Music Therapy in Depression: Exploring Mechanisms and Efficacy in Rat Models
Abstract
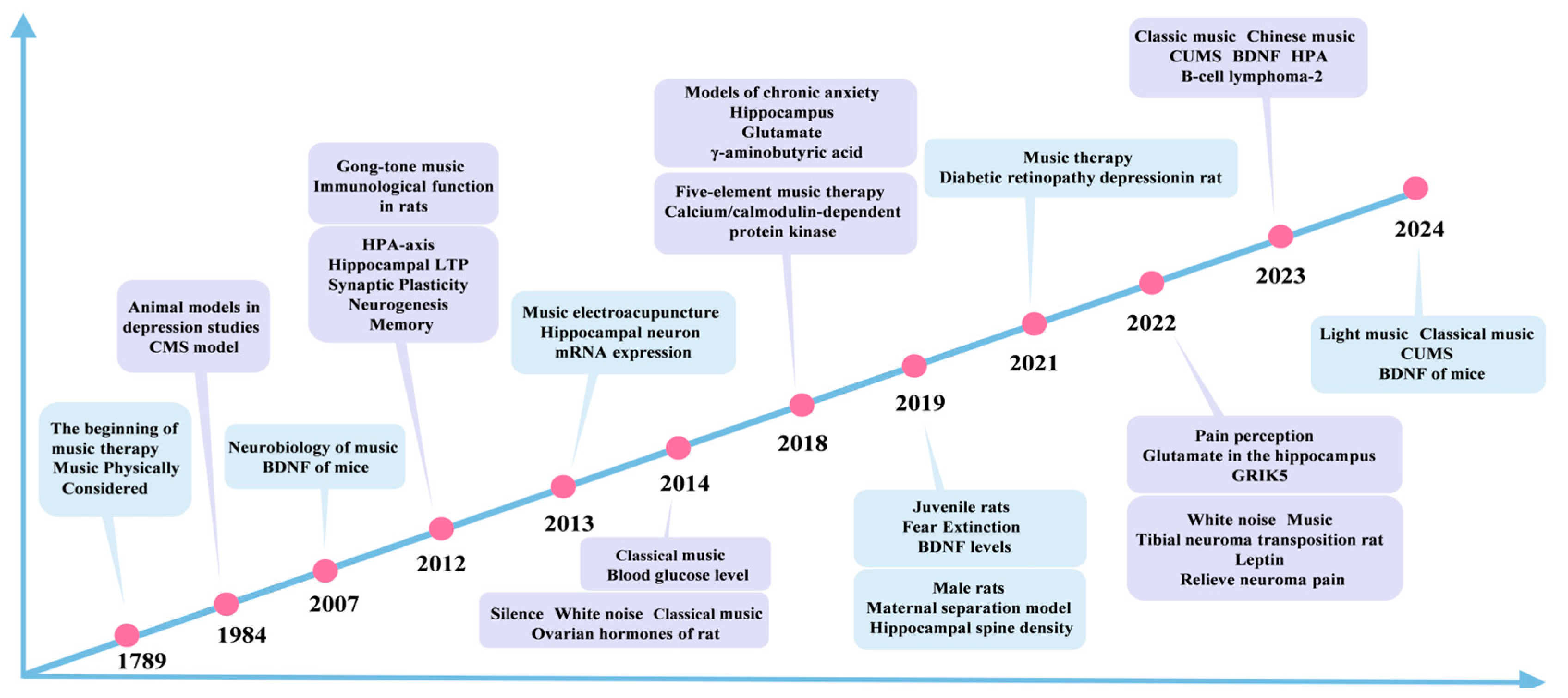
:1. Introduction
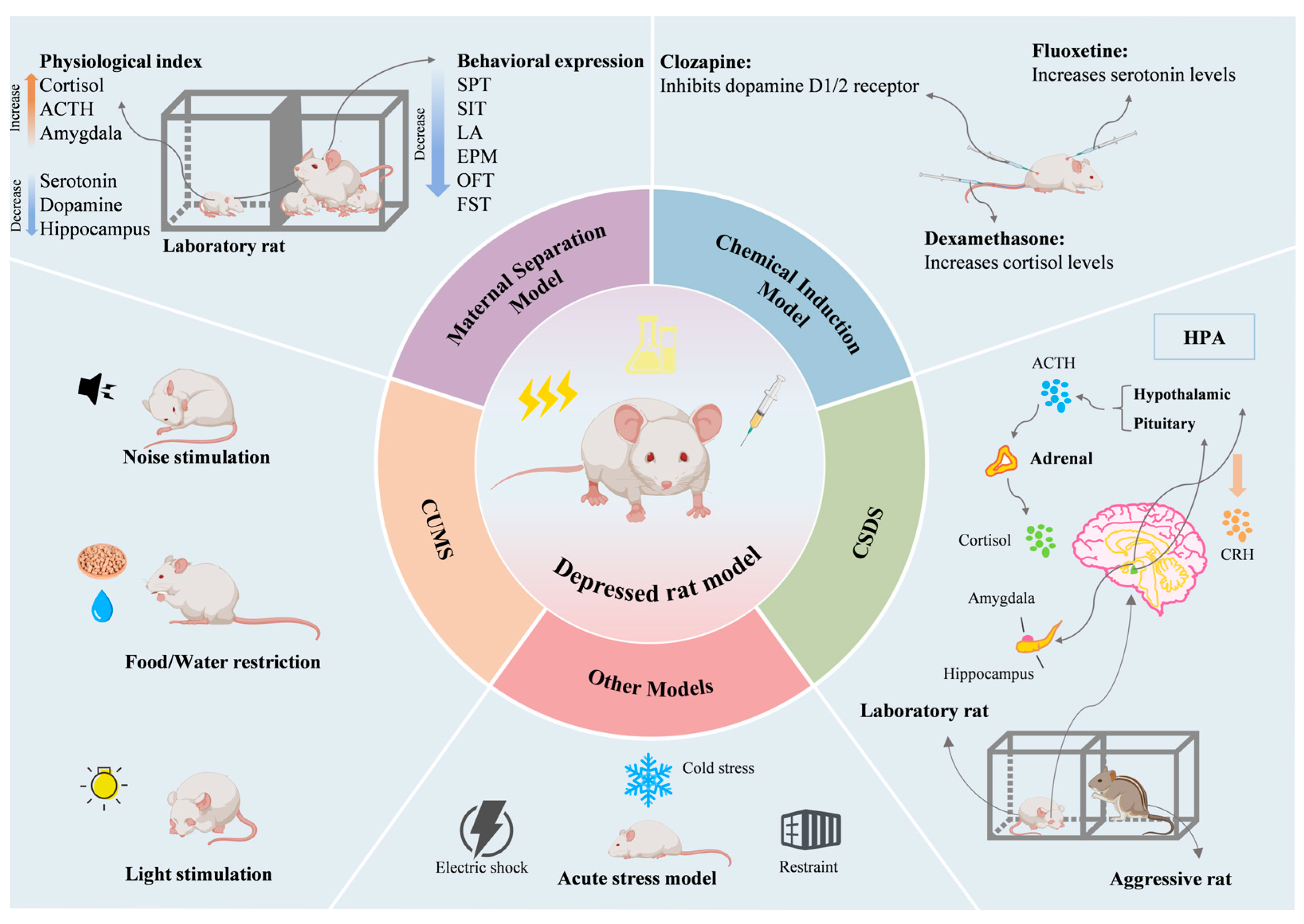
2. Construction of Rat Model of Depression
2.1. CUMS Model
2.2. CSDS Model
2.3. Maternal Separation Model
2.4. Chemically Induced Model
2.5. Other Models
3. Behavioral Test Methods of Rat Model of Depression
3.1. Forced Swim Test (FST)
3.2. Sucrose Preference Test (SPT)
3.3. Open Field Test (OFT)
3.4. Tail Suspension Test (TST)
4. Effects of Music on Physiological and Biochemical Indexes of Depressed Rats
4.1. Cortisol Levels
4.2. Neurotransmitter Levels
4.3. Other Indexes
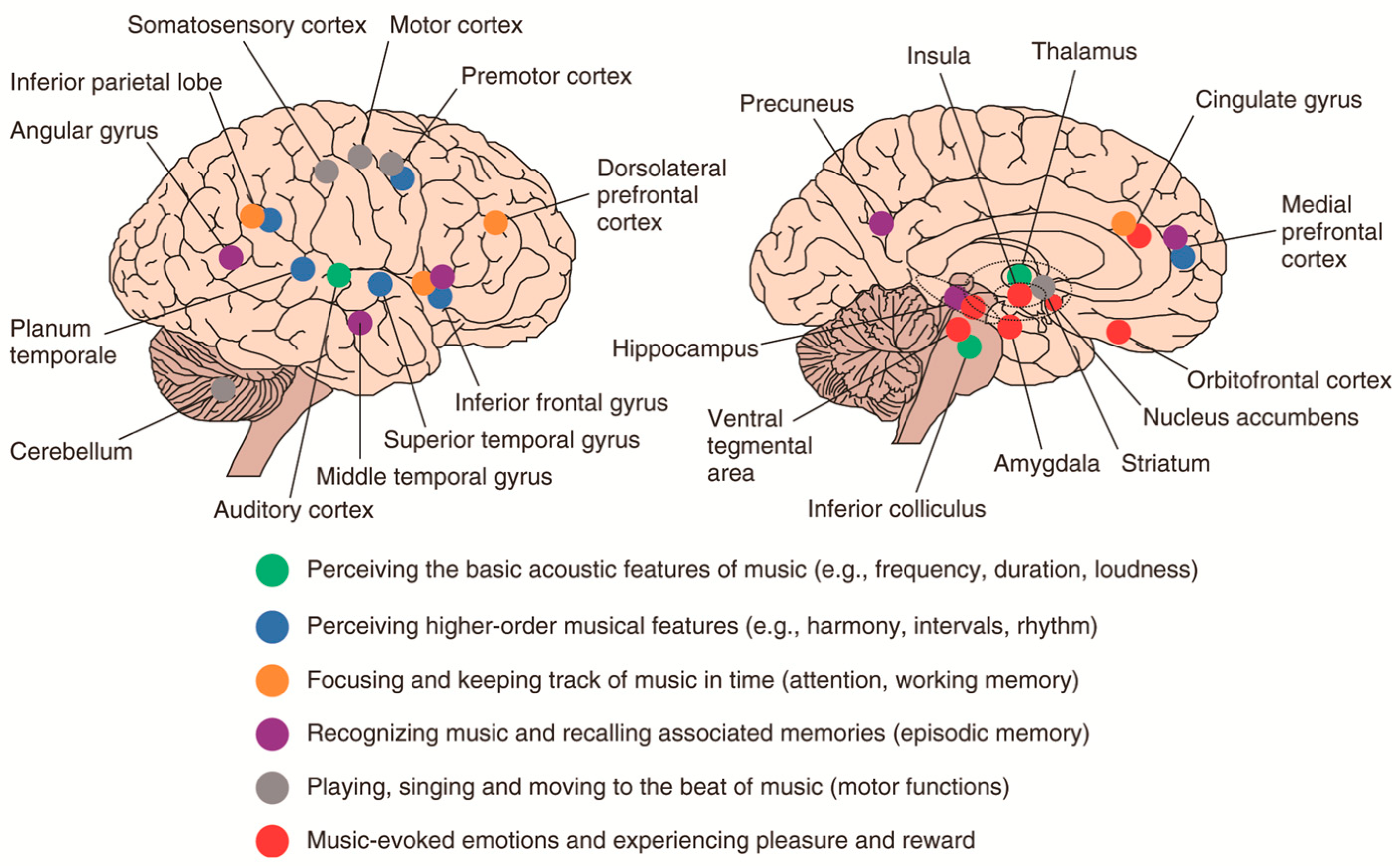
5. Effect of Music on Nervous Structure and Function in Depressed Rats
5.1. Brain Function and Adaptability
5.2. Neuroplasticity
5.3. BDNF
5.4. Neurotransmitters
6. Effects of Different Types of Music on Depressive Behavior in Rats
6.1. Classical Music
6.2. Light Music
6.3. Pop Music
6.4. Other Types of Music
7. Future Challenges and Perspectives
- (1)
- The personalization of music therapy is a key direction for future research. As individuals respond differently to music, future studies should explore how personalized music therapy programs can be tailored to a patient’s musical preferences, cultural background, and emotional state, while also considering the biophysical aspects of music such as volume, tempo, timbre, duration of tracks, and frequency range. This personalized treatment may be achievable through precision medicine, which combines genotypic and phenotypic information to optimize treatment outcomes [77,78]. With the development of big data and machine learning technologies, we can expect more accurate, personalized music therapy solutions to emerge.
- (2)
- Addressing Species-Specific Differences and Translational Limitations: Future research should also focus on understanding the species-specific differences in neurobiology and music perception between rodents and humans. This is crucial for translating the findings from rodent models to human applications. Strategies to address these limitations could include developing more sophisticated animal models that better mimic human conditions, as well as conducting parallel studies in both rodents and humans to validate the translational potential of music therapy.
- (3)
- Addressing Sample Size and Long-Term Effects: Future research should address the limitations of small sample sizes and short-term focus by conducting studies with larger cohorts and long-term follow-ups. This will enhance statistical power and provide clearer insights into the lasting effects of music therapy. Additionally, future studies should include appropriate controls, such as non-musical auditory stimuli or silence, to confirm the specificity of music’s effects. Meanwhile, with advances in remote monitoring technologies, it may be easier in future studies to assess the long-term effects of music therapy [79].
- (4)
- A deep understanding of the neurobiological mechanisms of music therapy is essential for improving therapeutic outcomes. Future research should focus on how music affects brain function and neurotransmitter levels, as well as how these changes correlate with improvements in depression. These studies may be conducted using neuroimaging techniques and molecular biological methods [80,81]. For example, music has been found to improve the behavior of depressed mice by regulating the levels of serotonin (5-HT) [47]. With advances in neuroscience, we hope to uncover the specific effects of music therapy on brain structure and function.
- (5)
- Combining music therapy with other therapeutic approaches (e.g., drug therapy, psychotherapy) may improve therapeutic outcomes. Future research is needed to explore the optimal combination and timing of these multimodal interventions to maximize the therapeutic effects. Technological innovations, such as virtual reality (VR) and artificial intelligence (AI), present new possibilities for music therapy. AI can analyze rats’ emotional responses in real time and adjust musical stimuli, while VR can provide an immersive environment for musical experiences.
- (6)
- The cost-effectiveness and acceptability of music therapy make it an important component of public health strategies, particularly in preventing depression and promoting mental health. With the development of Internet technology, remote music therapy has become possible, enabling more rats to receive treatment in experimental settings. The realization of teletherapy will depend on the advancements in web technology and mobile applications, which will make music therapy more convenient and accessible.
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Musleh-Vega, S.; Ojeda, J.; Vidal, P.M. Gut Microbiota-Brain Axis as a Potential Modulator of Psychological Stress after Spinal Cord Injury. Biomedicines 2022, 10, 847. [Google Scholar] [CrossRef]
- Correia, B.; Fernandes, J.; Botica, M.J.; Ferreira, C.; Quintas, A. Novel Psychoactive Substances: The Razor’s Edge between Therapeutical Potential and Psychoactive Recreational Misuse. Medicines 2022, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Li, Y.; Yuan, J.; Zhi, N.; Huang, Y.; Liu, Y.; Zhang, M.; Wu, S.; Zhao, X. New Insights into TETs in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 4909. [Google Scholar] [CrossRef]
- Fleischmann, A.; Arensman, E.; Berman, A.; Carli, V.; De Leo, D.; Hadlaczky, G.; Howlader, S.; Vijayakumar, L.; Wasserman, D.; Saxena, S. Overview evidence on interventions for population suicide with an eye to identifying best-supported strategies for LMICs. Glob. Ment. Health 2016, 3, e5. [Google Scholar] [CrossRef]
- Li, D.; Wu, Q.; Han, X. Application of Medial Ganglionic Eminence Cell Transplantation in Diseases Associated With Interneuron Disorders. Front. Cell. Neurosci. 2022, 16, 939294. [Google Scholar] [CrossRef]
- Lv, X.; Wang, Y.; Zhang, Y.; Ma, S.; Liu, J.; Ye, K.; Wu, Y.; Voon, V.; Sun, B. Auditory entrainment coordinates cortical-BNST-NAc triple time locking to alleviate the depressive disorder. Cell Rep. 2024, 43, 114474. [Google Scholar]
- Zhang, N.; Chen, S.; Li, Q.; He, Z.; Jiang, W. Efficacy of art therapy in enhancing mental health of clinical nurses: A meta-analysis. J. Psychiatr. Ment. Health Nurs. 2024, 31, 729–741. [Google Scholar] [CrossRef]
- Matziorinis, A.M.; Koelsch, S. The promise of music therapy for Alzheimer’s disease: A review. Ann. N. Y. Acad. Sci. 2022, 1516, 11–17. [Google Scholar] [CrossRef]
- Lin, D.; Huang, X.; Sun, Y.; Wei, C.; Wu, A. Perioperative Sleep Disorder: A Review. Front. Med. 2021, 8, 640416. [Google Scholar] [CrossRef]
- Kannan, M.A.; Ab Aziz, N.A.; Ab Rani, N.S.; Abdullah, M.W.; Mohd Rashid, M.H.; Shab, M.S.; Ismail, N.I.; Ab Ghani, M.A.; Reza, F.; Muzaimi, M. A review of the holy Quran listening and its neural correlation for its potential as a psycho-spiritual therapy. Heliyon 2022, 8, e12308. [Google Scholar] [CrossRef]
- Pasqualitto, F.; Panin, F.; Maidhof, C.; Thompson, N.; Fachner, J. Neuroplastic Changes in Addiction Memory—How Music Therapy and Music-Based Intervention May Reduce Craving: A Narrative Review. Brain Sci. 2023, 13, 259. [Google Scholar] [CrossRef] [PubMed]
- Talaee, N.; Azad Yekta, M.; Vaseghi, S. New insights into individual differences in response to chronic unpredictable mild stress (CUMS) in rats with respect to hippocampal BDNF and GSK3-β expression levels. Physiol. Behav. 2024, 287, 114718. [Google Scholar] [CrossRef]
- Lin, S.; Du, Y.; Xia, Y.; Xie, Y.; Xiao, L.; Wang, G. Advances in optogenetic studies of depressive-like behaviors and underlying neural circuit mechanisms. Front. Psychiatry 2022, 13, 950910. [Google Scholar] [CrossRef]
- Hao, J.; Jiang, K.; Wu, M.; Yu, J.; Zhang, X. The effects of music therapy on amino acid neurotransmitters: Insights from an animal study. Physiol. Behav. 2020, 224, 113024. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Erwood, K.; Ncomanzi, S.; Fischer, V.; O’Brien, D.; Lee, A. Management strategies for adult patients with dental anxiety in the dental clinic: A systematic review. Aust. Dent. J. 2022, 67 (Suppl. S1), S3–S13. [Google Scholar] [CrossRef]
- Yoon, H.; Baek, H.J. External Auditory Stimulation as a Non-Pharmacological Sleep Aid. Sensors 2022, 22, 1264. [Google Scholar] [CrossRef]
- Sharma, S.; Chawla, S.; Kumar, P.; Ahmad, R.; Kumar Verma, P. The chronic unpredictable mild stress (CUMS) Paradigm: Bridging the gap in depression research from bench to bedside. Brain Res. 2024, 1843, 149123. [Google Scholar] [CrossRef] [PubMed]
- Fei, F.; Chen, Z.; Tao, Y.; Jiang, X.; Xu, X.; Ma, Y.; Feng, P.; Wang, P. Comparison of CUMS at different pregnancy stages, maternal separation, and their effects on offspring in postpartum depression mouse models. Heliyon 2024, 10, e35363. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, Z.; Liang, Y.; Yang, R.; Tan, Y. Exploring the role and mechanism of sodium benzoate in CUMS-induced depression model of rats. Neuro Endocrinol. Lett. 2020, 41, 205–212. [Google Scholar]
- Huang, S.-H.; Liu, W.-Z.; Qin, X.; Guo, C.-Y.; Xiong, Q.-C.; Wang, Y.; Hu, P.; Pan, B.-X.; Zhang, W.-H. Association of Increased Amygdala Activity with Stress-Induced Anxiety but not Social Avoidance Behavior in Mice. Neurosci. Bull. 2022, 38, 16–28. [Google Scholar] [CrossRef]
- Kim, J.; Pokharel, K.; Sandali, M.; Kim, C.S. Establishment of the Mouse Model of Social Avoidance Induced by Female-Directed Female Aggression. Chronic Stress 2022, 6, 24705470221129288. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zheng, Y.; Zhai, Z.; Chen, Y.; Zhu, Y.; Qiu, G.; Wang, B.; Wang, S.; Chen, Y.; Yan, J. Electroacupuncture restores maternal separation-induced glutamatergic presynaptic deficits of the medial prefrontal cortex in adulthood. Neuroscience 2025, 570, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Raglan, G.B.; Schmidt, L.A.; Schulkin, J. The role of glucocorticoids and corticotropin-releasing hormone regulation on anxiety symptoms and response to treatment. Endocr. Connect. 2017, 6, R1–R7. [Google Scholar] [CrossRef]
- Pranav Chintamani, J.; Sugato, B. Effects of glucocorticoids in depression: Role of astrocytes. AIMS Neurosci. 2018, 5, 200–210. [Google Scholar] [CrossRef]
- Dehdar, K.; Raoufy, M.R. Effects of inhaled corticosteroids on brain volumetry, depression and anxiety-like behaviors in a rat model of asthma. Respir. Physiol. Neurobiol. 2023, 315, 104121. [Google Scholar] [CrossRef]
- Kawano, M.; Oshibuchi, H.; Kawano, T.; Muraoka, H.; Tsutsumi, T.; Yamada, M.; Inada, K.; Ishigooka, J. Dopamine dynamics during emotional cognitive processing: Implications of the specific actions of clozapine compared with haloperidol. Eur. J. Pharmacol. 2016, 781, 148–156. [Google Scholar] [CrossRef]
- Gumuslu, E.; Mutlu, O. Effects of Olanzapine and Clozapine on Depression and Anxiety in Mice: Alterations in Brain Neurotrophic Factor Expression Levels. J. Acad. Res. Med. 2019, 9, 97–101. [Google Scholar] [CrossRef]
- Ko, M.-C.; Lee, L.J.-H.; Li, Y.; Lee, L.-J. Long-term consequences of neonatal fluoxetine exposure in adult rats. Dev. Neurobiol. 2014, 74, 1038–1051. [Google Scholar] [CrossRef]
- Houwing, D.J.; de Waard, J.; Ramsteijn, A.S.; Woelders, T.; de Boer, S.F.; Wams, E.J.; Olivier, J.D.A. Perinatal fluoxetine exposure disrupts the circadian response to a phase-shifting challenge in female rats. Psychopharmacology 2020, 237, 2555–2568. [Google Scholar] [CrossRef]
- Moreines, J.L.; Owrutsky, Z.L.; Gagnon, K.G.; Grace, A.A. Divergent effects of acute and repeated quetiapine treatment on dopamine neuron activity in normal vs. chronic mild stress induced hypodopaminergic states. Transl. Psychiatry 2017, 7, 1275. [Google Scholar] [CrossRef]
- Estanislau, C.; Ramos, A.C.; Ferraresi, P.D.; Costa, N.F.; de Carvalho, H.M.C.P.; Batistela, S. Individual differences in the elevated plus-maze and the forced swim test. Behav. Process. 2011, 86, 46–51. [Google Scholar] [CrossRef]
- Mul, J.D.; Zheng, J.; Goodyear, L.J. Validity Assessment of 5 Day Repeated Forced-Swim Stress to Model Human Depression in Young-Adult C57BL/6J and BALB/cJ Mice. eNeuro 2016, 3, ENEURO.0201-16.2016. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Xu, S.; Wang, L.; Guo, L.; Zhou, X.; Wu, H.; Wang, W.; Liu, L. Salidroside exerts antidepressant-like action by promoting adult hippocampal neurogenesis through SIRT1/PGC-1α signalling. Acta Neuropsychiatr. 2024, 36, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.M.; Wang, B.; Wang, T.; Shao, J.; Chen, H.R.; Zhang, C.; Xu, L.H.; Li, J.J.; Wang, M.; Xu, D.X.; et al. Prenatal exposure to fenvalerate causes depressive-like behavior in adulthood by inhibiting brain-derived 5-HT synthesis. Environ. Pollut. 2024, 352, 124137. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, J.; Lan, J. Acute manipulation of Drd1 neurons in the prefrontal cortex bidirectionally regulates anxiety and depression-like behaviors. Neurosci. Lett. 2024, 832, 137805. [Google Scholar] [CrossRef]
- Yang, T.; Du, X.; Xu, L.X. Radioprotective effect of Ginkgolide B on brain: The mediating role of DCC/MST1 signaling. Int. J. Radiat. Biol. 2024, 100, 371–384. [Google Scholar] [CrossRef]
- Vadnie, C.A.; DePoy, L.M.; McClung, C.A. Measuring the Effects of Circadian Rhythm-Related Manipulations on Depression-Like Behavior in Rodents: Forced Swim and Tail Suspension Tests. In Circadian Clocks: Methods and Protocols; Brown, S.A., Ed.; Springer: New York, NY, USA, 2021; pp. 69–78. [Google Scholar]
- Bougarel, L.; Guitton, J.; Zimmer, L.; Vaugeois, J.-M.; El Yacoubi, M. Behaviour of a genetic mouse model of depression in the learned helplessness paradigm. Psychopharmacology 2011, 215, 595–605. [Google Scholar] [CrossRef]
- Gao, N.; Zheng, W.; Murezati, T.; Gu, W.; Li, X.; Jin, Z. GW117: A novel serotonin (5-HT2C) receptor antagonist and melatonin (MT1/MT2) receptor agonist with potential antidepressant-like activity in rodents. CNS Neurosci. Ther. 2021, 27, 702–713. [Google Scholar] [CrossRef]
- Jeon, S.-C.; Kim, H.-J.; Ko, E.-A.; Jung, S.-C. Prenatal Exposure to High Cortisol Induces ADHD-like Behaviors with Delay in Spatial Cognitive Functions during the Post-weaning Period in Rats. Exp. Neurobiol. 2021, 30, 87–100. [Google Scholar] [CrossRef]
- Shapero, B.G.; Curley, E.E.; Black, C.L.; Alloy, L.B. The interactive association of proximal life stress and cumulative HPA axis functioning with depressive symptoms. Depress. Anxiety 2019, 36, 1089–1101. [Google Scholar] [CrossRef]
- Fawzi, S.F.; Michel, H.E.; Menze, E.T.; Tadros, M.G.; George, M.Y. Clotrimazole ameliorates chronic mild stress-induced depressive-like behavior in rats; crosstalk between the HPA, NLRP3 inflammasome, and Wnt/β-catenin pathways. Int. Immunopharmacol. 2024, 127, 111354. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.B.; Pinna, G.; Barros, H.M.T. The Role of HPA Axis and Allopregnanolone on the Neurobiology of Major Depressive Disorders and PTSD. Int. J. Mol. Sci. 2021, 22, 5495. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Guo, W. Emotional Roles of Mono-Aminergic Neurotransmitters in Major Depressive Disorder and Anxiety Disorders. Front. Psychol. 2018, 9, 2201. [Google Scholar] [CrossRef]
- Ma, M.Y.; Chen, Q.X.; Cao, W.; Zhou, Y.B.; Yan, A.J.; Zhu, Y.R. LC-MS/MS-based Quantification of Ten Neurotransmitters in Rat Limbic System and Serum: Application to Chronic Unpredictable Mild Stress Induced Depression Rats. Mass. Spectrom. Lett. 2023, 14, 91–103. [Google Scholar] [CrossRef]
- Moraes, M.M.; Rabelo, P.C.R.; Pinto, V.A.; Pires, W.; Wanner, S.P.; Szawka, R.E.; Soares, D.D. Auditory stimulation by exposure to melodic music increases dopamine and serotonin activities in rat forebrain areas linked to reward and motor control. Neurosci. Lett. 2018, 673, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cai, D.; Lou, W. Music alleviates pain perception in depression mouse models by promoting the release of glutamate in the hippocampus of mice to act on GRIK5. Nucleosides Nucleotides Nucleic Acids 2022, 41, 463–473. [Google Scholar] [CrossRef]
- Liu, H.; Peng, X.G.; Gao, R.; Yang, K.; Zhao, Y.B. Comparative analysis of noise and music exposure on inflammatory responses on lipopolysaccharide-induced septic rats. Hum. Exp. Toxicol. 2024, 43, 9603271241282584. [Google Scholar] [CrossRef]
- Ribeiro, M.K.A.; Alcântara-Silva, T.R.M.; Oliveira, J.C.M.; Paula, T.C.; Dutra, J.B.R.; Pedrino, G.R.; Simões, K.; Sousa, R.B.; Rebelo, A.C.S. Music therapy intervention in cardiac autonomic modulation, anxiety, and depression in mothers of preterms: Randomized controlled trial. BMC Psychol. 2018, 6, 57. [Google Scholar] [CrossRef]
- Darki, C.; Riley, J.; Dadabhoy, D.P.; Darki, A.; Garetto, J. The Effect of Classical Music on Heart Rate, Blood Pressure, and Mood. Cureus 2022, 14, e27348. [Google Scholar] [CrossRef]
- Amagdei, A.; Balteş, F.R.; Avram, J.; Miu, A.C. Perinatal exposure to music protects spatial memory against callosal lesions. Int. J. Dev. Neurosci. 2010, 28, 105–109. [Google Scholar] [CrossRef]
- Sheikhi, S.; Saboory, E. Neuroplasticity Changes of Rat Brain by Musical Stimuli during Fetal Period. Cell J. 2015, 16, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Sihvonen, A.J.; Särkämö, T.; Leo, V.; Tervaniemi, M.; Altenmüller, E.; Soinila, S. Music-based interventions in neurological rehabilitation. Lancet Neurol. 2017, 16, 648–660. [Google Scholar] [CrossRef]
- Särkämö, T.; Tervaniemi, M.; Huotilainen, M. Music perception and cognition: Development, neural basis, and rehabilitative use of music. Wiley Interdiscip. Rev. Cogn. Sci. 2013, 4, 441–451. [Google Scholar] [CrossRef]
- Koelsch, S. Brain correlates of music-evoked emotions. Nat. Rev. Neurosci. 2014, 15, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Kim, B.-K.; Kim, T.-W.; Ji, E.-S.; Choi, H.-H. Music application alleviates short-term memory impairments through increasing cell proliferation in the hippocampus of valproic acid-induced autistic rat pups. J. Exerc. Rehabil. 2016, 12, 148–155. [Google Scholar] [CrossRef]
- Cheng, H.-Y.; Xie, H.-X.; Tang, Q.-L.; Yi, L.-T.; Zhu, J.-X. Light and classical music therapies attenuate chronic unpredictable mild stress-induced depression via BDNF signaling pathway in mice. Heliyon 2024, 10, e34196. [Google Scholar] [CrossRef]
- Xiong, X.; Han, L.; Liu, S.; Miao, J.; Luo, M.; Xue, M.; Wang, X.; Ni, L.; Yang, J.; Huang, C. Music intervention improves spatial learning and memory and alters serum proteomics profiling in rats. J. Neurosci. Res. 2018, 96, 1727–1736. [Google Scholar] [CrossRef]
- Fu, Q.; Qiu, R.; Chen, L.; Chen, Y.W.; Qi, W.; Cheng, Y. Music prevents stress-induced depression and anxiety-like behavior in mice. Transl. Psychiatry 2023, 13, 317. [Google Scholar] [CrossRef]
- Putra, A.; Irwanto; I’Tishom, R.; Setyoboedi, B.; Mustakim, M.R.D. Mozart music stimulation effect on wistar rats’ neurogenesis. Bali Med. J. 2023, 12, 921–925. [Google Scholar] [CrossRef]
- Gao, Y.N.; Zhang, Y.Q.; Wang, H.; Deng, Y.L.; Li, N.M. A New Player in Depression: MiRNAs as Modulators of Altered Synaptic Plasticity. Int. J. Mol. Sci. 2022, 23, 4555. [Google Scholar] [CrossRef]
- Dong, L.; Zhao, T.; Jin, Z.; Zheng, Y. Effect of music rhythm magnetic field on long-term potentiation of hippocampal Schaffer-CA1 synapse plasticity. Neurosci. Lett. 2024, 820, 137576. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, L.; Zhang, B.T.; Lu, A.; Wang, Y.; Yu, Y.; Zhang, G. Artificial Intelligence in Aptamer-Target Binding Prediction. Int. J. Mol. Sci. 2021, 22, 3605. [Google Scholar] [CrossRef] [PubMed]
- Korsós, G.; Horváth, K.; Lukács, A.; Vezér, T.; Glávits, R.; Fodor, K.; Fekete, S.G. Effects of accelerated human music on learning and memory performance of rats. Appl. Anim. Behav. Sci. 2018, 202, 94–99. [Google Scholar] [CrossRef]
- Rizzolo, L.; Leger, M.; Corvaisier, S.; Groussard, M.; Platel, H.; Bouet, V.; Schumann-Bard, P.; Freret, T. Long-Term Music Exposure Prevents Age-Related Cognitive Deficits in Rats Independently of Hippocampal Neurogenesis. Cereb. Cortex 2021, 31, 620–634. [Google Scholar] [CrossRef] [PubMed]
- Chen, R. Emotional Inhibitory Effect of Music Therapy on Anxiety Neurosis Based on Neural Content Analysis in Hippocampus. Neuroquantology 2018, 16, 53–59. [Google Scholar] [CrossRef]
- Xing, Y.S.; Chen, W.X.; Wang, Y.R.; Jing, W.; Gao, S.; Guo, D.Q.; Xia, Y.; Yao, D.Z. Music exposure improves spatial cognition by enhancing the BDNF level of dorsal hippocampal subregions in the developing rats. Brain Res. Bull. 2016, 121, 131–137. [Google Scholar] [CrossRef]
- Chen, W.G.; Zheng, J.X.; Shen, G.Y.; Ji, X.; Sun, L.L.; Li, X.; Xu, F.; Gu, J.H. Music Therapy Alleviates Motor Dysfunction in Rats With Focal Cerebral Ischemia-Reperfusion Injury by Regulating BDNF Expression. Front. Neurol. 2021, 12, 666311. [Google Scholar] [CrossRef]
- Luo, C.P.; Fan, H.M.; Li, S.J.; Zou, Y.L. Therapeutic of Candesartan and Music Therapy in Diabetic Retinopathy with Depression in Rats. Evid.-Based Complement. Altern. Med. 2021, 2021, 5570356. [Google Scholar] [CrossRef]
- Xing, Y.; Qin, Y.; Jing, W.; Zhang, Y.; Wang, Y.; Guo, D.; Xia, Y.; Yao, D. Exposure to Mozart music reduces cognitive impairment in pilocarpine-induced status epilepticus rats. Cogn. Neurodyn. 2016, 10, 23–30. [Google Scholar] [CrossRef]
- Papadakakis, A.; Sidiropoulou, K.; Panagis, G. Music exposure attenuates anxiety- and depression-like behaviors and increases hippocampal spine density in male rats. Behav. Brain Res. 2019, 372, 112023. [Google Scholar] [CrossRef]
- Fekete, S.; Sukikara, C.; Korsós, G. Influence of different environmental effects of human origin (socialization, music, noisemusic, noise) upon the rats’ behaviour. Part 3. Effect of different noises on the open-field test behaviour. Magy. Allatorvosok Lapja 2013, 135, 692–698. [Google Scholar]
- Singer, N.; Jacoby, N.; Lin, T.; Raz, G.; Shpigelman, L.; Gilam, G.; Granot, R.Y.; Hendler, T. Common modulation of limbic network activation underlies musical emotions as they unfold. NeuroImage 2016, 141, 517–529. [Google Scholar] [CrossRef]
- Escribano, B.; Quero, I.; Feijóo, M.; Tasset, I.; Montilla, P.; Túnez, I. Role of noise and music as anxiety modulators: Relationship with ovarian hormones in the rat. Appl. Anim. Behav. Sci. 2014, 152, 73–82. [Google Scholar] [CrossRef]
- Pranoto, A.; Wahyudi, E.; Prasetya, R.E.; Fauziyah, S.; Kinanti, R.G.; Sugiharto, S.; Rejeki, P.S. High intensity exercise increases brain derived neurotrophic factor expression and number of hippocampal neurons in rats. Comp. Exerc. Physiol. 2020, 16, 325–332. [Google Scholar] [CrossRef]
- Zhou, W.J.; Ye, C.H.; Wang, H.T.; Mao, Y.; Zhang, W.J.; Liu, A.; Yang, C.L.; Li, T.M.; Hayashi, L.R.; Zhao, W.; et al. Sound induces analgesia through corticothalamic circuits. Science 2022, 377, 198–204. [Google Scholar] [CrossRef]
- Dutta, E.; Bothra, A.; Chaspari, T.; Ioerger, T.; Mortazavi, B.J. Reinforcement Learning using EEG signals for Therapeutic Use of Music in Emotion Management. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 5553–5556. [Google Scholar]
- Chao, H.; Dong, L.; Liu, Y.; Lu, B. Emotion Recognition from Multiband EEG Signals Using CapsNet. Sensors 2019, 19, 2212. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Khan, A.A.; Nait-Abdesselam, F.; Dey, I. Anxiety and Depression Management For Elderly Using Internet of Things and Symphonic Melodies. In Proceedings of the ICC 2021—IEEE International Conference on Communications, Montreal, QC, Canada, 14–23 June 2021; pp. 1–6. [Google Scholar]
- Papagiannopoulou, E.A. Auditory Processing in ASD & Sound-Based Interventions. Music. Percept. 2015, 32, 515–529. [Google Scholar] [CrossRef]
- Jauset-Berrocal, J.A.; Soria-Urios, G. Cognitive neurorehabilitation: The foundations and applications of neurologic music therapy. Rev. Neurol. 2018, 67, 303–310. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, J.; Deng, W.; Le, T. Music Therapy in Depression: Exploring Mechanisms and Efficacy in Rat Models. Brain Sci. 2025, 15, 338. https://doi.org/10.3390/brainsci15040338
Le J, Deng W, Le T. Music Therapy in Depression: Exploring Mechanisms and Efficacy in Rat Models. Brain Sciences. 2025; 15(4):338. https://doi.org/10.3390/brainsci15040338
Chicago/Turabian StyleLe, Jingqi, Wangyan Deng, and Tao Le. 2025. "Music Therapy in Depression: Exploring Mechanisms and Efficacy in Rat Models" Brain Sciences 15, no. 4: 338. https://doi.org/10.3390/brainsci15040338
APA StyleLe, J., Deng, W., & Le, T. (2025). Music Therapy in Depression: Exploring Mechanisms and Efficacy in Rat Models. Brain Sciences, 15(4), 338. https://doi.org/10.3390/brainsci15040338






