Behavioral, Functional Imaging, and Neurophysiological Outcomes of Transcranial Direct Current Stimulation and Speech-Language Therapy in an Individual with Aphasia
Abstract
1. Introduction
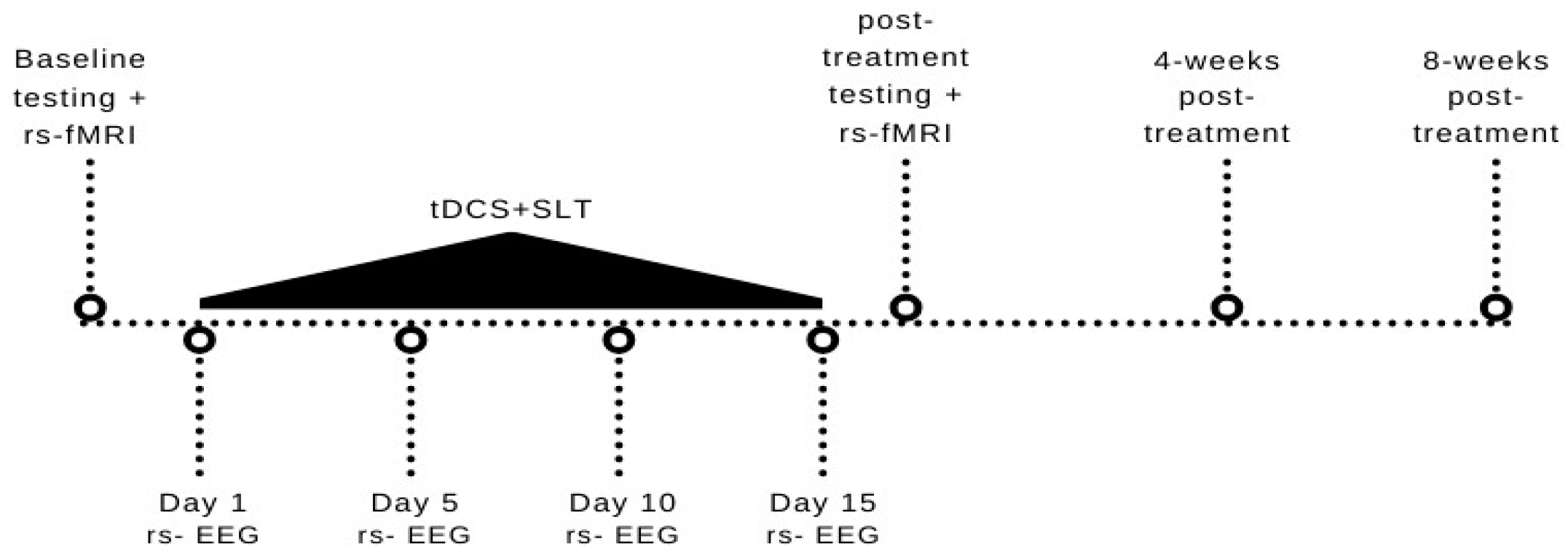
2. Method
2.1. Participant
2.2. Speech-Language Therapy
2.3. Transcranial Direct Current Stimulation
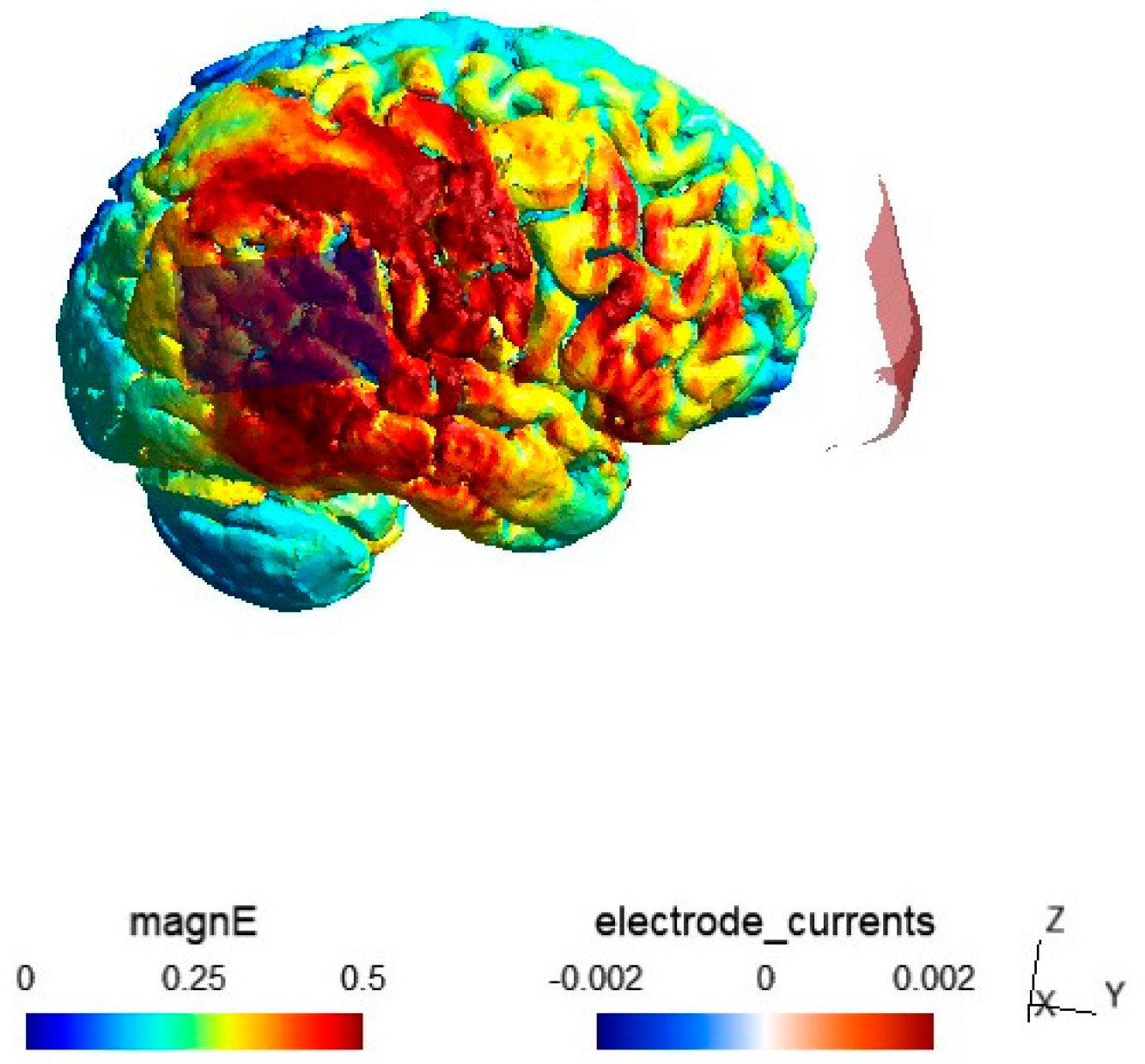
2.4. Electric Field Modeling
2.5. Outcome Measures
2.5.1. MRI and fMRI Recordings
2.5.2. MRI Data Preprocessing
2.5.3. Resting-State MRI (rs–fMRI) Preprocessing
2.5.4. Behavioral Outcomes
2.5.5. Resting-State EEG (rs–EEG) Recordings
2.5.6. EEG Preprocessing
2.5.7. Neuronavigation and Coordinate System Alignment between MRI, EEG, and tDCS
3. Analysis
3.1. Behavioral Data Analysis
3.2. Resting-State fMRI Analysis
3.3. EEG Analysis
4. Results
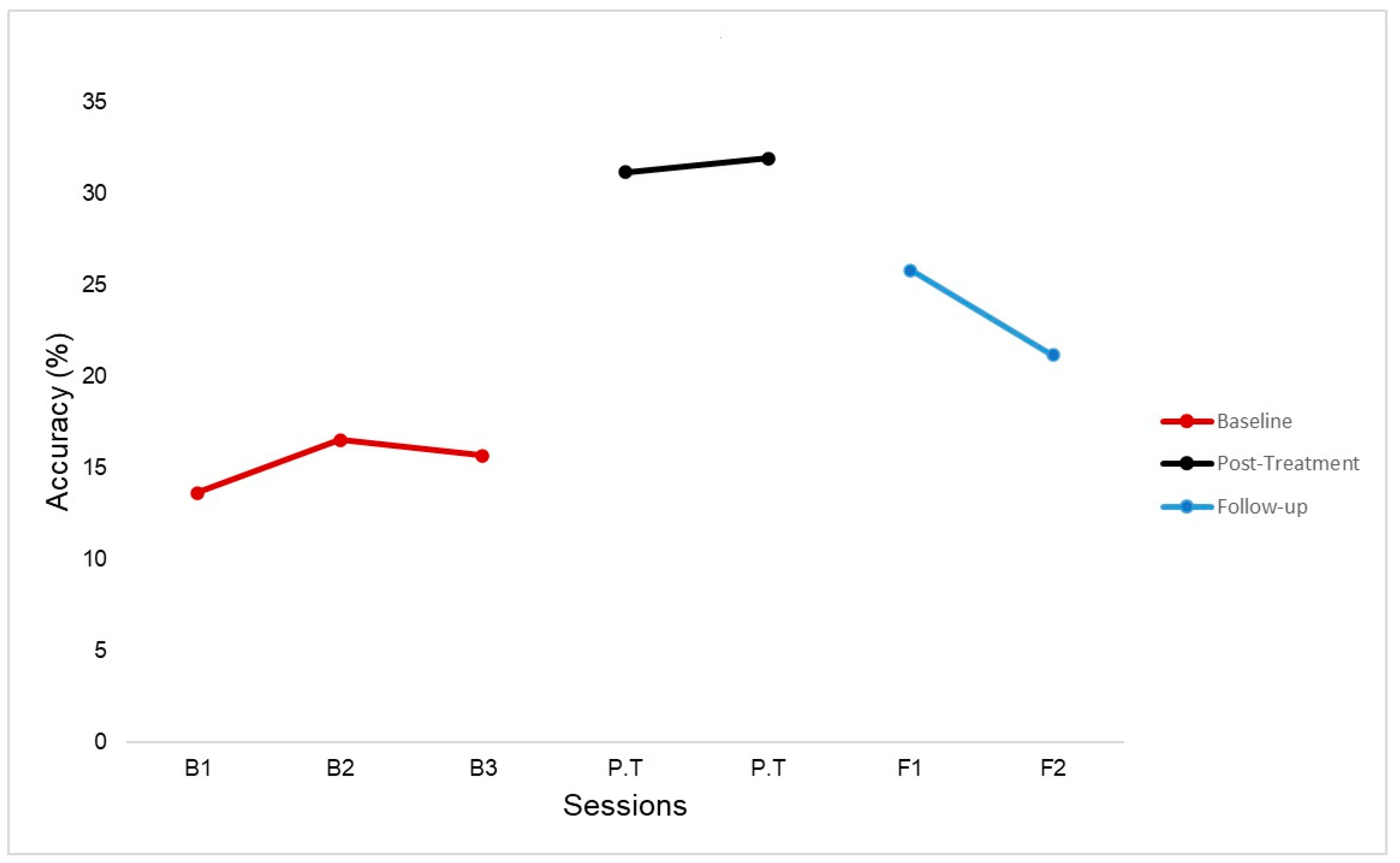
4.1. Behavioral Results
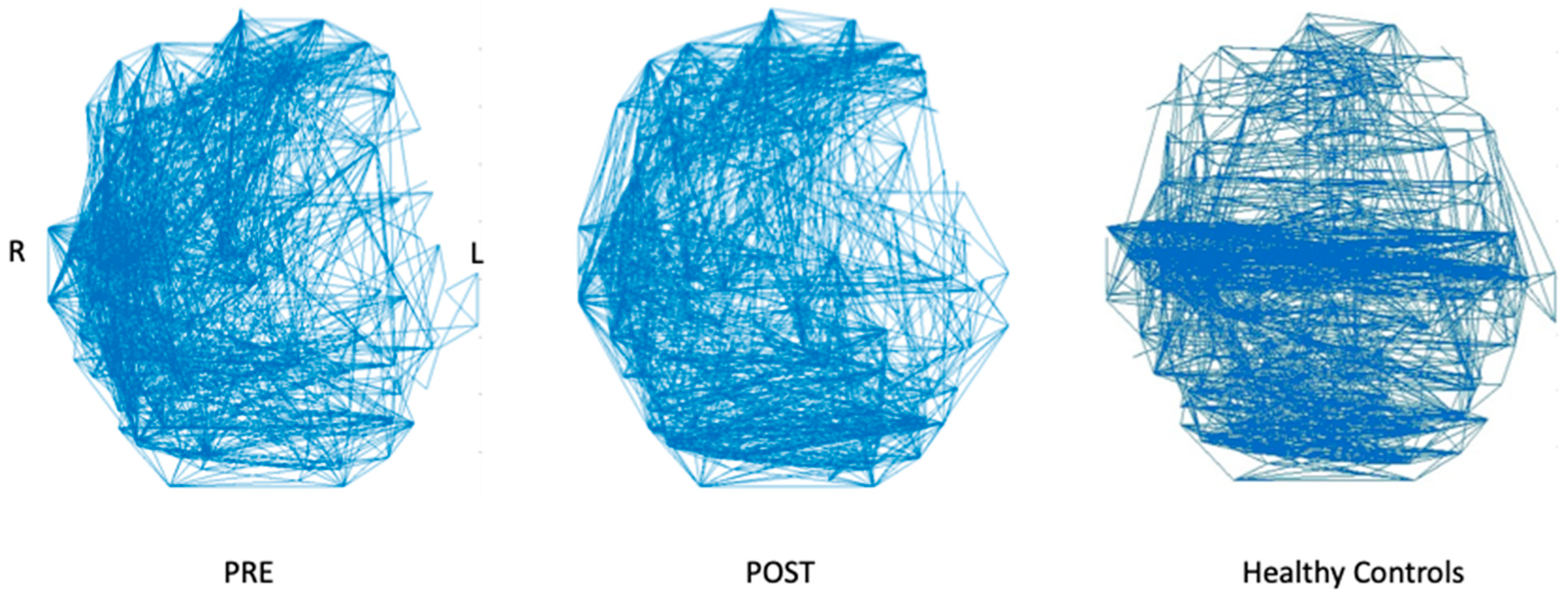
4.2. Resting-State fMRI Results
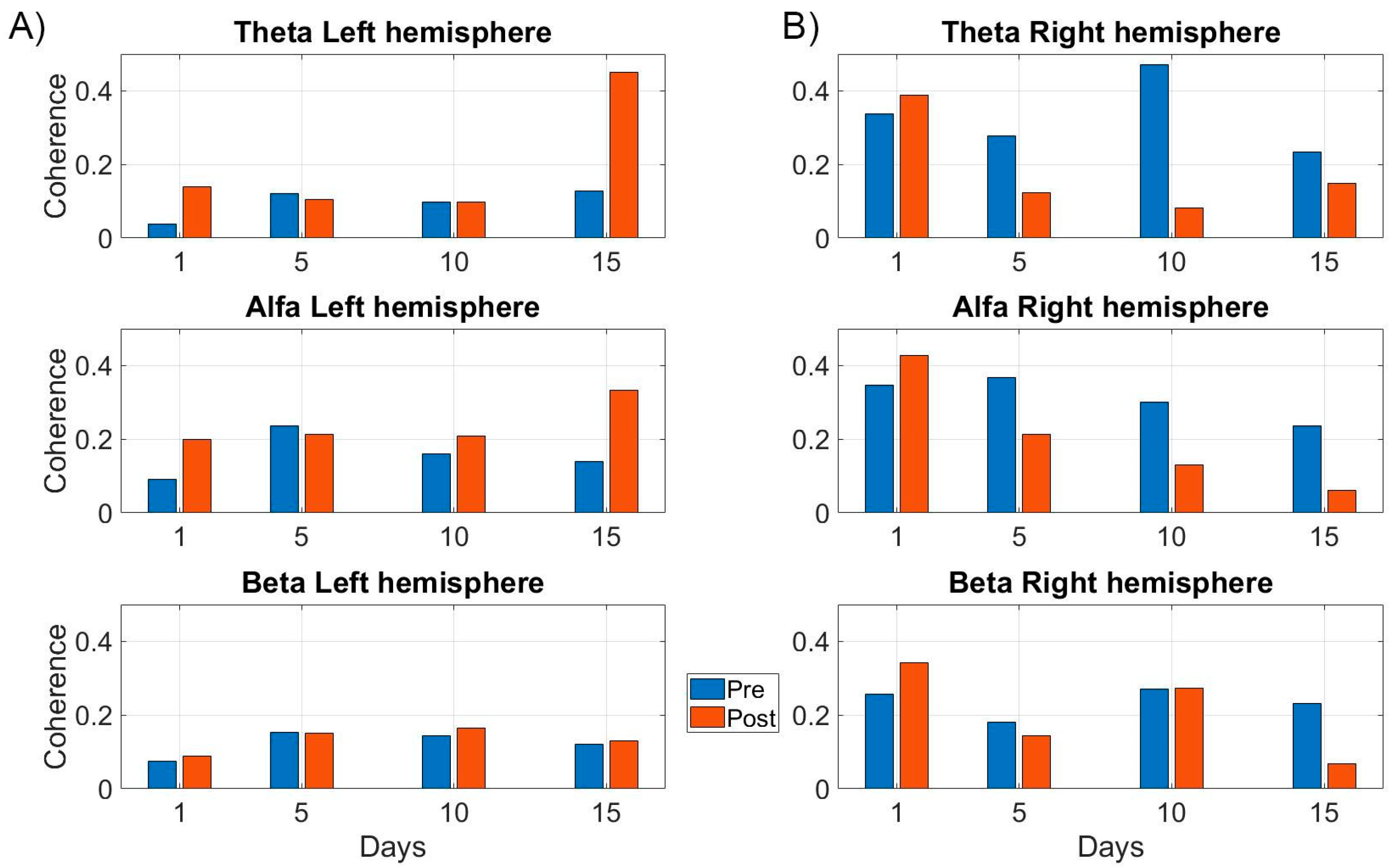
4.3. EEG Results
5. Discussion
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashaie, S.A.; Hurwitz, R.; Cherney, L.R. Depression and Subthreshold Depression in Stroke-Related Aphasia. Arch. Phys. Med. Rehabil. 2019, 100, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Hilari, K.; Needle, J.J.; Harrison, K.L. What Are the Important Factors in Health-Related Quality of Life for People with Aphasia? A Systematic Review. Arch. Phys. Med. Rehabil. 2012, 93, S86–S95. [Google Scholar] [CrossRef] [PubMed]
- Cruice, M.; Worrall, L.; Hickson, L.; Murison, R. Finding a Focus for Quality of Life with Aphasia: Social and Emotional Health, and Psychological Well-Being. Aphasiology 2003, 17, 333–353. [Google Scholar] [CrossRef]
- Brady, M.C.; Godwin, J.; Enderby, P.; Kelly, H.; Campbell, P. Speech and Language Therapy for Aphasia After Stroke. Stroke 2016, 47, e236–e237. [Google Scholar] [CrossRef]
- Cirillo, G.; Di Pino, G.; Capone, F.; Ranieri, F.; Florio, L.; Todisco, V.; Tedeschi, G.; Funke, K.; Di Lazzaro, V. Neurobiological After-Effects of Non-Invasive Brain Stimulation. Brain Stimul. 2017, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hartwigsen, G. Adaptive Plasticity in the Healthy Language Network: Implications for Language Recovery after Stroke. Neural Plast. 2016, 2016, 9674790. [Google Scholar] [CrossRef] [PubMed]
- Bestmann, S.; de Berker, A.O.; Bonaiuto, J. Understanding the Behavioural Consequences of Noninvasive Brain Stimulation. Trends Cogn. Sci. 2015, 19, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.H.; Chrysikou, E.G.; Coslett, B. Mechanisms of Aphasia Recovery after Stroke and the Role of Noninvasive Brain Stimulation. Brain Lang. 2011, 118, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Elsner, B.; Kugler, J.; Mehrholz, J. Transcranial Direct Current Stimulation (tDCS) for Improving Aphasia after Stroke: A Systematic Review with Network Meta-Analysis of Randomized Controlled Trials. J. Neuroeng. Rehabil. 2020, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S. A Technical Guide to tDCS, and Related Non-Invasive Brain Stimulation Tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- De Aguiar, V.; Paolazzi, C.L.; Miceli, G. tDCS in Post-Stroke Aphasia: The Role of Stimulation Parameters, Behavioral Treatment and Patient Characteristics. Cortex 2015, 63, 296–316. [Google Scholar] [CrossRef] [PubMed]
- Shah-Basak, P.; Boukrina, O.; Li, X.R.; Jebahi, F.; Kielar, A. Targeted Neurorehabilitation Strategies in Post-Stroke Aphasia. Restor. Neurol. Neurosci. 2023, 41, 129–191. [Google Scholar] [CrossRef] [PubMed]
- Turkeltaub, P.E.; Coslett, H.B.; Thomas, A.L.; Faseyitan, O.; Benson, J.; Norise, C.; Hamilton, R.H. The Right Hemisphere Is Not Unitary in Its Role in Aphasia Recovery. Cortex 2012, 48, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Anglade, C.; Thiel, A.; Ansaldo, A.I. The Complementary Role of the Cerebral Hemispheres in Recovery from Aphasia after Stroke: A Critical Review of Literature. Brain Inj. 2014, 28, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Heiss, W.-D.; Thiel, A. A Proposed Regional Hierarchy in Recovery of Post-Stroke Aphasia. Brain Lang. 2006, 98, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Keser, Z.; Sebastian, R.; Hasan, K.M.; Hillis, A.E. Right Hemispheric Homologous Language Pathways Negatively Predicts Poststroke Naming Recovery. Stroke 2020, 51, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.E.; Sghirripa, S.; Rogasch, N.C.; Hordacre, B.; Attrill, S. Non-Invasive Brain Stimulation in the Treatment of Post-Stroke Aphasia: A Scoping Review. Disabil. Rehabil. 2023, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, J.; Sharma, S.; Peper, M.; Brumberg, J. Investigating NIBS for Language Rehabilitation in Aphasia. Aphasiology 2023, 37, 1285–1314. [Google Scholar] [CrossRef]
- Galletta, E.E.; Conner, P.; Vogel-Eyny, A.; Marangolo, P. Use of tDCS in Aphasia Rehabilitation: A Systematic Review of the Behavioral Interventions Implemented with Noninvasive Brain Stimulation for Language Recovery. Am. J. Speech-Lang. Pathol. 2016, 25, S854–S867. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Li, Y.; Zhang, Y.; Fan, H.; Gao, Q.; Wang, L. Long-Term Effects of Transcranial Direct Current Stimulation (tDCS) Combined with Speech Language Therapy (SLT) on Post-Stroke Aphasia Patients: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. NeuroRehabilitation 2023, 53, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Biou, E.; Cassoudesalle, H.; Cogne, M.; Sibon, I.; De Gabory, I.; Dehail, P.; Aupy, J.; Glize, B. Transcranial Direct Current Stimulation in Post-Stroke Aphasia Rehabilitation: A Systematic Review. Ann. Phys. Rehabil. Med. 2019, 62, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Fridriksson, J.; Rorden, C.; Elm, J.; Sen, S.; George, M.S.; Bonilha, L. Transcranial Direct Current Stimulation vs Sham Stimulation to Treat Aphasia after Stroke: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Marangolo, P.; Fiori, V.; Calpagnano, M.A.; Campana, S.; Razzano, C.; Caltagirone, C.; Marini, A. tDCS over the Left Inferior Frontal Cortex Improves Speech Production in Aphasia. Front. Hum. Neurosci. 2013, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Marangolo, P.; Fiori, V.; Campana, S.; Calpagnano, M.A.; Razzano, C.; Caltagirone, C.; Marini, A. Something to Talk about: Enhancement of Linguistic Cohesion through tdCS in Chronic Non Fluent Aphasia. Neuropsychologia 2014, 53, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Marangolo, P.; Fiori, V.; Sabatini, U.; De Pasquale, G.; Razzano, C.; Caltagirone, C.; Gili, T. Bilateral Transcranial Direct Current Stimulation Language Treatment Enhances Functional Connectivity in the Left Hemisphere: Preliminary Data from Aphasia. J. Cogn. Neurosci. 2016, 28, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Alemanno, F.; Fedeli, D.; Monti, A.; Houdayer, E.; Della Rosa, P.A.; Zangrillo, F.; Emedoli, D.; Pelagallo, E.; Corbo, M.; Iannaccone, S. Increased Interhemispheric Functional Connectivity after Right Anodal tDCS in Chronic Non-Fluent Aphasia: Preliminary Findings. Front. Neurosci. 2024, 18, 1346095. [Google Scholar] [CrossRef] [PubMed]
- Raymer, A.M.; Johnson, R.K. Effectiveness of Transcranial Direct Current Stimulation as an Adjuvant to Aphasia Treatment Following Stroke: Evidence From Systematic Reviews and Meta-Analyses. Am. J. Speech-Lang. Pathol. 2024, 1–13. [Google Scholar] [CrossRef]
- Kertesz, A. WAB-R: Western Aphasia Battery-Revised; PsychCorp: London, UK, 2007; ISBN 0-15-400662-9. [Google Scholar]
- Walker, G.M.; Schwartz, M.F. Short-Form Philadelphia Naming Test: Rationale and Empirical Evaluation. Am. J. Speech-Language Pathol. 2012, 21, S140–S153. [Google Scholar] [CrossRef] [PubMed]
- Babbitt, E.M.; Heinemann, A.W.; Semik, P.; Cherney, L.R. Psychometric Properties of the Communication Confidence Rating Scale for Aphasia (CCRSA): Phase 2. Aphasiology 2011, 25, 727–735. [Google Scholar] [CrossRef]
- Cherney, L.R.; Babbitt, E.M.; Semik, P.; Heinemann, A.W. Psychometric Properties of the Communication Confidence Rating Scale for Aphasia (CCRSA): Phase 1. Top. Stroke Rehabil. 2011, 18, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Chiou, H.S.; Yu, V.Y. Measuring Life Participation, Communicative Confidence, Language, and Cognition in People with Aphasia. Perspect. ASHA Spec. Interest Groups 2018, 3, 4–12. [Google Scholar] [CrossRef]
- Eaton, W.W.; Smith, C.; Ybarra, M.; Muntaner, C.; Tien, A. Center for Epidemiologic Studies Depression Scale: Review and Revision (CESD and CESD-R). In The Use of Psychological Testing for Treatment Planning and Outcomes Assessment: Instruments for Adults; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 2004. [Google Scholar]
- Hilari, K.; Byng, S.; Lamping, D.L.; Smith, S.C. Stroke and Aphasia Quality of Life Scale-39 (SAQOL-39) Evaluation of Acceptability, Reliability, and Validity. Stroke 2003, 34, 1944–1950. [Google Scholar] [CrossRef] [PubMed]
- Castellucci, G.A.; Kovach, C.K.; Howard III, M.A.; Greenlee, J.D.; Long, M.A. A Speech Planning Network for Interactive Language Use. Nature 2022, 602, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Cherney, L.R.; Halper, A.S.; Holland, A.L.; Cole, R. Computerized Script Training for Aphasia: Preliminary Results. Am. J. Speech-Lang. Pathol. 2008, 17, 19–34. [Google Scholar] [CrossRef]
- Manheim, L.M.; Halper, A.S.; Cherney, L. Patient-Reported Changes in Communication after Computer-Based Script Training for Aphasia. Arch. Phys. Med. Rehabil. 2009, 90, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Kaye, R.C.; Cherney, L.R. Script Templates: A Practical Approach to Script Training in Aphasia. Top. Lang. Disord. 2016, 36, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Cherney, L.R.; Van Vuuren, S. Complexity and Feedback during Script Training in Aphasia: A Feasibility Study. Arch. Phys. Med. Rehabil. 2022, 103, S205–S214. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, H.I.; Nelson, L.A.; Richardson, J.D. Can Script Training Improve Narrative and Conversation in Aphasia across Etiology? Thieme Medical Publishers: New York, NY, USA, 2020; Volume 41, pp. 99–124. [Google Scholar]
- Robinaugh, G.; Henry, M.L. Behavioral Interventions for Primary Progressive Aphasia. Handb. Clin. Neurol. 2022, 185, 221–240. [Google Scholar] [PubMed]
- Ashaie, S.A.; Engel, S.; Cherney, L.R. Timing of Transcranial Direct Current Stimulation (tDCS) Combined with Speech and Language Therapy (SLT) for Aphasia: Study Protocol for a Randomized Controlled Trial. Trials 2022, 23, 668. [Google Scholar] [CrossRef] [PubMed]
- Thielscher, A.; Antunes, A.; Saturnino, G.B. Field Modeling for Transcranial Magnetic Stimulation: A Useful Tool to Understand the Physiological Effects of TMS? IEEE: Piscataway, NJ, USA, 2015; pp. 222–225. [Google Scholar]
- Geuzaine, C.; Remacle, J. Gmsh: A 3-D Finite Element Mesh Generator with Built-in Pre-and Post-processing Facilities. Int. J. Numer. Methods Eng. 2009, 79, 1309–1331. [Google Scholar] [CrossRef]
- Woolrich, M.W.; Jbabdi, S.; Patenaude, B.; Chappell, M.; Makni, S.; Behrens, T.; Beckmann, C.; Jenkinson, M.; Smith, S.M. Bayesian Analysis of Neuroimaging Data in FSL. Neuroimage 2009, 45, S173–S186. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.L.; Jenkinson, M.; Smith, S. Non-Linear Registration, Aka Spatial Normalisation FMRIB Technical Report TR07JA2. FMRIB Anal. Group Univ. Oxf. 2007, 2, 1–22. [Google Scholar]
- Azios, J.H.; Archer, B.; Simmons-Mackie, N.; Raymer, A.; Carragher, M.; Shashikanth, S.; Gulick, E. Conversation as an Outcome of Aphasia Treatment: A Systematic Scoping Review. Am. J. Speech-Lang. Pathol. 2022, 31, 2920–2942. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.F.; Madden, E.B.; Mozeiko, J.; Murray, L.L.; Patterson, J.P.; Purdy, M.; Sandberg, C.W.; Wallace, S.E. Generalization in Aphasia Treatment: A Tutorial for Speech-Language Pathologists. Am. J. Speech-Lang. Pathol. 2024, 33, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Cherney, L.R. Tau-U: A Quantitative Approach for Analysis of Single-Case Experimental Data in Aphasia. Am. J. Speech-Lang. Pathol. 2018, 27, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Jasper, H.H. Ten-Twenty Electrode System of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 371–375. [Google Scholar]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Parkkonen, L.; Hämäläinen, M.S. MNE Software for Processing MEG and EEG Data. Neuroimage 2014, 86, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Pitts, L.L.; Hurwitz, R.; Lee, J.B.; Carpenter, J.; Cherney, L.R. Validity, Reliability and Sensitivity of the NORLA-6: Naming and Oral Reading for Language in Aphasia 6-Point Scale. Int. J. Speech-Lang. Pathol. 2018, 20, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Power, J.D.; Cohen, A.L.; Nelson, S.M.; Wig, G.S.; Barnes, K.A.; Church, J.A.; Vogel, A.C.; Laumann, T.O.; Miezin, F.M.; Schlaggar, B.L. Functional Network Organization of the Human Brain. Neuron 2011, 72, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Rubinov, M.; Sporns, O. Complex Network Measures of Brain Connectivity: Uses and Interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.B.; Mennes, M.; Zuo, X.-N.; Gohel, S.; Kelly, C.; Smith, S.M.; Beckmann, C.F.; Adelstein, J.S.; Buckner, R.L.; Colcombe, S. Toward Discovery Science of Human Brain Function. Proc. Natl. Acad. Sci. USA 2010, 107, 4734–4739. [Google Scholar] [CrossRef] [PubMed]
- Baliki, M.N.; Babbitt, E.M.; Cherney, L.R. Brain Network Topology Influences Response to Intensive Comprehensive Aphasia Treatment. NeuroRehabilitation 2018, 43, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.W. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.W.; Hyde, J.S. Software Tools for Analysis and Visualization of fMRI Data. NMR Biomed. Int. J. Devoted Dev. Appl. Magn. Reson. In Vivo 1997, 10, 171–178. [Google Scholar] [CrossRef]
- Dale, A.M.; Sereno, M.I. Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. J. Cogn. Neurosci. 1993, 5, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.M.; Liu, A.K.; Fischl, B.R.; Buckner, R.L.; Belliveau, J.W.; Lewine, J.D.; Halgren, E. Dynamic Statistical Parametric Mapping: Combining fMRI and MEG for High-Resolution Imaging of Cortical Activity. Neuron 2000, 26, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.K.; Belliveau, J.W.; Dale, A.M. Spatiotemporal Imaging of Human Brain Activity Using Functional MRI Constrained Magnetoencephalography Data: Monte Carlo Simulations. Proc. Natl. Acad. Sci. USA 1998, 95, 8945–8950. [Google Scholar] [CrossRef] [PubMed]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, M.E.; Santana, M.B.; Baptista, A.F.; Datta, A.; Bikson, M.; Fregni, F.; Araujo, C.P. Transcranial DC Stimulation in Fibromyalgia: Optimized Cortical Target Supported by High-Resolution Computational Models. J. Pain 2011, 12, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Albizu, A.; Fang, R.; Indahlastari, A.; O’Shea, A.; Stolte, S.E.; See, K.B.; Boutzoukas, E.M.; Kraft, J.N.; Nissim, N.R.; Woods, A.J. Machine Learning and Individual Variability in Electric Field Characteristics Predict tDCS Treatment Response. Brain Stimul. 2020, 13, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Rorden, C.; Fridriksson, J. Using Transcranial Direct-Current Stimulation to Treat Stroke Patients with Aphasia. Stroke 2010, 41, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Cherney, L.R.; Babbitt, E.M.; Wang, X.; Pitts, L.L. Extended fMRI-Guided Anodal and Cathodal Transcranial Direct Current Stimulation Targeting Perilesional Areas in Post-Stroke Aphasia: A Pilot Randomized Clinical Trial. Brain Sci. 2021, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Bonilha, L.; Rorden, C.; Roth, R.; Sen, S.; George, M.S.; Fridriksson, J. Improved Naming in Patients with Broca’s Aphasia with tDCS. J. Neurol. Neurosurg. Psychiatry 2024, 95, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, A.; Socas, R.; Marrero, H.; Leon, N.; LLabres, J.; Enriquez, E. Transcranial Direct Current Stimulation Improves Word Production in Conduction Aphasia: Electroencephalographic and Behavioral Evidences. Int. J. Clin. Health Psychol. 2014, 14, 240–245. [Google Scholar] [CrossRef][Green Version]
- Turkeltaub, P.E.; Messing, S.; Norise, C.; Hamilton, R.H. Are Networks for Residual Language Function and Recovery Consistent across Aphasic Patients? Neurology 2011, 76, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, J.D.; Geranmayeh, F.; Lambon Ralph, M.A. The Multidimensional Nature of Aphasia Recovery Post-Stroke. Brain 2022, 145, 1354–1367. [Google Scholar] [CrossRef] [PubMed]
- Geranmayeh, F.; Wise, R.J.; Mehta, A.; Leech, R. Overlapping Networks Engaged during Spoken Language Production and Its Cognitive Control. J. Neurosci. 2014, 34, 8728–8740. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, F.R.; Doppelbauer, L.; Büscher, V.; Arndt, V.; Stahl, B.; Lucchese, G.; Hauk, O.; Mohr, B.; Pulvermüller, F. Increased Recruitment of Domain-General Neural Networks in Language Processing Following Intensive Language-Action Therapy: fMRI Evidence from People with Chronic Aphasia. Am. J. Speech-Lang. Pathol. 2021, 30, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Prigatano, G.P. The Importance of the Patient’s Subjective Experience in Stroke Rehabilitation. Top. Stroke Rehabil. 2011, 18, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Hole, E.; Stubbs, B.; Roskell, C.; Soundy, A. The Patient’s Experience of the Psychosocial Process That Influences Identity Following Stroke Rehabilitation: A Metaethnography. Sci. World J. 2014, 2014, 349151. [Google Scholar] [CrossRef] [PubMed]


| Assessment. | Baseline Scores |
|---|---|
| Western Aphasia Battery-Revised | |
| Information Content | 9/10 |
| Fluency | 5/10 |
| Object naming | 42/60 |
| Word fluency | 9/20 |
| Sentence completion | 6/10 |
| Responsive speech | 10/10 |
| Yes/no | 57/60 |
| Auditory word recognition | 41/60 |
| Sequential commands | 58/80 |
| Ravens Colored Progressive Matrices | 31/36 |
| Measure | Pre- | Post- | Healthy Controls: M, (Bootstrapped 95% CI) |
|---|---|---|---|
| Modularity | 0.483 | 0.544 | 0.548 (0.527–0.570) |
| Clustering Coefficient | 0.398 | 0.426 | 0.466 (0.455–0.478) |
| Efficiency | 0.302 | 0.293 | 0.300 (0.283–0.308) |
| Small Worldness | 5.511 | 5.694 | 6.296 (6.031–6.570) |
| Betweenness Centrality | 0.007 | 0.008 | 0.0099 (0.0094–0.0103) |
| Participation Coefficient | 0.530 | 0.542 | 0.514 (0.498–0.531) |
| Left intra-hemispheric connectivity | 0.030 | 0.041 | 0.053 (0.051–0.056) |
| Right intra-hemispheric connectivity | 0.101 | 0.088 | 0.066 (0.062–0.070) |
| Inter-hemispheric connectivity | 0.031 | 0.033 | 0.040 (0.038–0.042) |
| Lesion volume (cm3) | 231 | 231 | NA |
| White Matter Tract | Pre-Intervention | Post-Intervention |
|---|---|---|
| All white matter tracts | 0.303 | 0.328 |
| Left Superior Longitudinal Fasciculus | 0.238 | 0.249 |
| Right Superior Longitudinal Fasciculus | 0.348 | 0.375 |
| Left Cingulum | 0.380 | 0.404 |
| Right Cingulum | 0.369 | 0.395 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashaie, S.A.; Hernandez-Pavon, J.C.; Houldin, E.; Cherney, L.R. Behavioral, Functional Imaging, and Neurophysiological Outcomes of Transcranial Direct Current Stimulation and Speech-Language Therapy in an Individual with Aphasia. Brain Sci. 2024, 14, 714. https://doi.org/10.3390/brainsci14070714
Ashaie SA, Hernandez-Pavon JC, Houldin E, Cherney LR. Behavioral, Functional Imaging, and Neurophysiological Outcomes of Transcranial Direct Current Stimulation and Speech-Language Therapy in an Individual with Aphasia. Brain Sciences. 2024; 14(7):714. https://doi.org/10.3390/brainsci14070714
Chicago/Turabian StyleAshaie, Sameer A., Julio C. Hernandez-Pavon, Evan Houldin, and Leora R. Cherney. 2024. "Behavioral, Functional Imaging, and Neurophysiological Outcomes of Transcranial Direct Current Stimulation and Speech-Language Therapy in an Individual with Aphasia" Brain Sciences 14, no. 7: 714. https://doi.org/10.3390/brainsci14070714
APA StyleAshaie, S. A., Hernandez-Pavon, J. C., Houldin, E., & Cherney, L. R. (2024). Behavioral, Functional Imaging, and Neurophysiological Outcomes of Transcranial Direct Current Stimulation and Speech-Language Therapy in an Individual with Aphasia. Brain Sciences, 14(7), 714. https://doi.org/10.3390/brainsci14070714






