The Systemic Inflammation Response Index Efficiently Discriminates between the Failure Patterns of Patients with Isocitrate Dehydrogenase Wild-Type Glioblastoma Following Radiochemotherapy with FLAIR-Based Gross Tumor Volume Delineation
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Target Delineation and Systemic Treatment
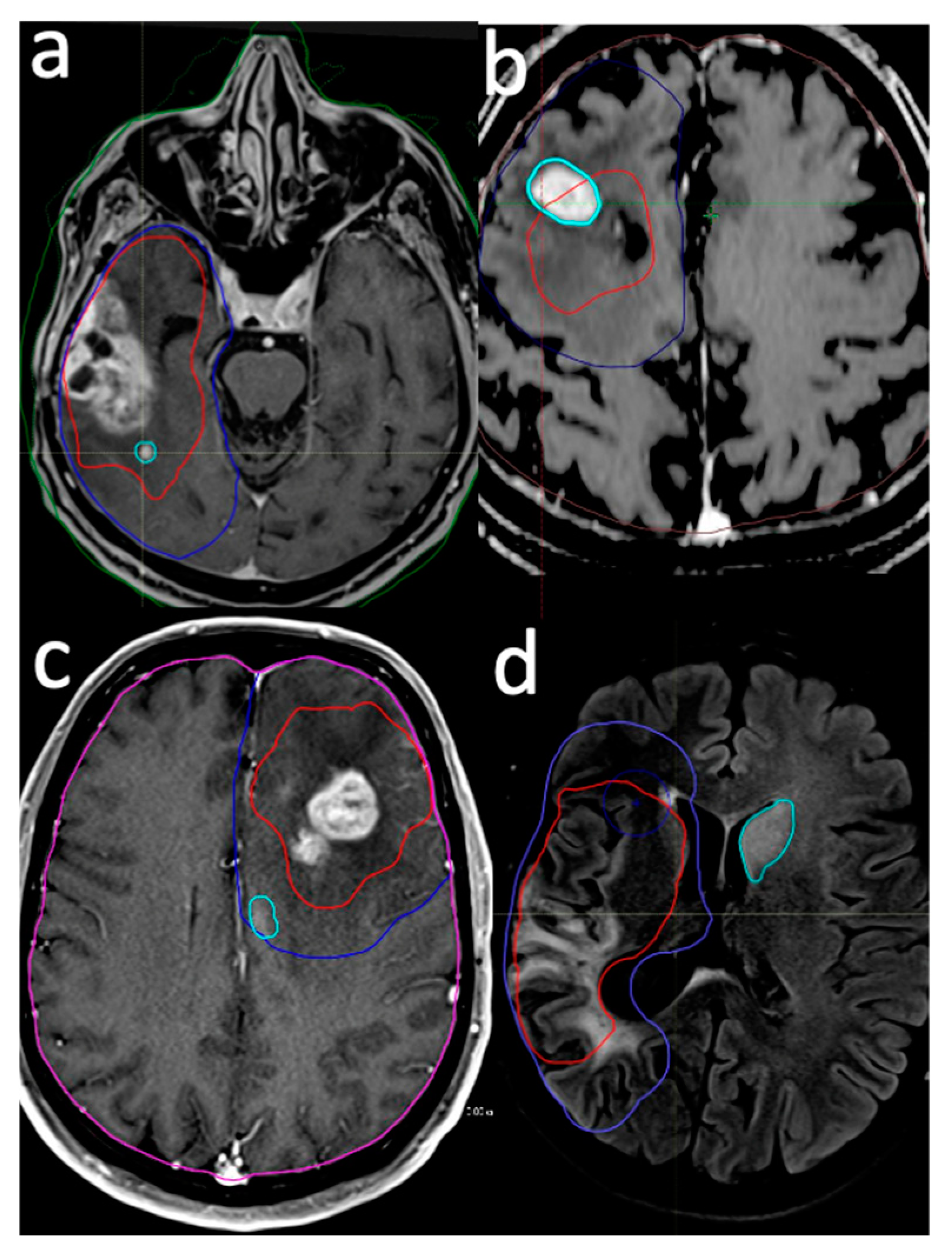
2.3. Delineation of Failure Pattern
- Intra-GTV: Recurrence is either entirely contained within the original GTV or originates within the GTV and extends beyond its boundaries.
- In-CTV/out-GTV: Recurrence occurs within the CTV but does not have any contact with the GTV, indicating that it is completely outside the GTV but still within the CTV.
- Distant: Recurrence is situated beyond the confines of the radiation field, signifying that the tumor has recurred at a location not encompassed within the original treatment area.
- Intra-GTV and distant: Recurrence is present both within the GTV and at a distant site outside the radiation field, implying multiple areas of tumor regrowth.
2.4. Systemic Inflammation Response Index Calculation
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Failure Pattern Outcomes
3.3. Failure Pattern Association Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Milano, M.T.; Okunieff, P.; Donatello, R.S.; Mohile, N.A.; Sul, J.; Walter, K.A.; Korones, D.N. Patterns and timing of recurrence after temozolomide-based chemoradiation for glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1147–1155. [Google Scholar] [CrossRef]
- Kruser, T.J.; Bosch, W.R.; Badiyan, S.N.; Bovi, J.A.; Ghia, A.J.; Kim, M.M.; Solanki, A.A.; Sachdev, S.; Tsien, C.; Wang, T.J.C.; et al. NRG brain tumor specialists consensus guidelines for glioblastoma contouring. J. Neurooncol. 2019, 143, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Niyazi, M.; Andratschke, N.; Bendszus, M.; Chalmers, A.J.; Erridge, S.C.; Galldiks, N.; Lagerwaard, F.J.; Navarria, P.; Munck Af Rosenschold, P.; Ricardi, U.; et al. ESTRO-EANO guideline on target delineation and radiotherapy details for glioblastoma. Radiother. Oncol. 2023, 184, 109663. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Arita, H.; Okita, Y.; Kagawa, N.; Kishima, H.; Hashimoto, N.; Tanaka, H.; Watanabe, Y.; Shimosegawa, E.; Hatazawa, J.; et al. Comparison of diffusion tensor imaging and (11)C-methionine positron emission tomography for reliable prediction of tumor cell density in gliomas. J. Neurosurg. 2016, 125, 1136–1142. [Google Scholar] [CrossRef]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non-Contrast-Enhanced Tumor With Survival Within Molecular Subgroups of Patients With Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef]
- Incekara, F.; Smits, M.; van der Voort, S.R.; Dubbink, H.J.; Atmodimedjo, P.N.; Kros, J.M.; Vincent, A.; van den Bent, M. The Association Between the Extent of Glioblastoma Resection and Survival in Light of MGMT Promoter Methylation in 326 Patients With Newly Diagnosed IDH-Wildtype Glioblastoma. Front. Oncol. 2020, 10, 1087. [Google Scholar] [CrossRef]
- Yeung, Y.T.; McDonald, K.L.; Grewal, T.; Munoz, L. Interleukins in glioblastoma pathophysiology: Implications for therapy. Br. J. Pharmacol. 2013, 168, 591–606. [Google Scholar] [CrossRef]
- Arbatskiy, M.; Balandin, D.; Churov, A.; Varachev, V.; Nikolaeva, E.; Mitrofanov, A.; Bekyashev, A.; Tkacheva, O.; Susova, O.; Nasedkina, T. Intratumoral Cell Heterogeneity in Patient-Derived Glioblastoma Cell Lines Revealed by Single-Cell RNA-Sequencing. Int. J. Mol. Sci. 2024, 25, 8472. [Google Scholar] [CrossRef]
- Ahmad, N.; Chen, L.; Yuan, Z.; Ma, X.; Yang, X.; Wang, Y.; Zhao, Y.; Jin, H.; Khaidamah, N.; Wang, J.; et al. Pyrimidine compounds BY4003 and BY4008 inhibit glioblastoma cells growth via modulating JAK3/STAT3 signaling pathway. Neurotherapeutics 2024, e00431, in press. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.; Tang, N.; Hao, Y.; Lee, M.; Peng, S.; Bybee, R.; Hartman, L.; Garcia-Mansfield, K.; Sharma, R.; Pirrotte, P.; et al. Glioblastoma vulnerability to neddylation inhibition is dependent on PTEN status, and dysregulation of the cell cycle and DNA replication. Neurooncol. Adv. 2024, 6, vdae104. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Song, H.W.; Cai, H.Q.; Kong, L.W.; Yao, K.; Jiang, T.; Li, S.W.; Yan, C.X. Preoperative inflammation markers and IDH mutation status predict glioblastoma patient survival. Oncotarget 2017, 8, 50117–50123. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Yuan, Y.; Li, T.; Zuo, M.; Liu, Y. Prognostic significance of preoperative systemic inflammation response index in newly diagnosed glioblastoma patients underwent gross total resection: A propensity score matching analysis. World J. Surg. Oncol. 2022, 20, 137. [Google Scholar] [CrossRef]
- Topkan, E.; Kucuk, A.; Ozdemir, Y.; Mertsoylu, H.; Besen, A.A.; Sezen, D.; Bolukbasi, Y.; Pehlivan, B.; Selek, U. Systemic Inflammation Response Index Predicts Survival Outcomes in Glioblastoma Multiforme Patients Treated with Standard Stupp Protocol. J. Immunol. Res. 2020, 2020, 8628540. [Google Scholar] [CrossRef]
- Topkan, E.; Besen, A.A.; Ozdemir, Y.; Kucuk, A.; Mertsoylu, H.; Pehlivan, B.; Selek, U. Prognostic Value of Pretreatment Systemic Immune-Inflammation Index in Glioblastoma Multiforme Patients Undergoing Postneurosurgical Radiotherapy Plus Concurrent and Adjuvant Temozolomide. Mediat. Inflamm. 2020, 2020, 4392189. [Google Scholar] [CrossRef] [PubMed]
- Jarmuzek, P.; Kozlowska, K.; Defort, P.; Kot, M.; Zembron-Lacny, A. Prognostic Values of Systemic Inflammatory Immunological Markers in Glioblastoma: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 3339. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Blasel, S.; Zagorcic, A.; Jurcoane, A.; Bähr, O.; Wagner, M.; Harter, P.N.; Hattingen, E. Perfusion MRI in the Evaluation of Suspected Glioblastoma Recurrence. J. Neuroimaging 2016, 26, 116–123. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, L.; Wang, Q.; Zheng, X.; Wu, C.; Xu, B.N. Role of magnetic resonance spectroscopy for the differentiation of recurrent glioma from radiation necrosis: A systematic review and meta-analysis. Eur. J. Radiol. 2014, 83, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Dobelbower, M.C.; Burnett Iii, O.L.; Nordal, R.A.; Nabors, L.B.; Markert, J.M.; Hyatt, M.D.; Fiveash, J.B. Patterns of failure for glioblastoma multiforme following concurrent radiation and temozolomide. J. Med. Imaging Radiat. Oncol. 2011, 55, 77–81. [Google Scholar] [CrossRef]
- Seaberg, M.H.; Kazda, T.; Youland, R.S.; Laack, N.N.; Pafundi, D.H.; Anderson, S.K.; Sarkaria, J.N.; Galanis, E.; Brown, P.D.; Brinkmann, D.H. Dosimetric patterns of failure in the era of novel chemoradiotherapy in newly-diagnosed glioblastoma patients. Radiother. Oncol. 2023, 188, 109768. [Google Scholar] [CrossRef]
- Mizuhata, M.; Takamatsu, S.; Shibata, S.; Sakurai, T.; Minamikawa, R.; Yamazaki, M.; Kinoshita, M.; Miyashita, K.; Kumano, T.; Nakada, M.; et al. Patterns of failure in glioblastoma multiforme following Standard (60 Gy) or Short course (40 Gy) radiation and concurrent temozolomide. Jpn. J. Radiol. 2023, 41, 660–668. [Google Scholar] [CrossRef]
- Langhans, M.; Popp, I.; Grosu, A.L.; Shusharina, N.; Binder, H.; Baltas, D.; Bortfeld, T. Recurrence analysis of glioblastoma cases based on distance and dose information. Radiother. Oncol. 2023, 183, 109600. [Google Scholar] [CrossRef]
- Tu, Z.; Xiong, H.; Qiu, Y.; Li, G.; Wang, L.; Peng, S. Limited recurrence distance of glioblastoma under modern radiotherapy era. BMC Cancer 2021, 21, 720. [Google Scholar] [CrossRef] [PubMed]
- Guram, K.; Smith, M.; Ginader, T.; Bodeker, K.; Pelland, D.; Pennington, E.; Buatti, J.M. Using Smaller-Than-Standard Radiation Treatment Margins Does Not Change Survival Outcomes in Patients with High-Grade Gliomas. Pract. Radiat. Oncol. 2019, 9, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, C.; Rudra, S.; Ma, S.; Brenneman, R.; Huang, Y.; Henke, L.; Abraham, C.; Campian, J.; Tsien, C.; Huang, J. Evaluation of interim MRI changes during limited-field radiation therapy for glioblastoma and implications for treatment planning. Radiother. Oncol. 2021, 158, 237–243. [Google Scholar] [CrossRef]
- McDonald, M.W.; Shu, H.K.; Curran, W.J., Jr.; Crocker, I.R. Pattern of failure after limited margin radiotherapy and temozolomide for glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 130–136. [Google Scholar] [CrossRef]
- Gebhardt, B.J.; Dobelbower, M.C.; Ennis, W.H.; Bag, A.K.; Markert, J.M.; Fiveash, J.B. Patterns of failure for glioblastoma multiforme following limited-margin radiation and concurrent temozolomide. Radiat. Oncol. 2014, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Niyazi, M.; Brada, M.; Chalmers, A.J.; Combs, S.E.; Erridge, S.C.; Fiorentino, A.; Grosu, A.L.; Lagerwaard, F.J.; Minniti, G.; Mirimanoff, R.O.; et al. ESTRO-ACROP guideline “target delineation of glioblastomas”. Radiother. Oncol. 2016, 118, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.T.; Kahvecioglu, A.; Yedekci, F.Y.; Yigit, E.; Ciftci, G.C.; Kertmen, N.; Zorlu, F.; Yazici, G. Comparison of different target volume delineation strategies based on recurrence patterns in adjuvant radiotherapy for glioblastoma. Neurooncol. Pract. 2024, 11, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, H.; Xu, Y.; Nyalali, A.M.K.; Li, F. The prognostic value of the preoperative inflammatory index on the survival of glioblastoma patients. Neurol. Sci. 2022, 43, 5523–5531. [Google Scholar] [CrossRef] [PubMed]
- Edward, T.H.; Joseph, P.A.; Horacio, S.; Robert, M.P.; Isaac, Y.; Noriyuki, K.; Linda, M.L.; Carol, A.K. Chronic inflammation drives glioma growth: Cellular and molecular factors responsible for an immunosuppressive microenvironment. Neuroimmunol. Neuroinflamm. 2014, 1, 66–76. [Google Scholar]
- Fossati, G.; Ricevuti, G.; Edwards, S.W.; Walker, C.; Dalton, A.; Rossi, M.L. Neutrophil infiltration into human gliomas. Acta Neuropathol. 1999, 98, 349–354. [Google Scholar] [CrossRef]
- Liang, J.; Piao, Y.; Holmes, L.; Fuller, G.N.; Henry, V.; Tiao, N.; de Groot, J.F. Neutrophils Promote the Malignant Glioma Phenotype through S100A4. Clin. Cancer Res. 2014, 20, 187–198. [Google Scholar] [CrossRef]
- Sun, C.; Wang, S.; Ma, Z.; Zhou, J.; Ding, Z.; Yuan, G.; Pan, Y. Neutrophils in glioma microenvironment: From immune function to immunotherapy. Front. Immunol. 2024, 15, 1393173. [Google Scholar] [CrossRef]
- Ye, X.-Z.; Xu, S.-L.; Xin, Y.-H.; Yu, S.-C.; Ping, Y.-F.; Chen, L.; Xiao, H.-L.; Wang, B.; Yi, L.; Wang, Q.-L.; et al. Tumor-Associated Microglia/Macrophages Enhance the Invasion of Glioma Stem-like Cells via TGF-β1 Signaling Pathway. J. Immunol. 2012, 189, 444–453. [Google Scholar] [CrossRef]
- Khan, F.; Pang, L.; Dunterman, M.; Lesniak, M.S.; Heimberger, A.B.; Chen, P. Macrophages and microglia in glioblastoma: Heterogeneity, plasticity, and therapy. J. Clin. Investig. 2023, 133, e163446. [Google Scholar] [CrossRef]
- Bambury, R.M.; Teo, M.Y.; Power, D.G.; Yusuf, A.; Murray, S.; Battley, J.E.; Drake, C.; O’Dea, P.; Bermingham, N.; Keohane, C.; et al. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J. Neurooncol. 2013, 114, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.; Ratliff, T.; Huppertz, A.; Ge, Y.; Dictus, C.; Ahmadi, R.; Grau, S.; Hiraoka, N.; Eckstein, V.; Ecker, R.C.; et al. Effector T-Cell Infiltration Positively Impacts Survival of Glioblastoma Patients and Is Impaired by Tumor-Derived TGF-β. Clin. Cancer Res. 2011, 17, 4296–4308. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | All Patients (N = 71) | SIRI < 3 (N = 36) | SIRI ≥ 3 (N = 35) | p-Value |
|---|---|---|---|---|
| Age group, N (%) | 0.49 | |||
| <50 | 23 (34.4) | 13 (36.1) | 10 (28.6) | |
| ≥50 | 48 (67.6) | 23 (63.9) | 25 (71.4) | |
| Gender, N (%) | 0.49 | |||
| Male | 48 (32.4) | 12 (33.3) | 11 (31.4) | |
| Female | 23 (67.6) | 24 (66.7) | 24 (68.6) | |
| ECOG, N (%) | 0.96 | |||
| 0–1 | 63 (88.7) | 32 (88.9) | 31 (88.6) | |
| 2 | 8 (11.3) | 4 (11.1) | 4 (11.4) | |
| Tumor, N (%) | 0.56 | |||
| Unicentric | 59 (83.1) | 29 (80.6) | 30 (85.7) | |
| Multicentric | 12 (16.9) | 7 (19.4) | 5 (14.3) | |
| Corpus callosum involvement, N (%) | 0.92 | |||
| Yes | 26 (36.6) | 13 (36.1) | 13 (37.4) | |
| No | 45 (63.4) | 23 (63.9) | 22 | |
| Thalamic involvement, N (%) | 0.32 | |||
| Yes | 20 (28.2) | 12 (33.3) | 8 (22.9) | |
| No | 51 (71.8) | 24 (66.7) | 27 (77.1) | |
| Surgery type, N (%) | 0.92 | |||
| Gross total | 15 (21.1) | 8 (22.2) | 7 (20.0) | |
| Subtotal | 47 (66.2) | 23 (63.9) | 24 (68.6) | |
| Biopsy | 9 (12.7) | 5 (13.9) | 4 (11.4) | |
| P53 mutation status, N (%) | 0.89 | |||
| Yes | 20 (28.2) | 11 (30.6) | 9 (25.7) | |
| No | 20 (28.2) | 10 (27.8) | 10 (28.6) | |
| Unknown | 31 (43.6) | 15 (41.6) | 16 (45.7) | |
| ATRX mutation status, N (%) | 0.82 | |||
| Yes | 3 (4.2) | 2 (5.5) | 1 (2.9) | |
| No | 39 (54.9) | 19 (52.8) | 20 (57.1) | |
| Unknown | 29 (40.9) | 15 (41.7) | 14 (40.0) | |
| Ki 67 status, N (%) | 0.56 | |||
| <30% | 18 (25.3) | 10 (27.9) | 8 (22.9) | |
| ≥30% | 25 (35.2) | 14 (38.8) | 11 (31.4) | |
| Unknown | 28 (39.5) | 12 (33.3) | 16 (45.7) |
| Characteristic | Patients (N = 71) | SIRI < 3 (N = 36) | SIRI ≥ 3 (N = 35) | p-Value |
|---|---|---|---|---|
| Whole brain volume (cm3) | 0.90 | |||
| Mean (range) | 1356 (861.4–1785.6) | 1359 (861.4–1785.6) | 1353 (1106–1750) | |
| GTV (cm3) | 0.60 | |||
| Mean (range) | 137.6 (14.2–339.3) | 138 (14.2–326) | 148 (45.4–339.3) | |
| CTV (cm3) | 0.46 | |||
| Mean (range) | 333 (85.5–706) | 317 (85.5–706) | 339 (149–613.53) | |
| Failure pattern, N (%) | 0.20 | |||
| Intra GTV | 40 (56.3%) | 16 (44.4) | 24 (68.6) | |
| In CTV/out GTV | 4 (5.6%) | 2 (5.6) | 2 (5.7) | |
| Distant | 18 (25.4%) | 12 (33.3) | 6 (17.1) | |
| Intra GTV + Distant | 9 (12.7%) | 6 (16.7) | 3 (8.6) | |
| Failure group, N (%) | 0.035 | |||
| Group 1 | 44 (62%) | 18 (50.0) | 26 (74.3) | |
| Group 2 | 27 (27%) | 18 (50.0) | 9 (25.7) | |
| MDR, mm | 0.75 | |||
| Mean (range) | 13.5 (3–36.9) | 1.31 (3–32.5) | 13.9 (3–36.9) |
| Characteristic | Failure Pattern Groups | |||
|---|---|---|---|---|
| Group 1 (Intra-GTV; in-CTV/out-GTV) N = 44 (%) | Group 2 (Distant; Intra-GTV + Distant) N = 27 (%) | p-Value | ||
| Age, N (%) | <55 years | 20 (45.4) | 14 (51.9) | 0.60 |
| ≥55 years | 24 (54.6) | 13 (48.1) | ||
| Gender, N (%) | Female | 13 (29.5) | 10 (37.1) | 0.51 |
| Male | 31 (70.5) | 17 (62.9) | ||
| Surgery, N (%) | GTR | 11 (25) | 4 (14.8) | 0.13 |
| STR | 30 (68.1) | 17 (62.9) | ||
| Biopsy | 3 (6.9) | 6 (22.3) | ||
| ECOG, N (%) | 0–1 | 40(90.9) | 23 (85.2) | 0.75 |
| 2 | 4 (9.1) | 4 (14.8) | ||
| P53 Mutation, N (%) | No | 13 (29.5) | 7 (25.9) | 0.873 |
| Yes | 13 (29.5) | 7 (25.9) | ||
| Unknown | 18 (41) | 13 (48.2) | ||
| ATRX Mutation, N (%) | No | 24 (54.6) | 15 (55.6) | 0.98 |
| Yes | 2 (4.4) | 1 (3.7) | ||
| Unknown | 18 (41) | 11 (40.7) | ||
| Ki 67, N (%) | <30% | 12 (27.3) | 6 (22.2) | 0.89 |
| ≥30% | 15 (34.1) | 10 (37.1) | ||
| Unknown | 17 (38.6) | 11 (40.7) | ||
| Tumor, N (%) | Unicentric | 37 (84.1) | 22 (81.4) | 0.77 |
| Multicentric | 7 (15.9) | 5 (18.6) | ||
| Corpus callosum involvement, N (%) | No | 29 (65.9) | 16 (59.3) | 0.57 |
| Yes | 15 (34.1) | 11 (40.7) | ||
| Thalamic involvement, N (%) | No | 33 (75) | 18 (66.7) | 0.44 |
| Yes | 11 (25) | 9 (33.3) | ||
| SIRI, N (%) | <3 | 18 (40) | 18 (66.7) | 0.03 |
| ≥3 | 26 (60) | 9 (33.3) | ||
| Yes | 26 (59) | 12 (44.4) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senyurek, S.; Aygun, M.S.; Kilic Durankus, N.; Akdemir, E.Y.; Sezen, D.; Topkan, E.; Bolukbasi, Y.; Selek, U. The Systemic Inflammation Response Index Efficiently Discriminates between the Failure Patterns of Patients with Isocitrate Dehydrogenase Wild-Type Glioblastoma Following Radiochemotherapy with FLAIR-Based Gross Tumor Volume Delineation. Brain Sci. 2024, 14, 922. https://doi.org/10.3390/brainsci14090922
Senyurek S, Aygun MS, Kilic Durankus N, Akdemir EY, Sezen D, Topkan E, Bolukbasi Y, Selek U. The Systemic Inflammation Response Index Efficiently Discriminates between the Failure Patterns of Patients with Isocitrate Dehydrogenase Wild-Type Glioblastoma Following Radiochemotherapy with FLAIR-Based Gross Tumor Volume Delineation. Brain Sciences. 2024; 14(9):922. https://doi.org/10.3390/brainsci14090922
Chicago/Turabian StyleSenyurek, Sukran, Murat Serhat Aygun, Nulifer Kilic Durankus, Eyub Yasar Akdemir, Duygu Sezen, Erkan Topkan, Yasemin Bolukbasi, and Ugur Selek. 2024. "The Systemic Inflammation Response Index Efficiently Discriminates between the Failure Patterns of Patients with Isocitrate Dehydrogenase Wild-Type Glioblastoma Following Radiochemotherapy with FLAIR-Based Gross Tumor Volume Delineation" Brain Sciences 14, no. 9: 922. https://doi.org/10.3390/brainsci14090922
APA StyleSenyurek, S., Aygun, M. S., Kilic Durankus, N., Akdemir, E. Y., Sezen, D., Topkan, E., Bolukbasi, Y., & Selek, U. (2024). The Systemic Inflammation Response Index Efficiently Discriminates between the Failure Patterns of Patients with Isocitrate Dehydrogenase Wild-Type Glioblastoma Following Radiochemotherapy with FLAIR-Based Gross Tumor Volume Delineation. Brain Sciences, 14(9), 922. https://doi.org/10.3390/brainsci14090922






