Higher Intensity of Salt Stress Accompanied by Heat Inhibits Stomatal Conductance and Induces ROS Accumulation in Tomato Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. Measurements
2.3.1. Leaf Photosynthetic Activity
2.3.2. Leaf H2O2 Content and O2·− Production Rate
2.3.3. Total RNA Extraction, Reverse Transcription, and qRT-PCR
2.3.4. Morphological Characterization of Plants
2.4. Data Analysis
3. Results
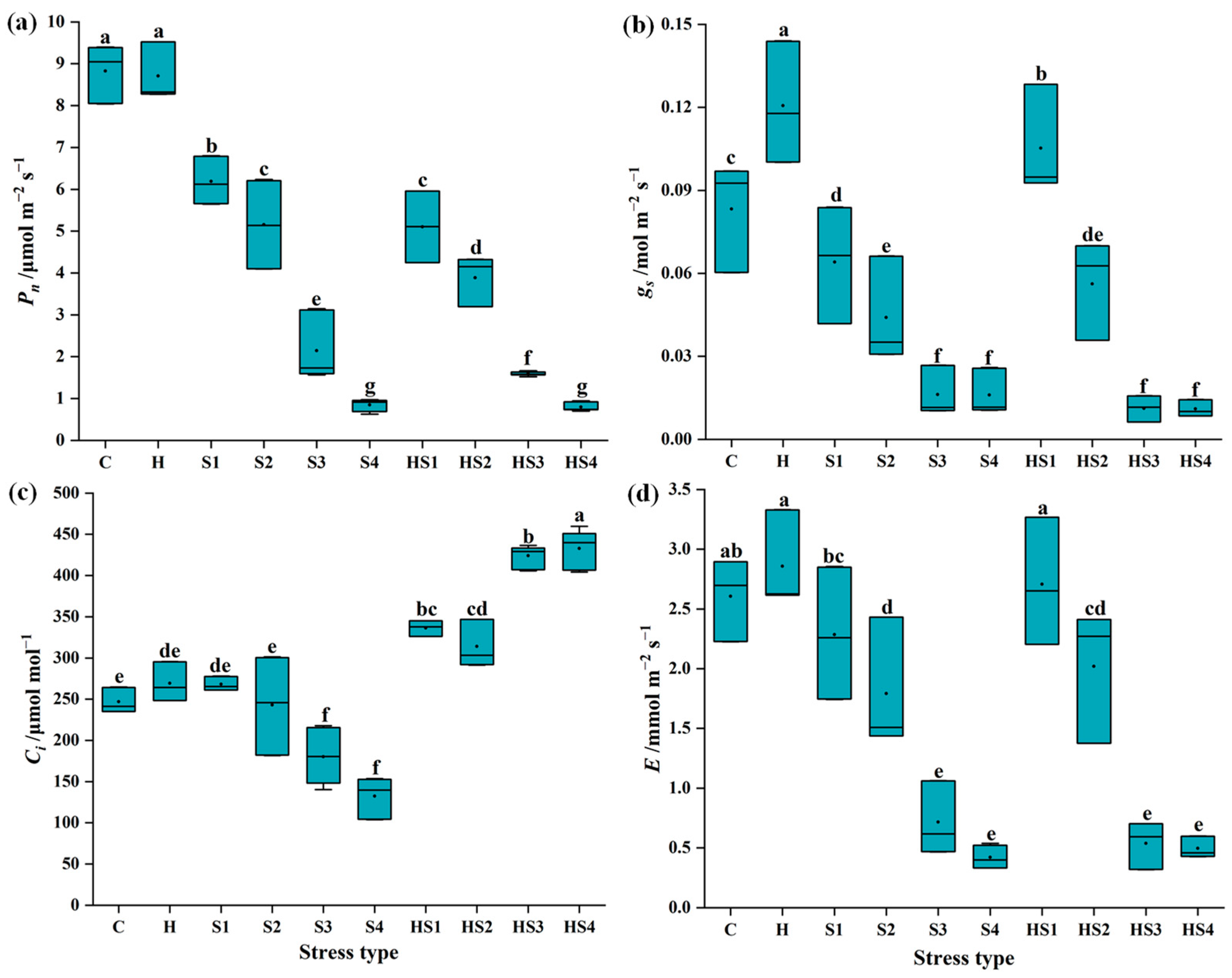
3.1. Changes in Photosynthetic Activity in Tomato Plants Subjected to Stress Combination
3.2. Changes of H2O2 and O2·− Levels of Tomato Plants Subjected to Stress Combination
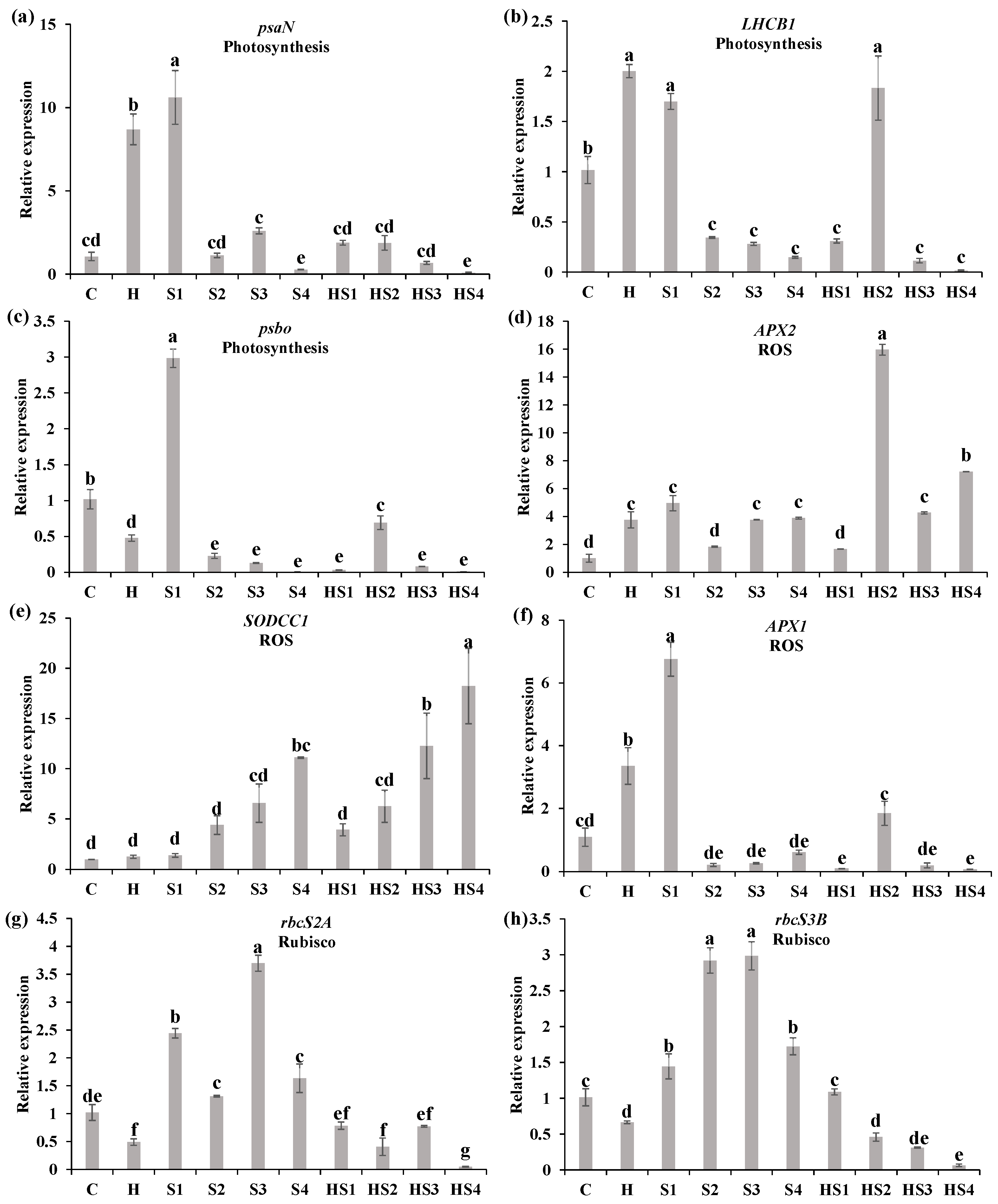
3.3. Changes in the Steady-State Levels of Transcripts Encoding Different Photosynthesis/ROS-Related Proteins
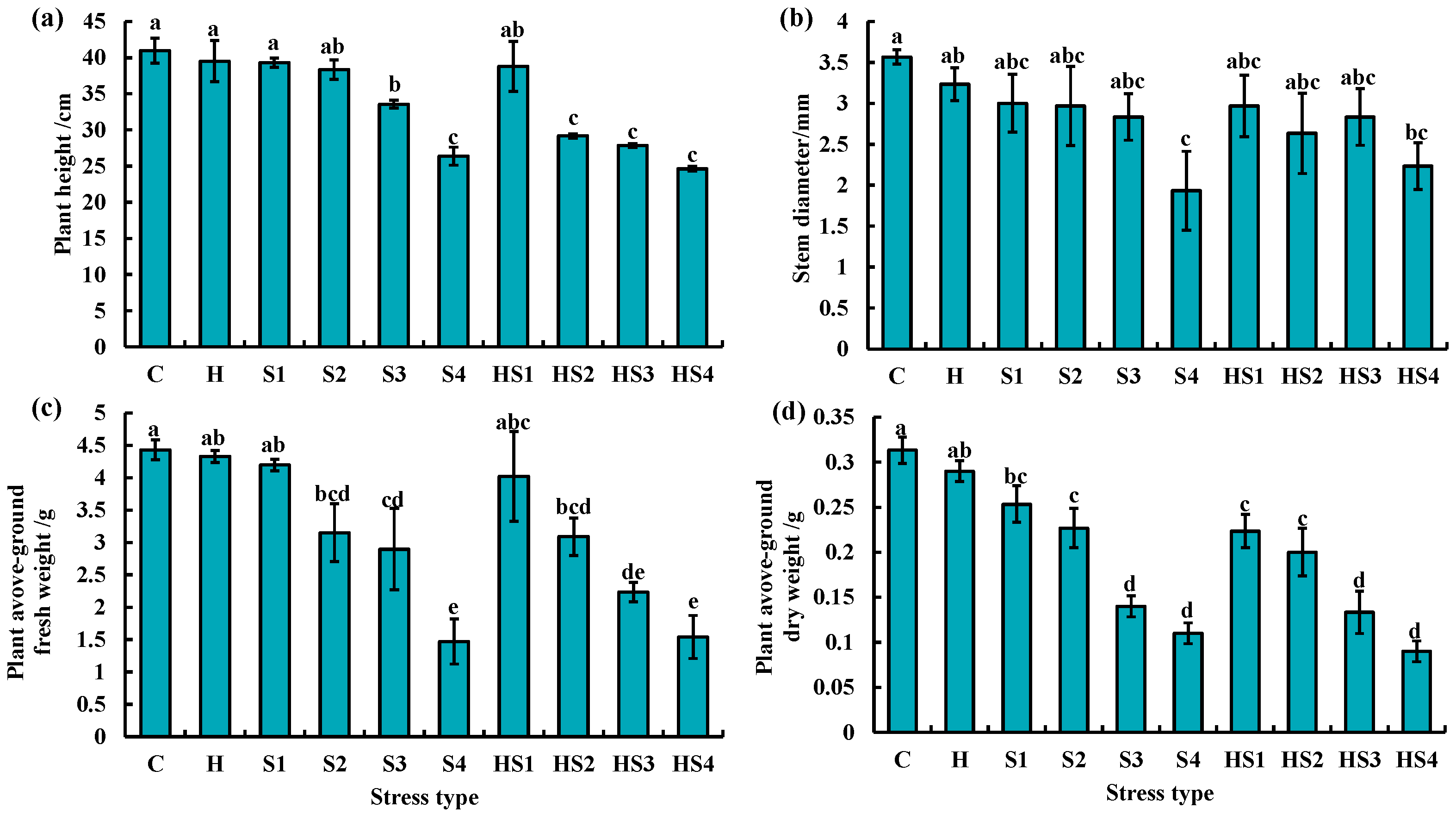
3.4. Morphological Changes in Tomato Plants Subjected to Stress Combination
4. Discussion
4.1. Physiological and Molecular Responses of Tomato to Combined Heat and Salt Stress Are Affected by Stress Intensity and Duration
4.2. The Dominant Stressor of the Combined Heat and Salt Stress Can Change with Increasing Salt Stress Intensity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diffenbaugh, N.S.; Singh, D.; Mankin, J.S.; Horton, D.E.; Swain, D.L.; Touma, D.; Charland, A.; Liu, Y.; Haugen, M.; Tsiang, M.; et al. Quantifying the influence of global warming on unprecedented extreme climate events. Proc. Natl. Acad. Sci. USA 2017, 114, 4881–4886. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Zhao, X.; Bürger, M.; Chory, J.; Wang, X.C. The role of ethylene in plant temperature stress response. Trends Plant Sci. 2023, 7, 808–824. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Rastogi, A.; Yadav, S. Effects of Heat stress and molecular mitigation approaches in orphan legume, Chickpea. Mol. Biol. Rep. 2020, 47, 4659–4670. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Xie, D.L.; Huang, H.M.; Zhou, C.Y.; Liu, C.X.; Kanwar, M.K.; Qi, Z.Y.; Zhou, J. HsfA1a confers pollen thermotolerance through upregulating antioxidant capacity, protein repair, and degradation in Solanum lycopersicum L. Hortic. Res. 2022, 9, uhac163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247273. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Colmenero Flores, J.M.; Rosales Villegas, M.Á. Interaction between salt and heat stress: When two wrongs make a right. Plant Cell Environ. 2014, 37, 1042–1045. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. Response and defence mechanisms of vegetable crops against drought, heat and salinity stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Song, T. Heat and drought priming induce tolerance to subsequent heat and drought stress by regulating leaf photosynthesis, root morphology, and antioxidant defense in maize seedlings. Environ. Exp. Bot. 2022, 202, 105010. [Google Scholar] [CrossRef]
- Ramegowda, V.; Senthil, A.; Senthil-Kumar, M. Stress combinations and their interactions in crop plants. Plant Physiol. Rep. 2024, 29, 1–5. [Google Scholar] [CrossRef]
- Li, Y.K.; Jiang, F.L.; Niu, L.F.; Wang, G.; Yin, J.; Song, X.M.; Ottosen, C.O.; Rosenqvist, E.; Mittler, R.; Wu, Z.; et al. Synergistic regulation at physiological, transcriptional and metabolic levels in tomato plants subjected to a combination of salt and heat stress. Plant J. 2024, 117, 1656–1675. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Kim, N.; Lee, S.H.; Khan, M.A.; Asaf, S.; Park, J.R.; Asif, S.; Lee, I.J.; Kim, K.M. Enhanced flavonoid accumulation reduces combined salt and heat stress through regulation of transcriptional and hormonal mechanisms. Front. Plant Sci. 2021, 12, 796956. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Bassil, E.; Hamilton, J.S.; Inupakutika, M.A.; Zandalinas, S.I.; Tripathy, D.; Mittler, R. ABA is required for plant acclimation to a combination of salt and heat stress. PLoS ONE 2016, 11, e0147625. [Google Scholar] [CrossRef] [PubMed]
- Pardo, H.M.; Arbona, V.; Simon, I.; Rosa, M.R. Specific ABA-independent tomato transcriptome reprogramming under abiotic stress combination. Plant J. 2024, 117, 1746–1763. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yu, X.Q.; Ottosen, C.O.; Rosenqvist, E.; Zhao, L.P.; Wang, Y.L.; Yu, W.G.; Zhao, T.M.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Carmo-Silva, A.E.; Gore, M.A.; Andrade-Sanchez, P.; French, A.N.; Hunsaker, D.J.; Salvucci, M.E. Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environ. Exp. Bot. 2012, 83, 1–11. [Google Scholar] [CrossRef]
- Balfagón, D.; Zandalinas, S.I.; Mittler, R.; Gómez-Cadenas, A. High temperatures modify plant responses to abiotic stress conditions. Physiol. Plantarum 2020, 170, 335–344. [Google Scholar] [CrossRef]
- Slowik, D.; Rossmann, M.; Konarev, P.V.; Irrgang, K.D.; Saenger, W. Structural investigation of PsbO from plant and cyanobacterial photosystem II. J. Mol. Biol. 2011, 407, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Najami, N.; Janda, T.; Barriah, W.; Kayam, G.; Tal, M.; Guy, G.; Volokita, M. Ascorbate peroxidase gene family in tomato: Its identification and characterization. Mol. Genet. Genom. 2008, 279, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, D.; Datta, S.K. Micropropagation of gerbera: Lipid peroxidation and antioxidant enzyme activities during acclimatization process. Acta Physiol. Plant. 2008, 30, 325–331. [Google Scholar] [CrossRef]
- Ke, D.; Sun, G.; Wang, Z. Effects of superoxide radicals on ACC synthase activity in chilling-stressed etiolated mungbean seedlings. Plant Growth Regul. 2007, 51, 83–91. [Google Scholar] [CrossRef]
- Melino, V.; Tester, M. Salt-tolerant crops: Time to deliver. Annu. Rev. Plant Biol. 2023, 74, 671–696. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R.; Lawson, T. Signal transduction networks during stress combination. J. Exp. Bot. 2020, 71, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Goswami, S.; Dubey, K.; Singh, K.; Singh, J.P.; Kumar, A.; Rai, G.K.; Singh, S.D.; Bakshi, S.; Singh, B.; et al. RuBisCo activase—A catalytic chaperone involved in modulating the RuBisCo activity and heat stress-tolerance in wheat. J. Plant Biochem. Biotechnol. 2019, 28, 63–75. [Google Scholar] [CrossRef]
- Chen, L.; Jia, H.; Tian, Q.; Du, L.; Gao, Y.; Miao, X.; Liu, Y. Protecting effect of phosphorylation on oxidative damage of D1 protein by downregulating the production of superoxide anion in photosystem II membranes under high light. Photosynth. Res. 2012, 112, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Delacalle, M.; Silva, C.J.; Mestre, T.C.; Martinez, V.; Blanco-Ulate, B.; Rivero, R.M. Synchronization of proline, ascorbate and oxidative stress pathways under the combination of salinity and heat in tomato plants. Environ. Exp. Bot. 2021, 183, 104351. [Google Scholar] [CrossRef]
- Jensen, N.B.; Ottosen, C.O.; Zhou, R. Exogenous Melatonin Alters Stomatal Regulation in Tomato Seedlings Subjected to Combined Heat and Drought Stress through Mechanisms Distinct from ABA Signaling. Plants 2023, 12, 1156. [Google Scholar] [CrossRef]
- Li, W.J.; Meng, R.; Liu, Y.; Chen, S.M.; Jiang, J.f.; Wang, L.K.; Zhao, S.; Wang, Z.X.; Fang, W.M.; Chen, F.D.; et al. Heterografted chrysanthemums enhance salt stress tolerance by integrating reactive oxygen species, soluble sugar, and proline. Hortic. Res. 2022, 9, uhac073. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Jiang, F.; He, Z.; Liu, Y.; Chen, Z.; Ottosen, C.-O.; Mittler, R.; Wu, Z.; Zhou, R. Higher Intensity of Salt Stress Accompanied by Heat Inhibits Stomatal Conductance and Induces ROS Accumulation in Tomato Plants. Antioxidants 2024, 13, 448. https://doi.org/10.3390/antiox13040448
Li Y, Jiang F, He Z, Liu Y, Chen Z, Ottosen C-O, Mittler R, Wu Z, Zhou R. Higher Intensity of Salt Stress Accompanied by Heat Inhibits Stomatal Conductance and Induces ROS Accumulation in Tomato Plants. Antioxidants. 2024; 13(4):448. https://doi.org/10.3390/antiox13040448
Chicago/Turabian StyleLi, Yankai, Fangling Jiang, Zhenxiang He, Yi Liu, Zheng Chen, Carl-Otto Ottosen, Ron Mittler, Zhen Wu, and Rong Zhou. 2024. "Higher Intensity of Salt Stress Accompanied by Heat Inhibits Stomatal Conductance and Induces ROS Accumulation in Tomato Plants" Antioxidants 13, no. 4: 448. https://doi.org/10.3390/antiox13040448
APA StyleLi, Y., Jiang, F., He, Z., Liu, Y., Chen, Z., Ottosen, C.-O., Mittler, R., Wu, Z., & Zhou, R. (2024). Higher Intensity of Salt Stress Accompanied by Heat Inhibits Stomatal Conductance and Induces ROS Accumulation in Tomato Plants. Antioxidants, 13(4), 448. https://doi.org/10.3390/antiox13040448









