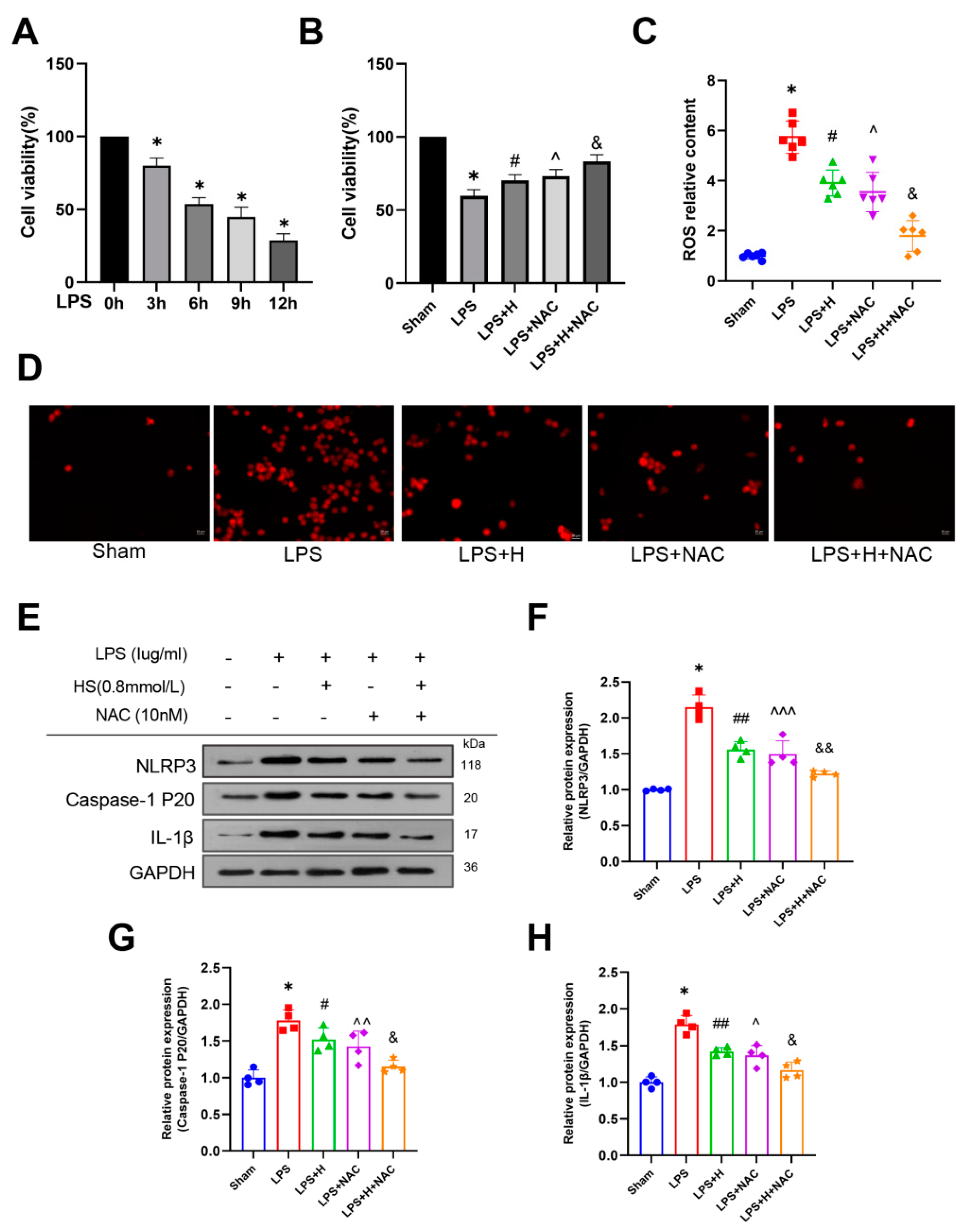
3.6. HS Exerted Anti-Inflammatory Action through Inhibiting the ROS-NLRP3 Inflammasome Pathway In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, L.; Zhang, D.; Chen, J.; Sun, C.; Ji, K.; Li, W.; Zhao, W.; Li, C.; Wu, C.; Li, M.; et al. Long-term outcome of endovascular therapy for acute basilar artery occlusion. J. Cereb. Blood Flow Metab. 2021, 41, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.W.; Tyrrell, P.; Sattar, N.; Warburton, E. Inflammation and ischaemic stroke. Curr. Opin. Neurol. 2007, 20, 334–342. [Google Scholar] [CrossRef]
- Li, H.; Luo, Y.; Yang, P.; Liu, J. Hydrogen as a complementary therapy against ischemic stroke: A review of the evidence. J. Neurol. Sci. 2019, 396, 240–246. [Google Scholar] [CrossRef]
- Domoki, F. Hydrogen-induced Neuroprotection in Neonatal Hypoxic-ischemic Encephalopathy. Curr. Pharm. Des. 2021, 27, 687–694. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Sun, H.; Chen, L.; Zhou, W.; Hu, L.; Li, L.; Tu, Q.; Chang, Y.; Liu, Q.; Sun, X.; Wu, M.; et al. The protective role of hydrogen-rich saline in experimental liver injury in mice. J. Hepatol. 2011, 54, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.F.; Zheng, X.F.; Cai, J.M.; You, X.M.; Deng, X.M.; Zhang, J.H.; Jiang, L.; Sun, X.J. Hydrogen-rich saline reduces lung injury induced by intestinal ischemia/reperfusion in rats. Biochem. Biophys. Res. Commun. 2009, 381, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Hui, K.; Zhang, L.; Sun, X.; Li, W.; Duan, M. The effect of hydrogen-rich saline on the brain of rats with transient ischemia. J. Surg. Res. 2011, 168, e95–e101. [Google Scholar] [CrossRef]
- Song, L.; Pei, L.; Yao, S.; Wu, Y.; Shang, Y. NLRP3 Inflammasome in Neurological Diseases, from Functions to Therapies. Front. Cell. Neurosci. 2017, 11, 63. [Google Scholar] [CrossRef] [Green Version]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Salminen, A.; Ojala, J.; Kaarniranta, K.; Kauppinen, A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: Impact on the aging process and age-related diseases. Cell. Mol. Life Sci. 2012, 69, 2999–3013. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.; Bartok, E.; Rieger, A.; Franchi, L.; Núñez, G.; Hornung, V. Cutting edge: Reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 2011, 187, 613–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, O.; Thomas, C.J.; Guarda, G.; Tschopp, J. The inflammasome: An integrated view. Immunol. Rev. 2011, 243, 136–151. [Google Scholar] [CrossRef]
- Catherine, D.; Virginie, P.; Van Bruggen, R.; Chad, S.; Mossman, B.T.; Tschopp, J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008, 320, 674–677. [Google Scholar] [CrossRef] [Green Version]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production. Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Stancu, I.C.; Cremers, N.; Vanrusselt, H.; Couturier, J.; Vanoosthuyse, A.; Kessels, S.; Lodder, C.; Brône, B.; Huaux, F.; Octave, J.N.; et al. Aggregated Tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathol. 2019, 137, 599–617. [Google Scholar] [CrossRef] [Green Version]
- He, X.F.; Zeng, Y.X.; Li, G.; Feng, Y.K.; Wu, C.; Liang, F.Y.; Zhang, Y.; Lan, Y.; Xu, G.Q.; Pei, Z. Extracellular ASC exacerbated the recurrent ischemic stroke in an NLRP3-dependent manner. J. Cereb. Blood Flow Metab. 2019, 40, 1048–1060. [Google Scholar] [CrossRef]
- Myagmar, B.O.; Chen, R.; Zhang, X.; Xu, R.; Jiang, W.; Cao, W.; Ji, H.; Zhang, X. Cerebroprotein hydrolysate injection is involved in promoting long-term angiogenesis, vessel diameter and density after cerebral ischemia in mice. Life Sci. 2022, 300, 120568. [Google Scholar] [CrossRef]
- Cui, L.; Murikinati, S.R.; Wang, D.; Zhang, X.; Duan, W.M.; Zhao, L.R. Reestablishing neuronal networks in the aged brain by stem cell factor and granulocyte-colony stimulating factor in a mouse model of chronic stroke. PLoS ONE 2013, 8, e64684. [Google Scholar] [CrossRef] [Green Version]
- Qiu, P.; Liu, Y.; Chen, K.; Dong, Y.; Liu, S.; Zhang, J. Hydrogen-rich saline regulates the polarization and apoptosis of alveolar macrophages and attenuates lung injury via suppression of autophagy in septic rats. Ann. Transl. Med. 2021, 9, 974. [Google Scholar] [CrossRef]
- Qiu, X.; Dong, K.; Guan, J.; He, J. Hydrogen attenuates radiation-induced intestinal damage by reducing oxidative stress and inflammatory response. Int. Immunopharmacol. 2020, 84, 106517. [Google Scholar] [CrossRef]
- Hayashi-Takagi, A.; Yagishita, S.; Nakamura, M.; Shirai, F.; Wu, Y.I.; Loshbaugh, A.L.; Kuhlman, B.; Hahn, K.M.; Kasai, H. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 2015, 525, 333–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.Z.; Zhang, X.J.; Zhang, C.; Yang, Y.; He, J.N.; Chen, Y.X. Protective effects of leonurine against ischemic stroke in mice by activating nuclear factor erythroid 2-related factor 2 pathway. CNS Neurosci. Ther. 2019, 25, 1006–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Cheng, J.; Shi, G.; Zhang, C.; Du, Y.; Chen, L.; Qiao, H.; Chen, R.; Zhang, X. Liraglutide Ameliorates Cerebral Ischemia in Mice via Antipyroptotic Pathways. Neurochem. Res. 2022, 47, 1904–1916. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Chen, J.; Song, D.; Zhang, C.; Chen, R.; Xu, R.; Jiang, W.; Li, L. Chrysophanol facilitates long-term neurological recovery through limiting microglia-mediated neuroinflammationafter ischemic stroke in mice. Int. Immunopharmacol. 2022, 112, 109220. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, X.; Liu, X.; Wang, H.; Xue, J.; Yu, J.; Kang, N.; Wang, X. Chrysophanol inhibits NALP3 inflammasome activation and ameliorates cerebral ischemia/reperfusion in mice. Mediat. Inflamm. 2014, 2014, 370530. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Ge, M.; Jin, J.; Xu, H.; Mao, L.; Geng, S.; Wu, J.; Zhu, J.; Li, X.; Zhong, C. Mechanism investigation on Bisphenol S-induced oxidative stress and inflammation in murine RAW264.7 cells: The role of NLRP3 inflammasome, TLR4, Nrf2 and MAPK. J. Hazard. Mater. 2020, 394, 122549. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Liu, X.; Zhang, C.; Shang, W.; Xue, J.; Chen, R.; Xing, Y.; Song, D.; Xu, R. Ginsenoside Rg1 promotes cerebral angiogenesis via the PI3K/Akt/mTOR signaling pathway in ischemic mice. Eur. J. Pharmacol. 2019, 856, 172418. [Google Scholar] [CrossRef]
- Qian, Y.; Lu, X.; Chen, L.; Sun, J.; Cao, K.; Yu, Q.; Shao, J. Effect of astaxanthin on neuron damage, inflammatory factors expressions and oxidative stress in mice with subarachnoid hemorrhage. Am. J. Transl. Res. 2021, 13, 13043–13050. [Google Scholar]
- Zhuang, W.; Wang, C.; Shi, X.; Qiu, S.; Zhang, S.; Xu, B.; Chen, M.; Jiang, W.; Dong, H.; Qiao, Y. MCMV triggers ROS/NLRP3-associated inflammasome activation in the inner ear of mice and cultured spiral ganglion neurons, contributing to sensorineural hearing loss. Int. J. Mol. Med. 2018, 41, 3448–3456. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Tao, J.; Duan, F.; Li, H.; Tan, H. HHcy Induces Pyroptosis and Atherosclerosis via the Lipid Raft-Mediated NOX-ROS-NLRP3 Inflammasome Pathway in apoE-/- Mice. Cells 2022, 11, 2438. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Li, T.T.; Cao, H.L.; Yang, W.C. Recent advances in the neuroprotective effects of medical gases. Med. Gas Res. 2019, 9, 80–87. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; He, Q.; Zhu, P.; Zhuang, Q.; Shen, J.; Zhang, X.; Zhao, M. Hydrogen: A Novel Option in Human Disease Treatment. Oxid. Med. Cell. Longev. 2020, 2020, 8384742. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J. Secondary stroke prevention. Lancet Neurol. 2014, 13, 178–194. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front. Mol. Neurosci. 2020, 13, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutin, H.; LeFeuvre, R.A.; Horai, R.; Asano, M.; Iwakura, Y.; Rothwell, N.J. Role of IL-1α and IL-1β in ischemic brain damage. J. Neurosci. 2001, 21, 5528–5534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, C.; Peng, Y.; Qin, C.; Fan, F.; Liu, J.; Long, J. Hydrogen-rich water improves cognitive impairment gender-dependently in APP/PS1 mice without affecting Aβ clearance. Free Radic. Res. 2018, 52, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Nagatani, K.; Wada, K.; Takeuchi, S.; Kobayashi, H.; Uozumi, Y.; Otani, N.; Fujita, M.; Tachibana, S.; Nawashiro, H. Effect of hydrogen gas on the survival rate of mice following global cerebral ischemia. Shock 2012, 37, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Nagatani, K.; Otani, N.; Nawashiro, H.; Sugawara, T.; Wada, K.; Mori, K. Hydrogen improves neurological function through attenuation of blood-brain barrier disruption in spontaneously hypertensive stroke-prone rats. BMC Neurosci. 2015, 16, 22. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, J.; Toth-Szuki, V.; Varga, V.; Kovacs, V.; Remzso, G.; Domoki, F. Molecular hydrogen affords neuroprotection in a translational piglet model of hypoxic-ischemic encephalopathy. J. Physiol. Pharmacol. 2016, 67, 677–689. [Google Scholar] [PubMed]
- Chen, K.; Wang, N.; Diao, Y.; Dong, W.; Sun, Y.; Liu, L.; Wu, X. Hydrogen-Rich Saline Attenuates Brain Injury Induced by Cardiopulmonary Bypass and Inhibits Microvascular Endothelial Cell Apoptosis Via the PI3K/Akt/GSK3β Signaling Pathway in Rats. Cell. Physiol. Biochem. 2017, 43, 1634–1647. [Google Scholar] [CrossRef]
- Conforti-Andreoni, C.; Ricciardi-Castagnoli, P.; Mortellaro, A. The inflammasomes in health and disease: From genetics to molecular mechanisms of autoinflammation and beyond. Cell. Mol. Immunol. 2011, 8, 135–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, M.; Shinohara, M.L. NLRP3 Inflammasome and MS/EAE. Autoimmune Dis. 2013, 2013, 859145. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Chen, S.; Hu, Q.; Feng, H.; Zhang, J.H.; Tang, J. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann. Neurol. 2014, 75, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.D.; Li, W.; Chen, Z.R.; Hu, Y.C.; Shen, W.; Zhou, M.L.; Zhu, L.; Hang, C.H. Expression of the NLRP3 inflammasome in cerebral cortexafter traumatic brain injury in a rat model. Neurochem. Res. 2013, 38, 2072–2083. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.S.; Yu, J.T.; Jiang, T.; Zhu, X.C.; Tan, L. The NLRP3 inflammasome in Alzheimer’s disease. Mol. Neurobiol. 2013, 48, 875–882. [Google Scholar] [CrossRef]
- Hoegen, T.; Tremel, N.; Klein, M.; Angele, B.; Wagner, H.; Kirschning, C.; Pfister, H.W.; Fontana, A.; Hammerschmidt, S.; Koedel, U. The NLRP3 inflammasome contributes to brain injury in pneumococcal meningitis and is activated through ATP-dependent lysosomal cathepsin B release. J. Immunol. 2011, 187, 5440–5451. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, J.H.; Chen, X.Y.; Hu, Q.H.; Wang, M.X.; Jin, R.; Zhang, Q.Y.; Wang, W.; Wang, R.; Kang, L.L.; et al. Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid. Redox Signal. 2015, 22, 848–870. [Google Scholar] [CrossRef] [Green Version]
- Yatsiv, I.; Morganti-Kossmann, M.C.; Perez, D.; Dinarello, C.A.; Novick, D.; Rubinstein, M.; Otto, V.I.; Rancan, M.; Kossmann, T.; Redaelli, C.A.; et al. Elevated intracranial IL-18 in humans and mice after traumatic brain injury and evidence of neuroprotective effects of IL-18-binding protein after experimental closed head injury. J. Cereb. Blood Flow Metab. 2002, 22, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, S.; Luo, J.; Liu, A.; Tang, S.; Liu, S.; Yu, M.; Zhang, Y. Helicobacter pylori induces IL-1β and IL-18 production in human monocytic cell line through activation of NLRP3 inflammasome via ROS signaling pathway. Pathog. Dis. 2015, 73, ftu024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Q.; Ren, Y.; Reinach, P.S.; She, Y.; Xiao, B.; Hua, S.; Qu, J.; Chen, W. Reactive oxygen species activated NLRP3 inflammasomes prime environment-induced murine dry eye. Exp. Eye Res. 2014, 125, 1–8. [Google Scholar] [CrossRef]
- Wang, C.; An, Y.; Wang, Y.; Shen, K.; Wang, X.; Luan, W.; Ma, F.; Ni, L.; Liu, M.; Yu, L. Insulin-like growth factor-I activates NFκB and NLRP3 inflammatory signalling via ROS in cancer cells. Mol. Cell. Probes. 2020, 52, 101583. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Wu, H.; Hong, Y.; Tu, S.; Sun, X.; Wu, Q.; Zhao, Q.; Zhang, J.; Sheng, J. Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome. Mol. Neurobiol. 2016, 53, 3462–3476. [Google Scholar] [CrossRef] [PubMed]
- Iida, A.; Nosaka, N.; Yumoto, T.; Knaup, E.; Naito, H.; Nishiyama, C.; Yamakawa, Y.; Tsukahara, K.; Terado, M.; Sato, K.; et al. The Clinical Application of Hydrogen as a Medical Treatment. Acta Med. Okayama 2016, 70, 331–337. [Google Scholar] [CrossRef]
- Nakao, A.; Toyoda, Y.; Sharma, P.; Evans, M.; Guthrie, N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome-an open label pilot study. J. Clin. Biochem. Nutr. 2010, 46, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.M.; Kang, Y.N.; Choi, I.B.; Gu, Y.; Kawamura, T.; Toyoda, Y.; Nakao, A. Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors. Med. Gas Res. 2011, 1, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene | Primers | Sequences |
|---|---|---|
| NLRP3 | Forward | 5′-CCAGCCAGAGTGGAATGACA-3′ |
| Reverse | 5′-AGCGGGAGACAAATGGAGAT-3′ | |
| Caspase-1 | Forward | 5′-GGGACCCTCAAGTTTTGCC-3′ |
| Reverse | 5′-GACGTGTACGAGTGGTTGTATT-3′ | |
| IL-1β | Forward | 5′-AACTCAACTGTGAAATGCCACC-3′ |
| Reverse | 5′-CATCAGGACAGCCCAGGTC-3′ | |
| GAPDH | Forward | 5′-AGGAGCGAGACCCCACTAACA-3′ |
| Reverse | 5′-AGGGGGGCTAAGCAGTTGGT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Chen, L.; Qiao, H.; Zhang, L.; Yang, L.; Zhang, P.; Wang, J.; Zhang, C.; Jiang, W.; Xu, R.; et al. Hydrogen-Rich Saline—A Novel Neuroprotective Agent in a Mouse Model of Experimental Cerebral Ischemia via the ROS-NLRP3 Inflammasome Signaling Pathway In Vivo and In Vitro. Brain Sci. 2023, 13, 939. https://doi.org/10.3390/brainsci13060939
Du Y, Chen L, Qiao H, Zhang L, Yang L, Zhang P, Wang J, Zhang C, Jiang W, Xu R, et al. Hydrogen-Rich Saline—A Novel Neuroprotective Agent in a Mouse Model of Experimental Cerebral Ischemia via the ROS-NLRP3 Inflammasome Signaling Pathway In Vivo and In Vitro. Brain Sciences. 2023; 13(6):939. https://doi.org/10.3390/brainsci13060939
Chicago/Turabian StyleDu, Yuanyuan, Linyu Chen, Huimin Qiao, Lan Zhang, Lan Yang, Peipei Zhang, Jing Wang, Cong Zhang, Wei Jiang, Renhao Xu, and et al. 2023. "Hydrogen-Rich Saline—A Novel Neuroprotective Agent in a Mouse Model of Experimental Cerebral Ischemia via the ROS-NLRP3 Inflammasome Signaling Pathway In Vivo and In Vitro" Brain Sciences 13, no. 6: 939. https://doi.org/10.3390/brainsci13060939






