The Global Monkeypox (Mpox) Outbreak: A Comprehensive Review
Abstract
1. Introduction
2. Transmission Route Associated with Mpox
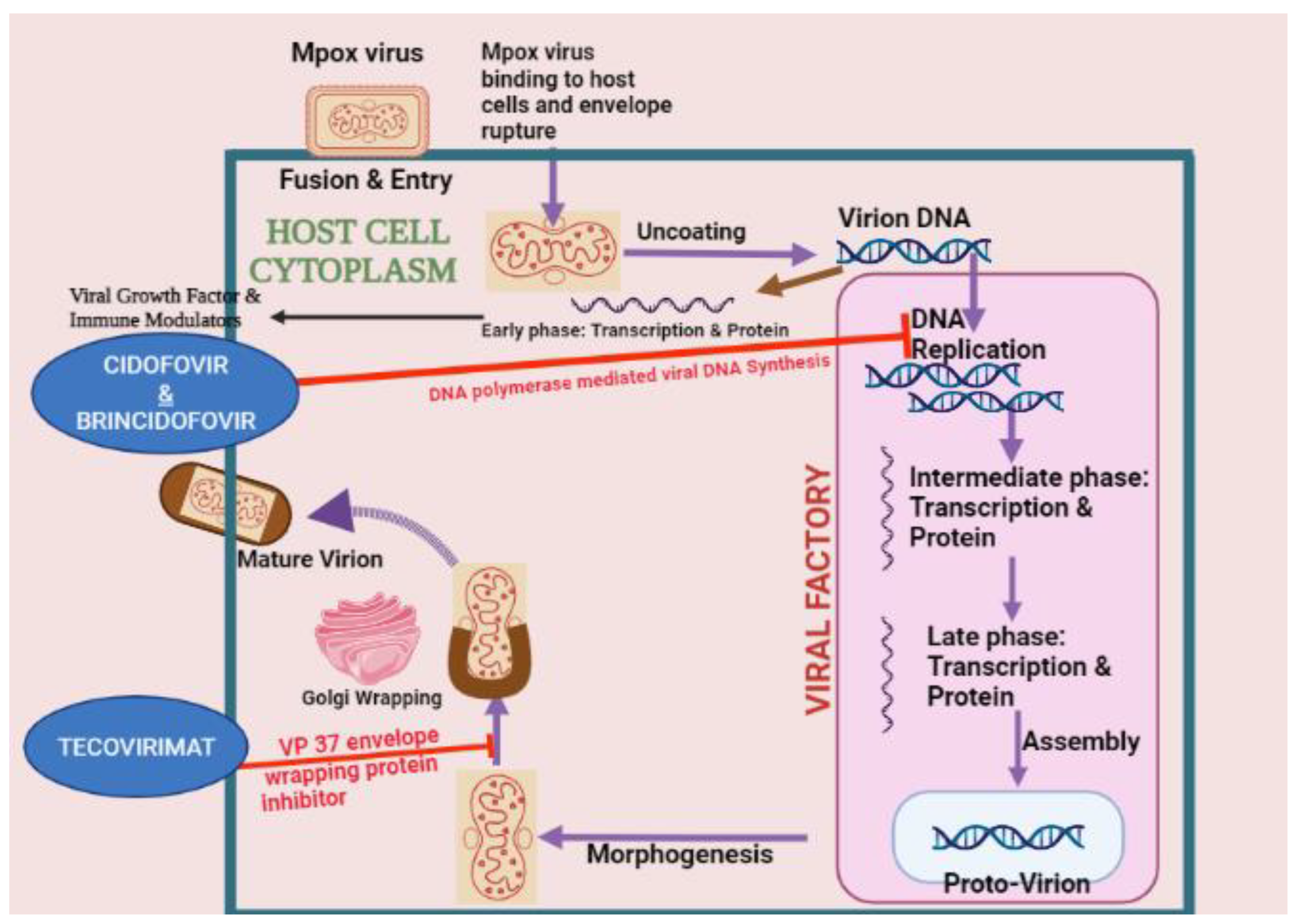
3. Proposed Pathophysiology of Mpox Virus
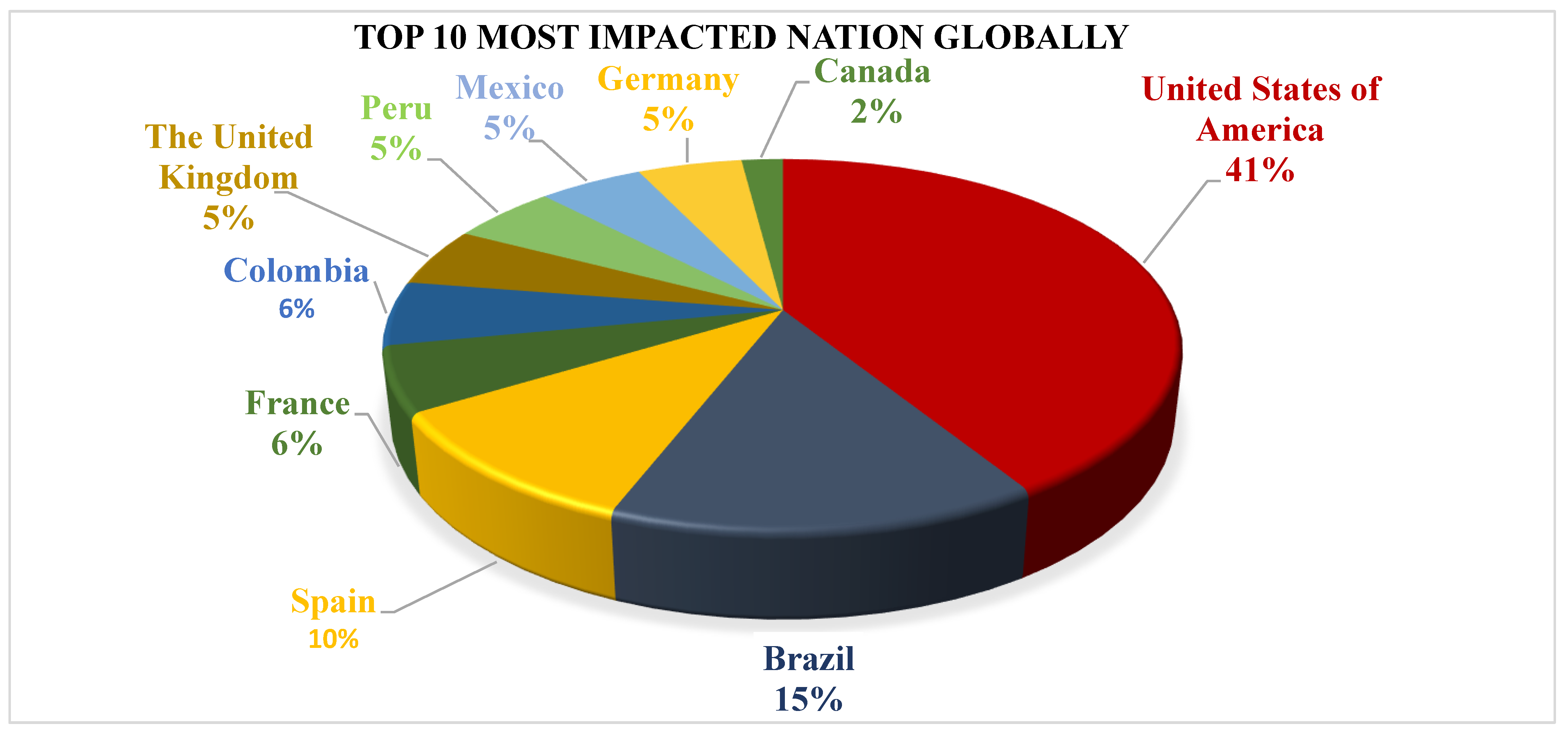
4. Epidemiology
5. Clinical Symptoms and Diagnosis of Mpox Virus
6. Phylogenetic Clades of Mpox Virus and Their Evolutionary Divergence
7. Vaccines for Mpox Virus
8. Treatment for Mpox
9. Challenges
10. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Srivastava, G.; Srivastava, G. Human monkeypox disease. Clin. Dermatol. 2022, 40, 604–612. [Google Scholar] [CrossRef]
- Durski, K.N.; McCollum, A.M.; Nakazawa, Y.; Petersen, B.W.; Reynolds, M.G.; Briand, S.; Djingarey, M.H.; Olson, V.; Damon, I.K.; Khalakdina, A. Emergence of monkeypox-west and central Africa, 1970–2017. Morb. Mortal. Wkly. Rep. 2018, 67, 306. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.H.; Guagliardo, S.A.J.; Doty, J.B.; Babeaux, A.D.; Matheny, A.; Burgado, J.; Townsend, M.B.; Morgan, C.N.; Satheshkumar, P.S.; Ndakala, N. Epidemiologic and ecologic investigations of monkeypox, Likouala Department, Republic of the Congo, 2017. Emerg. Infect. Dis. 2019, 25, 273. [Google Scholar] [CrossRef] [PubMed]
- Kava, C.M.; Rohraff, D.M.; Wallace, B.; Mendoza-Alonzo, J.L.; Currie, D.W.; Munsey, A.E.; Roth, N.M.; Bryant-Genevier, J.; Kennedy, J.L.; Weller, D.L. Epidemiologic features of the monkeypox outbreak and the public health response-United States, May 17–October 6, 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 1449–1456. [Google Scholar] [CrossRef]
- Yinka-Ogunleye, A.; Aruna, O.; Dalhat, M.; Ogoina, D.; McCollum, A.; Disu, Y.; Mamadu, I.; Akinpelu, A.; Ahmad, A.; Burga, J. Outbreak of human monkeypox in Nigeria in 2017–18: A clinical and epidemiological report. Lancet Infect. Dis. 2019, 19, 872–879. [Google Scholar] [CrossRef]
- Sale, T.A.; Melski, J.W.; Stratman, E.J. Monkeypox: An epidemiologic and clinical comparison of African and US disease. J. Am. Acad. Dermatol. 2006, 55, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Hammerschlag, Y.; MacLeod, G.; Papadakis, G.; Sanchez, A.A.; Druce, J.; Taiaroa, G.; Savic, I.; Mumford, J.; Roberts, J.; Caly, L. Monkeypox infection presenting as genital rash, Australia, May 2022. Eurosurveillance 2022, 27, 2200411. [Google Scholar] [CrossRef]
- Patrocinio-Jesus, R.; Peruzzu, F. Monkeypox genital lesions. N. Engl. J. Med. 2022, 387, 66. [Google Scholar] [CrossRef]
- Patel, A.; Bilinska, J.; Tam, J.C.; Fontoura, D.D.S.; Mason, C.Y.; Daunt, A.; Snell, L.B.; Murphy, J.; Potter, J.; Tuudah, C. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: Descriptive case series. EbioMed. 2022, 378, e072410. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Ding, K.; Wang, X.-H.; Sun, G.-Y.; Liu, Z.-X.; Luo, Y. The evolving epidemiology of monkeypox virus. Cytokine Growth Factor Rev. 2022, 68, 1–12. [Google Scholar] [CrossRef]
- Patauner, F.; Gallo, R.; Durante-Mangoni, E. Monkeypox infection: An update for the practicing physician: Monkeypox infection. Eur. J. Intern. Med. 2022, 104, 1–6. [Google Scholar] [CrossRef]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef]
- Smith, S.A.; Kotwa, G.J. Immune response to poxvirus infections in various animals. Crit. Rev. Microbiol. 2002, 28, 149–185. [Google Scholar] [CrossRef]
- Alzhanova, D.; Früh, K. Modulation of the host immune response by cowpox virus. Microbes Infect. 2010, 12, 900–909. [Google Scholar] [CrossRef]
- Li, H.; Huang, Q.-Z.; Zhang, H.; Liu, Z.-X.; Chen, X.-H.; Ye, L.-L.; Luo, Y. The land-scape of immune response to monkeypox virus. EBioMedicine 2023, 87, 104424. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/news-room/fact-sheets/detail/monkeypox (accessed on 23 April 2023).
- Choudhary, G.; Prabha, P.K.; Gupta, S.; Prakash, A.; Medhi, B. Monkeypox infection: A quick glance. Indian J. Pharmacol. 2022, 54, 161. [Google Scholar] [PubMed]
- Brasil, P.; Martins, E.B.; Calvet, G.A.; Werneck, G.L. What do we need to know about the monkeypox virus infection in humans? Cad. Saúde Pública 2022, 38, e00129222. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Thapliyal, K.; Singh, V.P. Monkeypox, a Re-emerging Infection: A Narrative Review. J. Indian Acad. Clin. Med. 2022, 23. [Google Scholar]
- Sah, R.; Abdelaal, A.; Reda, A.; Katamesh, B.E.; Manirambona, E.; Abdelmonem, H.; Rodriguez-Morales, A.J. Monkeypox and its possible sexual transmission: Where are we now with its evidence? Pathogens 2022, 11, 924. [Google Scholar] [CrossRef]
- Assessment, R.R. Monkeypox multi-country outbreak. Eur. Cent. Dis-Ease Prev. Control 2022. Available online: http://www.sepexpal.org/wp-content/uploads/2022/05/23-mayo.-ECDC.-Monkeypox-multi-country-outbreak.pdf (accessed on 23 April 2023).
- Lapa, D.; Carletti, F.; Mazzotta, V.; Matusali, G.; Pinnetti, C.; Meschi, S.; Gagliardini, R.; Colavita, F.; Mondi, A.; Minosse, C. Monkeypox virus isolation from a semen sample collected in the early phase of infection in a patient with prolonged seminal viral shedding. Lancet Infect. Dis. 2022, 22, 1267–1269. [Google Scholar] [CrossRef]
- Vaughan, A.; Aarons, E.; Astbury, J.; Brooks, T.; Chand, M.; Flegg, P.; Hardman, A.; Harper, N.; Jarvis, R.; Mawdsley, S. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg. Infect. Dis. 2020, 26, 782. [Google Scholar] [CrossRef] [PubMed]
- Kantele, A.; Chickering, K.; Vapalahti, O.; Rimoin, A. Emerging diseases-The monkeypox epidemic in the Democratic Republic of the Congo. Clin. Microbiol. Infect. 2016, 22, 658–659. [Google Scholar] [CrossRef]
- Kozlov, M. Monkeypox goes global: Why scientists are on alert. Nature 2022, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Page, M. First monkeypox genome from latest outbreak shows links to 2018 strain. NewScientist 2022. [Google Scholar]
- Farahat, R.A.; Abdelaal, A.; Shah, J.; Ghozy, S.; Sah, R.; Bonilla-Aldana, D.K.; Leblebicioglu, H. Monkeypox outbreaks during COVID-19 pandemic: Are we looking at an independent phenomenon or an overlapping pandemic? Ann. Clin. Microbiol. Antimicrob. 2022, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Awan, U.A.; Riasat, S.; Naeem, W.; Kamran, S.; Khattak, A.A.; Khan, S. Monkeypox: A new threat at our doorstep! J. Infect. 2022, 85, e47–e48. [Google Scholar] [CrossRef]
- Schmidt, F.I.; Bleck, C.K.E.; Mercer, J. Poxvirus host cell entry. Curr. Opin. Virol. 2012, 2, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Kugelman, J.R.; Johnston, S.C.; Mulembakani, P.M.; Kisalu, N.; Lee, M.S.; Koroleva, G.; McCarthy, S.E.; Gestole, M.C.; Wolfe, N.D.; Fair, J.N. Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg. Infect. Dis. 2014, 20, 232. [Google Scholar] [CrossRef]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox virus in Nigeria: Infection biology, epidemiology, and evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef]
- Adalja, A.; Inglesby, T. A novel international monkeypox outbreak. Am. Coll. Physicians 2022, 175, 1175–1176. [Google Scholar] [CrossRef]
- Mauldin, M.R.; McCollum, A.M.; Nakazawa, Y.J.; Mandra, A.; Whitehouse, E.R.; Davidson, W.; Zhao, H.; Gao, J.; Li, Y.; Doty, J. Exportation of monkeypox virus from the African continent. J. Infect. Dis. 2022, 225, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Velavan, T.P.; Meyer, C.G. Monkeypox 2022 outbreak: An update. Trop. Med. Int. Health 2022, 27, 604–605. [Google Scholar] [CrossRef] [PubMed]
- Damon, I.K. Status of human monkeypox: Clinical disease, epidemiology and research. Vaccine 2011, 29, D54–D59. [Google Scholar] [CrossRef]
- Saijo, M.; Ami, Y.; Suzaki, Y.; Nagata, N.; Iwata, N.; Hasegawa, H.; Iizuka, I.; Shiota, T.; Sakai, K.; Ogata, M. Virulence and pathophysiology of the Congo Basin and West African strains of monkeypox virus in non-human primates. J. Gen. Virol. 2009, 90, 2266–2271. [Google Scholar] [CrossRef]
- Kozlov, M. Monkeypox outbreaks: 4 key questions researchers have. Nature 2022, 606, 238–239. [Google Scholar] [CrossRef]
- Hammarlund, E.; Dasgupta, A.; Pinilla, C.; Norori, P.; Früh, K.; Slifka, M.K. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc. Natl. Acad. Sci. USA 2008, 105, 14567–14572. [Google Scholar] [CrossRef]
- Estep, R.D.; Messaoudi, I.; O’Connor, M.A.; Li, H.; Sprague, J.; Barron, A.; Engelmann, F.; Yen, B.; Powers, M.F.; Jones, J.M. Deletion of the monkeypox virus inhibitor of complement enzymes locus impacts the adaptive immune response to monkeypox virus in a nonhuman primate model of infection. J. Virol. 2011, 85, 9527–9542. [Google Scholar] [CrossRef]
- Lepers, A.; Shaw, L.; Schneckenburger, P.; Cacan, R.; Verbert, A.; Schauer, R. A study on the regulation of N-glycoloylneuraminic acid biosynthesis and utilization in rat and mouse liver. Eur. J. Biochem. 1990, 193, 715–723. [Google Scholar] [CrossRef]
- Kindrachuk, J.; Arsenault, R.; Kusalik, A.; Kindrachuk, K.N.; Trost, B.; Napper, S.; Jahrling, P.B.; Blaney, J.E. Systems kinomics demonstrates Congo Basin monkeypox virus infection selectively modulates host cell signaling responses as compared to West African monkeypox virus. Mol. Cell. Proteom. 2012, 11. [Google Scholar] [CrossRef]
- Weaver, J.R.; Isaacs, S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol. Rev. 2008, 225, 96–113. [Google Scholar] [CrossRef]
- Kaler, J.; Hussain, A.; Flores, G.; Kheiri, S.; Desrosiers, D. Monkeypox: A comprehensive review of transmission, pathogenesis, and manifestation. Cureus 2022, 14, e26531. [Google Scholar] [CrossRef]
- Farahat, R.A.; Sah, R.; El-Sakka, A.A.; Benmelouka, A.Y.; Kundu, M.; Labieb, F.; Shaheen, R.S.; Abdelaal, A.; Abdelazeem, B.; Bonilla-Aldana, D.K.; et al. Human monkeypox disease (MPX). Le Infez. Med. 2022, 30, 372–391. [Google Scholar] [CrossRef]
- Weinstein, R.A.; Nalca, A.; Rimoin, A.W.; Bavari, S.; Whitehouse, C.A. Reemergence of monkeypox: Prevalence, diagnostics, and countermeasures. Clin. Infect. Dis. 2005, 41, 1765–1771. [Google Scholar] [CrossRef]
- Chen, W.; Bibby, K. Model-based theoretical evaluation of the feasibility of using wastewater-based epidemiology to monitor monkeypox. Environ. Sci. Technol. Lett. 2022, 9, 772–778. [Google Scholar] [CrossRef]
- Vaughan, A.; Aarons, E.; Astbury, J.; Balasegaram, S.; Beadsworth, M.; Beck, C.R.; Chand, M.; O’connor, C.; Dunning, J.; Ghebrehewet, S. Two cases of monkeypox imported to the United Kingdom, September 2018. Eurosurveillance 2018, 23, 1800509. [Google Scholar] [CrossRef]
- Yinka-Ogunleye, A.; Aruna, O.; Ogoina, D.; Aworabhi, N.; Eteng, W.; Badaru, S.; Mohammed, A.; Agenyi, J.; Etebu, E.; Numbere, T.-W. Reemergence of human monkeypox in Nigeria, 2017. Emerg. Infect. Dis. 2018, 24, 1149. [Google Scholar] [CrossRef]
- Silenou, B.C.; Tom-Aba, D.; Adeoye, O.; Arinze, C.C.; Oyiri, F.; Suleman, A.K.; Yinka-Ogunleye, A.; Dörrbecker, J.; Ihekweazu, C.; Krause, G. Use of surveillance outbreak response management and analysis system for human monkeypox outbreak, Nigeria, 2017–2019. Emerg. Infect. Dis. 2020, 26, 345. [Google Scholar] [CrossRef]
- Hobson, G.; Adamson, J.; Adler, H.; Firth, R.; Gould, S.; Houlihan, C.; Johnson, C.; Porter, D.; Rampling, T.; Ratcliffe, L. Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Eurosurveillance 2021, 26, 2100745. [Google Scholar] [CrossRef]
- Ng, O.T.; Lee, V.; Marimuthu, K.; Vasoo, S.; Chan, G.; Lin, R.T.P.; Leo, Y.S. A case of imported Monkeypox in Singapore. Lancet Infect. Dis. 2019, 19, 1166. [Google Scholar] [CrossRef]
- Erez, N.; Achdout, H.; Milrot, E.; Schwartz, Y.; Wiener-Well, Y.; Paran, N.; Politi, B.; Tamir, H.; Israely, T.; Weiss, S. Diagnosis of imported monkeypox, Israel, 2018. Emerg. Infect. Dis. 2019, 25, 980. [Google Scholar] [CrossRef]
- Vivancos, R.; Anderson, C.; Blomquist, P.; Balasegaram, S.; Bell, A.; Bishop, L.; Brown, C.S.; Chow, Y.; Edeghere, O.; Florence, I. Community transmission of monkeypox in the United Kingdom, April to May 2022. Eurosurveillance 2022, 27, 2200422. [Google Scholar] [CrossRef]
- Núñez, I.; García-Grimshaw, M.; Ceballos-Liceaga, S.E.; Toledo-Salinas, C.; Carbajal-Sandoval, G.; Sosa-Laso, L.; García-Rodríguez, G.; Cortés-Alcalá, R.; de la Torre, A.; Fragoso-Saavedra, S. Epidemiological and clinical characteristics of patients with human monkeypox infection in Mexico: A nationwide observational study. Lancet Reg. Health–Am. 2023, 17, 100392. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Kaler, J.; Tabrez, E.; Tabrez, S.; Tabrez, S.S. Novel COVID-19: A comprehensive review of transmission, manifestation, and pathogenesis. Cureus 2020, 12, e8184. [Google Scholar] [CrossRef] [PubMed]
- Okyay, R.A.; Bayrak, E.; Kaya, E.; Şahin, A.R.; Koçyiğit, B.F.; Taşdoğan, A.M.; Avcı, A.; Sümbül, H.E. Another epidemic in the shadow of COVID 19 pandemic: A review of monkeypox. Proteins 2022, 7, 95–99. [Google Scholar] [CrossRef]
- Grant, R.; Nguyen, L.-B.L.; Breban, R. Modelling human-to-human transmission of monkeypox. Bull. World Health Organ. 2020, 98, 638. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://worldhealthorg.shinyapps.io/mpx_global/ (accessed on 23 April 2023).
- Altindis, M.; Puca, E.; Shapo, L. Diagnosis of monkeypox virus–An overview. Travel Med. Infect. Dis. 2022, 102459. [Google Scholar] [CrossRef] [PubMed]
- Lounis, M.; Riad, A. Monkeypox (MPOX)-Related Knowledge and Vaccination Hesitancy in Non-Endemic Countries: Concise Literature Review. Vaccines 2023, 11, 229. [Google Scholar] [CrossRef]
- McCollum, A.M.; Damon, I.K. Human monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. [Google Scholar] [CrossRef]
- Cheema, A.Y.; Ogedegbe, O.J.; Munir, M.; Alugba, G.; Ojo, T.K. Monkeypox: A review of clinical features, diagnosis, and treatment. Cureus 2022, 14, e26756. [Google Scholar] [CrossRef]
- Chen, N.; Li, G.; Liszewski, M.K.; Atkinson, J.P.; Jahrling, P.B.; Feng, Z.; Schriewer, J.; Buck, C.; Wang, C.; Lefkowitz, E.J. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 2005, 340, 46–63. [Google Scholar] [CrossRef]
- Knipe, D.; Howley, P.; Griffin, D.; Lamb, R.; Martin, M.; Roizman, B.; Straus, S. Fields Virology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 1–2. [Google Scholar]
- McFadden, G. Poxvirus tropism. Nat. Rev. Microbiol. 2005, 3, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.P.; Rodrigues, R.A.L.; Lima, M.T.; Drumond, B.P.; Abrahão, J.S. Poxvirus host range genes and virus–host spectrum: A critical review. Viruses 2017, 9, 331. [Google Scholar] [CrossRef]
- Ghaseminia, M. Preventing monkeypox outbreaks: Focus on diagnosis, care, treatment, and vaccination. J. Clin. Transl. Sci. 2023, 7, e60. [Google Scholar] [CrossRef] [PubMed]
- Chumakov, K.; Brechot, C.; Gallo, R.C.; Plotkin, S. Choosing the Right Path toward Polio Eradication. N. Engl. J. Med. 2023, 388, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Modis, Y. Class II fusion proteins. Viral Entry Host Cells 2013, 150–166. [Google Scholar] [CrossRef]
- Haller, S.L.; Peng, C.; McFadden, G.; Rothenburg, S. Poxviruses and the evolution of host range and virulence. Infect. Genet. Evol. 2014, 21, 15–40. [Google Scholar] [CrossRef]
- Fine, P.; Jezek, Z.; Grab, B.; Dixon, H. The transmission potential of monkeypox virus in human populations. Int. J. Epidemiol. 1988, 17, 643–650. [Google Scholar] [CrossRef]
- Pitkänen, K.J.; Mielke, J.H.; Jorde, L.B. Smallpox and its eradication in Finland: Implications for disease control. Popul. Stud. 1989, 43, 95–111. [Google Scholar] [CrossRef]
- Esposito, J.J.; Sammons, S.A.; Frace, A.M.; Osborne, J.D.; Olsen-Rasmussen, M.; Zhang, M.; Govil, D.; Damon, I.K.; Kline, R.; Laker, M. Genome sequence diversity and clues to the evolution of variola (smallpox) virus. Science 2006, 313, 807–812. [Google Scholar] [CrossRef]
- Shchelkunov, S.N. An increasing danger of zoonotic orthopoxvirus infections. PLoS Pathog. 2013, 9, e1003756. [Google Scholar] [CrossRef] [PubMed]
- Rimoin, A.W.; Mulembakani, P.M.; Johnston, S.C.; Lloyd Smith, J.O.; Kisalu, N.K.; Kinkela, T.L.; Blumberg, S.; Thomassen, H.A.; Pike, B.L.; Fair, J.N. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. USA 2010, 107, 16262–16267. [Google Scholar] [CrossRef] [PubMed]
- Ježek, Z.; Grab, B.; Szczeniowski, M.; Paluku, K.; Mutombo, M. Human monkeypox: Secondary attack rates. Bull. World Health Organ. 1988, 66, 465. [Google Scholar] [PubMed]
- Petersen, B.W.; Harms, T.J.; Reynolds, M.G.; Harrison, L.H. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupational exposure to orthopoxviruses-Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 257–262. [Google Scholar] [CrossRef]
- von Krempelhuber, A.; Vollmar, J.; Pokorny, R.; Rapp, P.; Wulff, N.; Petzold, B.; Handley, A.; Mateo, L.; Siersbol, H.; Kollaritsch, H. A randomized, double-blind, dose-finding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE®. Vaccine 2010, 28, 1209–1216. [Google Scholar] [CrossRef]
- Stittelaar, K.J.; van Amerongen, G.; Kondova, I.; Kuiken, T.; van Lavieren, R.F.; Pistoor, F.H.; Niesters, H.G.; van Doornum, G.; van der Zeijst, B.A.; Mateo, L. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J. Virol. 2005, 79, 7845–7851. [Google Scholar] [CrossRef]
- Frey, S.E.; Newman, F.K.; Kennedy, J.S.; Sobek, V.; Ennis, F.A.; Hill, H.; Yan, L.K.; Chaplin, P.; Vollmar, J.; Chaitman, B.R. Clinical and immunologic responses to multiple doses of IMVAMUNE®(Modified Vaccinia Ankara) followed by Dryvax® challenge. Vaccine 2007, 25, 8562–8573. [Google Scholar] [CrossRef]
- Jackson, L.A.; Frey, S.E.; El Sahly, H.M.; Mulligan, M.J.; Winokur, P.L.; Kotloff, K.L.; Campbell, J.D.; Atmar, R.L.; Graham, I.; Anderson, E.J. Safety and immunogenicity of a modified vaccinia Ankara vaccine using three immunization schedules and two modes of delivery: A randomized clinical non-inferiority trial. Vaccine 2017, 35, 1675–1682. [Google Scholar] [CrossRef]
- Overton, E.T.; Lawrence, S.J.; Stapleton, J.T.; Weidenthaler, H.; Schmidt, D.; Koenen, B.; Silbernagl, G.; Nopora, K.; Chaplin, P. A randomized phase II trial to compare safety and immunogenicity of the MVA-BN smallpox vaccine at various doses in adults with a history of AIDS. Vaccine 2020, 38, 2600–2607. [Google Scholar] [CrossRef]
- Overton, E.T.; Stapleton, J.; Frank, I.; Hassler, S.; Goepfert, P.A.; Barker, D.; Wagner, E.; von Krempelhuber, A.; Virgin, G.; Weigl, J. Safety and immunogenicity of modified vaccinia Ankara-Bavarian Nordic smallpox vaccine in vaccinia-naive and experienced human immunodeficiency virus-infected individuals: An open-label, controlled clinical phase II trial. In Open Forum Infectious Diseases; Oxford University Press US: New York, NY, USA, 2015; Volume 2, p. ofv040. [Google Scholar]
- Kennedy, J.S.; Gurwith, M.; Dekker, C.L.; Frey, S.E.; Edwards, K.M.; Kenner, J.; Lock, M.; Empig, C.; Morikawa, S.; Saijo, M. Safety and immunogenicity of LC16m8, an attenuated smallpox vaccine in vaccinia-naive adults. J. Infect. Dis. 2011, 204, 1395–1402. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Fujii, T.; Kanatani, Y.; Shinmura, Y.; Yokote, H.; Hashizume, S. Freeze-dried live attenuated smallpox vaccine prepared in cell culture “LC16-KAKETSUKEN”: Post-marketing surveillance study on safety and efficacy compliant with Good Clinical Practice. Vaccine 2015, 33, 6120–6127. [Google Scholar] [CrossRef]
- World Health Organization. Vaccines and Immunization for Monkeypox: Interim Guidance, 14 June 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Petersen, B.W.; Kabamba, J.; McCollum, A.M.; Lushima, R.S.; Wemakoy, E.O.; Tamfum, J.-J.M.; Nguete, B.; Hughes, C.M.; Monroe, B.P.; Reynolds, M.G. Vaccinating against monkeypox in the Democratic Republic of the Congo. Antivir. Res. 2019, 162, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.K.; Petersen, B.W.; Whitehill, F.; Razeq, J.H.; Isaacs, S.N.; Merchlinsky, M.J.; Campos-Outcalt, D.; Morgan, R.L.; Damon, I.; Sánchez, P.J. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices-United States, 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 734. [Google Scholar] [CrossRef]
- Yang, Z. Monkeypox: A potential global threat? J. Med. Virol. 2022, 94, 4034–4036. [Google Scholar] [CrossRef] [PubMed]
- Lansiaux, E.; Jain, N.; Laivacuma, S.; Reinis, A. The virology of human monkeypox virus (hMPXV): A brief overview. Virus Res. 2022, 322, 198932. [Google Scholar] [CrossRef]
- Pittman, P.R.; Hahn, M.; Lee, H.S.; Koca, C.; Samy, N.; Schmidt, D.; Hornung, J.; Weidenthaler, H.; Heery, C.R.; Meyer, T.P. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N. Engl. J. Med. 2019, 381, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J. Interim guidance for prevention and treatment of monkeypox in persons with HIV infection—United States, August 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71. [Google Scholar] [CrossRef]
- Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/index.html (accessed on 12 January 2023).
- Center for Disease Control and Prevention. Monkeypox in the U.S—Guidance for Tecovirimat Use Under Expanded Access Investigational New Drug Protocol during 2022 U.S. Monkeypox Cases. Available online: https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html (accessed on 9 August 2022).
- SIGA Technologies. A Double-Blind, Randomized, Placebo-Controlled, Multicenter Study to Assess the Safety, Tolerability, and Pharmacokinetics of TPOXX When Administered Orally for 28 Days in Adult Subjects; SIGA Technologies: New York, NY, USA, 2022. [Google Scholar]
- Hutson, C.L.; Kondas, A.V.; Mauldin, M.R.; Doty, J.B.; Grossi, I.M.; Morgan, C.N.; Ostergaard, S.D.; Hughes, C.M.; Nakazawa, Y.; Kling, C. Pharmacokinetics and efficacy of a potential smallpox therapeutic, brincidofovir, in a lethal monkeypox virus animal model. MSphere 2021, 6, e00927-20. [Google Scholar] [CrossRef]
- Smee, D.F. Progress in the discovery of compounds inhibiting orthopoxviruses in animal models. Antivir. Chem. Chemother. 2008, 19, 115–124. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S. Monkeypox virus infection in humans across 16 countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Rajsri, K.S.; Rao, M. A Review of Monkeypox: The New Global Health Emergency. Venereology 2022, 1, 199–211. [Google Scholar] [CrossRef]


| Scheme | Types of Mpox Tests | Description | Specimen Taken |
|---|---|---|---|
| 1. | Polymerase chain reaction (PCR) | In nucleic-acid-amplification testing, also known as molecular testing or PCR, the laboratory technician extracts genetic material from a patient specimen and subsequently amplifies it using pathogen-specific primers. Upon amplification, if the virus is present in the sample, the test detects it, thereby revealing whether the patient is actively infected at the time of testing. PCR is the preferred laboratory test for monkeypox diagnosis due to its high sensitivity and accuracy. | Lesion biopsy |
| 2. | Viral culture | Routine diagnostic procedures do not include virus isolation, and it should only be conducted in laboratories that possess adequate expertise and containment facilities. Virus isolation is not a standard diagnostic approach. | Lesion fluid |
| 3. | Electron microscopy | In evaluating the sample for a potential poxvirus, electron microscopy is an option, but due to the high technical skills and facility required and the availability of molecular assays, this method is not routinely used for the diagnosis of poxviruses. | Biopsy specimen, scab material, vesicular fluid |
| 4. | Immunohistochemistry | A check for orthopoxvirus-specific antigens is done through testing. | Biopsy specimen |
| 5. | Anti-Orthopoxvirus IgG and IgM tests | These tests can be utilized to assess either recent or past exposure to orthopoxvirus. | Blood specimen |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, S.; Kumar, S.; Jain, S.; Mohanty, A.; Thapa, N.; Poudel, P.; Bhusal, K.; Al-qaim, Z.H.; Barboza, J.J.; Padhi, B.K.; et al. The Global Monkeypox (Mpox) Outbreak: A Comprehensive Review. Vaccines 2023, 11, 1093. https://doi.org/10.3390/vaccines11061093
Srivastava S, Kumar S, Jain S, Mohanty A, Thapa N, Poudel P, Bhusal K, Al-qaim ZH, Barboza JJ, Padhi BK, et al. The Global Monkeypox (Mpox) Outbreak: A Comprehensive Review. Vaccines. 2023; 11(6):1093. https://doi.org/10.3390/vaccines11061093
Chicago/Turabian StyleSrivastava, Shriyansh, Sachin Kumar, Shagun Jain, Aroop Mohanty, Neeraj Thapa, Prabhat Poudel, Krishna Bhusal, Zahraa Haleem Al-qaim, Joshuan J. Barboza, Bijaya Kumar Padhi, and et al. 2023. "The Global Monkeypox (Mpox) Outbreak: A Comprehensive Review" Vaccines 11, no. 6: 1093. https://doi.org/10.3390/vaccines11061093
APA StyleSrivastava, S., Kumar, S., Jain, S., Mohanty, A., Thapa, N., Poudel, P., Bhusal, K., Al-qaim, Z. H., Barboza, J. J., Padhi, B. K., & Sah, R. (2023). The Global Monkeypox (Mpox) Outbreak: A Comprehensive Review. Vaccines, 11(6), 1093. https://doi.org/10.3390/vaccines11061093









