An Alternative Serological Measure for Assessing Foot-and-Mouth Disease Vaccine Efficacy against Homologous and Heterologous Viral Challenges in Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Preparation of Vaccines
2.3. Vaccine Efficacy Study and Challenge Protocol
2.4. Serological Assays
2.5. Statistical Analysis
3. Results
3.1. In Vivo Efficacy Results
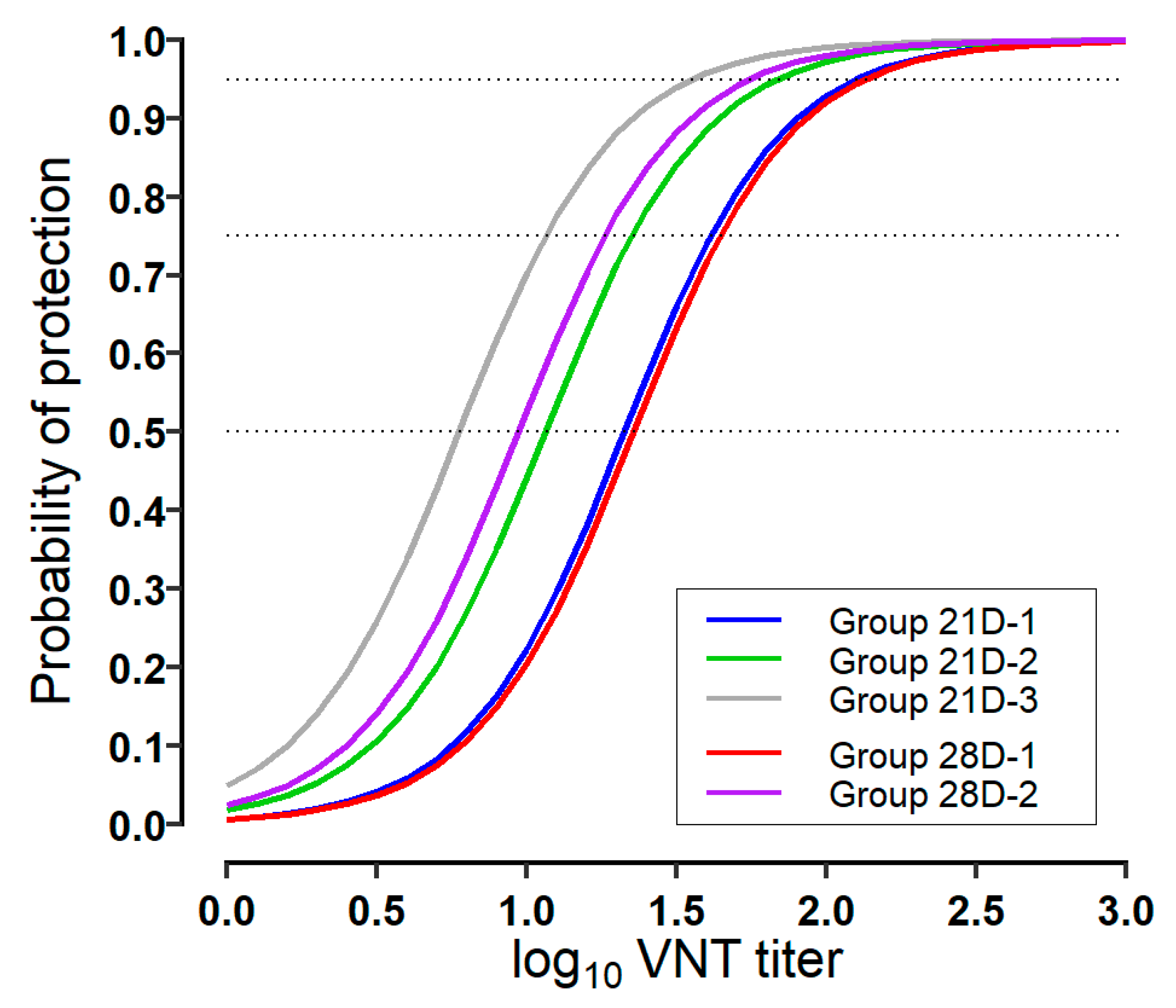
3.2. Estimation of Protection Probability to VNT Titer
3.3. The Characteristics of the Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bachrach, H.L. Foot-and-mouth disease. Annu. Rev. Microbiol. 1968, 22, 201–244. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H. Foot-and-mouth disease. In Virus Diseases of Food Animals; Gibbs, E.P.G., Ed.; Academic Press Inc.: New York, NY, USA, 1981; Volume 2, pp. 333–363. [Google Scholar]
- Paton, D.J.; Reeve, R.; Capozzo, A.V.; Ludi, A. Estimating the protection afforded by foot-and-mouth disease vaccines in the laboratory. Vaccine 2019, 37, 5515–5524. [Google Scholar] [CrossRef] [PubMed]
- WOAH. WOAH Terrestrial Manual 2022. In Foot and Mouth Disease (Infection with Foot and Mouth Disease Virus); WOAH: Paris, France, 2022. [Google Scholar]
- Waters, R.; Ludi, A.B.; Fowler, V.L.; Wilsden, G.; Browning, C.; Gubbins, S.; Statham, B.; Bin-Tarif, A.; Mioulet, V.; King, D.J.; et al. Efficacy of a high-potency multivalent foot-and-mouth disease virus vaccine in cattle against heterologous challenge with a field virus from the emerging A/ASIA/G-VII lineage. Vaccine 2018, 36, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Brehm, K.E.; Kumar, N.; Thulke, H.H.; Haas, B. High potency vaccines induce protection against heterologous challenge with foot-and-mouth disease virus. Vaccine 2008, 26, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Nagendrakumar, S.B.; Srinivasan, V.A.; Madhanmohan, M.; Yuvaraj, S.; Parida, S.; Di Nardo, A.; Horsington, J.; Paton, D.J. Evaluation of cross-protection between O1 Manisa and O1 Campos in cattle vaccinated with foot-and-mouth disease virus vaccine incorporating different payloads of inactivated O1 Manisa antigen. Vaccine 2011, 29, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Fishbourne, E.; Ludi, A.B.; Wilsden, G.; Hamblin, P.; Statham, B.; Bin-Tarif, A.; Brocchi, E.; Grazioli, S.; Dekker, A.; Eble, P.; et al. Efficacy of a high potency O1 Manisa foot-and-mouth disease vaccine in cattle against heterologous challenge with a field virus from the O/ME-SA/Ind-2001 lineage collected in North Africa. Vaccine 2017, 35, 2761–2765. [Google Scholar] [CrossRef] [PubMed]
- Horsington, J.; Perez, C.B.; Maradei, E.; Novo, S.G.; Gonzales, J.L.; Singanallur, N.B.; Bonastre, P.; Vosloo, W. Protective effects of high-potency FMDV O1 Manisa monovalent vaccine in cattle challenged with FMDV O/SKR/2010 at 7 or 4 days post vaccination. Vaccine 2017, 35, 5179–5185. [Google Scholar] [CrossRef]
- Goris, N.; Merkelbach-Peters, P.; Diev, V.I.; Verloo, D.; Zakharov, V.M.; Kraft, H.P.; De Clercq, K. European Pharmacopoeia foot-and-mouth disease vaccine potency testing in cattle: Between test variability and its consequences. Vaccine 2007, 25, 3373–3379. [Google Scholar] [CrossRef]
- Goris, N.; Maradei, E.; D’Aloia, R.; Fondevila, N.; Mattion, N.; Perez, A.; Smitsaart, E.; Nauwynck, H.J.; La Torre, J.; Palma, E.; et al. Foot-and-mouth disease vaccine potency testing in cattle using homologous and heterologous challenge strains: Precision of the “Protection against Podal Generalisation” test. Vaccine 2008, 26, 3432–3437. [Google Scholar] [CrossRef]
- Hingley, P.J.; Pay, T.W. Sources of variability in foot and mouth disease vaccine potency estimates based on serum neutralizing antibody assay. J. Biol. Stand. 1987, 15, 127–142. [Google Scholar] [CrossRef]
- Vianna Filho, Y.L.; Astudillo, V.; Gomes, I.; Fernandez, G.; Rozas, C.E.; Ravison, J.A.; Alonso, A. Potency control of foot-and-mouth disease vaccine in cattle. Comparison of the 50% protective dose and the protection against generalization. Vaccine 1993, 11, 1424–1428. [Google Scholar] [CrossRef] [PubMed]
- Periolo, O.H.; Seki, C.; Grigera, P.R.; Robiolo, B.; Fernandez, G.; Maradei, E.; D’Aloia, R.; La Torre, J.L. Large-scale use of liquid-phase blocking sandwich ELISA for the evaluation of protective immunity against aphthovirus in cattle vaccinated with oil-adjuvanted vaccines in Argentina. Vaccine 1993, 11, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Robiolo, B.; Grigera, P.R.; Periolo, O.H.; Seki, C.; Bianchi, T.; Maradei, E.; La Torre, J.L. Assessment of foot and mouth disease vaccine potency by liquid-phase blocking ELISA: A proposal for an alternative to the challenge procedure in Argentina. Vaccine 1995, 13, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Barnett, P.V.; Statham, R.J.; Vosloo, W.; Haydon, D.T. Foot-and-mouth disease vaccine potency testing: Determination and statistical validation of a model using a serological approach. Vaccine 2003, 21, 3240–3248. [Google Scholar] [CrossRef] [PubMed]
- Goris, N.; Willems, T.; Diev, V.I.; Merkelbach-Peters, P.; Vanbinst, T.; Van der Stede, Y.; Kraft, H.P.; Zakharov, V.M.; Borisov, V.V.; Nauwynck, H.J.; et al. Indirect foot-and-mouth disease vaccine potency testing based on a serological alternative. Vaccine 2008, 26, 3870–3879. [Google Scholar] [CrossRef] [PubMed]
- Maradei, E.; La Torre, J.; Robiolo, B.; Esteves, J.; Seki, C.; Pedemonte, A.; Iglesias, M.; D’Aloia, R.; Mattion, N. Updating of the correlation between lpELISA titers and protection from virus challenge for the assessment of the potency of polyvalent aphtovirus vaccines in Argentina. Vaccine 2008, 26, 6577–6586. [Google Scholar] [CrossRef]
- Willems, T.; Lefebvre, D.J.; Goris, N.; Diev, V.I.; Kremenchugskaya, S.R.; Paul, G.; Haas, B.; De Clercq, K. Characteristics of serology-based vaccine potency models for foot-and-mouth disease virus. Vaccine 2012, 30, 5849–5855. [Google Scholar] [CrossRef] [PubMed]
- Gubbins, S.; Paton, D.J.; Dekker, A.; Ludi, A.B.; Wilsden, G.; Browning, C.F.J.; Eschbaumer, M.; Barnabei, J.; Duque, H.; Pauszek, L.L.; et al. Predicting cross-protection against foot-and-mouth disease virus strains by serology after vaccination. Front. Vet. Sci. 2022, 9, 1027006. [Google Scholar] [CrossRef]
- Black, L.; Francis, M.J.; Rweyemamu, M.M.; Umebara, O.; Boge, A. The relationship between serum antibody titres and protection from foot and mouth disease in pigs after oil emulsion vaccination. J. Biol. Stand. 1984, 12, 379–389. [Google Scholar] [CrossRef]
- Galdo Novo, S.; Malirat, V.; Maradei, E.D.; Pedemonte, A.R.; Espinoza, A.M.; Smitsaart, E.; Lee, K.N.; Park, J.H.; Bergmann, I.E. Efficacy of a high quality O1/Campos foot-and-mouth disease vaccine upon challenge with a heterologous Korean O Mya98 lineage virus in pigs. Vaccine 2018, 36, 1570–1576. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, Y.J.; Kim, R.H.; Park, J.N.; Park, M.E.; Ko, M.K.; Choi, J.H.; Chu, J.Q.; Lee, K.N.; Kim, S.M.; et al. Rapid Engineering of Foot-and-Mouth Disease Vaccine and Challenge Viruses. J. Virol. 2017, 91, e00155-17. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lu, Z.J.; Xie, B.X.; Sun, P.; Chen, Y.L.; Fu, Y.F.; Liu, Z.X. Alternative way to test the efficacy of swine FMD vaccines: Measurement of pigs median infected dose (PID50) and regulation of live virus challenge dose. Virol. J. 2010, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.M.; Belsham, G.J. Molecular epidemiology, evolution and phylogeny of foot-and-mouth disease virus. Infect. Genet. Evol. 2018, 59, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.K.; Jo, H.E.; Choi, J.H.; You, S.H.; Shin, S.H.; Jo, H.; Lee, M.J.; Kim, S.M.; Kim, B.; Park, J.H. Chimeric vaccine strain of type O foot-and-mouth disease elicits a strong immune response in pigs against ME-SA and SEA topotypes. Vet. Microbiol. 2019, 229, 124–129. [Google Scholar] [CrossRef]
- Alves, M.P.; Guzylack-Piriou, L.; Juillard, V.; Audonnet, J.C.; Doel, T.; Dawson, H.; Golde, W.T.; Gerber, H.; Peduto, N.; McCullough, K.C.; et al. Innate immune defenses induced by CpG do not promote vaccine-induced protection against foot-and-mouth disease virus in pigs. Clin. Vaccine Immunol. 2009, 16, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Spiegelhalter, D.J.; Best, N.G.; Carlin, B.P.; Van Der Linde, A. Bayesian measures of model complexity and fit. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2002, 64, 583–639. [Google Scholar] [CrossRef]
- Zweig, M.H.; Campbell, G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin. Chem. 1993, 39, 561–577. [Google Scholar] [CrossRef]
- Jamal, S.M.; Bouma, A.; van den Broek, J.; Stegeman, A.; Chenard, G.; Dekker, A. Foot-and-mouth disease vaccine potency testing: The influence of serotype, type of adjuvant, valency, fractionation method, and virus culture on the dose-response curve in cattle. Vaccine 2008, 26, 6317–6321. [Google Scholar] [CrossRef]
- Salt, J.S.; Barnett, P.V.; Dani, P.; Williams, L. Emergency vaccination of pigs against foot-and-mouth disease: Protection against disease and reduction in contact transmission. Vaccine 1998, 16, 746–754. [Google Scholar] [CrossRef]
- Francis, M.J.; Black, L. Antibody response in pig nasal fluid and serum following foot-and-mouth disease infection or vaccination. J. Hyg. 1983, 91, 329–334. [Google Scholar] [CrossRef]
- Park, J.N.; Lee, S.Y.; Chu, J.Q.; Lee, Y.J.; Kim, R.H.; Lee, K.N.; Kim, S.M.; Tark, D.S.; Kim, B.; Park, J.H. Protection to homologous and heterologous challenge in pigs immunized with vaccine against foot-and-mouth disease type O caused an epidemic in East Asia during 2010/2011. Vaccine 2014, 32, 1882–1889. [Google Scholar] [CrossRef]
- Eble, P.L.; Bouma, A.; de Bruin, M.G.; van Hemert-Kluitenberg, F.; van Oirschot, J.T.; Dekker, A. Vaccination of pigs two weeks before infection significantly reduces transmission of foot-and-mouth disease virus. Vaccine 2004, 22, 1372–1378. [Google Scholar] [CrossRef]
- Li, Y.; Stirling, C.M.; Denyer, M.S.; Hamblin, P.; Hutchings, G.; Takamatsu, H.H.; Barnett, P.V. Dramatic improvement in FMD DNA vaccine efficacy and cross-serotype antibody induction in pigs following a protein boost. Vaccine 2008, 26, 2647–2656. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Pacheco, J.M.; Rodriguez, L.L.; Arzt, J. Infection dynamics of foot-and-mouth disease virus in pigs using two novel simulated-natural inoculation methods. Res. Vet. Sci. 2014, 96, 396–405. [Google Scholar] [CrossRef]
- Kim, T.; Hong, J.K.; Oem, J.K.; Lee, K.N.; Lee, H.S.; Kim, Y.J.; Ryoo, S.; Ko, Y.J.; Park, J.H.; Choi, J.; et al. Cross-protective efficacy of the O1 Manisa+O 3039 bivalent vaccine and the O 3039 monovalent vaccine against heterologous challenge with FMDV O/Jincheon/SKR/2014 in pig. Vaccine 2019, 37, 1702–1709. [Google Scholar] [CrossRef]
- Choi, J.; Jo, H.J.; Jung, S.S.; Choi, J.; Lee, S.H.; Kim, H.H.; Kim, Y.J.; Kim, B.; Park, J.H.; Kim, J. Evaluation of swine protection with foot-and-mouth disease O(1)/Campos and O/Primorsky/2014 vaccines against the O Mya-98 lineage virus from East Asia. Vaccine 2021, 39, 1701–1707. [Google Scholar] [CrossRef]
- Burrows, R. The infectivity assay of foot-and-mouth disease virus in pigs. J. Hyg. 1966, 64, 419–429. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Diaz-San Segundo, F.; de Los Santos, T.; Rodriguez, L.L.; Arzt, J. The Pathogenesis of Foot-and-Mouth Disease in Pigs. Front. Vet. Sci. 2016, 3, 41. [Google Scholar] [CrossRef]
- Pacheco, J.M.; Tucker, M.; Hartwig, E.; Bishop, E.; Arzt, J.; Rodriguez, L.L. Direct contact transmission of three different foot-and-mouth disease virus strains in swine demonstrates important strain-specific differences. Vet. J. 2012, 193, 456–463. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Pacheco, J.M.; Rodriguez, L.L.; Arzt, J. Early events in the pathogenesis of foot-and-mouth disease in pigs; identification of oropharyngeal tonsils as sites of primary and sustained viral replication. PLoS ONE 2014, 9, e106859. [Google Scholar] [CrossRef]
- Nishi, T.; Morioka, K.; Kawaguchi, R.; Yamada, M.; Ikezawa, M.; Fukai, K. Quantitative analysis of infection dynamics of foot-and-mouth disease virus strain O/CATHAY in pigs and cattle. PLoS ONE 2021, 16, e0245781. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.M.; Mason, P.W. Evaluation of infectivity and transmission of different Asian foot-and-mouth disease viruses in swine. J. Vet. Sci. 2010, 11, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Lian, K.; Yang, F.; Jin, Y.; Zhu, Z.; Guo, J.; Cao, W.; Liu, H.; He, J.; Zhang, K.; et al. Cross-protective efficacy of engineering serotype A foot-and-mouth disease virus vaccine against the two pandemic strains in swine. Vaccine 2015, 33, 5772–5778. [Google Scholar] [CrossRef] [PubMed]

| Serotype | Vaccine Strain | Challenge Strain | No. of Challenged Animals | No. of Protected Animals | VNT Titer ± SD 1 (log 10) | |||
|---|---|---|---|---|---|---|---|---|
| 21 dpv 2 | 28 dpv | 4 dpc 3 | 7 dpc | |||||
| O | O/Jincheon | O/Jincheon | 29 | 9 | 0.96 ± 0.48 1 | 0.78 ± 0.74 | 1.59 ± 0.39 | 2.44 ± 0.22 |
| O/Primorsky | O/Jincheon | 67 | 49 | 1.42 ± 0.62 | 1.30 ± 0.50 | 1.53 ± 0.40 | 2.15 ± 0.42 | |
| A | A/Yeoncheon | A/Yeoncheon | 15 | 4 | 0.33 ± 0.69 | 0.48 ± 0.86 | 0.74 ± 0.95 | 2.63 ± 0.57 |
| A/Pocheon | A/Yeoncheon | 54 | 35 | 0.88 ± 0.90 | 1.17 ± 1.02 | 1.78 ± 0.78 | 2.24 ± 0.48 | |
| Asia-1 | Asia-1/Shamir | Asia-1/Shamir | 74 | 54 | 1.26 ± 0.81 | 1.39 ± 0.86 | 1.72 ± 0.59 | 2.16 ± 0.27 |
| Model | Group | Probability of Protection | ||||
|---|---|---|---|---|---|---|
| 0.50 | 0.625 | 0.75 | 0.85 | 0.95 | ||
| 21-dpv | 21D-1 | 1.33 (1.09–1.56) 1 | 1.46 (1.23–1.72) | 1.62 (1.38–1.92) | 1.78 (1.54–2.14) | 2.10 (1.82–2.60) |
| 21D-2 | 1.06 (0.93–1.20) | 1.19 (1.06–1.33) | 1.35 (1.22–1.51) | 1.52 (1.37–1.69) | 1.83 (1.66–2.13) | |
| 21D-3 | 0.78 (0.59–0.97) | 0.91 (0.73–1.12) | 1.06 (0.88–1.29) | 1.23 (1.04–1.53) | 1.55 (1.32–1.94) | |
| 28-dpv | 28D-1 | 1.36 (1.12–1.59) | 1.49 (1.26–1.75) | 1.64 (1.41–1.94) | 1.81 (1.57–2.17) | 2.12 (1.85–2.60) |
| 28D-2 | 0.97 (0.87–1.09) | 1.10 (0.99–1.22) | 1.26 (1.15–1.39) | 1.42 (1.30–1.58) | 1.74 (1.57–2.00) | |
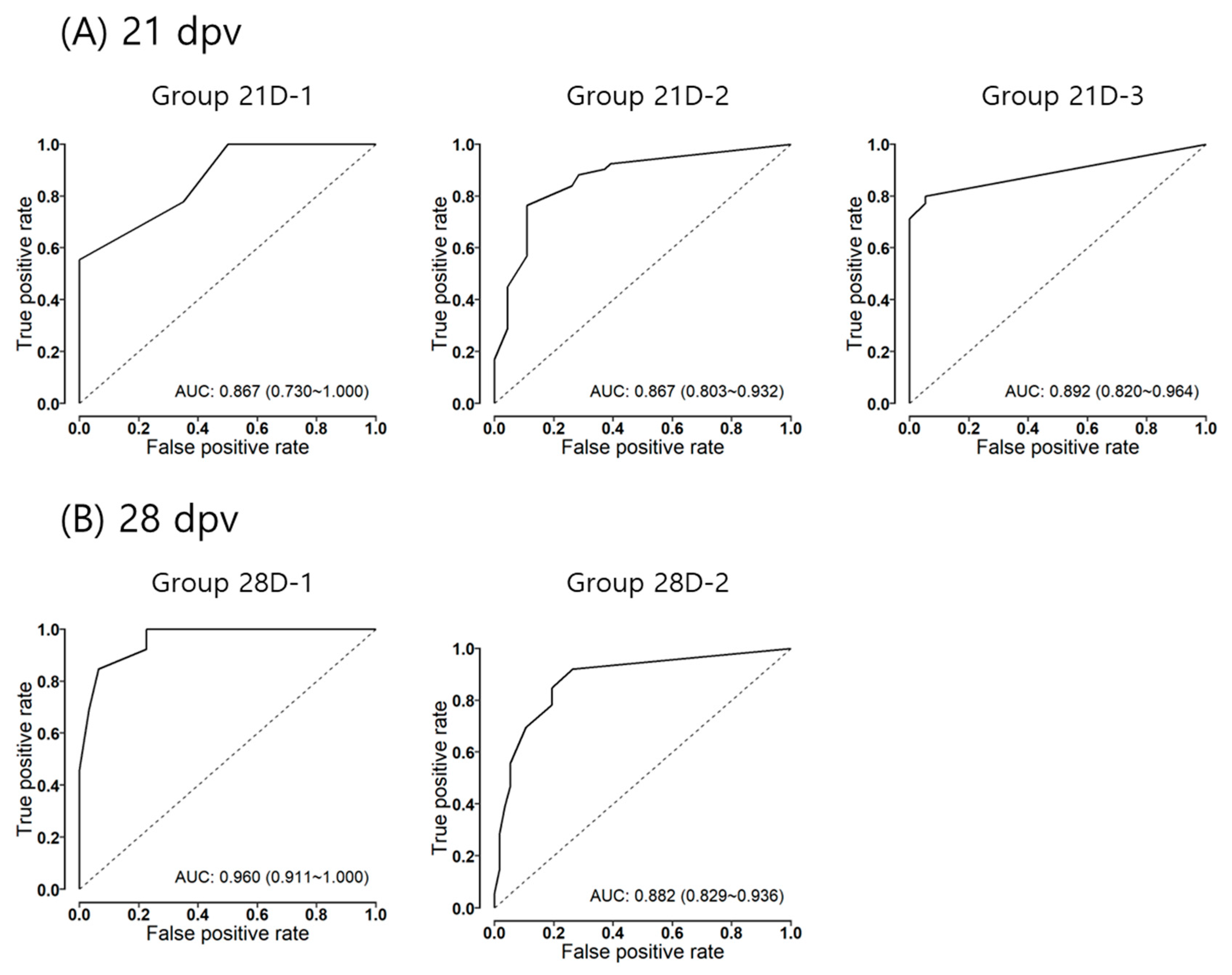
| Model | Group | VNT Titer Cutoff | Probability of Protection (95% CI 1) | Sens. (%) | Spec. (%) | ACC (%) |
|---|---|---|---|---|---|---|
| 21-dpv | 21D-1 | 1.27 | 0.45 (0.24–0.65) | 55.6 | 100.0 | 86.2 |
| 21D-2 | 1.43 | 0.80 (0.71–0.88) | 76.3 | 89.1 | 80.6 | |
| 21D-3 | 0.75 | 0.48 (0.30–0.65) | 80.0 | 94.7 | 85.2 | |
| 28-dpv | 28D-1 | 1.27 | 0.42 (0.22–0.63) | 84.6 | 93.5 | 90.9 |
| 28D-2 | 0.97 | 0.50 (0.39–0.61) | 92.0 | 73.7 | 86.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, S.H.; Kim, H.-H.; Shin, S.-H.; Park, S.-H.; Park, J.-H.; Park, C.-K. An Alternative Serological Measure for Assessing Foot-and-Mouth Disease Vaccine Efficacy against Homologous and Heterologous Viral Challenges in Pigs. Vaccines 2024, 12, 10. https://doi.org/10.3390/vaccines12010010
Kim J, Lee SH, Kim H-H, Shin S-H, Park S-H, Park J-H, Park C-K. An Alternative Serological Measure for Assessing Foot-and-Mouth Disease Vaccine Efficacy against Homologous and Heterologous Viral Challenges in Pigs. Vaccines. 2024; 12(1):10. https://doi.org/10.3390/vaccines12010010
Chicago/Turabian StyleKim, Jaejo, Seung Heon Lee, Ha-Hyun Kim, Sung-Ho Shin, Sang-Hyun Park, Jong-Hyeon Park, and Choi-Kyu Park. 2024. "An Alternative Serological Measure for Assessing Foot-and-Mouth Disease Vaccine Efficacy against Homologous and Heterologous Viral Challenges in Pigs" Vaccines 12, no. 1: 10. https://doi.org/10.3390/vaccines12010010
APA StyleKim, J., Lee, S. H., Kim, H.-H., Shin, S.-H., Park, S.-H., Park, J.-H., & Park, C.-K. (2024). An Alternative Serological Measure for Assessing Foot-and-Mouth Disease Vaccine Efficacy against Homologous and Heterologous Viral Challenges in Pigs. Vaccines, 12(1), 10. https://doi.org/10.3390/vaccines12010010







