Short-Term Adverse Effects Following Booster Dose of Inactivated-Virus vs. Adenoviral-Vector COVID-19 Vaccines in Algeria: A Cross-Sectional Study of the General Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
- Z2 = 1.96 for α = 0.05 (confidence interval of 95%);
- p is the proportion (p = 0.5; in the 2022 study of Lounis et al. [14], approximately 50% of the vaccinated population developed at least one local or systemic side effect);
- q = 1 − p; and
- e is the accepted margin of error (ME) (here, we chose an ME of 7%, or 0.07);
2.3. Instrument
2.4. Ethical Considerations
2.5. Statistical Analysis
3. Results
3.1. Demographic and Anamnestic Characteristics of the Study Population
3.2. COVID-19 Infection History and Vaccine Primer and Booster Characteristics
3.3. Booster Side Effects
3.3.1. Local Side Effects
3.3.2. Systemic Side Effects
3.4. Risk Factors of the Side Effects of Vaccines
4. Discussion
4.1. Limitations
4.2. Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aouissi, H.A.; Kechebar, M.S.A.; Ababsa, M.; Roufayel, R.; Neji, B.; Petrisor, A.-I.; Hamimes, A.; Epelboin, L.; Ohmagari, N. The Importance of Behavioral and Native Factors on COVID-19 Infection and Severity: Insights from a Preliminary Cross-Sectional Study. Healthcare 2022, 10, 1341. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 13 September 2022).
- Aouissi, H.A.; Hamimes, A.; Ababsa, M.; Bianco, L.; Napoli, C.; Kebaili, F.K.; Krauklis, A.E.; Bouzekri, H.; Dhama, K. Bayesian Modeling of COVID-19 to Classify the Infection and Death Rates in a Specific Duration: The Case of Algerian Provinces. Int. J. Environ. Res. Public Health 2022, 19, 9586. [Google Scholar] [CrossRef] [PubMed]
- Alzarea, A.I.; Khan, Y.H.; Alatawi, A.D.; Alanazi, A.S.; Alzarea, S.I.; Butt, M.H.; Almalki, Z.S.; Alahmari, A.K.; Mallhi, T.H. Surveillance of Post-Vaccination Side Effects of COVID-19 Vaccines among Saudi Population: A Real-World Estimation of Safety Profile. Vaccines 2022, 10, 924. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Batista, C.; Ben Amor, Y.; Ergonul, O.; Figueroa, J.P.; Gilbert, S.; Gursel, M.; Hassanain, M.; Kang, G.; Kaslow, D.C.; et al. Global public health security and justice for vaccines and therapeutics in the COVID-19 pandemic. EClinicalMedicine 2021, 39, 101053. [Google Scholar] [CrossRef]
- World Health Organization (WHO). COVID-19 Vaccine Tracker and Landscape. 2022. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 13 September 2022).
- Kunal, S.; Sakthivel, P.; Gupta, N.; Ish, P. Mix and match COVID-19 vaccines: Potential benefit and perspective from India. Postgrad. Med. J. 2022, 98, e99–e101. [Google Scholar] [CrossRef]
- Menni, C.; May, A.; Polidori, L.; Louca, P.; Wolf, J.; Capdevila, J.; Hu, C.; Ourselin, S.; Steves, C.J.; Valdes, A.M.; et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: A prospective community study from the ZOE COVID Study. Lancet Infect. Dis. 2022, 22, 1002–1010. [Google Scholar] [CrossRef]
- Al-Hanawi, M.K.; Keetile, M.; Kadasah, N.A.; Alshareef, N.; Qattan, A.M.N.; Alsharqi, O. Side Effects and Perceptions of COVID-19 Vaccination in Saudi Arabia: A Cross-Sectional Study. Front. Med. 2022, 9, 899517. [Google Scholar] [CrossRef]
- Riad, A.; Hocková, B.; Kantorová, L.; Slávik, R.; Spurná, L.; Stebel, A.; Havriľak, M.; Klugar, M. Side Effects of mRNA-Based COVID-19 Vaccine: NationwidePhase IV Study among Healthcare Workers in Slovakia. Pharmaceuticals 2021, 14, 873. [Google Scholar] [CrossRef]
- Alshahrani, M.M.; Alqahtani, A. Side Effects of Mixing Vaccines against COVID-19 Infection among Saudi Population. Vaccines 2022, 10, 519. [Google Scholar] [CrossRef]
- Orebi, H.A.; Emara, H.E.; Alhindi, A.A.; Shahin, M.R.; Hegazy, A.H.; Kabbash, I.A.; Saied, S.M. Perceptions and experiences of COVID-19 vaccines’ side effects among healthcare workers at an Egyptian University Hospital: A cross-sectional study. Trop. Med. Health 2022, 50, 37. [Google Scholar] [CrossRef]
- Hatmal, M.M.; Al-Hatamleh, M.A.I.; Olaimat, A.N.; Mohamud, R.; Fawaz, M.; Kateeb, E.T.; Alkhairy, O.K.; Tayyem, R.; Lounis, M.; Al-Raeei, M.; et al. Reported adverse effects and attitudes among Arab populations following COVID-19 vaccination: A large-scale multinational study implementing machine learning tools in predicting post-vaccination adverse effects based on predisposing factors. Vaccines 2022, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Lounis, M.; Rais, M.A.; Bencherit, D.; Aouissi, H.A.; Oudjedi, A.; Klugarová, J.; Pokorná, A.; Klugar, M.; Riad, A. Side Effects of COVID-19 Inactivated Virus vs. Adenoviral Vector Vaccines: Experience of Algerian Healthcare Workers. Front. Public Health 2022, 10, 896343. [Google Scholar] [CrossRef]
- Dziedzic, A.; Riad, A.; Attia, S.; Klugar, M.; Tanasiewicz, M. Self-Reported Adverse Events of COVID-19 Vaccines in Polish Healthcare Workers and Medical Students. Cross-Sectional Study and Pooled Analysis of CoVaST Project Results in Central Europe. J. Clin. Med. 2021, 10, 5338. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.C.; Dao, T.L.; Truong, T.M.D.; Nguyen, T.H.; Phan, T.N.; Nguyen, H.M.; Pham, T.D.; Nguyen, X.B.; Nguyen, T.B.; Hoang, V.T. Short-Term Adverse Effects Immediately after the Start of COVID-19 Booster Vaccination in Vietnam. Vaccines 2022, 10, 1325. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Shekhar, R.; Garg, I.; Pal, S.; Kottewar, S.; Sheikh, A.B. COVID-19 vaccine booster: To boost or not to boost. Infect. Dis. Rep. 2021, 13, 924–929. [Google Scholar] [CrossRef]
- Tang, P.; Hasan, M.R.; Chemaitelly, H.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; AlMukdad, S.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat. Med. 2021, 27, 2136–2143. [Google Scholar] [CrossRef]
- Rzymski, P.; Sikora, D.; Zeyland, J.; Poniedziałek, B.; Kiedik, D.; Falfushynska, H.; Fal, A. Frequency and Nuisance Level of Adverse Events in Individuals Receiving Homologous and Heterologous COVID-19 Booster Vaccine. Vaccines 2022, 10, 754. [Google Scholar] [CrossRef]
- Pugliese, M.E.; Battaglia, R.; Raso, M.G.; Chiaravalloti, R.; Coschignano, F.; Pagliuso, A.; Bruschetta, R.; Pugliese, G.; Scola, P.; Tonin, P.; et al. Heterologous COVID-19 Booster Vaccination in the Chronic Disorder of Consciousness: A Pilot Study. Clin. Pract. 2022, 12, 318–325. [Google Scholar] [CrossRef]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Saltoğlu, N.; Dinç, H.; Balkan, I.I.; Can, G.; Özbey, D.; Beytur, A.N.; Keskin, E.; Budak, B.; Aydoğan, O.; Mete, B.; et al. Heterologous booster COVID-19 vaccination elicited potent immune responses in HCWs. Diagn. Microbiol. Infect. Dis. 2022, 104, 115758. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.M.; Xu, Q.Y.; Jia, Z.J.; Wu, M.J.; Liu, Y.Y.; Lin, L.R.; Liu, L.L.; Yang, T.C. A Third Dose of an Inactivated Vaccine Dramatically Increased the Levels and Decay Times of Anti-SARSCoV-2 Antibodies, but Disappointingly Declined Again: A Prospective, Longitudinal, Cohort Study at 18 Serial Time Points Over 368 Days. Front. Immunol. 2022, 13, 876037. [Google Scholar] [CrossRef] [PubMed]
- Ourworlindata. 2022. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 13 September 2022).
- Lounis, M.; Bencherit, D.; Rais, M.A.; Riad, A. COVID-19 Vaccine Booster Hesitancy (VBH) and Its Drivers in Algeria: National Cross-Sectional Survey-Based Study. Vaccines 2022, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Ourworlindata. Coronavirus (COVID-19) Vaccinations. 2022. Available online: https://ourworldindata.org/grapher/cumulative-covid-vaccine-boosterdoses?country=~{}DZA (accessed on 13 September 2022).
- Del Águila, M.R.; González-Ramírez, A.R. Sample size calculation. Allergol. Immunopathol. 2014, 42, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Lounis, M.; Abdelhadi, S.; Rais, M.A.; Bencherit, D.; Sallam, M. Intention to get COVID-19 vaccination and its associated predictors: A cross-sectional study among the general public in Algeria. Vacunas 2022, 23, S52–S59. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Wyka, K.; White, T.M.; Picchio, C.A.; Rabin, K.; Ratzan, S.C.; Leigh, J.P.; Hu, J.; El-Mohandes, A. Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021. Nat. Commun. 2022, 13, 3801. [Google Scholar] [CrossRef]
- Klugar, M.; Riad, A.; Mohanan, L.; Pokorná, A. COVID-19 Vaccine Booster Hesitancy (VBH) of Healthcare Workers in Czechia: National Cross-Sectional Study. Vaccines 2021, 9, 1437. [Google Scholar] [CrossRef]
- Chansaenroj, J.; Suntronwong, N.; Kanokudom, S.; Assawakosri, S.; Yorsaeng, R.; Vichaiwattana, P.; Klinfueng, S.; Wongsrisang, L.; Srimuan, D.; Thatsanatorn, T.; et al. Immunogenicity Following Two Doses of the BBIBP-CorV Vaccine and a Third Booster Dose with a Viral Vector and mRNA COVID-19 Vaccines against Delta and Omicron Variants in Prime Immunized Adults with Two Doses of the BBIBP-CorV Vaccine. Vaccines 2022, 10, 1071. [Google Scholar] [CrossRef]
- Lin, Z.; Cheng, M.; Zhu, F.; Yang, X.; Zuo, J.; He, S. Immunogenicity and safety of different platforms of COVID-19 vaccines given as a third (booster) dose in healthy adults. J. Med. Virol. 2022, 94, 4047. [Google Scholar] [CrossRef]
- Alrudian, N.; Mokhatrish, M.; Alghuwainem, M.A.; Alhaddad, A.T.; Alanazi, S.S.; Altamimi, M.A.; Alahmadi, L.M.; Alenezi, A.K.; Ali, A.H.A. Side effects of COVID-19 third booster dose among healthcare workers in Saudi Arabia. Med. Sci. 2022, 26, ms284e2350. [Google Scholar] [CrossRef]
- Ali, M.D.; Almadan, L.Z.; Alghamdi, R.A.; Alghamdi, A.S.; Almarhoon, S.A.; Hassan, Y.A.; Ahmad, A.; Ghosn, S.A.; Banu, N.; Eltrafi, Z. Evaluation of Prevalence of Side-Effects Associated with Booster Dose of mRNA-Based COVID-19 Vaccine Among Healthcare Workers in Eastern Province, Saudi Arabia: A Descriptive Cross-Sectional Study. Infect. Drug Resist. 2022, 15, 4335. [Google Scholar] [CrossRef] [PubMed]
- El-Shitany, N.A.; Bagher, A.M.; Binmahfouz, L.S.; Eid, B.G.; Almukadi, H.; Badr-Eldin, S.M.; El-Hamamsy, M.; Mohammedsaleh, Z.M.; Saleh, F.M.; Almuhayawi, M.S.; et al. The Adverse Reactions of Pfizer BioNTech COVID-19 Vaccine Booster Dose are Mild and Similar to the Second Dose Responses: A Retrospective Cross-Sectional Study. Int. J. Gen. Med. 2022, 15, 6821–6836. [Google Scholar] [CrossRef] [PubMed]
- Matula, Z.; Gönczi, M.; Beko, G.; Kádár, B.; Ajzner, É.; Uher, F.; Vályi-Nagy, I. Antibody and T Cell Responses against SARS-CoV-2 Elicited by the Third Dose of BBIBP-CorV (Sinopharm) and BNT162b2 (Pfizer-BioNTech) Vaccines Using a Homologous or Heterologous Booster Vaccination Strategy. Vaccines 2022, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Hause, A.M. Safety Monitoring of an Additional Dose of COVID-19 Vaccine—United States, 12 August–19 September 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1379. [Google Scholar] [CrossRef]
- Hause, A.M. Safety Monitoring of COVID-19 Vaccine Booster Doses Among Persons Aged 12–17 Years—United States, 9 December 2021–20 February 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 347. [Google Scholar] [CrossRef]
- Hause, A.M.; Baggs, J.; Marquez, P.; Myers, T.R.; Su, J.R.; Blanc, P.G.; Baumblatt, J.A.G.; Woo, E.J.; Gee, J.; Shimabukuro, T.T.; et al. Safety Monitoring of COVID-19 Vaccine Booster Doses Among Adults—United States, 22 September 2021–6 February 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 249–254. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and Immunogenicity of the ChAdOx1 NCoV-19 Vaccine against SARS-CoV-2: A Preliminary Report of a Phase 1/2, Single-Blind, Randomised Controlled Trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Doedée, A.M.C.M.; Boland, G.J.; Pennings, J.L.; De Klerk, A.; Berbers, G.A.M.; Van Der Klis, F.R.M.; De Melker, H.E.; Van Loveren, H.; Janssen, R. Effects of Prophylactic and Therapeutic Paracetamol Treatment during Vaccination on Hepatitis B Antibody Levels in Adults: Two Open-Label, Randomized Controlled Trials. PLoS ONE 2014, 9, e98175. [Google Scholar] [CrossRef] [Green Version]
- Coronavirus (COVID-19) Vaccines Side Effects and Safety. Available online: https://www.nhs.uk/conditions/coronavirus-COVID-19/coronavirus-vaccination/safety-and-side-effects/ (accessed on 13 September 2022).
- Flanagan, K.L.; Fink, A.L.; Plebanski, M.; Klein, S.L. Sex and Gender Differences in the Outcomes of Vaccination over the Life Course. Annu. Rev. Cell Dev. Biol. 2017, 33, 577–599. [Google Scholar] [CrossRef]
- Nohl, A.; Brune, B.; Weichert, V.; Standl, F.; Stang, A.; Dudda, M. COVID-19: Vaccination Side Effects and Sick Leave in Frontline Healthcare-Workers—A Web-Based Survey in Germany. Vaccines 2022, 10, 411. [Google Scholar] [CrossRef]
- Aouissi, H.A.; Belhaouchet, I. What about Rheumatic Diseases and COVID-19? New Microbes New Infect. 2021, 41, 100846. [Google Scholar] [CrossRef] [PubMed]
- De Sarro, C.; Papadopoli, R.; Morgante, M.C.; Nobile, C.G.A.; De Sarro, G.; Pileggi, C. Vaccinations Status against Vaccine-Preventable Diseases and Willingness to Be Vaccinated in an Italian Sample of Frail Subjects. Vaccines 2022, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Iguacel, I.; Maldonado, A.L.; Ruiz-Cabello, A.L.; Casaus, M.; Moreno, L.A.; Martínez-Jarreta, B. Association between COVID-19 Vaccine Side Effects and Body Mass Index in Spain. Vaccines 2021, 9, 1321. [Google Scholar] [CrossRef] [PubMed]

| Variable | Adenoviral-Virus Vaccines (n = 102) | Inactivated-Virus Vaccines (n = 94) | Total (n = 196) | |
|---|---|---|---|---|
| Number (%) | Number (%) | Number (%) | ||
| Age | 18–30 years old | 30 (29.4) | 35 (37.2) | 65 (33.2) |
| 31–40 years old | 29 (28.4) | 25 (26.6) | 54 (27.6) | |
| 41–50 years old | 22 (21.6) | 20 (21.3) | 42 (21.4) | |
| Over 50 years old | 21 (20.6) | 14 (14.9) | 35 (17.9) | |
| Sex | Male | 48 (47.1) | 49 (52.1) | 97 (49.5) |
| Female | 54 (52.9) | 45 (47.9) | 99 (50.5) | |
| ABO group (n = 194) | A | 30 (29.4) | 30 (31.9) | 60 (30.6) |
| B | 13 (12.7) | 3 (3.2) | 16 (8.2) | |
| AB | 19 (18.6) | 19 (20.2) | 38 (19.4) | |
| O | 39 (38.2) | 41 (43.6) | 80 (40.8) | |
| BMI | Normal | 45 (44.1) | 47 (50) | 92 (46.9) |
| Obese | 38 (37.3) | 35 (37.2) | 73 (37.2) | |
| Over Obese | 19 (18.6) | 12 (12.8) | 31 (15.8) | |
| Allergy | (Yes) | 38 (37.3) | 35 (37.2) | 73 (37.2) |
| Smokers | (Yes) | 13 (12.7) | 19 (20.2 | 32 (16.3 |
| Chronic diseases | (Yes) | 28 (27.5) | 16 (17) | 44 (22.4) |
| Type | Hypertension | 9 (8.8) | 9 (9.6) | 18 (9.2) |
| Diabetis | 10 (9.8) | 6 (6.4) | 16 (8.2) | |
| COPD | 6 (5.9) | 2 (2.1) | 8 (4.1) | |
| Cardiovascular | 2 (2) | 2 (2.1) | 4 (2.0) | |
| Thyroid | 4 (3.9) | 0 (0) | 4 (2.0) | |
| Others | 5 (4.9) | 6 (6.4) | 11 (5.6) | |
| Medication | (Yes) | 58 (56.9) | 38 (40.4) | 96 (49) |
| Type | Antihistamines | 19 (18.6) | 11 (11.7) | 30 (15.3) |
| Antibiotics | 17 (16.7) | 10 (10.6) | 27 (13.8) | |
| Antihypertensive | 8 (7.8) | 9 (9.6) | 17 (8.7) | |
| Anti-reflux | 9 (8.8) | 5 (5.3) | 14 (7.1) | |
| Anti-diabetes | 7 (6.9) | 5 (5.3) | 12 (6.1) | |
| Anti-sthma | 8 (7.8) | 3 (3.2) | 10 (5.1) | |
| Contraceptives | 6 (5.9) | 2 (2.1) | 8 (4.1) | |
| Antdepressants | 3 (2.9) | 4 (4.3) | 7 (3.6) | |
| Thyroid hormones | 4 (3.9) | 3 (3.2) | 7 (3.6) | |
| Corticoids | 3 (2.9) | 4 (4.3) | 7 (3.6) | |
| Cholesterol lowering | 3 (2.9) | 1 (1.1) | 4 (2) | |
| Anticoagulant | 1 (1) | 1 (1.1) | 2 (1) | |
| Variable | Number | Frequency (%) | |
|---|---|---|---|
| COVID-19 infection | No | 55 | 28.1 |
| Yes | 141 | 71.9 | |
| Number of infections | One time | 113 | 80.1 |
| Two times | 23 | 16.3 | |
| Three times | 4 | 2.8 | |
| Onset | Before vaccination | 122 | 86.5 |
| After the first dose | 19 | 13.5 | |
| After the second dose | 35 | 24.8 | |
| After the third dose | 27 | 19.1 | |
| Vaccination timing | From a month to 3 months ago | 39 | 19.9 |
| From a week to one month ago | 12 | 6.1 | |
| Less than a week ago | 5 | 2.6 | |
| More than 3 months ago | 140 | 71.4 | |
| Vaccine first dose | AstraZeneeca (ChAdOx1, AZD1222) | 37 | 18.9 |
| Janssen (Ad26.CoV2.S) | 5 | 2.6 | |
| Sinopharm (BBIBP-CorV) | 20 | 10.2 | |
| Sinovac (CoronaVac) | 99 | 50.5 | |
| Sputnik V (Gam-COVID-Vac) | 35 | 17.9 | |
| Vaccine second dose | AstraZeneeca (ChAdOx1, AZD1222) | 36 | 18.4 |
| Janssen (Ad26.CoV2.S) | 5 | 2.6 | |
| Sinopharm (BBIBP-CorV) | 23 | 11.7 | |
| Sinovac (CoronaVac) | 96 | 49.0 | |
| Sputnik V (Gam-COVID-Vac) | 36 | 18.4 | |
| Vaccine booster | AstraZeneeca (ChAdOx1, AZD1222) | 28 | 14.3 |
| Janssen (Ad26.CoV2.S) | 71 | 36.2 | |
| Sinopharm (BBIBP-CorV) | 13 | 6.6 | |
| Sinovac (CoronaVac) | 81 | 41.3 | |
| Sputnik V (Gam-COVID-Vac) | 3 | 1.5 | |
| Primer doses type | Adenoviral-vector virus | 77 | 39.3 |
| Inactivated virus | 119 | 60.7 | |
| Booster dose type | Adenoviral-vector virus | 102 | 52.0 |
| Inactivated virus | 94 | 48.0 | |
| Time between primer and booster doses (months) | 1 month | 19 | 9.7 |
| 1 to 3 months | 33 | 16.8 | |
| 3 to 6 months | 92 | 46.9 | |
| More than 6 months | 51 | 26.0 | |
| State of mind during booster vaccination | Anxious | 12 | 6.1 |
| Worried | 18 | 9.2 | |
| Indifferent | 79 | 40.3 | |
| Serene | 85 | 43.4 | |
| Variable | Booster Vaccine Type | Sig. | |||
|---|---|---|---|---|---|
| Adenoviral-Vector-Virus | Inactivated-Virus | Total | |||
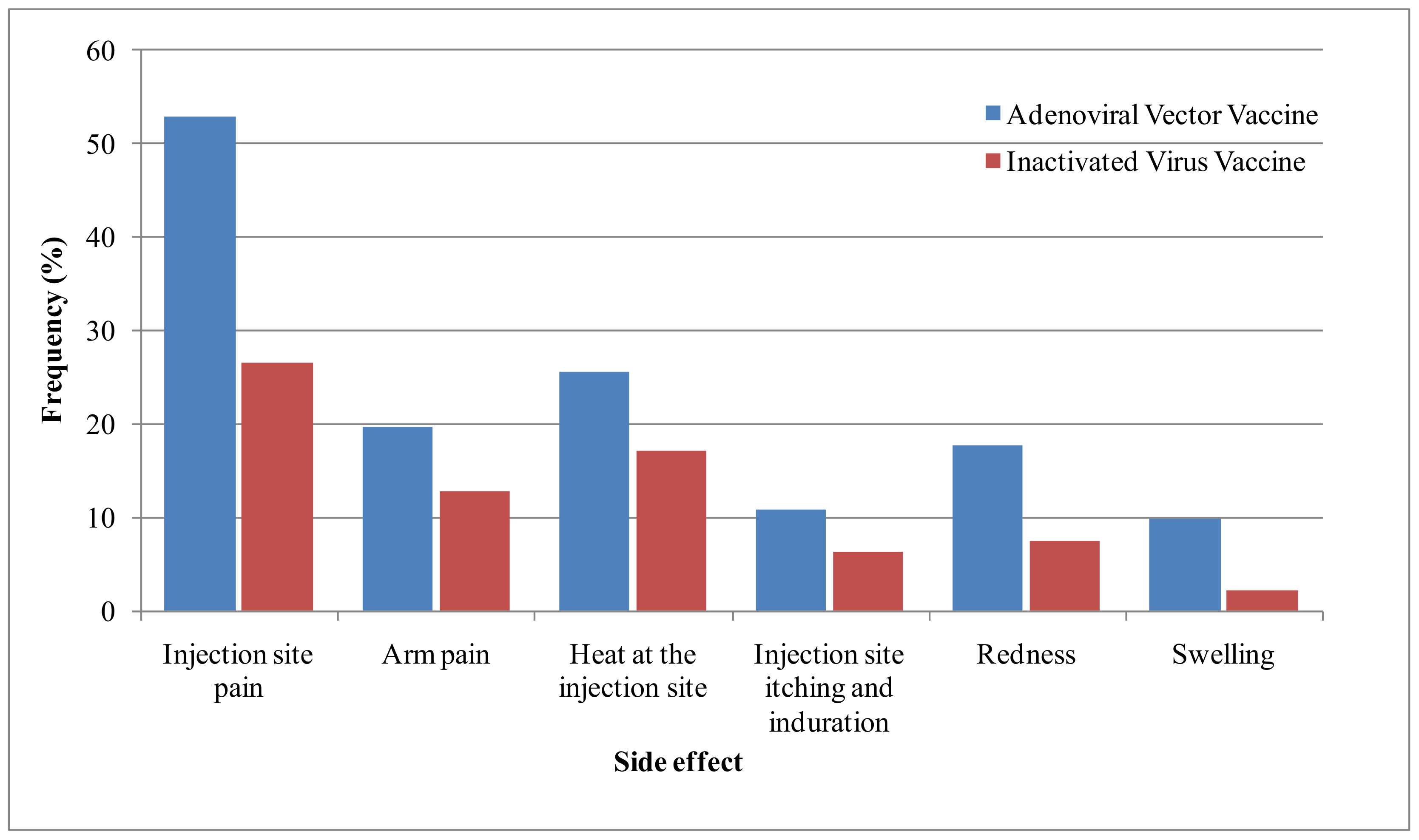
| Local side effects | Injection-site pain | 54 (52.9) | 25 (26.6) | 70 (40.3) | 0.000 |
| Arm pain | 20 (19.6) | 12 (12.8) | 32 (16.3) | 0.195 | |
| Heat at the injection site | 26 (25.5) | 16 (17) | 42 (21.4) | 0.149 | |
| Injection-site itching and induration | 11 (10.8) | 6 (6.4) | 17 (8.7) | 0.274 * | |
| Redness | 18 (17.6) | 7 (7.4) | 25 (12.8) | 0.032 | |
| Swelling | 10 (9.8) | 3 (2.1) | 12 (6.1) | 0.052 * | |
| Onset | <12 h | 58 (75.3) | 29 (70.7) | 87 (73.7) | 0.589 |
| >12 h | 10 (13) | 10 (24.4) | 20 (16.9) | 0.129 * | |
| >24 h | 9 (11.7) | 2 (4.9) | 11 (9.3) | 0.327 * | |
| Duration | Less than 24 h | 27 (34.2) | 12 (30) | 39 (32.8) | 0.647 |
| From 24 to 72 h | 30 (38) | 13 (32.5) | 43 (36.1) | 0.557 | |
| From 3 days to a week | 15(19) | 7 (17.5) | 22 (18.5) | 1 * | |
| More than a week | 7 (8.9) | 8 (20) | 15 (12.6) | 0.141 * | |
| Similarity | Not the same signs | 45 (53.6) | 21 (40.4) | 66 (48.5) | 0.135 |
| The same signs as the first dose | 19 (22.6) | 15 (28.8) | 34 (25) | 0.415 | |
| The same signs as the second dose | 2 (2.4) | 5 (9.6) | 7 (5.1) | 0.106 * | |
| The same signs as the two primer doses | 19 (21.4) | 11 (21.2) | 29 (21.3) | 0.970 | |
| Severity | The same severity as the two primer doses | 20 (25.6) | 19 (38.8) | 39 (30.7) | 0.118 |
| Less severe than the two primer doses | 24 (30.8) | 17 (34.7) | 41 (32.3) | 0.645 | |
| More severe than the two primer doses | 34 (43.6) | 13 (26.5) | 47 (37) | 0.052 | |
| Variable | Outcomes | Booster Vaccine Type | Sig. | ||
|---|---|---|---|---|---|
| Adenoviral-Vector Virus | Inactivated Virus | Total | |||
| Systemic side effects | Fever | 54 (52.9) | 27 (28.7) | 81 (41.3) | 0.001 |
| Fatigue | 51 (50) | 31 (33) | 82 (41.8) | 0.016 | |
| Headache | 34 (33.3) | 25 (26.6) | 59 (30.1) | 0.304 | |
| Chills | 32 (31.4) | 11 (11.7) | 43 (21.9) | 0.001 | |
| Arthralgia | 30 (29.4) | 12 (12.8) | 42 (21.4) | 0.005 | |
| Dizziness | 22 (21.6) | 13 (13.8) | 35 (17.9) | 0.158 | |
| Sweating | 12 (21.6) | 12 (12.8) | 34 (17.3) | 0.104 | |
| Somnolence | 12 (11.8) | 6 (6.4) | 18 (9.2) | 0.223 * | |
| Cough | 10 (9.8) | 4 (4.3) | 14 (7.1) | 0.169 * | |
| Insomnia | 10 (9.8) | 7 (7.4) | 17 (8.7) | 0.619 * | |
| Dyspnea | 8 (7.8) | 2 (2.1) | 10 5.1) | 0.103 * | |
| Nasal discharge | 7 (6.9) | 4 (4.3) | 11 (5.6) | 0.541 * | |
| Chest pain | 6 (5.9) | 2 (2.1) | 8 (4.1) | 0.282 * | |
| Abdominal pain | 6 (5.9) | 2 (2.1) | 8 (4.1) | 0.282 * | |
| Diarrhea | 7 (6.9) | 9 (9.6) | 16 (8.2) | 0.604 * | |
| Loss of taste/smell | 6 (5.9) | 4 (4.3) | 10 (5.1) | 0.75 * | |
| Halitosis | 7 (6.9) | 2 (2.1) | 9 (4.6) | 0.173 * | |
| Myalgia | 1 (1) | 0 (0) | 0 (0.5) | 1 * | |
| Onset | Immediately | 5 (6.6) | 8 (17.8) | 13 (10.7) | 0.071 * |
| The first day | 52 (68.4) | 25 (55.6) | 77 (63.6) | 0.155 | |
| The first week | 12 (15.8) | 8 (17.8) | 20 (16.5) | 0.804 * | |
| The second week | 3 (3.9) | 2 (4.4) | 5 (4.1) | 1 * | |
| After the first month | 4 (5.3) | 2 (4.4) | 6 (5) | 1 * | |
| Duration | Less than 2 days | 41 (54.7) | 20 (44.4) | 61 (50.8) | 0.278 |
| From 2 days to a week | 18 (24) | 16 (35.6) | 34 (28.3) | 0.174 | |
| From a week to 2 weeks | 7 (9.3) | 2 (4.4) | 9 (7.5) | 0.481 * | |
| From 2 weeks to 4 weeks | 2 (2.7) | 1 (2.2) | 3 (2.5) | 1 * | |
| More than 4 weeks | 7 (9.3) | 6 (13.3) | 13 (10.8) | 0.551 * | |
| Similarity | Not the same signs | 47 (56) | 21 (37.5) | 68 (48.6) | 0.032 |
| The same as the first dose | 21 (25) | 18 (32.1) | 39 (27.9) | 0.356 | |
| The same as the second dose | 3 (3.6) | 7 (12.5) | 10 (7.1) | 0.089 * | |
| The same as the primer doses | 13 (15.5) | 10 (17.9) | 23 (16.4) | 0.817 * | |
| Severity | The same severity as the primer doses | 19 (24.7) | 18 (37.5) | 37 (29.6) | 0.127 |
| Less severe than the primer doses | 20 (26) | 20 (41.7) | 40 (32) | 0.067 | |
| More severe than the primer doses | 38 (49.4) | 10 (20.8) | 48 (38.4) | 0.001 | |
| Variable | Local Side Effects | Systemic Side Effects | Total Side Effects | ||||
|---|---|---|---|---|---|---|---|
| Number (%) | Sig. | Number (%) | Sig. | Number (%) | Sig. | ||
| Age | 18–30 years old | 39 (60) | 0.574 | 37 (56.9) | 0.160 | 44 (67.7) | 0.124 |
| 31–40 years old | 32 (59.3) | 0.532 | 36 (66.7) | 0.604 | 39 (72.2) | 0.653 | |
| 41–50 years old | 29 (69) | 0.341 | 25 (59.5) | 0.518 | 32 (76.2) | 0.775 | |
| Over 50 years old | 23 (65.7) | 0.689 | 27 (77.1) | 0.036 | 31 (88.6) | 0.114 | |
| Sex | Male | 53 (54.6) | 0.02 | 52 (53.6) | 0.003 | 65 (67) | 0.017 |
| Female | 70 (70.7) | 73 (73.7) | 81 (81.8) | ||||
| ABO group | A | 37 (61.7) | 0.703 | 40 (66.7) | 0.528 | 45 (75) | 0.869 |
| AB | 10 (62.5) | 1 | 10 (62.5) | 1 | 11 (68.8) | 0.563 | |
| B | 27 (71.1) | 0.245 | 27 (71.1) | 0.275 | 31 (81.6) | 0.304 | |
| O | 48 (60) | 0.486 | 46 (57.5) | 0.153 | 57 (71.3) | 0.427 | |
| BMI class | Normal | 52 (56.5) | 0.090 | 54 (58.7) | 0.164 | 61 (66.3) | 0.013 |
| Obese | 47 (64.4) | 0.716 | 46 (63) | 0.864 | 58 (79.5) | 0.220 | |
| Overly obese | 24 (77.4) | 0.072 | 25 (80.6) | 0.041 | 27 (87.1) | 0.114 | |
| Chronic diseases | Yes | 32 (72.7) | 0.12 | 34 (77.3) | 0.034 | 39 (88.6) | 0.015 |
| No | 91 (59.9) | 91 (59.9) | 107 (70.4) | ||||
| Allergy | Yes | 49 (67.1) | 0.33 | 50 (68.5) | 0.290 | 57 (78.1) | 0.374 |
| No | 74 (60.2) | 75 (61) | 89 (72.4) | ||||
| Smoking | Yes | 19 (59.4) | 0.665 | 18 (56.3) | 0.333 | 21 (65.6) | 0.374 |
| No | 104 (63.4) | 107 (65.2) | 125 (76.2) | ||||
| Medication | Yes | 74 (77.1) | 0.000 | 67 (69.8) | 0.086 | 80 (83.3) | 0.005 |
| No | 49 (49) | 58 (58) | 66 (66) | ||||
| COVID-19 infection | Yes | 71 (58.7) | 0.134 | 77 (63.6) | 0.959 | 88 (73.8) | 0.707 |
| No | 52 (69.3) | 48 (64) | 58 (76.4) | ||||
| Booster type | Adenoviral-virus | 79 (77.5) | 0.000 | 77 (75.5) | 0.000 | 89 (87.3) | 0.000 |
| Inactivated-virus | 44 (46.8) | 48 (51.1) | 57 (60.6) | ||||
| Mixing/matching (primers-booster) | AA + | 43 (79.6) | 0.003 | 40 (74.1) | 0.064 | 50 (92.6) | 0.000 |
| AI + | 11 (47.8) | 0.115 | 11 (47.8) | 0.09 | 13 (56.5) | 0.035 | |
| IA + | 38 (74.5) | 0.044 | 39 (76.5) | 0.028 | 40 (81.6) | 0.256 | |
| II + | 31 (45.6) | 0.000 | 35 (51.5) | 0.009 | 43 (61.4) | 0.002 | |
| Time between primers and booster doses (months) | 1 month or less | 11 (57.9) | 0.619 | 12 (63.2) | 1.000 | 14 (73.7) | 1 |
| 1 to 3 months | 19 (57.6) | 0.472 | 19 (57.6) | 0.431 | 21 (63.6) | 0.122 | |
| 3 to 6 months | 64 (69.6) | 0.076 | 62 (67.4) | 0.297 | 74 (80.4) | 0.066 | |
| More than 6 mths | 29 (56.9) | 0.285 | 31 (60.8) | 0.628 | 36 (70.6) | 0.473 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lounis, M.; Aouissi, H.A.; Abdelhadi, S.; Rais, M.A.; Belkessa, S.; Bencherit, D. Short-Term Adverse Effects Following Booster Dose of Inactivated-Virus vs. Adenoviral-Vector COVID-19 Vaccines in Algeria: A Cross-Sectional Study of the General Population. Vaccines 2022, 10, 1781. https://doi.org/10.3390/vaccines10111781
Lounis M, Aouissi HA, Abdelhadi S, Rais MA, Belkessa S, Bencherit D. Short-Term Adverse Effects Following Booster Dose of Inactivated-Virus vs. Adenoviral-Vector COVID-19 Vaccines in Algeria: A Cross-Sectional Study of the General Population. Vaccines. 2022; 10(11):1781. https://doi.org/10.3390/vaccines10111781
Chicago/Turabian StyleLounis, Mohamed, Hani Amir Aouissi, Samir Abdelhadi, Mohammed Amir Rais, Salem Belkessa, and Djihad Bencherit. 2022. "Short-Term Adverse Effects Following Booster Dose of Inactivated-Virus vs. Adenoviral-Vector COVID-19 Vaccines in Algeria: A Cross-Sectional Study of the General Population" Vaccines 10, no. 11: 1781. https://doi.org/10.3390/vaccines10111781
APA StyleLounis, M., Aouissi, H. A., Abdelhadi, S., Rais, M. A., Belkessa, S., & Bencherit, D. (2022). Short-Term Adverse Effects Following Booster Dose of Inactivated-Virus vs. Adenoviral-Vector COVID-19 Vaccines in Algeria: A Cross-Sectional Study of the General Population. Vaccines, 10(11), 1781. https://doi.org/10.3390/vaccines10111781








