Increased Risk for Clinically Significant Sleep Disturbances in Mild Traumatic Brain Injury: An Approach to Leveraging the Federal Interagency Traumatic Brain Injury Research Database
Abstract
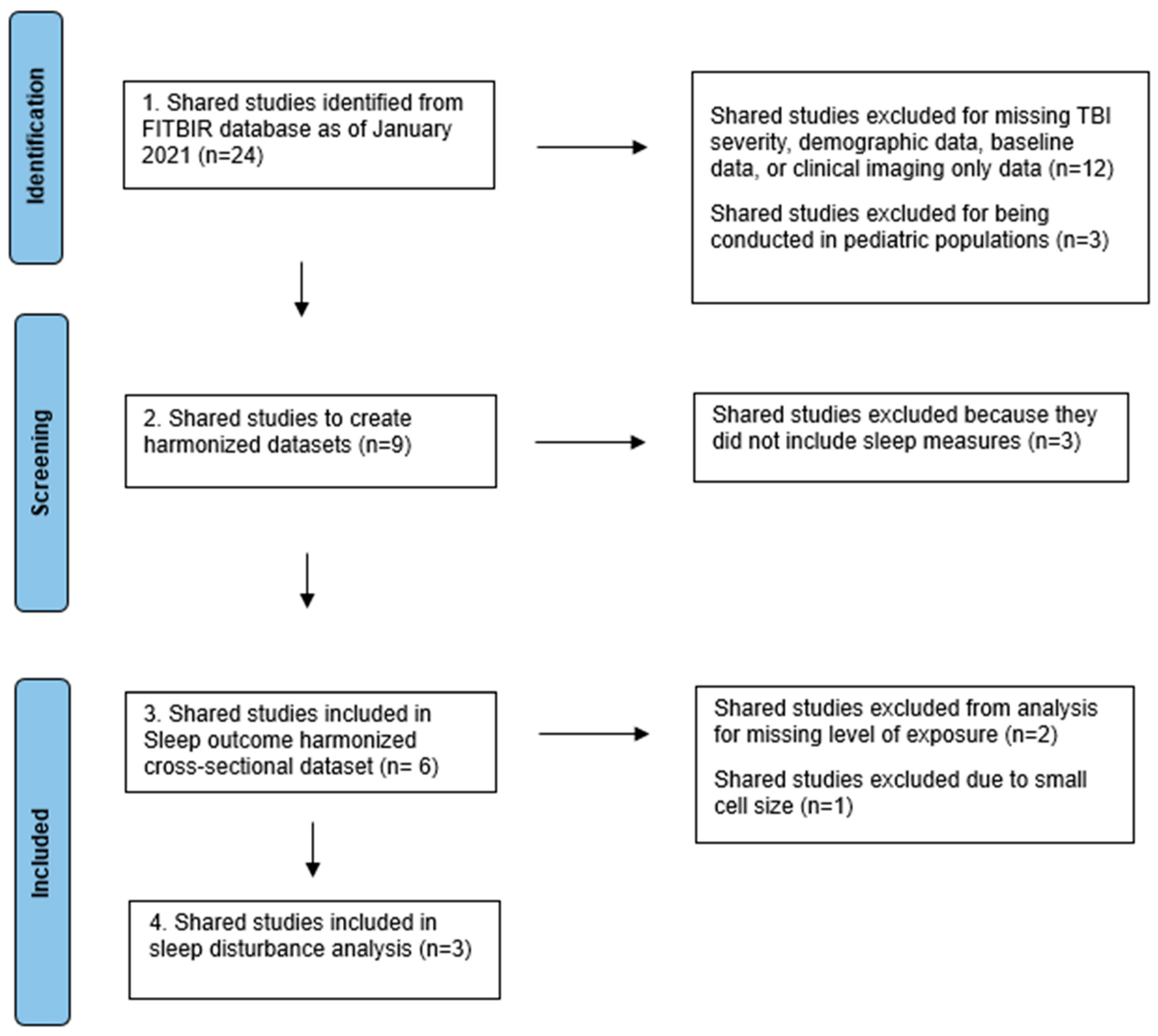
:1. Introduction
1.1. Addressing Methodological Limitations in the Extant Literature
1.2. Objectives
- Aim 1: Develop a model system for harmonizing data for key variables across FITBIR study datasets resulting in an integrated database containing data for participants with and without a history of TBI.
- Aim 2: Use merged datasets to estimate rates of sleep disturbance and identify outcome risk factors.
- Aim 3: Develop and share the methodologic products (e.g., the code and quantitative analysis syntax) created for this research project that can be used to facilitate more rapid synthesis of FITBIR data in the future as more studies are added to the publicly available database.
2. Methods
2.1. Study Approval
2.2. Study Inclusion/Exclusion Criteria
2.3. Measures
2.4. Data Analysis
3. Results
4. Discussion
Strengths/Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grima, N.; Ponsford, J.; Rajaratnam, S.M.; Mansfield, D.; Pase, M.P. Sleep Disturbances in Traumatic Brain Injury: A Meta-Analysis. J. Clin. Sleep Med. 2016, 12, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Zuzuárregui, J.R.P.; Bickart, K.; Kutscher, S.J. A Review of Sleep Disturbances Following Traumatic Brain Injury. Sleep Sci. Pract. 2018, 2, 2. [Google Scholar] [CrossRef]
- Nakase-Richardson, R.; Schwartz, D.J.; Drasher-Phillips, L.; Ketchum, J.M.; Calero, K.; Dahdah, M.N.; Monden, K.R.; Bell, K.; Magalang, U.; Hoffman, J.M.; et al. Comparative Effectiveness of Sleep Apnea Screening Instruments During Inpatient Rehabilitation Following Moderate to Severe TBI. Arch. Phys. Med. Rehabil. 2020, 101, 283–296. [Google Scholar] [CrossRef]
- Montgomery, M.C.; Baylan, S.; Gardani, M. Prevalence of Insomnia and Insomnia Symptoms Following Mild-Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2022, 61, 101563. [Google Scholar] [CrossRef]
- Noyes, E.T.; Tang, X.; Sander, A.M.; Silva, M.A.; Walker, W.C.; Finn, J.A.; Cooper, D.B.; Nakase-Richardson, R. Relationship of Medical Comorbidities to Psychological Health at 2 and 5 Years Following Traumatic Brain Injury (TBI). Rehabil. Psychol. 2021, 66, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Hammond, F.M.; Corrigan, J.D.; Ketchum, J.M.; Malec, J.F.; Dams-O’Connor, K.; Hart, T.; Novack, T.A.; Bogner, J.; Dahdah, M.N.; Whiteneck, G.G. Prevalence of Medical and Psychiatric Comorbidities Following Traumatic Brain Injury. J. Head Trauma Rehabil. 2019, 34, E1–E10. [Google Scholar] [CrossRef]
- Leng, Y.; Byers, A.L.; Barnes, D.E.; Peltz, C.B.; Li, Y.; Yaffe, K. Traumatic Brain Injury and Incidence Risk of Sleep Disorders in Nearly 200,000 US Veterans. Neurology 2021, 96, e1792–e1799. [Google Scholar] [CrossRef]
- Wickwire, E.M.; Schnyer, D.M.; Germain, A.; Williams, S.G.; Lettieri, C.J.; McKeon, A.B.; Scharf, S.M.; Stocker, R.; Albrecht, J.; Badjatia, N.; et al. Sleep, Sleep Disorders, and Circadian Health Following Mild Traumatic Brain Injury in Adults: Review and Research Agenda. J. Neurotrauma 2018, 35, 2615–2631. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; Yamakawa, G.R.; Shultz, S.R.; Mychasiuk, R. Is the Glymphatic System the Missing Link between Sleep Impairments and Neurological Disorders? Examining the Implications and Uncertainties. Prog. Neurobiol. 2021, 198, 101917. [Google Scholar] [CrossRef]
- Herrero Babiloni, A.; Baril, A.A.; Charlebois-Plante, C.; Jodoin, M.; Sanchez, E.; De Baets, L.; Arbour, C.; Lavigne, G.J.; Gosselin, N.; De Beaumont, L. The Putative Role of Neuroinflammation in the Interaction between Traumatic Brain Injuries, Sleep, Pain and Other Neuropsychiatric Outcomes: A State-of-the-Art Review. J. Clin. Med. 2023, 12, 1793. [Google Scholar] [CrossRef]
- Lowe, A.; Neligan, A.; Greenwood, R. Sleep Disturbance and Recovery during Rehabilitation after Traumatic Brain Injury: A Systematic Review. Disabil. Rehabil. 2020, 42, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Duclos, C.; Beauregard, M.P.; Bottari, C.; Ouellet, M.C.; Gosselin, N. The Impact of Poor Sleep on Cognition and Activities of Daily Living after Traumatic Brain Injury: A Review. Aust. Occup. Ther. J. 2015, 62, 2–12. [Google Scholar] [CrossRef]
- Holcomb, E.M.; Towns, S.; Kamper, J.E.; Barnett, S.D.; Sherer, M.; Evans, C.; Nakase-Richardson, R. The Relationship Between Sleep-Wake Cycle Disturbance and Trajectory of Cognitive Recovery During Acute Traumatic Brain Injury. J. Head Trauma Rehabil. 2016, 31, 108–116. [Google Scholar] [CrossRef]
- Wilde, M.C.; Castriotta, R.J.; Lai, J.M.; Atanasov, S.; Masel, B.E.; Kuna, S.T. Cognitive Impairment in Patients with Traumatic Brain Injury and Obstructive Sleep Apnea. Arch. Phys. Med. Rehabil. 2007, 88, 1284–1288. [Google Scholar] [CrossRef]
- Nakase-Richardson, R.; Sherer, M.; Barnett, S.D.; Yablon, S.A.; Evans, C.C.; Kretzmer, T.; Schwartz, D.J.; Modarres, M. Prospective Evaluation of the Nature, Course, and Impact of Acute Sleep Abnormality after Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2013, 94, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Sandsmark, D.K.; Kumar, M.A.; Woodward, C.S.; Schmitt, S.E.; Park, S.; Lim, M.M. Sleep Features on Continuous Electroencephalography Predict Rehabilitation Outcomes After Severe Traumatic Brain Injury. J. Head Trauma Rehabil. 2016, 31, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Nakase-Richardson, R.; Sherer, M.; Barnett, S.D.; Evans, C.C.; Yablon, S.A. Posttraumatic Confusion Predicts Patient Cooperation during Traumatic Brain Injury Rehabilitation. Am. J. Phys. Med. Rehabil. 2012, 91, 890–893. [Google Scholar] [CrossRef]
- Ponsford, J.; Schönberger, M.; Rajaratnam, S.M. A Model of Fatigue Following Traumatic Brain Injury. J. Head Trauma Rehabil. 2015, 30, 277–282. [Google Scholar] [CrossRef]
- Wickwire, E.M.; Albrecht, J.S.; Griffin, N.R.; Schnyer, D.M.; Yue, J.K.; Markowitz, A.J.; Okonkwo, D.O.; Valadka, A.B.; Badjatia, N.; Manley, G.T. Sleep Disturbances Precede Depressive Symptomatology Following Traumatic Brain Injury. Curr. Neurobiol. 2019, 10, 49–55. [Google Scholar]
- Martindale, S.L.; Konst, M.J.; Bateman, J.R.; Arena, A.; Rowland, J.A. The Role of PTSD and TBI in Post-Deployment Sleep Outcomes. Mil. Psychol. 2020, 32, 212–221. [Google Scholar] [CrossRef]
- Sullan, M.J.; Crocker, L.D.; Thomas, K.R.; Orff, H.J.; Davey, D.K.; Jurick, S.M.; Twamley, E.W.; Norman, S.B.; Schiehser, D.M.; Aupperle, R.; et al. Baseline Sleep Quality Moderates Symptom Improvement in Veterans with Comorbid PTSD and TBI Receiving Trauma-Focused Treatment. Behav. Res. Ther. 2021, 143, 103892. [Google Scholar] [CrossRef]
- Bomyea, J.; Lang, A.J.; Delano-Wood, L.; Jak, A.; Hanson, K.L.; Sorg, S.; Clark, A.L.; Schiehser, D.M. Neuropsychiatric Predictors of Post-Injury Headache After Mild-Moderate Traumatic Brain Injury in Veterans. Headache 2016, 56, 699–710. [Google Scholar] [CrossRef]
- Pattinson, C.L.; Brickell, T.A.; Bailie, J.; Hungerford, L.; Lippa, S.M.; French, L.M.; Lange, R.T. Sleep Disturbances Following Traumatic Brain Injury Are Associated with Poor Neurobehavioral Outcomes in US Military Service Members and Veterans. J. Clin. Sleep Med. 2021, 17, 2425–2438. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wen, Y.T.; Thompson, H.J.; Liu, C.Y.; Su, Y.K.; Chen, P.Y.; Chen, C.Y.; Chuang, Y.H.; Lin, Y.J.; Chen, C.T.; et al. Sleep Disturbances Following Traumatic Brain Injury in Older Adults: A Comparison Study. J. Head Trauma Rehabil. 2020, 35, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.A.; Edmed, S.L.; Allan, A.C.; Karlsson, L.J.; Smith, S.S. Characterizing Self-Reported Sleep Disturbance after Mild Traumatic Brain Injury. J. Neurotrauma 2015, 32, 474–486. [Google Scholar] [CrossRef]
- Albrecht, J.S.; Wickwire, E.M. Sleep Disturbances among Older Adults Following Traumatic Brain Injury. Int. Rev. Psychiatry 2020, 32, 31–38. [Google Scholar] [CrossRef]
- Rauchman, S.H.; Zubair, A.; Jacob, B.; Rauchman, D.; Pinkhasov, A.; Placantonakis, D.G.; Reiss, A.B. Traumatic Brain Injury: Mechanisms, Manifestations, and Visual Sequelae. Front. Neurosci. 2023, 17, 1090672. [Google Scholar] [CrossRef]
- Balba, N.M.; Elliott, J.E.; Weymann, K.B.; Opel, R.A.; Duke, J.W.; Oken, B.S.; Morasco, B.J.; Heinricher, M.M.; Lim, M.M. Increased Sleep Disturbances and Pain in Veterans with Comorbid Traumatic Brain Injury and Posttraumatic Stress Disorder. J. Clin. Sleep Med. 2018, 14, 1865–1878. [Google Scholar] [CrossRef]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.-W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR Guiding Principles for Scientific Data Management and Stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef]
- Stewart, L.A.; Clarke, M.; Rovers, M.; Riley, R.D.; Simmonds, M.; Stewart, G.; Tierney, J.F. Preferred Reporting Items for Systematic Review and Meta-Analyses of Individual Participant Data: The PRISMA-IPD Statement. JAMA 2015, 313, 1657–1665. [Google Scholar] [CrossRef]
- Dietch, J.R.; Furst, A.J. Perspective: Cognitive Behavioral Therapy for Insomnia Is a Promising Intervention for Mild Traumatic Brain Injury. Front. Neurol. 2020, 11, 530273. [Google Scholar] [CrossRef]
- Martin, A.M.; Pinto, S.M.; Tang, X.; Hoffman, J.M.; Wittine, L.; Walker, W.C.; Schwartz, D.J.; Kane, G.; Takagishi, S.C.; Nakase-Richardson, R. Associations between Early Sleep-Disordered Breathing Following Moderate-to-Severe Traumatic Brain Injury and Long-Term Chronic Pain Status: A Traumatic Brain Injury Model Systems Study. J. Clin. Sleep Med. 2023, 19, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Mysliwiec, V.; Martin, J.L.; Ulmer, C.S.; Chowdhuri, S.; Brock, M.S.; Spevak, C.; Sall, J. The Management of Chronic Insomnia Disorder and Obstructive Sleep Apnea: Synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann. Intern. Med. 2020, 172, 325–336. [Google Scholar] [CrossRef]
- Silva, M.A.; Calvo, D.; Brennan, E.M.; Reljic, T.; Drasher-Phillips, L.; Schwartz, D.J.; Kumar, A.; Cotner, B.A.; Taylor, D.J.; Nakase-Richardson, R. Incidence and Predictors of Adherence to Sleep Apnea Treatment in Rehabilitation Inpatients with Acquired Brain Injury. Sleep Med. 2020, 69, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Lequerica, A.H.; Watson, E.; Dijkers, M.P.; Goldin, Y.; Hoffman, J.M.; Niemeier, J.P.; Silva, M.A.; Rabinowitz, A.; Chiaravalloti, N.D. The Utility of the Patient Health Questionnaire (PHQ-9) Sleep Disturbance Item as a Screener for Insomnia in Individuals with Moderate to Severe Traumatic Brain Injury. J. Head Trauma Rehabil. 2022, 37, E383–E389. [Google Scholar] [CrossRef]
- O’Neil, M.E. FITBIR: Accelerating Synthesis of TBI Research Using Novel Methods. Available online: https://fitbir.nih.gov/meta_study_profile/223 (accessed on 1 February 2023). [CrossRef]
- Chan, L. Effect of Aerobic Exercise Training on Cardiorespiratory Function in Patients with TBI (CNRM). Available online: https://fitbir.nih.gov/study_profile/205 (accessed on 21 September 2021).
- Broglio, S.P. Concussion Assessment, Research and Education (CARE) Consortium. Available online: https://fitbir.nih.gov/study_profile/310 (accessed on 21 September 2021).
- Rivara, F.P.; Koepsell, T.D.; Wang, J.; Temkin, N.; Dorsch, A.; Vavilala, M.S.; Durbin, D.; Jaffe, K.M. Disability 3, 12, and 24 Months After Traumatic Brain Injury Among Children and Adolescents. Pediatrics 2011, 128, e1129–e1138. [Google Scholar] [CrossRef]
- Zafonte, R.; Friedewald, W.T.; Lee, S.M.; Levin, B.; Diaz-Arrastia, R.; Ansel, B.; Eisenberg, H.; Timmons, S.D.; Temkin, N.; Novack, T.; et al. The Citicoline Brain Injury Treatment (COBRIT) Trial: Design and Methods. J. Neurotrauma 2009, 26, 2207–2216. [Google Scholar] [CrossRef]
- Lathan, C. An Independent, Prospective, Head to Head Study of the Reliability and Validity of Neurocognitive Test Batteries for the Assessment of Mild Traumatic Brain Injury. Available online: https://fitbir.nih.gov/study_profile/244 (accessed on 21 September 2021).
- Yue, J.K.; Phelps, R.R.L.; Winkler, E.A.; Deng, H.; Upadhyayula, P.S.; Vassar, M.J.; Madhok, D.Y.; Schnyer, D.M.; Puccio, A.M.; Lingsma, H.F.; et al. Substance Use on Admission Toxicology Screen Is Associated with Peri-Injury Factors and Six-Month Outcome after Traumatic Brain Injury: A TRACK-TBI Pilot Study. J. Clin. Neurosci. 2020, 75, 149–156. [Google Scholar] [CrossRef]
- Wright, D.W.; Yeatts, S.D.; Silbergleit, R.; Palesch, Y.Y.; Hertzberg, V.S.; Frankel, M.; Goldstein, F.C.; Caveney, A.F.; Howlett-Smith, H.; Bengelink, E.M.; et al. Very Early Administration of Progesterone for Acute Traumatic Brain Injury. N. Engl. J. Med. 2014, 371, 2457–2466. [Google Scholar] [CrossRef]
- Robertson, C. Effects of Erythropoietin on Cerebral Vascular Dysfunction and Anemia in Traumatic Brain Injury. Available online: https://ctv.veeva.com/study/effects-of-erythropoietin-on-cerebral-vascular-dysfunction-and-anemia-in-traumatic-brain-injury (accessed on 23 July 2021).
- Roy, M.J.; Costanzo, M.; Gill, J.; Leaman, S.; Law, W.; Ndiongue, R.; Taylor, P.; Kim, H.S.; Bieler, G.S.; Garge, N.; et al. Predictors of Neurocognitive Syndromes in Combat Veterans. Cureus 2015, 7, e293. [Google Scholar] [CrossRef]
- Harrison-Felix, C. Integrating Traumatic Brain Injury Model Systems Data into the Federal Interagency Traumatic Brain Injury Research Informatics System. Available online: https://fitbir.nih.gov/study_profile/255 (accessed on 21 September 2021).
- Walker, W.C.; Carne, W.; Franke, L.M.; Nolen, T.; Dikmen, S.D.; Cifu, D.X.; Wilson, K.; Belanger, H.G.; Williams, R. The Chronic Effects of Neurotrauma Consortium (CENC) Multi-Centre Observational Study: Description of Study and Characteristics of Early Participants. Brain Inj. 2016, 30, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Mac Donald, C.L.; Barber, J.; Patterson, J.; Johnson, A.M.; Dikmen, S.; Fann, J.R.; Temkin, N. Association Between 5-Year Clinical Outcome in Patients with Nonmedically Evacuated Mild Blast Traumatic Brain Injury and Clinical Measures Collected within 7 Days Postinjury in Combat. JAMA Netw. Open 2019, 2, e186676. [Google Scholar] [CrossRef]
- McKenzie, L. Evaluation of Spot Light: A Concussion Injury Management App for Youth. Available online: https://ctv.veeva.com/study/evaluation-of-spot-light-a-concussion-injury-management-app-for-youth-sports (accessed on 23 July 2021).
- Damiano, D. Effects of Rapid-Resisted Exercise on Ambulatory Adults with Traumatic Brain Injury (CNRM). Available online: https://fitbir.nih.gov/study_profile/271 (accessed on 21 September 2021).
- Gill, J. Biomarkers-Driven Development of Experiemental Therapeutics for Traumatic Brain Injury (CNRM). Available online: https://fitbir.nih.gov/study_profile/272 (accessed on 21 September 2021).
- Gullapalli, R. Traumatic Brain Injury Data for FITBIR Informatics System: Maryland Retrospective Dataset. Available online: https://fitbir.nih.gov/study_profile/313 (accessed on 21 September 2021).
- Gullapalli, R. Traumatic Brain Injury Data for FITBIR Informatics System: Maryland MagNeTS Prospective Dataset. Available online: https://fitbir.nih.gov/study_profile/314 (accessed on 21 September 2021).
- Sours, C. Traumatic Brain Injury Data for FITBIR Informatics System: Maryland MAGNETS Dataset-FMRI TBI Subset. Available online: https://fitbir.nih.gov/study_profile/315 (accessed on 21 September 2021).
- McKee, A. Tauopathy Consensus Study of Pathology Images. Available online: https://fitbir.nih.gov/study_profile/319 (accessed on 21 September 2021).
- Okonkwo, D.O. Targeted Evaluation, Action, and Monitoring of Traumatic Brain Injury (TEAM-TBI). Available online: https://fitbir.nih.gov/study_profile/321 (accessed on 21 September 2021).
- Capo-Aponte, J.E. Automated Comprehensive Evaluation of Mild Traumatic Brain Injury Visual Dysfunction. Available online: https://fitbir.nih.gov/study_profile/326 (accessed on 21 September 2021).
- Weaver, L. Brain Injury and Mechanisms of Action of Hyperbaric Oxygen for Persistent Post-Concussive Symptoms after Mild Traumatic Brain Injury (BIMA). Available online: https://fitbir.nih.gov/study_profile/363 (accessed on 21 September 2021).
- Weaver, L. Development of Normative Datasets for Assessments Used in Patients with Post Concussive Symptoms Due to Mild Traumatic Brain Injury (NORMAL). Available online: https://fitbir.nih.gov/study_profile/364 (accessed on 21 September 2021).
- Stewart, L.A.; Tierney, J.F. To IPD or Not to IPD? Advantages and Disadvantages of Systematic Reviews Using Individual Patient Data. Eval. Health Prof. 2002, 25, 76–97. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.; Patall, E.A. The Relative Benefits of Meta-Analysis Conducted with Individual Participant Data versus Aggregated Data. Psychol. Methods 2009, 14, 165–176. [Google Scholar] [CrossRef]
- Simmonds, M.; Stewart, G.; Stewart, L. A Decade of Individual Participant Data Meta-Analyses: A Review of Current Practice. Contemp. Clin. Trials 2015, 45, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Tierney, J.F.; Stewart, L.A.; Clarke, M.; Cochrane Individual Participant Data Meta-analysis Methods Group. Chapter 26: Individual Participant Data. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2022. [Google Scholar]
- Morin, C.M. Insomnia: Psychological Assessment and Management; Guilford Press: New York, NY, USA, 1993; ISBN 0-89862-210-7. [Google Scholar]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an Outcome Measure for Insomnia Research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Kaufmann, C.N.; Orff, H.J.; Moore, R.C.; Delano-Wood, L.; Depp, C.A.; Schiehser, D.M. Psychometric Characteristics of the Insomnia Severity Index in Veterans with History of Traumatic Brain Injury. Behav. Sleep Med. 2019, 17, 12–18. [Google Scholar] [CrossRef]
- Simon, G.E.; Coleman, K.J.; Rossom, R.C.; Beck, A.; Oliver, M.; Johnson, E.; Whiteside, U.; Operskalski, B.; Penfold, R.B.; Shortreed, S.M.; et al. Risk of Suicide Attempt and Suicide Death Following Completion of the Patient Health Questionnaire Depression Module in Community Practice. J. Clin. Psychiatry 2016, 77, 221–227. [Google Scholar] [CrossRef]
- Simon, G.E.; Rutter, C.M.; Peterson, D.; Oliver, M.; Whiteside, U.; Operskalski, B.; Ludman, E.J. Does Response on the PHQ-9 Depression Questionnaire Predict Subsequent Suicide Attempt or Suicide Death? Psychiatr. Serv. 2013, 64, 1195–1202. [Google Scholar] [CrossRef]
- Wu, Y.; Levis, B.; Riehm, K.E.; Saadat, N.; Levis, A.W.; Azar, M.; Rice, D.B.; Boruff, J.; Cuijpers, P.; Gilbody, S.; et al. Equivalency of the Diagnostic Accuracy of the PHQ-8 and PHQ-9: A Systematic Review and Individual Participant Data Meta-Analysis. Psychol. Med. 2020, 50, 1368–1380. [Google Scholar] [CrossRef]
- Weathers, F.; Litz, B.; Huska, J.; Keane, T. PTSD Checklist-Specific Version. National Center for PTSD. 1994. Available online: https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp (accessed on 14 March 2022).
- Weathers, F.W. The PTSD Checklist: Reliability, Validity and Diagnostic Utility. In Proceedings of the Annual Meeting of the International Society for Traumatic Stress Studies, San Antonio, TX, USA, October 1993. [Google Scholar]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Fictenberg, N.L.; Putnam, S.H.; Mann, N.R.; Zafonte, R.D.; Millard, A.E. Insomnia Screening in Postacute Traumatic Brain Injury: Utility and Validity of the Pittsburgh Sleep Quality Index. Am. J. Phys. Med. Rehabil. 2001, 80, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Matsangas, P.; Mysliwiec, V. The Utility of the Pittsburgh Sleep Quality Index in US Military Personnel. Mil. Psychol. 2018, 30, 360–369. [Google Scholar] [CrossRef]
- Guskiewicz, K.M.; Register-Mihalik, J.; McCrory, P.; McCrea, M.; Johnston, K.; Makdissi, M.; Dvorák, J.; Davis, G.; Meeuwisse, W. Evidence-Based Approach to Revising the SCAT2: Introducing the SCAT3. Br. J. Sports Med. 2013, 47, 289–293. [Google Scholar] [CrossRef] [PubMed]
- King, N.S.; Crawford, S.; Wenden, F.J.; Moss, N.E.; Wade, D.T. The Rivermead Post Concussion Symptoms Questionnaire: A Measure of Symptoms Commonly Experienced after Head Injury and Its Reliability. J. Neurol. 1995, 242, 587–592. [Google Scholar] [CrossRef]
- Riley, R.D.; Lambert, P.C.; Abo-Zaid, G. Meta-Analysis of Individual Participant Data: Rationale, Conduct, and Reporting. BMJ 2010, 340, c221. [Google Scholar] [CrossRef]
- Ventresca, M.; Schünemann, H.J.; Macbeth, F.; Clarke, M.; Thabane, L.; Griffiths, G.; Noble, S.; Garcia, D.; Marcucci, M.; Iorio, A.; et al. Obtaining and Managing Data Sets for Individual Participant Data Meta-Analysis: Scoping Review and Practical Guide. BMC Med. Res. Methodol. 2020, 20, 113. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Lobo, J.M.; Logan, J.G.; Kang, H.; Kwon, Y.; Sohn, M.W. A Scoping Review of Racial/Ethnic Disparities in Sleep. Sleep Med. 2021, 81, 169–179. [Google Scholar] [CrossRef]
- Rao, V.; Bergey, A.; Hill, H.; Efron, D.; McCann, U. Sleep Disturbance after Mild Traumatic Brain Injury: Indicator of Injury? J. Neuropsychiatry Clin. Neurosci. 2011, 23, 201–205. [Google Scholar] [CrossRef]
- Fogelberg, D.J.; Hoffman, J.M.; Dikmen, S.; Temkin, N.R.; Bell, K.R. Association of Sleep and Co-Occurring Psychological Conditions at 1 Year after Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2012, 93, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.N.; Hansen, S.L.; Capener, D.C.; Matsangas, P.; Mysliwiec, V. Gender Differences in Sleep Disorders in the US Military. Sleep Health 2017, 3, 336–341. [Google Scholar] [CrossRef] [PubMed]
| FITBIR Shared Studies and Measures—Sleep Disorder Outcome | |||||||
|---|---|---|---|---|---|---|---|
| Study ID | Title | Sample Size | TBI Severity | Sleep Measure | Sleep Variable | Cut Point for Dichotomous Sleep Variable | Included in Analysis |
| 246 [42] | Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Pilot | 599 | mild TBI, moderate/severe TBI | Rivermead Post-Concussion Symptoms Questionnaire (RPQ) | RPQSleepDistScale | No = 0–2 Yes = 3–4 | No |
| 246 [42] | Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Pilot | 599 | mild TBI, moderate/severe TBI | Posttraumatic Stress Disorder Checklist Civilian Version (PCLC_Standard) | PCLSFallStayAsleepInd | No = 1 Yes = 2–5 | No |
| 246 [42] | Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Pilot | 599 | mild TBI, moderate/severe TBI | TRACK-TBI Neurologic Assessment | NeuroAssmtSlpMoreInd | No = 0 Yes = 1 | No |
| 246 [42] | Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Pilot | 599 | mild TBI, moderate/severe TBI | TRACK-TBI Neurologic Assessment | NeuroAssmtSlpTrblFallInd | No = 0 Yes = 1 | No |
| 248 [43] | Progesterone for the Treatment of Traumatic Brain Injury (ProTECT III | 882 | moderate/severe TBI | Patient Health Questionnaire 8 Item (PHQ 8) | PHQ9SleepImpairScore | No = 0 Yes = 1–3 | No |
| 254 [45] | Predictors of PTSD and Post-Concussive Syndrome of OIF/OEF Veterans | 80 | mild TBI, no TBI | Pittsburgh Sleep Quality Index (PSQI) | PSQITotalScore | No = 0–7 Yes = 8–18 | No |
| 263 [47] | CENC Study 1: Observational Study on Late Neurologic Effects of OEF/OIF/OND Combat | 1539 | mild TBI, no TBI | Pittsburgh Sleep Quality Index (PSQI) | PSQITotalScore | No = 0–7 Yes = 8–18 | Yes |
| 264 [48] | CENC Study 25: Assessment of Long-Term Outcome and Disability in Active-Duty Military Prospectively Examined Following Concussive TBI | 94 | mild TBI, no TBI | Insomnia Severity Index (ISI) | ISITotalScore | No = 0–9 Yes = 10–28 | Yes |
| 326 [57] | Automated Comprehensive Evaluation of Mild Traumatic Brain Injury Visual Dysfunction | 120 | mild TBI, no TBI | Sport Concussion Assessment Tool 3 (SCAT3) | Scat3TroublFallAsleep | No = 0 Yes = 1–6 | Yes |
| TBI Category | ||||
|---|---|---|---|---|
| Overall N = 3314 1 | No TBI N = 491 1 | Mild TBI N = 1920 1 | Moderate/Severe TBI N = 903 1 | |
| Sleep disorder | ||||
| No | 1195 (45%) | 294 (62%) | 640 (39%) | 261 (48%) |
| Yes | 1476 (55%) | 181 (38%) | 1007 (61%) | 288 (52%) |
| Missing | 643 | 16 | 273 | 354 |
| Gender | ||||
| Male | 2674 (81%) | 396 (81%) | 1612 (84%) | 666 (74%) |
| Female | 640 (19%) | 95 (19%) | 308 (16%) | 237 (26%) |
| Age category | ||||
| <25 | 480 (15%) | 69 (14%) | 173 (9.0%) | 238 (26%) |
| 25–39 | 1387 (42%) | 268 (55%) | 851 (44%) | 268 (30%) |
| 40–49 | 688 (21%) | 93 (19%) | 458 (24%) | 137 (15%) |
| 50–64 | 574 (17%) | 54 (11%) | 346 (18%) | 174 (19%) |
| 65+ | 180 (5.4%) | 5 (1.0%) | 89 (4.6%) | 86 (9.5%) |
| Missing | 5 | 2 | 3 | 0 |
| Race/ethnicity | ||||
| White | 1894 (63%) | 163 (53%) | 1155 (65%) | 576 (65%) |
| Other | 1094 (37%) | 144 (47%) | 635 (35%) | 315 (35%) |
| Missing | 326 | 184 | 130 | 12 |
| Population type | ||||
| Veteran/Military | 1833 (55%) | 491 (100%) | 1342 (70%) | 0 (0%) |
| Civilian | 1481 (45%) | 0 (0%) | 578 (30%) | 903 (100%) |
| TBI Category | |||
| Overall, N = 1753 1 | No TBI, N = 418 1 | Mild TBI, N = 1335 1 | |
| Sleep disturbance | |||
| No | 724 (42%) | 239 (58%) | 485 (37%) |
| Yes | 1001 (58%) | 172 (42%) | 829 (63%) |
| Missing | 28 | 7 | 21 |
| Gender | |||
| Male | 1527 (87%) | 334 (80%) | 1193 (89%) |
| Female | 226 (13%) | 84 (20%) | 142 (11%) |
| Age category | |||
| <25 | 103 (5.9%) | 63 (15%) | 40 (3.0%) |
| 25–39 | 928 (53%) | 217 (52%) | 711 (53%) |
| 40–49 | 444 (25%) | 81 (19%) | 363 (27%) |
| 50–64 | 259 (15%) | 50 (12%) | 209 (16%) |
| 65+ | 14 (0.8%) | 5 (1.2%) | 9 (0.7%) |
| Missing | 5 | 2 | 3 |
| Race/ethnicity | |||
| Non-Hispanic White | 929 (61%) | 163 (53%) | 766 (63%) |
| Other | 595 (39%) | 144 (47%) | 451 (37%) |
| Missing | 229 | 111 | 118 |
| Population type | |||
| Veteran/Military | 1753 (100%) | 418 (100%) | 1335 (100%) |
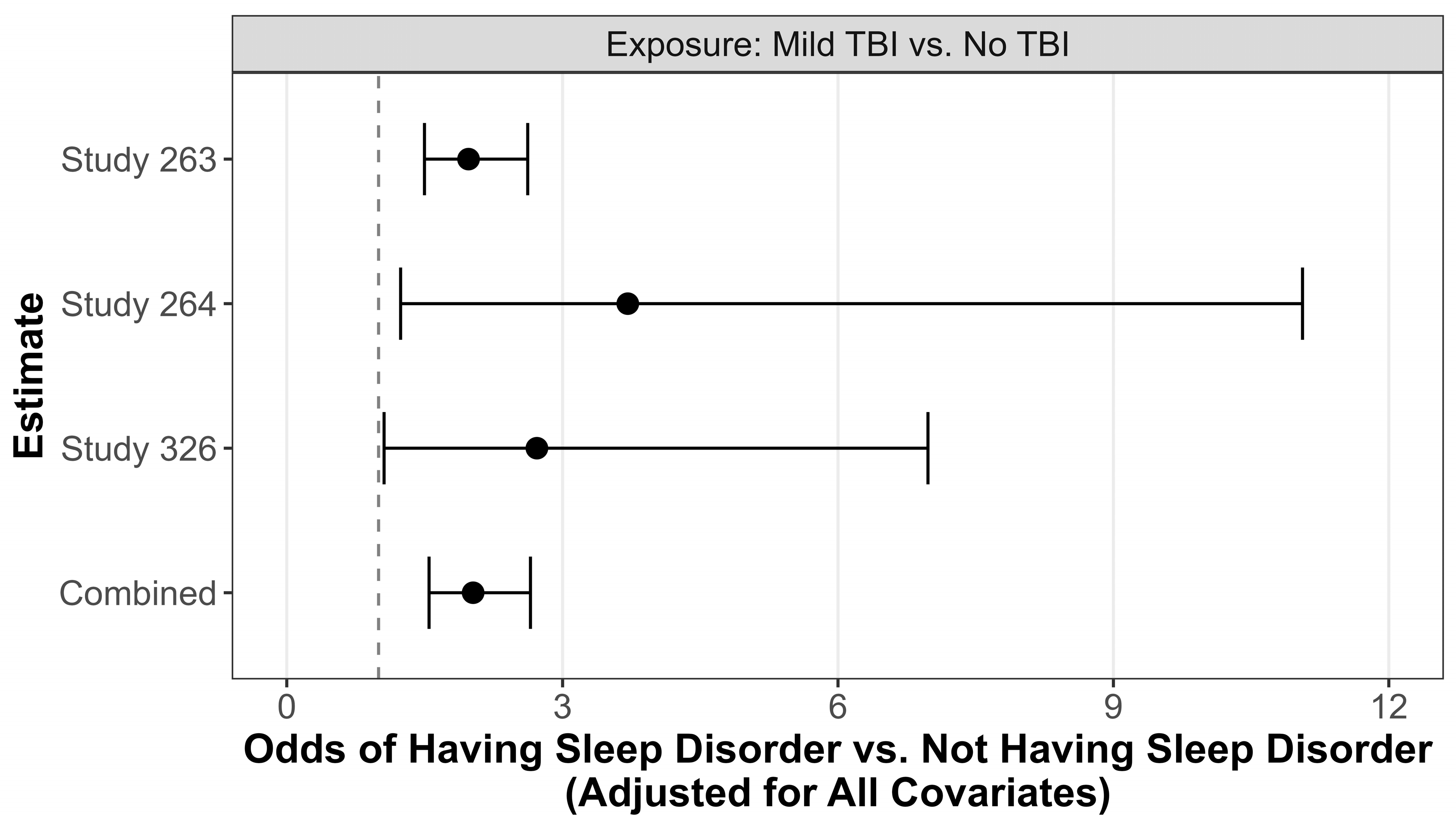
| OR | CI | p Value | |
|---|---|---|---|
| Study 263 | |||
| Male | 0.76 | (0.54, 1.07) | 0.11 |
| Non-Hispanic White | 0.50 | (0.40, 0.63) | <0.0001 |
| TBI | 1.98 | (1.50, 2.62) | <0.0001 |
| Age | 0.99 | (0.98, 0.99) | 0.02 |
| Study 264 | |||
| Male | 0.76 | (0.10, 5.58) | 0.78 |
| Non-Hispanic White | 1.90 | (0.53, 6.81) | 0.32 |
| TBI | 3.71 | (1.24, 11.06) | 0.02 |
| Age | 1.07 | (0.99, 1.15) | 0.08 |
| Study 326 | |||
| Male | 0.54 | (0.17, 1.72) | 0.30 |
| Non-Hispanic White | |||
| TBI | 2.72 | (1.06, 6.98) | 0.04 |
| Age | 1.06 | (0.96, 1.17) | 0.28 |
| Combined | |||
| Male | 0.77 | (0.55, 1.07) | 0.12 |
| Non-Hispanic White | 0.52 | (0.42, 0.65) | <0.0001 |
| TBI | 2.03 | (1.55, 2.65) | <0.0001 |
| Age | 0.988 | (0.98, 0.99) | 0.04 |
| 263 vs. 264 | 0.99 | (0.61, 1.61) | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Neil, M.E.; Krushnic, D.; Walker, W.C.; Cameron, D.; Baker-Robinson, W.; Hannon, S.; Clauss, K.; Cheney, T.P.; Cook, L.J.; Niederhausen, M.; et al. Increased Risk for Clinically Significant Sleep Disturbances in Mild Traumatic Brain Injury: An Approach to Leveraging the Federal Interagency Traumatic Brain Injury Research Database. Brain Sci. 2024, 14, 921. https://doi.org/10.3390/brainsci14090921
O’Neil ME, Krushnic D, Walker WC, Cameron D, Baker-Robinson W, Hannon S, Clauss K, Cheney TP, Cook LJ, Niederhausen M, et al. Increased Risk for Clinically Significant Sleep Disturbances in Mild Traumatic Brain Injury: An Approach to Leveraging the Federal Interagency Traumatic Brain Injury Research Database. Brain Sciences. 2024; 14(9):921. https://doi.org/10.3390/brainsci14090921
Chicago/Turabian StyleO’Neil, Maya E., Danielle Krushnic, William C. Walker, David Cameron, William Baker-Robinson, Sara Hannon, Kate Clauss, Tamara P. Cheney, Lawrence J. Cook, Meike Niederhausen, and et al. 2024. "Increased Risk for Clinically Significant Sleep Disturbances in Mild Traumatic Brain Injury: An Approach to Leveraging the Federal Interagency Traumatic Brain Injury Research Database" Brain Sciences 14, no. 9: 921. https://doi.org/10.3390/brainsci14090921
APA StyleO’Neil, M. E., Krushnic, D., Walker, W. C., Cameron, D., Baker-Robinson, W., Hannon, S., Clauss, K., Cheney, T. P., Cook, L. J., Niederhausen, M., Kaplan, J., Pappas, M., & Martin, A. M. (2024). Increased Risk for Clinically Significant Sleep Disturbances in Mild Traumatic Brain Injury: An Approach to Leveraging the Federal Interagency Traumatic Brain Injury Research Database. Brain Sciences, 14(9), 921. https://doi.org/10.3390/brainsci14090921





