Flavonol Composition and Antioxidant Activity of Onions (Allium cepa L.) Based on the Development of New Analytical Ultrasound-Assisted Extraction Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Materials
2.2. Chemicals and Solvents
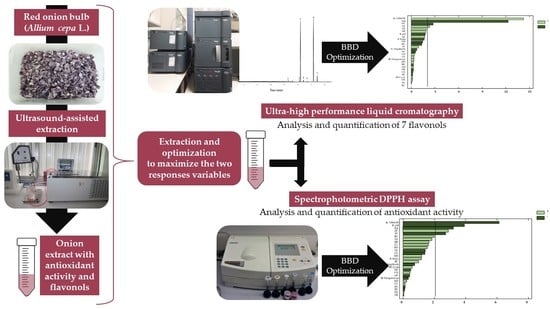
2.3. Ultrasound-Assisted Extraction
2.3.1. Ultrasound-Assisted Extraction Equipment
2.3.2. Ultrasound-Assisted Extraction Procedure
2.4. Flavonols Identification
2.5. Flavonols Quantification
2.6. Antioxidant Activity
2.7. Applying a Box-Behnken Design (BBD) to UAE Optimization
3. Results and Discussion
3.1. Stability Study
3.2. Development of the Individual UAE Method for Total Flavonols
3.2.1. Box–Behnken Design for Individual Total Flavonols
0.17·X6 − 1.94·X12 − 0.050·X1X2 − 0.099·X1X3 + 0.037·X1X4 + 0.41·X1X5 +
0.34·X1X6 − 0.046·X2 2 + 0.00057·X2X3 + 0.053·X2X4 − 0.17·X2X5 − 0.035·X2X6 −
0.44·X32 + 0.070·X3X4 − 0.038·X3X5 + 0.045·X3X6 − 0.23·X4 2 + 0.031·X4X5 +
0.34·X4X6 − 0.0082·X5 2 + 0.31·X5X6 − 0.15·X6 2,
3.2.2. Optimal Conditions for the Individual Extraction of Total Flavonols
3.2.3. Optimal Time and Precision for the Extraction of Individual Total Flavonols
3.3. Development of Individual UAE Method for Antioxidant Activity
3.3.1. Box–Behnken Design for Individual Antioxidant Activity
1.10·X12 − 0.017 ·X1X2 − 0.048·X1X3 + 0.063·X1X4 + 0.71·X1X5 − 0.16·X1X6 +
0.57·X2 2 + 0.77·X2X3 + 0.59·X2X4 − 0.028·X2X5+ 0.38·X2X6 + 0.55·X32 + 0.17·X3X4
− 0.53·X3X5 + 0.037·X3X6 + 0.41·X4 2 + 0.020·X4X5 − 0.19·X4X6 − 0.48·X5 2 +
1.11·X5X6 − 0.93·X6 2,
3.3.2. Optimal Conditions for Individual Antioxidant Activity
3.3.3. Optimal Extraction Time and Precision for Individual Antioxidant Activity
3.4. Development of a Simultaneous Extraction Method
3.5. Application of the Developed Methods to Different Onion Varieties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fredotovíc, Ž.; Šprung, M.; Soldo, B.; Ljubenkov, I.; Bidic-Leto, I.; Bilusic, T.; Cikes-Culic, V.; Puizina, J. Chemical composition and biological activity of allium cepa L. and Allium × cornutum (Clementi ex Visiani 1842) methanolic extracts. Molecules 2017, 22, 448. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, J.; Gonçalves, J.L.; Alves, V.L.; Câmara, J.S. Chemical Fingerprint of Free Polyphenols and Antioxidant Activity in Dietary Fruits and Vegetables Using a Non-Targeted Approach Based on QuEChERS Ultrasound-Assisted Extraction Combined with UHPLC-PDA. Antioxidants 2020, 9, 305. [Google Scholar] [CrossRef] [Green Version]
- Galani, J.H.Y.; Patel, J.S.; Patel, N.J.; Talati, J.G. Storage of fruits and vegetables in refrigerator increases their phenolic acids but decreases the total phenolics, anthocyanins and vitamin C with subsequent loss of their antioxidant capacity. Antioxidants 2017, 6, 59. [Google Scholar] [CrossRef] [Green Version]
- Böttcher, C.; Krähmer, A.; Stürtz, M.; Widder, S.; Schulz, H. Comprehensive metabolite profiling of onion bulbs (Allium cepa) using liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry. Metabolomics 2017, 13, 1–15. [Google Scholar] [CrossRef]
- Ko, E.Y.; Nile, S.H.; Jung, Y.S.; Keum, Y.S. Antioxidant and antiplatelet potential of different methanol fractions and flavonols extracted from onion (Allium cepa L.). 3 Biotech 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Block, E. The Organosulfur Chemistry of the Genus Allium—Implications for the Organic Chemistry of Sulfur. Angew. Chem. Int. Ed. Engl. 1992, 31, 1135–1178. [Google Scholar] [CrossRef]
- Higuchi, O.; Tateshita, K.; Nishimura, H. Antioxidative Activity of Sulfur-Containing Compounds in Allium Species for Human Low-Density Lipoprotein (LDL) Oxidation in Vitro. J. Agric. Food Chem. 2003, 51, 7208–7214. [Google Scholar] [CrossRef]
- Xiao, H.; Parkin, K.L. Antioxidant functions of selected Allium thiosulfinates and S-alk(en)yl-L-cysteine sulfoxides. J. Agric. Food Chem. 2002, 50, 2488–2493. [Google Scholar] [CrossRef]
- Ko, E.Y.; Sharma, K.; Nile, S.H. Effect of harvesting practices, lifting time, curing methods, and irrigation on quercetin content in onion (Allium cepa L.) cultivars. Emir. J. Food Agric. 2016, 28, 594–600. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.; Rok Lee, Y.; Park, S.W.; Nile, S.H. Importance of growth hormones and temperature for physiological regulation of dormancy and sprouting in onions. Food Rev. Int. 2016, 32, 233–255. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Hollman, P.C.H.; Venema, D.P. Optimization of a Quantitative HPLC Determination of Potentially Anticarcinogenic Flavonoids in Vegetables and Fruits. J. Agric. Food Chem. 1992, 40, 1591–1598. [Google Scholar] [CrossRef]
- Benmalek, Y.; Yahia, O.A.; Belkebir, A.; Fardeau, M.L. Anti-microbial and anti-oxidant activities of illicium verum, Crataegus oxyacantha ssp monogyna and Allium cepa red and white varieties. Bioengineered 2013, 4, 244–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khiari, Z.; Makris, D.P.; Kefalas, P. An investigation on the recovery of antioxidant phenolics from onion solid wastes employing water/ethanol-based solvent systems. Food Bioprocess Technol. 2009, 2, 337–343. [Google Scholar] [CrossRef]
- Kiassos, E.; Mylonaki, S.; Makris, D.P.; Kefalas, P. Implementation of response surface methodology to optimise extraction of onion (Allium cepa) solid waste phenolics. Innov. Food Sci. Emerg. Technol. 2009, 10, 246–252. [Google Scholar] [CrossRef]
- Mason, T.J.; Paniwnyk, L.; Lorimer, J.P. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996, 3, S253–S260. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef]
- González de Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Amores-Arrocha, A.; Palma, M.; Barbero, G.F.; Jiménez-Cantizano, A. Alternative Ultrasound-Assisted Method for the Extraction of the Bioactive Compounds Present in Myrtle (Myrtus communis L.). Molecules 2019, 24, 882. [Google Scholar] [CrossRef] [Green Version]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Zapata, L.M.; Heredia, A.M.; Quinteros, C.F.; Malleret, A.D.; Clemente, G.; Cárcel, J.A. Optimización de la extracción de antocianinas de arándanos. Cienc. Docencia y Tecnol. 2014, 25, 166–192. [Google Scholar]
- El Hami, A.; Philippe, P. Chapter 5: Electro-Thermo-Mechanical Modeling. In Embedded Mechatronic Systems–Analysis of Failures, Modeling, Simulation and Optimizationook, 2nd ed.; ISTE Press-Elsevier: London, UK, 2012; Volume 2, pp. 107–150. [Google Scholar]
- Hu, Z.; Cai, M.; Liang, H.H. Desirability function approach for the optimization of microwave-assisted extraction of saikosaponins from Radix Bupleuri. Sep. Purif. Technol. 2008, 61, 266–275. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Carrera, C.; Palma, M.; Barroso, C.G. Alternative Extraction Method of Bioactive Compounds from Mulberry (Morus nigra L.) Pulp Using Pressurized-Liquid Extraction. Food Anal. Methods 2018, 11, 2384–2395. [Google Scholar] [CrossRef]
- González de Peredo, A.V.; Vázquez-Espinosa, M.; Piñeiro, Z.; Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Development of a rapid and accurate UHPLC-PDA-FL method for the quantification of phenolic compounds in grapes. Food Chem. 2021, 334, 127569. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterisation of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant. J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandao, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Maran, J.; Manikandan, S.; Thirugnanasambandham, K.; Vigna Nivetha, C.; Dinesh, R. Box-Behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide. Carbohydr. Polym. 2013, 92, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Setyaningsih, W.; Saputro, I.E.; Carrera, C.A.; Palma, M. Optimisation of an ultrasound-assisted extraction method for the simultaneous determination of phenolics in rice grains. Food Chem. 2019, 288, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barbero, G.; Pinedo, C.; Espada-Bellido, E.; Carrera, C.; Palma, M.; García-Barroso, C. Optimization of ultrasound-assisted extraction of bioactive compounds from jabuticaba (Myrciaria cauliflora) fruit through a Box-Behnken experimental design. Food Sci. Technol. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Kidak, R.; Ince, N.H. Ultrasonic destruction of phenol and substituted phenols: A review of current research. Ultrason. Sonochem. 2006, 13, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pereira, A.L.D.; Barbero, G.F.; Martínez, J. Recovery of anthocyanins from residues of Rubus fruticosus, Vaccinium myrtillus and Eugenia brasiliensis by ultrasound assisted extraction, pressurized liquid extraction and their combination. Food Chem. 2017, 231, 1–10. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Wijngaard, H.H.; Ballay, M.; Brunton, N. The optimisation of extraction of antioxidants from potato peel by pressurised liquids. Food Chem. 2012, 133, 1123–1130. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Howard, L.R. Effects of Solvent and Temperature on Pressurized Liquid Extraction of Anthocyanins and Total Phenolics from Dried Red Grape Skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef]
- Ivanovic, J.; Tadic, V.; Dimitrijevic, S.; Stamenic, M.; Petrovic, S.; Zizovic, I. Antioxidant properties of the anthocyanin-containing ultrasonic extract from blackberry cultivar “Čačanska Bestrna”. Ind. Crops Prod. 2014, 53, 274–281. [Google Scholar] [CrossRef]
- Biesaga, M. Influence of extraction methods on stability of flavonoids. J. Chromatogr. A 2011, 1218, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Luque de Castro, M.D.; Priego-Capote, F. Ultrasound assistance to liquid-liquid extraction: A debatable analytical tool. Anal. Chim. Acta 2007, 583, 2–9. [Google Scholar] [CrossRef]
- Stipcovich, T.; Barbero, G.F.; Ferreiro-González, M.; Palma, M.; Barroso, C.G. Fast analysis of capsaicinoids in Naga Jolokia extracts (Capsicum chinense) by high-performance liquid chromatography using fused core columns. Food Chem. 2018, 239, 217–224. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists. Peer Verified Methods Advisory Committee. In AOAC Peer Verified Methods Program; AOAC International: Gaithersburg, MD, USA, 1998; pp. 1–35. [Google Scholar]
- Hossain, M.B.; Lebelle, J.; Birsan, R.; Rai, D.K. Enrichment and assessment of the contributions of the major polyphenols to the total antioxidant activity of onion extracts: A fractionation by flash chromatography approach. Antioxidants 2018, 7, 175. [Google Scholar] [CrossRef] [Green Version]
- Campone, L.; Celano, R.; Piccinelli, A.L.; Pagano, I.; Carabetta, S.; Di Sanzo, R.; Russo, M.; Ibañez, E.; Cifuentes, A.; Rastrelli, L. Response surface methodology to optimize supercritical carbon dioxide/co-solvent extraction of brown onion skin by-product as source of nutraceutical compounds. Food Chem. 2018, 269, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Vilkhu, K.; Manasseh, R.; Mawson, R.; Ashokkumar, M. Ultrasound Technologies for Food and Bioprocessing; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lanchos-Perez, D.; Torres-Mayanga, P.C.; da Fonsecha Machado, A.P.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, P.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Flavonol glucosides in Allium species: A comparative study by means of HPLC-DAD-ESI-MS-MS. Food Chem. 2008, 107, 1668–1673. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Espada-Bellido, E.; Ferreiro-González, M.; Toledo-Domínguez, J.J.; Carrera, C.; Palma, M.; Barbero, G.F. Ultrasound-Assisted Extraction of Two Types of Antioxidant Compounds (TPC and TA) from Black Chokeberry (Aronia melanocarpa L.): Optimization of the Individual and Simultaneous Extraction Methods. Agronomy 2019, 9, 456. [Google Scholar] [CrossRef] [Green Version]
- Corell, L.; Armenta, S.; Esteve-Turrillas, F.A.; de la Guardia, M. Flavonoid determination in onion, chili and leek by hard cap espresso extraction and liquid chromatography with diode array detection. Microchem. J. 2018, 140, 74–79. [Google Scholar] [CrossRef]
- Jin, E.Y.; Lim, S.; oh Kim, S.; Park, Y.-S.; Jang, K.Y.; Chung, M.-S.; Park, H.; Shim, K.-S.; Choi, Y.J. Optimization of various extraction methods for quercetin from onion skin using response surface methodology. Food Sci. Biotechnol. 2011, 20, 1727–1733. [Google Scholar] [CrossRef]
- Martino, K.G.; Guyer, D. Supercritical fluid extraction of quercetin from onion skins. J. Food Process Eng. 2004, 27, 17–28. [Google Scholar] [CrossRef]
- Turner, C.; Turner, P.; Jacobson, G.; Almgren, K.; Waldeback, M.; Sjoberg, P.; Karlsson, E.N.; Markides, K.E. Subcritical water extraction and β-glucosidase-catalyzed hydrolysis of quercetin glycosides in onion waste. Green Chem. 2006, 8, 949–959. [Google Scholar] [CrossRef]








| Run | Factors | Responses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | X6 | YTF (mg Flavonols g−1 DW) | YDPPH (mg TE g−1 DW) | |||
| Experimental | Predicted | Experimental | Predicted | |||||||
| 1 | 0 | 0 | −1 | 0 | −1 | −1 | 8.00 | 7.94 | 10.41 | 10.02 |
| 2 | 0 | 0 | 1 | 0 | −1 | −1 | 7.59 | 7.78 | 10.41 | 10.69 |
| 3 | 0 | 0 | −1 | 0 | 1 | −1 | 7.15 | 7.03 | 9.80 | 7.12 |
| 4 | 0 | 0 | 1 | 0 | 1 | −1 | 7.09 | 6.72 | 5.05 | 5.65 |
| 5 | 0 | 0 | −1 | 0 | −1 | 1 | 6.67 | 6.89 | 8.27 | 8.23 |
| 6 | 0 | 0 | 1 | 0 | −1 | 1 | 6.65 | 6.92 | 6.93 | 9.05 |
| 7 | 0 | 0 | −1 | 0 | 1 | 1 | 7.56 | 7.22 | 9.51 | 9.79 |
| 8 | 0 | 0 | 1 | 0 | 1 | 1 | 6.88 | 7.09 | 8.63 | 8.47 |
| 9 | 0 | −1 | 0 | −1 | −1 | 0 | 7.25 | 7.26 | 11.00 | 11.37 |
| 10 | 0 | 1 | 0 | −1 | −1 | 0 | 7.33 | 7.57 | 10.68 | 10.09 |
| 11 | 0 | −1 | 0 | 1 | −1 | 0 | 6.90 | 7.13 | 11.11 | 10.41 |
| 12 | 0 | 1 | 0 | 1 | −1 | 0 | 7.51 | 7.65 | 11.06 | 11.51 |
| 13 | 0 | −1 | 0 | −1 | 1 | 0 | 7.10 | 7.17 | 9.88 | 9.65 |
| 14 | 0 | 1 | 0 | −1 | 1 | 0 | 7.24 | 6.79 | 7.77 | 8.26 |
| 15 | 0 | −1 | 0 | 1 | 1 | 0 | 7.19 | 7.16 | 7.96 | 8.76 |
| 16 | 0 | 1 | 0 | 1 | 1 | 0 | 7.22 | 7.00 | 10.34 | 9.75 |
| 17 | −1 | 0 | −1 | −1 | 0 | 0 | 2.96 | 3.47 | 10.60 | 10.92 |
| 18 | 1 | 0 | −1 | −1 | 0 | 0 | 6.88 | 6.55 | 8.31 | 8.16 |
| 19 | −1 | 0 | 1 | −1 | 0 | 0 | 3.16 | 3.39 | 10.44 | 10.35 |
| 20 | 1 | 0 | 1 | −1 | 0 | 0 | 6.52 | 6.07 | 7.98 | 7.39 |
| 21 | −1 | 0 | −1 | 1 | 0 | 0 | 2.77 | 3.30 | 10.17 | 10.72 |
| 22 | 1 | 0 | −1 | 1 | 0 | 0 | 6.82 | 6.52 | 8.08 | 8.21 |
| 23 | −1 | 0 | 1 | 1 | 0 | 0 | 3.09 | 3.49 | 10.72 | 10.83 |
| 24 | 1 | 0 | 1 | 1 | 0 | 0 | 6.91 | 6.32 | 8.41 | 8.13 |
| 25 | 0 | −1 | −1 | 0 | 0 | −1 | 7.36 | 7.38 | 10.89 | 10.84 |
| 26 | 0 | 1 | −1 | 0 | 0 | −1 | 7.25 | 7.52 | 5.96 | 8.40 |
| 27 | 0 | −1 | 1 | 0 | 0 | −1 | 6.74 | 7.14 | 8.75 | 8.91 |
| 28 | 0 | 1 | 1 | 0 | 0 | −1 | 6.99 | 7.28 | 10.41 | 9.53 |
| 29 | 0 | −1 | −1 | 0 | 0 | 1 | 7.16 | 7.02 | 10.20 | 10.52 |
| 30 | 0 | 1 | −1 | 0 | 0 | 1 | 7.27 | 7.02 | 10.32 | 9.60 |
| 31 | 0 | −1 | 1 | 0 | 0 | 1 | 7.38 | 6.96 | 10.61 | 8.73 |
| 32 | 0 | 1 | 1 | 0 | 0 | 1 | 7.14 | 6.97 | 10.27 | 10.88 |
| 33 | −1 | −1 | 0 | 0 | −1 | 0 | 4.65 | 4.37 | 11.82 | 11.44 |
| 34 | 1 | −1 | 0 | 0 | −1 | 0 | 6.80 | 6.59 | 6.86 | 7.33 |
| 35 | −1 | 1 | 0 | 0 | −1 | 0 | 5.68 | 4.88 | 12.51 | 11.38 |
| 36 | 1 | 1 | 0 | 0 | −1 | 0 | 6.88 | 6.90 | 7.63 | 7.20 |
| 37 | −1 | −1 | 0 | 0 | 1 | 0 | 3.75 | 3.51 | 8.13 | 8.34 |
| 38 | 1 | −1 | 0 | 0 | 1 | 0 | 6.81 | 7.39 | 6.14 | 7.06 |
| 39 | −1 | 1 | 0 | 0 | 1 | 0 | 2.92 | 3.34 | 8.43 | 8.17 |
| 40 | 1 | 1 | 0 | 0 | 1 | 0 | 6.53 | 7.02 | 6.22 | 6.82 |
| 41 | −1 | 0 | 0 | −1 | 0 | −1 | 5.56 | 4.86 | 8.37 | 8.55 |
| 42 | 1 | 0 | 0 | −1 | 0 | −1 | 6.60 | 7.07 | 5.71 | 6.02 |
| 43 | −1 | 0 | 0 | 1 | 0 | −1 | 4.15 | 4.14 | 8.64 | 9.07 |
| 44 | 1 | 0 | 0 | 1 | 0 | −1 | 6.89 | 6.49 | 7.18 | 6.79 |
| 45 | −1 | 0 | 0 | −1 | 0 | 1 | 2.70 | 3.17 | 9.42 | 9.77 |
| 46 | 1 | 0 | 0 | −1 | 0 | 1 | 6.79 | 6.72 | 6.97 | 6.59 |
| 47 | −1 | 0 | 0 | 1 | 0 | 1 | 4.36 | 3.82 | 9.80 | 9.53 |
| 48 | 1 | 0 | 0 | 1 | 0 | 1 | 6.74 | 7.52 | 6.81 | 6.60 |
| 49 | 0 | 0 | 0 | 0 | 0 | 0 | 7.35 | 7.50 | 9.30 | 9.49 |
| 50 | 0 | 0 | 0 | 0 | 0 | 0 | 7.64 | 7.50 | 9.65 | 9.49 |
| 51 | 0 | 0 | 0 | 0 | 0 | 0 | 7.75 | 7.50 | 9.51 | 9.49 |
| 52 | 0 | 0 | 0 | 0 | 0 | 0 | 7.53 | 7.50 | 9.44 | 9.49 |
| 53 | 0 | 0 | 0 | 0 | 0 | 0 | 7.31 | 7.50 | 9.48 | 9.49 |
| 54 | 0 | 0 | 0 | 0 | 0 | 0 | 7.41 | 7.50 | 9.53 | 9.49 |
| Source | Source Code | Coefficients | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| Model | 7.50 | 112.43 | 27 | 4.16 | 16.23 | <0.001 | |
| A: %MeoH | X1 | 1.48 | 52.23 | 1 | 52.23 | 203.52 | <0.001 |
| B: Temperature | X2 | 0.036 | 0.030 | 1 | 0.030 | 0.12 | 0.734 |
| C: Amplitude | X3 | −0.072 | 0.12 | 1 | 0.12 | 0.48 | 0.494 |
| D: Cycle | X4 | 0.019 | 0.0090 | 1 | 0.0090 | 0.04 | 0.853 |
| E: pH | X5 | −0.19 | 0.82 | 1 | 0.82 | 3.21 | 0.0849 |
| F: Ratio | X6 | −0.17 | 0.69 | 1 | 0.69 | 2.67 | 0.114 |
| AA | X12 | −1.94 | 38.79 | 1 | 38.79 | 151.15 | <0.001 |
| AB | X1X2 | −0.050 | 0.020 | 1 | 0.020 | 0.08 | 0.780 |
| AC | X1X3 | −0.10 | 0.079 | 1 | 0.079 | 0.31 | 0.584 |
| AD | X1X4 | 0.037 | 0.022 | 1 | 0.022 | 0.08 | 0.774 |
| AE | X1X5 | 0.41 | 1.37 | 1 | 1.37 | 5.35 | 0.0288 |
| AF | X1X6 | 0.34 | 0.90 | 1 | 0.90 | 3.53 | 0.0717 |
| BB | X22 | −0.046 | 0.022 | 1 | 0.022 | 0.09 | 0.772 |
| BC | X2X3 | <0.001 | <0.001 | 1 | <0.001 | 0.00 | 0.997 |
| BD | X2X4 | 0.053 | 0.023 | 1 | 0.023 | 0.09 | 0.769 |
| BE | X2X5 | −0.17 | 0.47 | 1 | 0.47 | 1.84 | 0.187 |
| BF | X2X6 | −0.035 | 0.0097 | 1 | 0.0097 | 0.04 | 0.848 |
| CC | X32 | −0.44 | 1.99 | 1 | 1.99 | 7.75 | 0.00990 |
| CD | X3X4 | 0.070 | 0.039 | 1 | 0.039 | 0.15 | 0.699 |
| CE | X3X5 | −0.038 | 0.011 | 1 | 0.011 | 0.04 | 0.836 |
| CF | X3X6 | 0.045 | 0.033 | 1 | 0.033 | 0.13 | 0.723 |
| DD | X42 | −0.23 | 0.54 | 1 | 0.54 | 2.11 | 0.159 |
| DE | X4X5 | 0.031 | 0.0075 | 1 | 0.0075 | 0.03 | 0.865 |
| DF | X4X6 | 0.34 | 0.94 | 1 | 0.94 | 3.67 | 0.066 |
| EE | X52 | −0.0082 | <0.001 | 1 | <0.001 | 0.00 | 0.960 |
| EF | X5X6 | 0.31 | 0.76 | 1 | 0.76 | 2.96 | 0.0970 |
| FF | X62 | −0.14 | 0.22 | 1 | 0.22 | 0.86 | 0.361 |
| Residual | 6.67 | 26 | 0.26 | ||||
| Lack-of-Fit | 6.52 | 21 | 0.31 | 10.55 | 0.0079 | ||
| Pure Error | 0.1472 | 5 | 0.029 | ||||
| Cor Total | 119.10 | 53 |
| Source | Source Code | Coefficients | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| Model | 9.49 | 118.69 | 27 | 4.40 | 3.69 | <0.001 | |
| A: %MeoH | X1 | −1.37 | 44.79 | 1 | 44.79 | 37.61 | <0.001 |
| B: Temperature | X2 | −0.073 | 0.13 | 1 | 0.13 | 0.11 | 0.747 |
| C: Amplitude | X3 | −0.16 | 0.64 | 1 | 0.64 | 0.54 | 0.470 |
| D: Cycle | X4 | 0.13 | 0.42 | 1 | 0.42 | 0.35 | 0.559 |
| E: pH | X5 | −0.87 | 18.12 | 1 | 18.12 | 15.22 | <0.001 |
| F: Ratio | X6 | 0.26 | 1.60 | 1 | 1.60 | 1.34 | 0.258 |
| AA | X12 | −1.10 | 12.53 | 1 | 12.53 | 10.52 | 0.00320 |
| AB | X1X2 | −0.017 | 0.0023 | 1 | 0.0023 | 0.00 | 0.966 |
| AC | X1X3 | −0.048 | 0.020 | 1 | 0.020 | 0.02 | 0.902 |
| AD | X1X4 | 0.063 | 0.063 | 1 | 0.063 | 0.05 | 0.820 |
| AE | X1X5 | 0.71 | 3.99 | 1 | 3.99 | 3.35 | 0.079 |
| AF | X1X6 | −0.16 | 0.21 | 1 | 0.21 | 0.18 | 0.676 |
| BB | X22 | 0.57 | 3.31 | 1 | 3.31 | 2.78 | 0.108 |
| BC | X2X3 | 0.77 | 4.71 | 1 | 4.71 | 3.95 | 0.0575 |
| BD | X2X4 | 0.59 | 2.83 | 1 | 2.83 | 2.37 | 0.136 |
| BE | X2X5 | −0.028 | 0.012 | 1 | 0.012 | 0.01 | 0.920 |
| BF | X2X6 | 0.38 | 1.16 | 1 | 1.16 | 0.98 | 0.332 |
| CC | X32 | 0.55 | 3.09 | 1 | 3.09 | 2.60 | 0.119 |
| CD | X3X4 | 0.17 | 0.24 | 1 | 0.24 | 0.20 | 0.660 |
| CE | X3X5 | −0.53 | 2.29 | 1 | 2.29 | 1.92 | 0.178 |
| CF | X3X6 | 0.037 | 0.022 | 1 | 0.022 | 0.02 | 0.893 |
| DD | X42 | 0.41 | 1.69 | 1 | 1.69 | 1.42 | 0.244 |
| DE | X4X5 | 0.020 | 0.0033 | 1 | 0.0033 | 0.00 | 0.959 |
| DF | X4X6 | −0.19 | 0.29 | 1 | 0.29 | 0.24 | 0.629 |
| EE | X52 | −0.48 | 2.40 | 1 | 2.40 | 2.02 | 0.168 |
| EF | X5X6 | 1.11 | 9.93 | 1 | 9.93 | 8.34 | 0.00770 |
| FF | X62 | −0.93 | 8.81 | 1 | 8.81 | 7.40 | 0.0115 |
| Residual | 30.9 | 26 | 1.19 | ||||
| Lack-of-fit | 30.90 | 21 | 1.47 | 111.66 | <0.001 | ||
| Pure Error | 0.0659 | 5 | 0.0132 | ||||
| Cor total | 149.66 | 53 |
| Factor | Total Flavonols | Antioxidant Activity | Total Flavonols and Antioxidant Activity |
|---|---|---|---|
| % MeOH | 79 | 62.5 | 76.9 |
| Temperature (°C) | 60 | 57 | 58.8 |
| Amplitude (%) | 53.5 | 90 | 85 |
| Cycle (s) | 0.54 | 0.96 | 0.94 |
| pH | 2 | 2 | 2 |
| Ratio (g mL−1) | 0.2:10.8 | 0.2:13.6 | 0.2:12.8 |
| Result (mg g−1) ± SD (n = 3) | 8.92 ± 0.02 | 12.00 ± 0.07 | 8.78 ± 0.03 and 11.85 ± 0.11 |
| Multiresponse UAE Method | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak 1 a | Peak 2 a | Peak 3 a | Peak 4 a | Peak 5 a | Peak 6 a | Peak 7 a | Total Flavonols b | Antioxidant Activity b | |
| Units | mg Flavonols g−1 DW | mg TE g−1 DW | |||||||
| 1. White onion I “spring” | 0.16 ± 0.00 | 0.71 ± 0.00 | 2.39 ± 0.19 | 0.54 ± 0.00 | 0.58 ± 0.00 | 1.67 ± 0.14 | 0.41 ± 0.02 | 6.45 ± 0.35 | 9.74 ± 0.38 |
| 2. Red onion I | 2.75 ± 0.01 | 0.84 ± 0.01 | 3.82 ± 0.47 | 0.65 ± 0.00 | 0.39 ± 0.01 | 2.08 ± 0.29 | 0.47 ± 0.02 | 9.40 ± 0.08 | 12.51 ± 0.04 |
| 3. White onion II “French” | 1.19 ± 0.00 | 0.70 ± 0.00 | 2.76 ± 0.06 | 0.48 ± 0.00 | 1.01 ± 0.02 | 2.94 ± 0.07 | 0.33 ± 0.00 | 11.01 ± 0.51 | 9.37 ± 0.44 |
| 4. White onion III “sweet” | 0.83 ± 0.00 | 0.60 ± 0.00 | 1.95 ± 0.06 | 0.46 ± 0.01 | 0.51 ± 0.00 | 2.05 ± 0.04 | 0.23 ± 0.01 | 1.89 ± 0.09 | 7.32 ± 0.17 |
| 5. Red onion II “Label” | 0.29 ± 0.01 | <LOQ | 0.84 ± 0.02 | <LOQ | 0.44 ± 0.00 | 0.32 ± 0.00 | <LOQ | 10.56 ± 0.01 | 12.65 ± 0.03 |
| 6. Yellow onion | 1.80 ± 0.08 | 0.83 ± 0.03 | 3.58 ± 0.22 | 0.75 ± 0.00 | 0.57 ± 0.00 | 2.09 ± 0.10 | 0.61 ± 0.02 | 6.63 ± 0.12 | 11.36 ± 0.17 |
| 7. Red onion III | 1.05 ± 0.05 | 1.16 ± 0.02 | 4.08 ± 0.15 | 0.70 ± 0.01 | 0.58 ± 0.00 | 2.49 ± 0.09 | 0.49 ± 0.00 | 10.23 ± 0.21 | 10.80 ± 0.47 |
| 8. Chalota onion | <LOQ | 0.65 ± 0.01 | 1.48 ± 0.07 | 0.44 ± 0.00 | 0.49 ± 0.00 | 1.01 ± 0.05 | 0.25 ± 0.00 | 11.39 ± 0.21 | 9.78 ± 0.41 |
| 9. White onion IV | 2.66 ± 0.15 | 0.87 ± 0.00 | 4.78 ± 0.03 | 0.62 ± 0.01 | 0.91 ± 0.00 | 3.67 ± 0.02 | 0.57 ± 0.01 | 14.08 ± 0.25 | 9.38 ± 0.43 |
| 10. White onion V “sweet” | <LOQ | <LOQ | 0.71 ± 0.13 | <LOQ | 3.20 ± 0.12 | 0.12 ± 0.01 | <LOQ | 2.64 ± 0.14 | 7.82 ± 0.36 |
| 11. White onion VI “spring” | 1.77 ± 0.16 | 1.22 ± 0.01 | 4.11 ± 0.24 | 0.85 ± 0.00 | 0.63 ± 0.00 | 2.19 ± 0.16 | 0.61 ± 0.02 | 4.22 ± 0.05 | 6.85 ± 0.08 |
| 12. Red onion IV | 0.94 ± 0.09 | 0.77 ± 0.00 | 2.46 ± 0.02 | 0.81 ± 0.01 | 0.58 ± 0.00 | 2.43 ± 0.01 | 0.79 ± 0.00 | 8.78 ± 0.03 | 11.85 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Carrera, C.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Flavonol Composition and Antioxidant Activity of Onions (Allium cepa L.) Based on the Development of New Analytical Ultrasound-Assisted Extraction Methods. Antioxidants 2021, 10, 273. https://doi.org/10.3390/antiox10020273
González-de-Peredo AV, Vázquez-Espinosa M, Espada-Bellido E, Carrera C, Ferreiro-González M, Barbero GF, Palma M. Flavonol Composition and Antioxidant Activity of Onions (Allium cepa L.) Based on the Development of New Analytical Ultrasound-Assisted Extraction Methods. Antioxidants. 2021; 10(2):273. https://doi.org/10.3390/antiox10020273
Chicago/Turabian StyleGonzález-de-Peredo, Ana V., Mercedes Vázquez-Espinosa, Estrella Espada-Bellido, Ceferino Carrera, Marta Ferreiro-González, Gerardo F. Barbero, and Miguel Palma. 2021. "Flavonol Composition and Antioxidant Activity of Onions (Allium cepa L.) Based on the Development of New Analytical Ultrasound-Assisted Extraction Methods" Antioxidants 10, no. 2: 273. https://doi.org/10.3390/antiox10020273
APA StyleGonzález-de-Peredo, A. V., Vázquez-Espinosa, M., Espada-Bellido, E., Carrera, C., Ferreiro-González, M., Barbero, G. F., & Palma, M. (2021). Flavonol Composition and Antioxidant Activity of Onions (Allium cepa L.) Based on the Development of New Analytical Ultrasound-Assisted Extraction Methods. Antioxidants, 10(2), 273. https://doi.org/10.3390/antiox10020273