Brain Magnetic Resonance Imaging in Wilson’s Disease—Significance and Practical Aspects—A Narrative Review
Abstract
:1. Introduction
2. Materials and Methods
3. Brain MRI in WD—Sequences and Its Significance
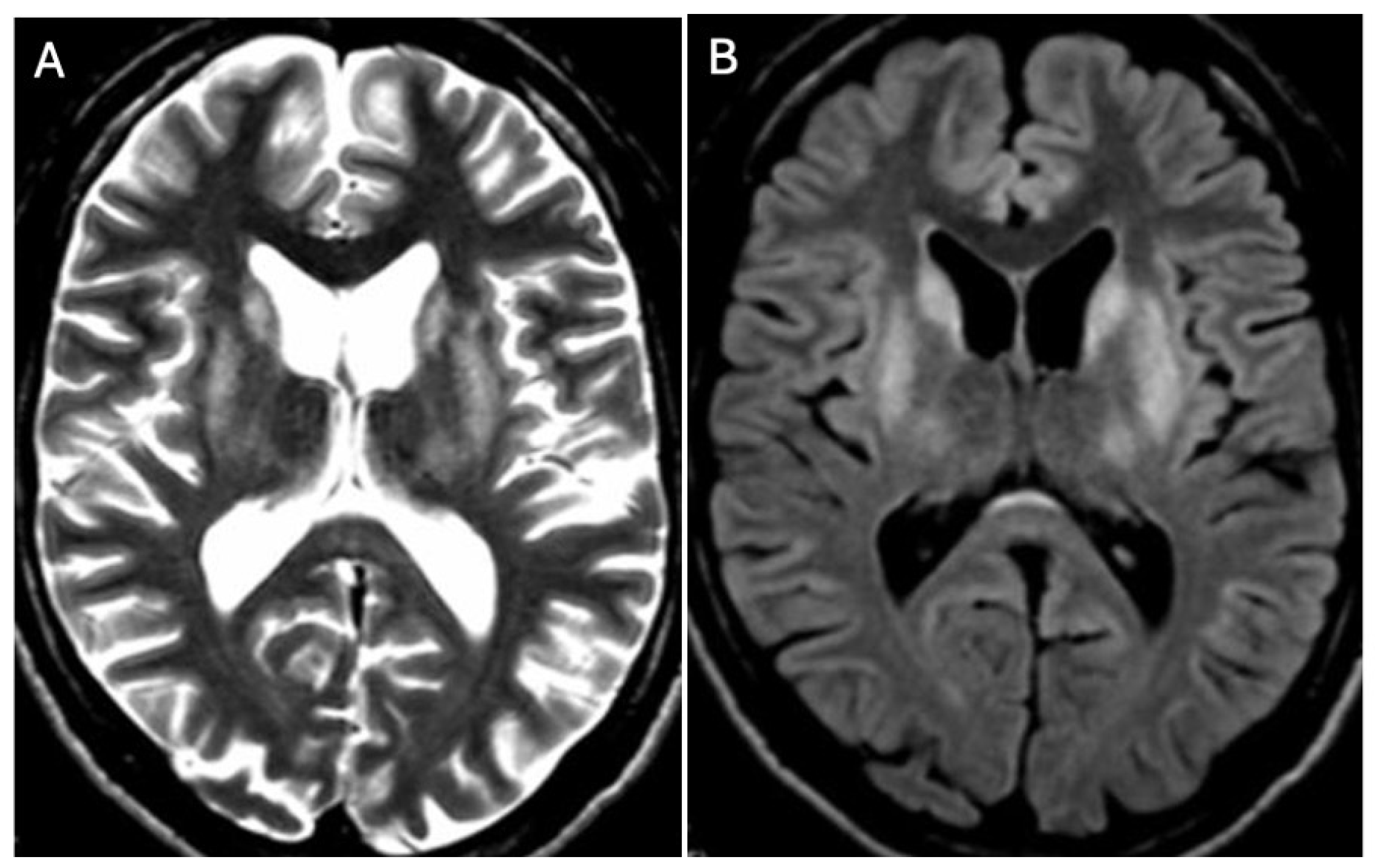
3.1. Classical Brain MRI Examination in WD Patients
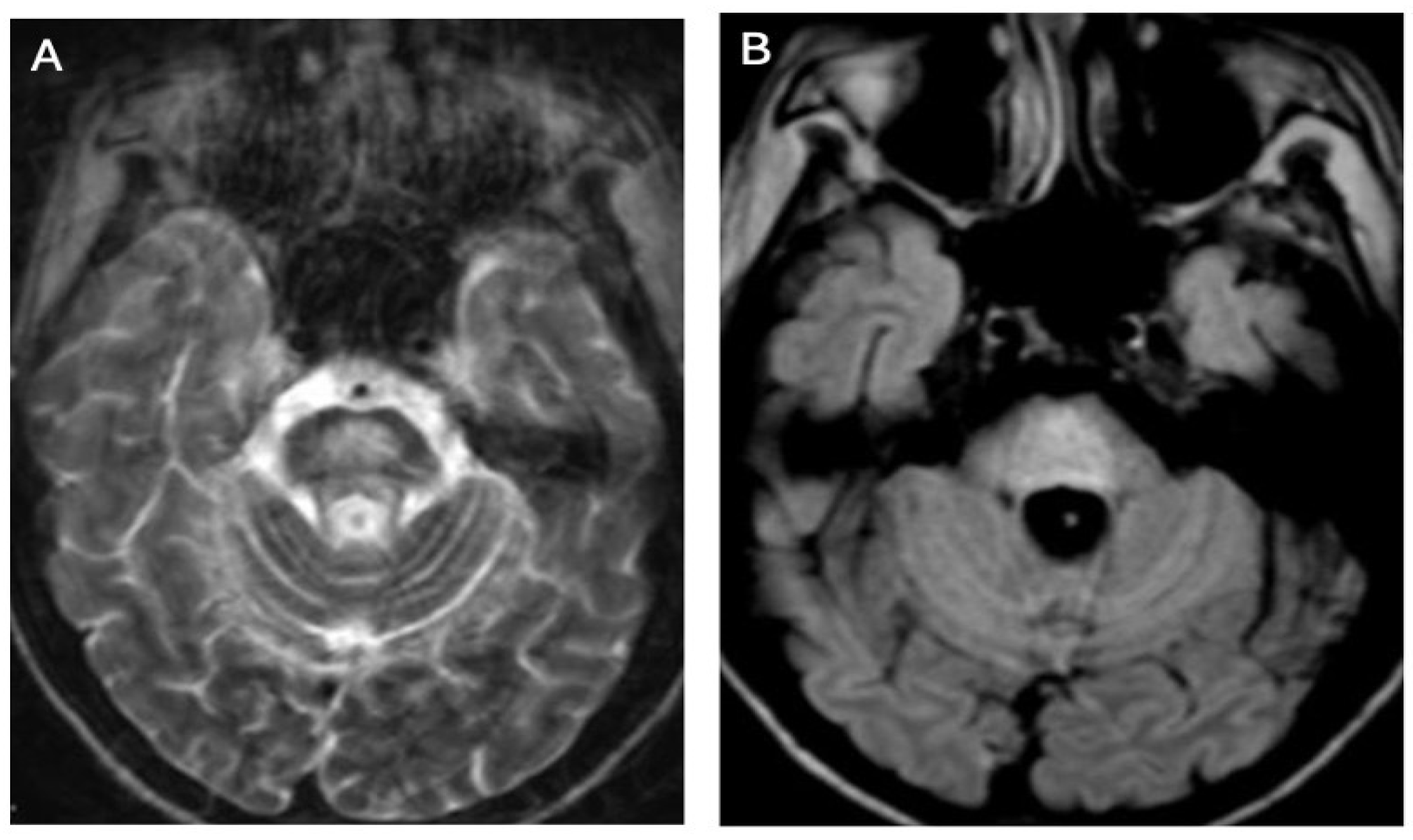
3.2. Diffusion MRI in WD
3.3. Diffusion Tensor Imaging (DTI)
3.4. Brain Iron Accumulation in WD (SWI, T2*, and QSM)
3.5. Volumetric Studies in WD
4. Brain MRI Scales in WD
Brain MRI Scoring Systems in WD
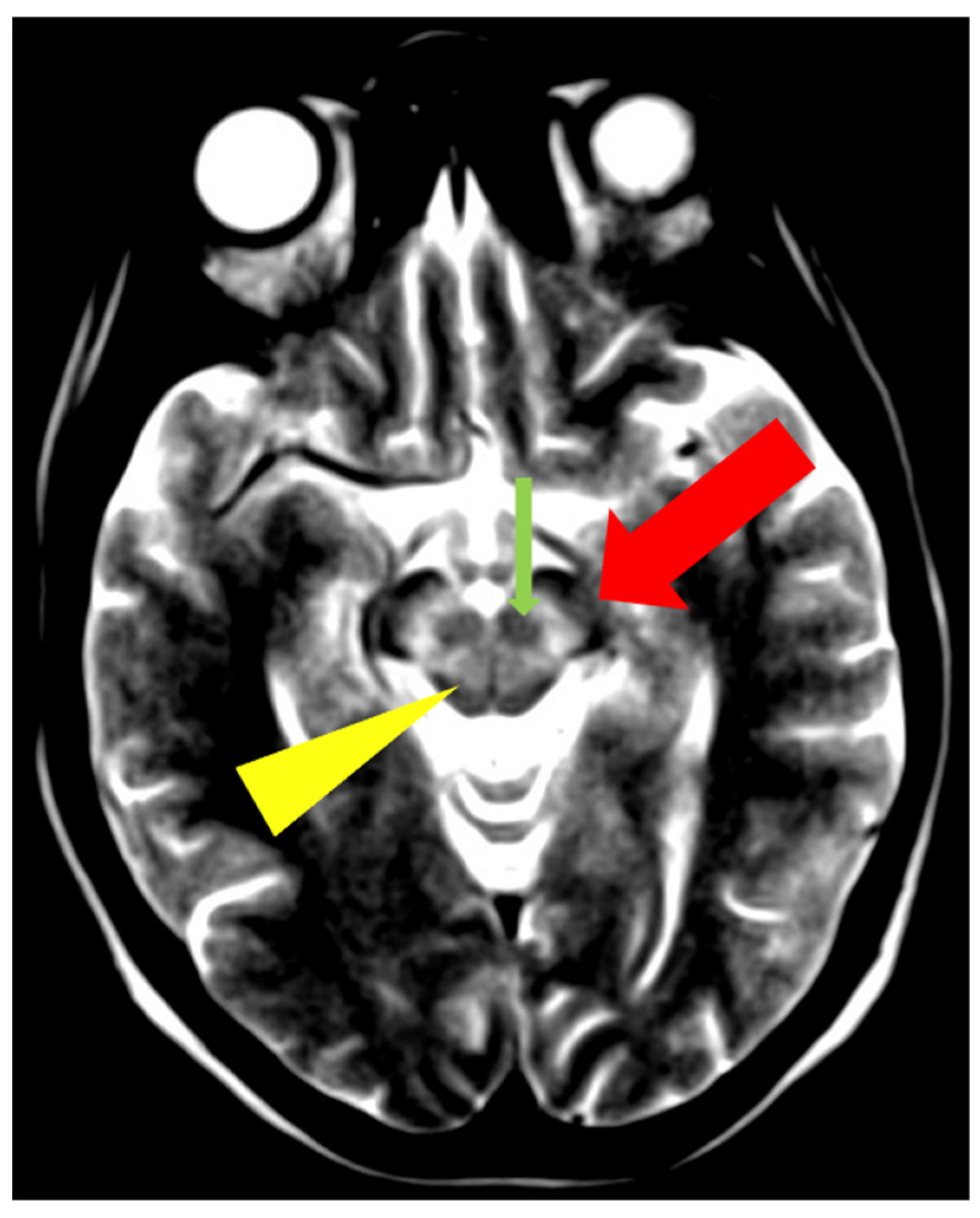
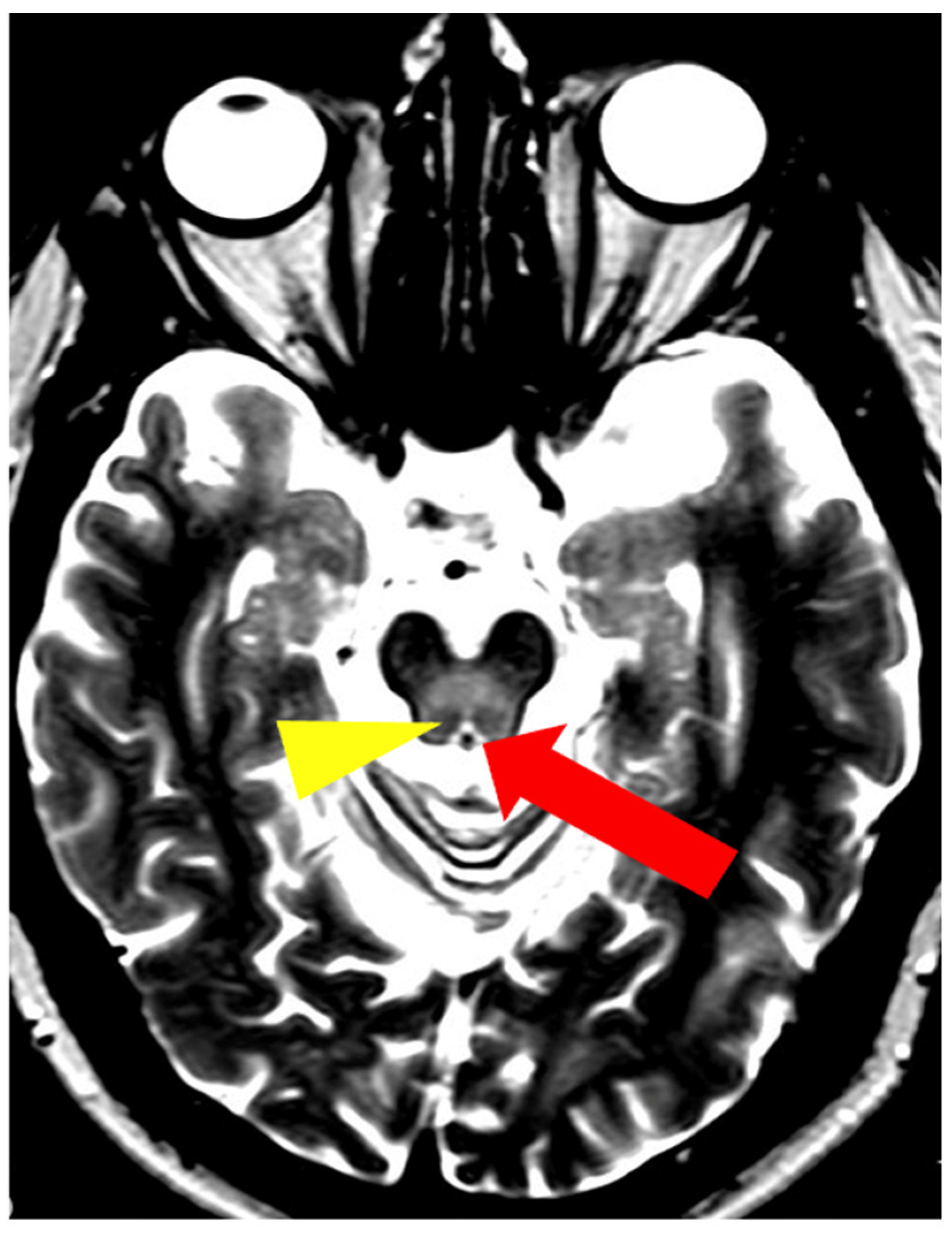
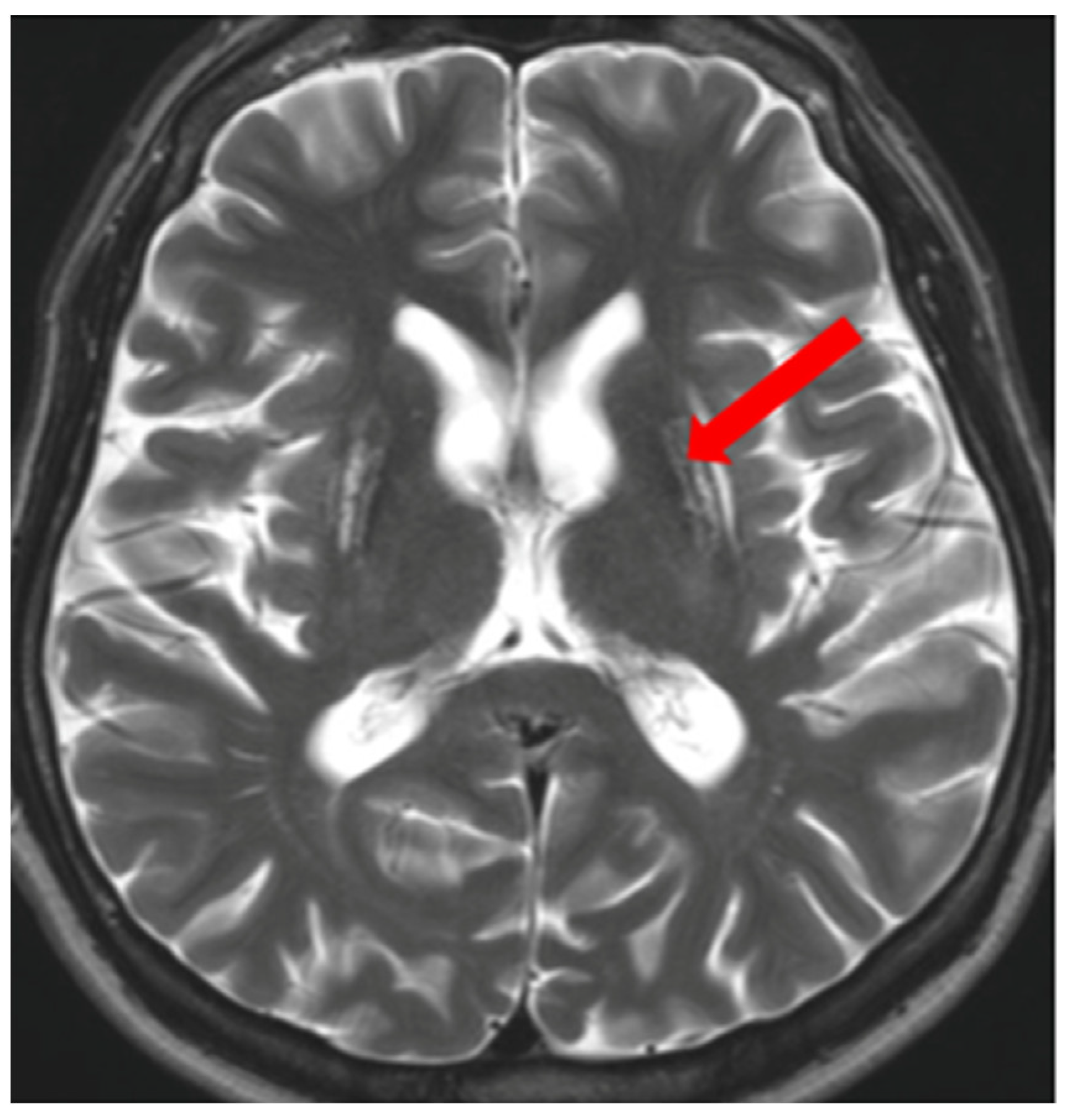
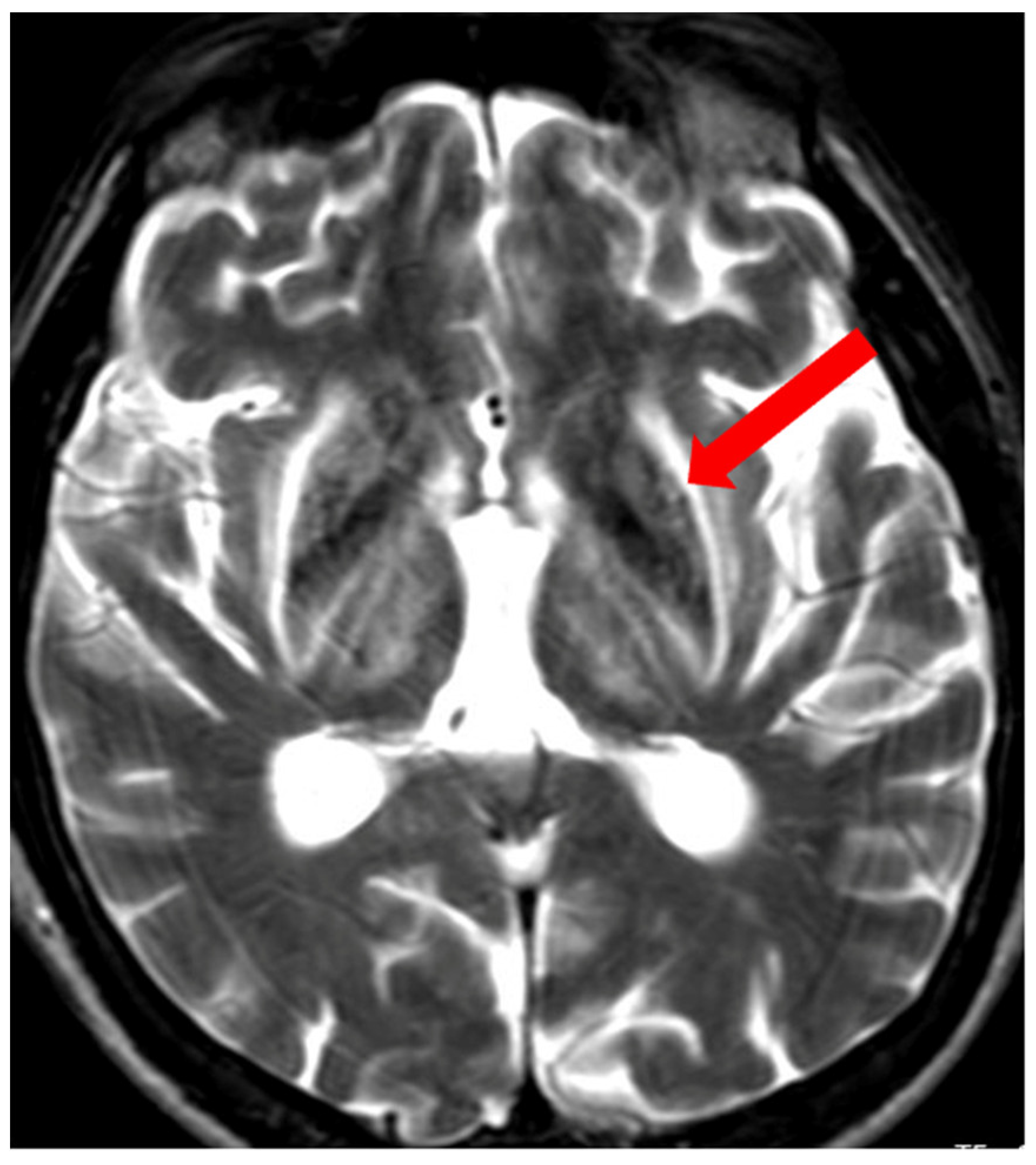
5. Neuroradiological Pathognomonic Signs of WD
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Association for Study of Liver. EASL Clinical Practice Guidelines: Wilson’s disease. J. Hepatol. 2012, 56, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Walshe, J.M. History of Wilson disease. Mov. Disord. 2006, 21, 2. [Google Scholar] [CrossRef]
- Schilsky, M.L.; Roberts, E.A.; Bronstein, J.M.; Dhawan, A.; Hamilton, J.P.; Rivard, A.M.; Washington, M.K.; Weiss, K.H.; Zimbrean, P.C. A multidisciplinary approach to the diagnosis and management of Wilson disease: Executive summary of the 2022 Practice Guidance on Wilson disease from the American Association for the Study of Liver Disease. Hepatology 2023, 77, 1428–1455. [Google Scholar] [CrossRef] [PubMed]
- Członkowska, A.; Litwin, T. Wilson disease—Currently used anticopper therapy. In Handbook of Clinical Neurology, Wilson Disease; Członkowska, A., Schilsky, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 142, pp. 181–191. [Google Scholar] [CrossRef]
- Ala, A.; Walker, A.P.; Ashkan, K.; Dooley, J.S.; Schilsky, M.L. Wilson’s disease. Lancet 2007, 369, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Antos, A.; Członkowska, A.; Bembenek, J.; Skowrońska, M.; Kurkowska-Jastrzębska, I.; Litwin, T. Blood based biomarkers of central nervous system involvement in Wilson’s disease. Diagnostics 2023, 13, 1554. [Google Scholar] [CrossRef] [PubMed]
- Belz, E.E.; Mullins, M.E. Radiological reasoning: Hyperintensity of the basal ganglia and cortex on FLAIR and diffusion-weighted imaging. AJR Am. J. Roentgenol. 2010, 195, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Bruha, R.; Marecek, Z.; Pospisilova, L.; Nevsimalova, S.; Vitek, L.; Martasek, P.; Nevoral, J.; Petrtyl, J.; Urbanek, P.; Jiraskova, A.; et al. Long-term follow-up of Wilson disease: Natural history, treatment, mutations analysis and phenotypic correlation. Liver Int. 2011, 31, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Członkowska, A.; Litwin, T.; Chabik, G. Wilson disease: Neurological features. In Handbook of Clinical Neurology, Wilson Disease; Członkowska, A., Schilsky, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 142, pp. 101–119. [Google Scholar] [CrossRef]
- Członkowska, A.; Litwin, T.; Dušek, P.; Ferenci, P.; Lutsenko, S.; Medici, V.; Rybakowski, J.K.; Weiss, K.H.; Schilsky, M.L. Wilson disease. Nat. Rev. Dis. Prim. 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Denny-Brown, D. Hepatolenticular degeneration (Wilson disease). Two different components. N. Engl. J. Med. 1964, 22, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- King, A.D.; Walshe, J.M.; Kendall, B.E.; Chinn, R.J.; Paley, M.N.; Wilkinson, I.D.; Halligan, S.; Hall-Craggs, M.A. Cranial MR imaging in Wilson’s disease. AJR Am. J. Roentgenol. 1996, 167, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Prayer, L.; Wimberger, D.; Kramer, J.; Grimm, G.; Oder, W.; Imhof, H. Cranial MRI in Wilson’s disease. Neuroradiology 1990, 32, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Van Wassenaer-van Hall, H.N.; Van den Heuvel, A.G.; Jansen, G.H.; Hoogenraad, T.U.; Mali, W.P. Cranial MR in Wilson disease: Abnormal white matter in extrapyramidal and pyramidal tracts. AJNR Am. J. Neuroradiol. 1995, 16, 2021–2027. [Google Scholar] [PubMed]
- Sinha, S.; Taly, A.B.; Prashanth, L.K.; Ravishankar, S.; Arunodaya, G.R.; Vasudev, M.K. Sequential MRI changes in Wilson’s disease with de-coppering therapy: A study of 50 patients. Br. J. Radiol. 2007, 80, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Taly, A.B.; Ravishankar, S.; Prashanth, L.K.; Venugopal, K.S.; Arunodaya, G.R.; Vasudev, M.K.; Swamy, H.S. Wilson’s disease: Cranial MRI observations and clinical correlation. Neuroradiology 2006, 48, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Svetel, M.; Kozić, D.; Stefanova, E.; Semnic, R.; Dragasević, N.; Kostić, V.S. Dystonia in Wilson’s Disease. Mov. Disord. 2001, 16, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Poujois, A.; Mikol, J.; Woimant, F. Wilson disease: Brain pathology. In Handbook of Clinical Neurology, Wilson Disease; Członkowska, A., Schilsky, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 142, pp. 77–89. [Google Scholar] [CrossRef]
- Prashanth, L.K.; Taly, A.B.; Sinha, S.; Ravishankar, S.; Arunodaya, G.R.; Vasudev, M.K.; Swamy, H.S. Prognostic factors in patients presenting with severe neurological forms of Wilson’s disease. Neurol. India 2005, 53, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.; Pinto, C.; Freixo, P.; Alves, H.; Ramos, C.; Santos Silva, E.; Nery, F.; Gandara, J.; Lopes, V.; Ferreira, S.; et al. Correlation between neuroimaging, neurological phenotype and functional outcomes in Wilson’s disease. Neurol. Sci. 2024, 45, 3201–3208. [Google Scholar] [CrossRef]
- Kalita, J.; Kumar, V.; Parashar, V.; Misra, U.K. Neuropsychiatric manifestations of Wilson disease: Correlation with MRI and glutamate excitotoxicity. Mol. Neurobiol. 2021, 58, 6020–6031. [Google Scholar] [CrossRef] [PubMed]
- Litwin, T.; Gromadzka, G.; Członkowska, A.; Gołębiowski, M.; Poniatowska, R. The effect of gender on brain MRI pathology in Wilson’s disease. Metab. Brain Dis. 2013, 28, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Ferenci, P. Diagnosis of Wilson disease. In Handbook of Clinical Neurology, Wilson Disease; Członkowska, A., Schilsky, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 142, pp. 171–180. [Google Scholar] [CrossRef]
- Litwin, T.; Dzieżyc, K.; Karliński, M.; Chabik, G.; Czepiel, W.; Członkowska, A. Early neurological worsening in patients with Wilson’s disease. J. Neurol. Sci. 2015, 355, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi-Sundaram, S.; Mahadevan, A.; Taly, A.B.; Arunodaya, G.R.; Swamy, H.S.; Shankar, S.K. Wilson’s disease: A clinico-neuropathological autopsy study. J. Neurosci. 2008, 15, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Mikol, J.; Vital, C.; Wassef, M.; Chappuis, P.; Poupon, J.; Lecharpentier, M.; Woimant, F. Extensive cortico-subcortical lesions in Wilson’s disease: Clinicopathological study of two cases. Acta Neuropathol. 2005, 110, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, A.C.; Caramelli, P.; Menezes, J.R.; Lo, L.S.; Bacheschi, L.A.; Barbosa, E.R.; Rosemberg, L.A.; Magalhaes, A. Wilson disease: MRI with clinical correlation. Neuroradiology 1994, 36, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Page, A.R.; Davie, C.A.; MacManus, D.; Miszkiel, K.A.; Walshe, J.M.; Miller, D.H.; Lees, A.J.; Schapira, A.H. Clinical correlation of brain MRI and MRS abnormalities in patients with Wilson disease. Neurology 2004, 63, 638–643. [Google Scholar] [CrossRef]
- Van Cauter, S.; Severino, M.; Ammendola, R.; Van Berkel, B.; Vavro, H.; van den Hauwe, L.; Rumboldt, Z. Bilateral lesions of the basal ganglia and thalami (central grey matter)-pictorial review. Neuroradiology 2020, 62, 1565–1605. [Google Scholar] [CrossRef] [PubMed]
- Winklewski, P.J.; Sabisz, A.; Naumczyk, P.; Jodzio, K.; Szurowska, E.; Szarmach, A. Understanding the Physiopathology Behind Axial and Radial Diffusivity Changes—What Do We Know? Front. Neurol. 2018, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Figley, C.R.; Uddin, M.N.; Wong, K.; Kornelsen, J.; Puig, J.; Figley, T.D. Potential Pitfalls of Using Fractional Anisotropy, Axial Diffusivity, and Radial Diffusivity as Biomarkers of Cerebral White Matter Microstructure. Front. Neurosci. 2022, 14, 799576. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D. Looking into the functional architecture of the brain with diffusion MRI. Nat. Rev. Neurosci. 2003, 4, 469–480. [Google Scholar] [CrossRef]
- Jadav, R.; Saini, J.; Sinha, S.; Bagepally, B.; Rao, S.; Taly, A.B. Diffusion tensor imaging (DTI) and its clinical correlates in drug naïve Wilson’s disease. Metab. Brain Dis. 2013, 28, 455–462. [Google Scholar] [CrossRef]
- Sener, R.N. Wilson’s disease: MRI demonstration of cavitations in basal ganglia and thalami. Pediatr. Radiol. 1993, 23, 157. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.E.; Gao, S.; Yang, R.M.; Han, Y.Z. MR imaging of the brain in neurologic Wilson disease. AJNR Am. J. Neuroradiol. 2019, 40, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Favrole, P.; Chabriat, H.; Guichard, J.P.; Woimant, F. Clinical correlates of cerebral water diffusion in Wilson disease. Neurology 2006, 66, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Sener, R.N. Diffusion MRI findings in Wilson’s disease. Comput. Med. Imaging Graph. 2003, 27, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Saatci, I.; Topcu, M.; Baltaoglu, F.F.; Kose, G.; Yalaz, K.; Renda, Y.; Besim, A. Cranial MR findings in Wilson’s disease. Acta Radiol. 1997, 38, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Kizkin, S.; Sarac, K.; Ozisik, H.I.; Ozcan, C. Central pontine myelinolysis in Wilson’s disease: MR spectroscopy findings. Magn. Reson. Imaging 2004, 22, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Litwin, T.; Gromadzka, G.; Szpak, G.M.; Jabłonka-Salach, K.; Bulska, E.; Członkowska, A. Brain metal accumulation in Wilson’s disease. J. Neurol. Sci. 2013, 329, 55–58. [Google Scholar] [CrossRef]
- Medici, V.; Rossaro, L.; Sturniolo, G.C. Wilson disease—A practical approach to diagnosis, treatment and follow-up. Dig. Liver Dis. 2007, 39, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Kozić, D.B.; Petrović, I.; Svetel, M.; Pekmezović, T.; Ragaji, A.; Kostić, V.S. Reversible lesions in the brain parenchyma in Wilson’s disease confirmed by magnetic resonance imaging: Earlier administration of chelating therapy can reduce the damage to the brain. Neural Regen. Res. 2014, 9, 1912–1916. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; Mazziotta, J.C.; Phelps, M.E. Wilson’s disease studied with FDG and positron emission tomography. Neurology 1987, 37, 1707–1711. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Yano, M.; Fujita, Y.; Wakusawa, S. Compound overload of copper and iron in patients with Wilson’s disease. Med. Mol. Morphol. 2006, 39, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ho, V.B.; Fitz, C.R.; Chuang, S.H.; Geyer, C.A. Bilateral basal ganglia lesions: Pediatric differential considerations. Radiographics 1993, 13, 269–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Kim, I.O.; Kim, W.S.; Cheon, J.E.; Moon, S.G.; Kwon, J.W.; Seo, J.K.; Yeon, K.M. MR imaging of the brain in Wilson disease of childhood: Findings before and after treatment with clinical correlation. AJNR Am. J. Neuroradiol. 2006, 27, 1373–1378. [Google Scholar] [PubMed]
- Klos, K.J.; Ahlskog, J.E.; Kumar, N.; Cambern, S.; Butz, J.; Burritt, M.; Fealey, R.D.; Cowl, C.T.; Parisi, J.E.; Josephs, K.A. Brain metal concentrations in chronic liver failure patients with pallidal T1 MRI hyperintensity. Neurology 2006, 67, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Dušek, P.; Bahn, E.; Litwin, T.; Jabłonka-Salach, K.; Łuciuk, A.; Huelnhagen, T.; Madai, V.I.; Dieringer, M.A.; Bulska, E.; Knauth, M.; et al. Brain iron accumulation in Wilson disease: A post mortem 7 Tesla MRI—Histopathological study. Neuropathol. Appl. Neurobiol. 2017, 43, 514–532. [Google Scholar] [CrossRef] [PubMed]
- Dušek, P.; Lescinskij, A.; Ruzicka, F.; Acosta-Cabronero, J.; Bruha, R.; Sieger, T.; Hajek, M.; Dezortova, M. Associations of Brain Atrophy and Cerebral Iron Accumulation at MRI with Clinical Severity in Wilson Disease. Radiology 2021, 299, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Svetel, M.; Potrebić, A.; Pekmezović, T.; Tomić, A.; Kresojević, N.; Jesić, R.; Dragasević, N.; Kostić, V.S. Neuropsychiatric aspects of treated Wilson’s disease. Park. Relat. Disord. 2009, 15, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Pulai, S.; Biswas, A.; Roy, A.; Guin, D.S.; Pandit, A.; Gangopadhyay, G.; Ghorai, P.K.; Sarkhel, S.; Senapati, A.K. Clinical features, MRI brain, and MRS abnormalities of drug-naive neurologic Wilson’s disease. Neurol. India 2014, 62, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.K.; Lee, T.G.; Wie, B.A.; Lee, S.B.; Park, S.H.; Chang, K.H. Initial and follow-up brain MRI findings and correlation with the clinical course in Wilson’s disease. Neurology 1994, 44, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Dušek, P.; Smolinski, Ł.; Redzia-Ogrodnik, B.; Gołębiowski, M.; Skowrońska, M.; Poujois, A.; Laurencin, C.; Jastrzębska-Kurkowska, I.; Litwin, T.; Członkowska, A. Semiquantitative Scale for Assessing Brain MRI Abnormalities in Wilson Disease: A Validation Study. Mov. Disord. 2020, 35, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Song, Y.; Zhou, X.; Chu, J.; Tang, X. Regional morphometric abnormalities and clinical relevance in Wilson’s disease. Mov. Disord. 2019, 34, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Shribman, S.; Bocchetta, M.; Sudre, C.H.; Acosta-Cabronero, J.; Burrows, M.; Cook, P.; Thomas, D.L.; Gillett, G.T.; Tsochatzis, E.A.; Bandmann, O.; et al. Neuroimaging correlates of brain injury in Wilson’s disease: A multimodal, whole brain MRI study. Brain 2022, 145, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Smoliński, Ł.; Ziemssen, T.; Akgun, K.; Antos, A.; Skowrońska, M.; Kurkowska-Jastrzębska, I.; Członkowska, A.; Litwin, T. Brain Atrophy Is Substantially Accelerated in Neurological Wilson’s Disease: A Longitudinal Study. Mov. Disord. 2022, 37, 2446–2451. [Google Scholar] [CrossRef] [PubMed]
- Diao, S.P.; Lu, C.X.; Huang, Y.Q.; Zhou, Z.H.; Liu, A.Q.; Hong, M.F. Linear structural features of Wilson’s disease and its correlation with neurological symptoms. Medicine 2022, 101, e31703. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Mohammadi, S.; Salehi, M.A.; Dager, S.R. Brain microstructural abnormalities in patients with Wilson’s disease: A systematic review of diffusion tensor imaging studies. Brain Imaging Behav. 2022, 16, 2809–2840. [Google Scholar] [CrossRef] [PubMed]
- Smoliński, Ł.; Litwin, T.; Rędzia-Ogrodnik, B.; Dzieżyc, K.; Kurkowska-Jastrzębska, I.; Członkowska, A. Brain volume is related to neurological impairment and to copper overload in Wilson’s disease. Neurol. Sci. 2019, 40, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Tinaz, S.; Arora, J.; Nalamada, K.; Vives-Rodriguez, A.; Sezgin, M.; Robakis, D.; Patel, A.; Constable, R.T.; Schilsky, M.L. Structural and functional brain changes in hepatic and neurological Wilson disease. Brain Imaging Behav. 2021, 15, 2269–2282. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zhang, J.; Yang, J.; Wang, Z.; Pan, Z.; Yue, X.; Zhao, R.; Qian, Y.; Yu, Y.; Li, X. Changes in brain susceptibility in Wilson’s disease patients: A quantitative susceptibility mapping study. Clin. Radiol. 2024, 79, e282–e286. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.Z.; Yuan, X.Z.; Li, G.Y.; Chen, J.L.; Wu, R.; Yang, L.L.; Zhang, S.Y.; Wang, X.P.; Li, J.Q. Increased magnetic susceptibility in the deep gray matter nuclei of Wilson’s disease: Have we been ignoring atrophy? Front. Neurosci. 2022, 16, 794375. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.-Z.; Li, G.Y.; Wu, Y.P.; Yuan, X.Z.; Luo, X.G.; Chen, J.L.; Taximaimaiti, R.; Wang, X.P.; Li, J.Q. Free water imaging as a novel biomarker in Wilson’s disease: A cross-sectional study. Park. Relat. Disord. 2023, 106, 105234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Schneider, T.; Wheeler-Kingshott, C.A.; Alexander, D.C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012, 61, 1000–1016. [Google Scholar] [CrossRef] [PubMed]
- Zaino, D.; Chiarotti, I.; Battisti, C.; Salvatore, S.; Federico, A.; Cerase, A. Six-year clinical and MRI quantitative susceptibility mapping (QSM) follow-up in neurological Wilson’s disease under zinc therapy: A case report. Neurol. Sci. 2019, 40, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Poujois, A.; Trocello, J.M.; Djebrani-Oussedik, N.; Poupon, J.; Collet, C.; Girardot-Tinant, N.; Sobesky, R.; Habes, D.; Debray, D.; Vanlemmens, C.; et al. Exchangeable copper: A reflection of the neurological severity in Wilson’s disease. Eur. J. Neurol. 2017, 24, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Litwin, T.; Rędzia-Ogrodnik, B.; Skowrońska, M.; Smoliński, Ł.; Antos, A.; Kraśniej-Dębkowska, A.; Kurkowska-Jastrzębska, I.; Członkowska, A. Brain magnetic resonance imaging and severity of neurological disease in Wilson’s disease. Park. Relat. Disord. 2020, 79, e97. [Google Scholar] [CrossRef]
- Rędzia-Ogrodnik, B.; Członkowska, A.; Bembenek, J.; Antos, A.; Kurkowska-Jastrzębska, I.; Skowrońska, M.; Smoliński, Ł.; Litwin, T. Brain magnetic resonance imaging and severity of neurological disease in Wilson’s disease—The neuroradiological correlations. Neurol. Sci. 2022, 43, 4405–4412. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, T.; Smoliński, Ł.; Członkowska, A.; Akgun, K.; Antos, A.; Bembenek, J.; Kurkowska-Jastrzębska, I.; Przybyłkowski, A.; Skowrońska, M.; Redzia-Ogrodnik, B.; et al. Serum neurofilament light chain and initial severity of neurological disease predict the early neurological deterioration in Wilson’s disease. Acta Neurol. Belg. 2023, 123, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Geng, H.; Cheng, S.R.; Xu, C.C.; Zhang, R.Q.; Wang, Y.; Wu, T.; Li, B.; Wang, T.; Han, Y.S.; et al. A weighted cranial diffusion-weighted imaging scale for Wilson’s disease. Front. Neurosci. 2023, 17, 1186053. [Google Scholar] [CrossRef] [PubMed]
- Rędzia-Ogrodnik, B.; Członkowska, A.; Antos, A.; Bembenek, J.; Kurkowska-Jastrzębska, I.; Przybyłkowski, A.; Skowrońska, M.; Smoliński, Ł.; Litwin, T. Pathognomonic neuroradiological signs in Wilson’s disease—Truth or myth? Park. Relat. Disord. 2023, 107, 105247. [Google Scholar] [CrossRef] [PubMed]
- Hitoshi, S.; Iwata, M.; Yoshikawa, K. Mid-brain pathology of Wilson’s disease: MRI analysis of three cases. J. Neurol. Neurosurg. Psychiatry 1991, 54, 624–626. [Google Scholar] [CrossRef] [PubMed]
- George, U.; Varte, N.; Rathore, S.; Jain, V.; Goyal, S. “Split thalamus”: Internal medullary involvement in Wilson’s disease. Neurol. India 2010, 58, 680. [Google Scholar] [CrossRef] [PubMed]
- Sener, R.N. The claustrum on MRI: Normal anatomy, and the bright claustrum as a new sign in Wilson’s disease. Pediatr. Radiol. 1993, 23, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Zhang, Z.; Zhang, Z.; Zheng, S.; Yao, T.; Dong, Y.; Zhu, W.; Wei, N.; Suo, Y.; Liu, X.; et al. Distinctive Pattern of Metal Deposition in Neurologic Wilson Disease: Insights From 7T Susceptibility-Weighted Imaging. Neurology 2024, 102, e209478. [Google Scholar] [CrossRef]
- Prashanth, L.K.; Sinha, S.; Taly, A.B.; Vasudev, M.K. Do MRI features distinguish Wilson’s disease from other early onset extrapyramidal disorders? An analysis of 100 cases. Mov. Disord. 2010, 25, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Vella, S.; Grech, R. Highlighting an atypical cause of the Face of the Giant Panda sign. BJR Case Rep. 2018, 4, 20170046. [Google Scholar] [CrossRef] [PubMed]
- Sonam, K.; Bindu, P.S.; Gayathri, N.; Khan, N.A.; Govindaraju, C.; Arvinda, H.R.; Nagappa, M.; Sinha, S.; Thangaraj, K.; Taly, A.B. The “double panda” sign in Leigh disease. J. Child. Neurol. 2014, 29, 980–982. [Google Scholar] [CrossRef] [PubMed]
- Litwin, T.; Dziezyc, K.; Poniatowska, R.; Czlonkowska, A. Effect of liver transplantation on brain magnetic resonance imaging pathology in Wilson’s disease: A case report. Neurol. Neurochir. Pol. 2013, 47, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Litwin, T.; Antos, A.; Bembenek, J.; Przybyłkowski, A.; Kurkowska-Jastrzębska, I.; Skowrońska, M.; Członkowska, A. Copper deficiency as Wilson’s disease overteratment: A systematic review. Diagnostics 2023, 13, 2424. [Google Scholar] [CrossRef] [PubMed]
- Gasparotti, R.; Pinelli, L.; Liserre, R. New MR sequences in daily practice: Susceptibility weighted imaging. A pictorial essay. Insights Imaging 2011, 2, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Imakita, S.; Sakuma, T.; Takamiya, M. Intracranial calcification on gradient-echo phase image: Depiction of diamagnetic susceptibility. Radiology 1996, 198, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, J.R.; Schweser, F.; Serres, B.; Deistung, A. Quantitative Susceptibility Mapping: Concepts and Applications. Clin. Neuroradiol. 2015, 25, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, T. Quantitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker. Magn. Reson. Med. 2015, 73, 82–101. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, A.D.; Liu, T.; Gupta, A.; Zheng, K.; Seedial, S.; Shtilbans, A.; Shahbazi, M.; Lange, D.; Wang, Y.; Tsiouris, A.J. Quantitative susceptibility mapping of the motor cortex in amyotrophic lateral sclerosis and primary lateral sclerosis. Am. J. Roentgenol. 2015, 204, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Langkammer, C.; Schweser, F.; Shmueli, K.; Kames, C.; Li, X.; Guo, L.; Milovic, C.; Kim, J.; Wei, H.; Bredies, K.; et al. Quantitative susceptibility mapping: Report from the 2016 reconstruction challenge. Magn. Reson. Med. 2018, 79, 1661–1673. [Google Scholar] [CrossRef] [PubMed]





| Normal/Absent | Mild/Moderate | Severe | |
|---|---|---|---|
| Acute toxicity score (evaluated as hyperintensity on T2-weighted and FLAIR sequences) | |||
| Putamen | 0 | 1 | 2 |
| Caudate nucleus | 0 | 1 | 2 |
| Thalamus | 0 | 1 | 2 |
| Mesencephalon | 0 | 1 | 2 |
| Pons | 0 | 1 | 2 |
| Other areas (sepcify) | 0 | 1 | 2 |
| Chronic damage score (evaluated as hypointensity on T2/T2*/SWI sequences) | |||
| Globus pallidus | 0 | 1 | |
| Putamen | 0 | 1 | |
| Caudate nucleus | 0 | 1 | |
| Thalamus | 0 | 1 | |
| Dentate nucleus | 0 | 1 | |
| Atrophy (assessed on T1 sequences) | |||
| Cortical | 0 | 1 | 2 |
| Central | 0 | 1 | 2 |
| Midbrain | 0 | 1 | 2 |
| Cerebellar | 0 | 1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litwin, T.; Rędzia-Ogrodnik, B.; Antos, A.; Przybyłkowski, A.; Członkowska, A.; Bembenek, J.P. Brain Magnetic Resonance Imaging in Wilson’s Disease—Significance and Practical Aspects—A Narrative Review. Brain Sci. 2024, 14, 727. https://doi.org/10.3390/brainsci14070727
Litwin T, Rędzia-Ogrodnik B, Antos A, Przybyłkowski A, Członkowska A, Bembenek JP. Brain Magnetic Resonance Imaging in Wilson’s Disease—Significance and Practical Aspects—A Narrative Review. Brain Sciences. 2024; 14(7):727. https://doi.org/10.3390/brainsci14070727
Chicago/Turabian StyleLitwin, Tomasz, Barbara Rędzia-Ogrodnik, Agnieszka Antos, Adam Przybyłkowski, Anna Członkowska, and Jan Paweł Bembenek. 2024. "Brain Magnetic Resonance Imaging in Wilson’s Disease—Significance and Practical Aspects—A Narrative Review" Brain Sciences 14, no. 7: 727. https://doi.org/10.3390/brainsci14070727







