Sequential Vaccination Against Streptococcus pneumoniae Appears as Immunologically Safe in Clinically Stable Kidney Transplant Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Vaccines and Vaccination
2.3. Determination of HLA and MICA Antibodies
2.4. Determination of Antibodies Against Pneumococci
2.5. Statistical Analysis
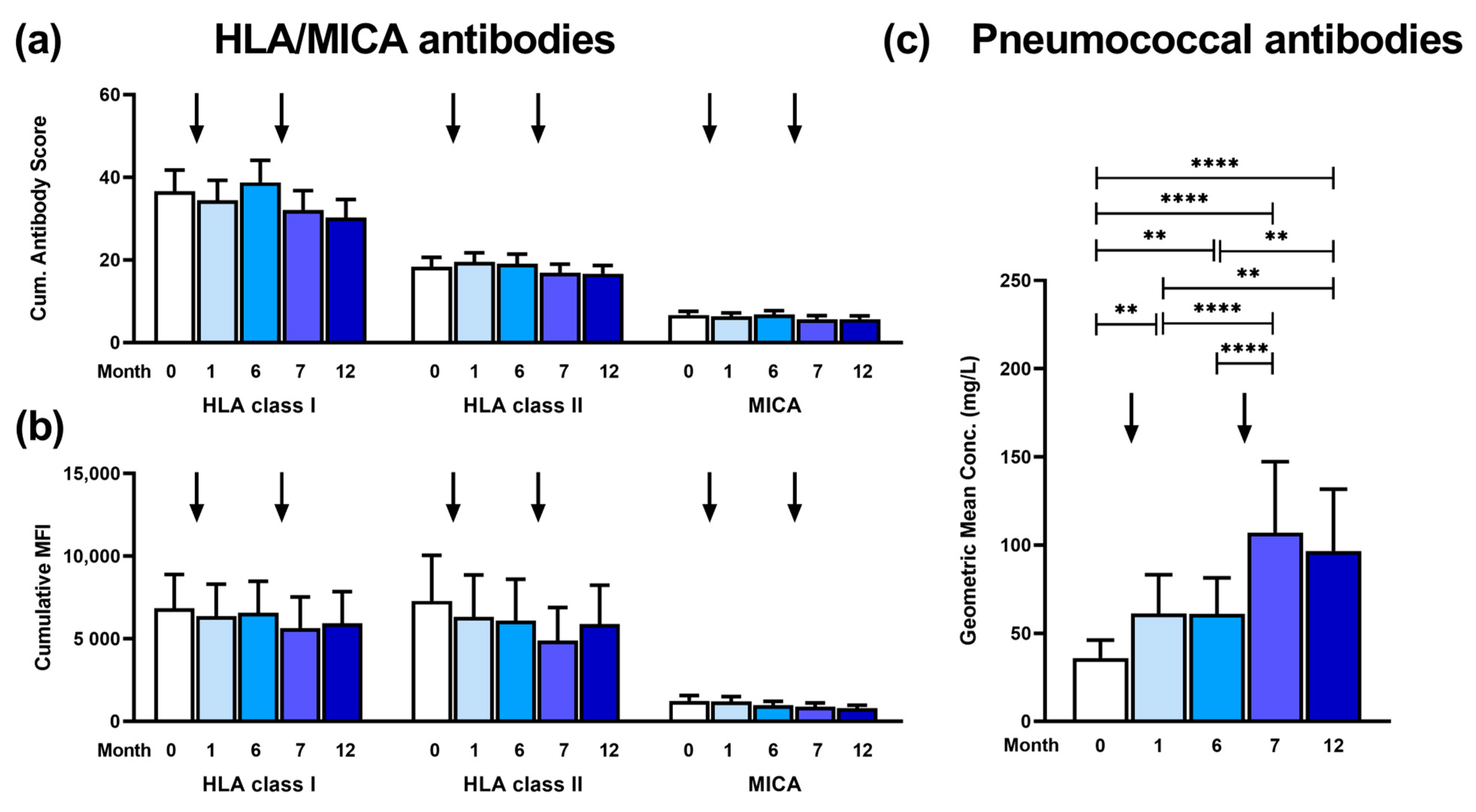
3. Results
3.1. Kinetics of Antibody Responses
3.2. Patterns of Antibodies Prior to and Post Vaccination
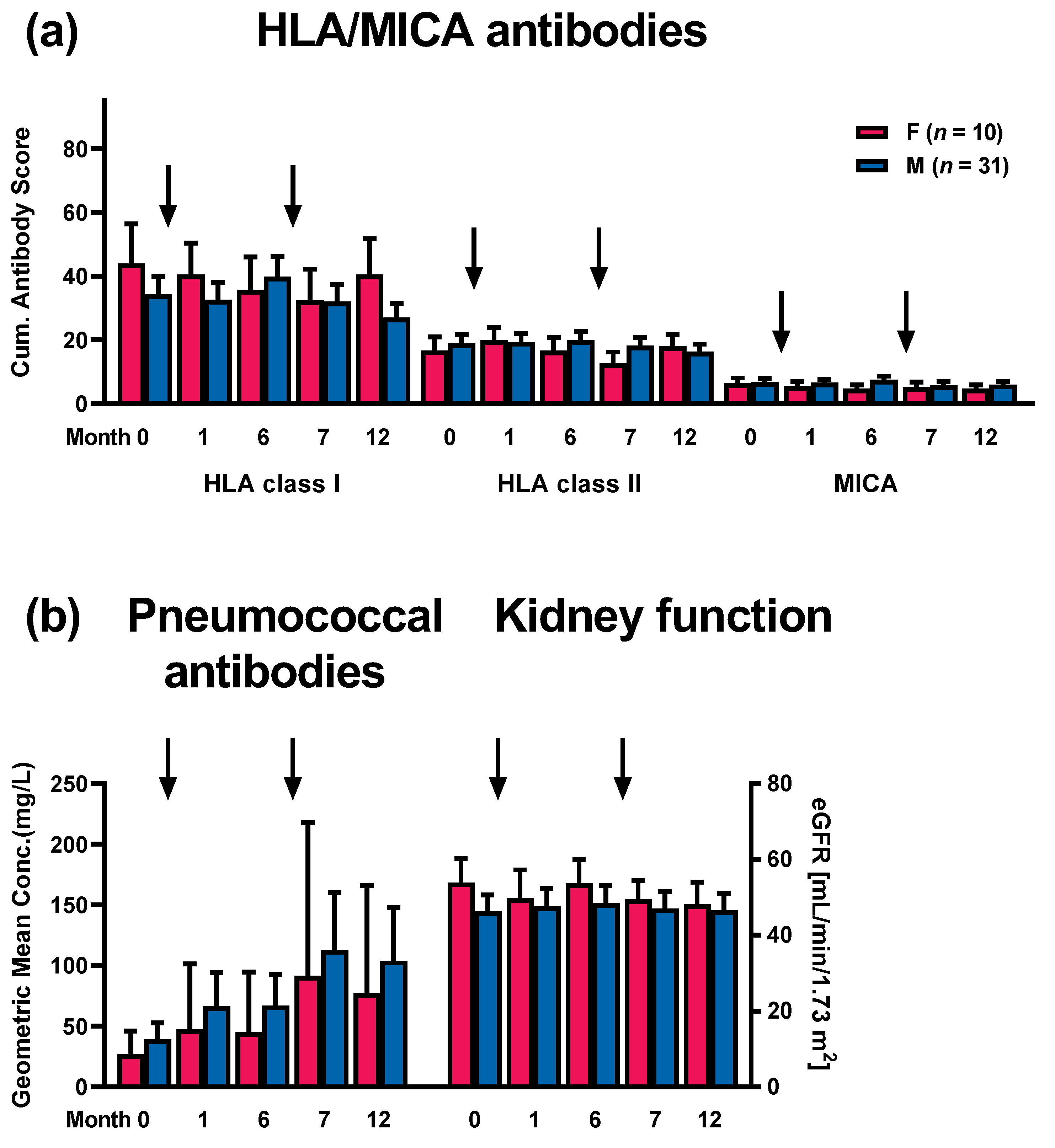
3.3. Correlation of HLA and MICA Antibodies with Clinical Outcome and with Patient Characteristics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simell, B.; Auranen, K.; Kayhty, H.; Goldblatt, D.; Dagan, R.; O’Brien, K.L.; Pneumococcal Carriage, G. The fundamental link between pneumococcal carriage and disease. Expert. Rev. Vaccines 2012, 11, 841–855. [Google Scholar] [CrossRef] [PubMed]
- van der Poll, T.; Opal, S.M. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 2009, 374, 1543–1556. [Google Scholar] [CrossRef] [PubMed]
- National Vaccine Program Office. Adult Immunization Plans. Available online: http://www.hhs.gov/nvpo/national-adult-immunization-plan/ (accessed on 9 July 2024).
- Arora, S.; Kipp, G.; Bhanot, N.; Sureshkumar, K.K. Vaccinations in kidney transplant recipients: Clearing the muddy waters. World J. Transplant. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine for Adults with Immunocompromising Conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 2012, 61, 816–819. [Google Scholar]
- Laws, H.J.; Baumann, U.; Bogdan, C.; Burchard, G.; Christopeit, M.; Hecht, J.; Heininger, U.; Hilgendorf, I.; Kern, W.; Kling, K.; et al. Impfen bei Immundefizienz [Vaccination in immunodeficiency]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2020, 63, 588–644. [Google Scholar] [CrossRef]
- Todar, K.G. Todar’s Online Textbook of Bacteriology; Kenneth Todar University of Wisconsin-Madison Department of Bacteriology: Madison, WI, USA, 2004. [Google Scholar]
- Rubins, J.B.; Puri, A.K.; Loch, J.; Charboneau, D.; MacDonald, R.; Opstad, N.; Janoff, E.N. Magnitude, duration, quality, and function of pneumococcal vaccine responses in elderly adults. J. Infect. Dis. 1998, 178, 431–440. [Google Scholar] [CrossRef]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef]
- Robert-Koch-Institut. Empfehlungen der Ständigen Impfkommission beim Robert Koch-Institut 2023. Epid. Bull. 2023, 4, 1–41. [Google Scholar]
- Pletz, M.W.; Maus, U.; Krug, N.; Welte, T.; Lode, H. Pneumococcal vaccines: Mechanism of action, impact on epidemiology and adaption of the species. Int. J. Antimicrob. Agents 2008, 32, 199–206. [Google Scholar] [CrossRef]
- Mond, J.J.; Lees, A.; Snapper, C.M. T cell-independent antigens type 2. Annu. Rev. Immunol. 1995, 13, 655–692. [Google Scholar] [CrossRef]
- Larsen, L.; Bistrup, C.; Sorensen, S.S.; Boesby, L.; Jorgensen, C.S.; Johansen, I.S. Immunogenicity and safety of double dosage of pneumococcal vaccines in adult kidney transplant recipients and waiting list patients: A non-blinded, randomized clinical trial. Vaccine 2022, 40, 3884–3892. [Google Scholar] [CrossRef] [PubMed]
- Mülling, N.; van de Sand, L.; Völk, K.; Aufderhorst, U.W.; van der Linden, M.; Horn, P.A.; Kribben, A.; Wilde, B.; Krawczyk, A.; Witzke, O.; et al. Antibody responses after sequential vaccination with PCV13 and PPSV23 in kidney transplant recipients. Infection 2023, 51, 1703–1716. [Google Scholar] [CrossRef] [PubMed]
- Hurst, F.P.; Lee, J.J.; Jindal, R.M.; Agodoa, L.Y.; Abbott, K.C. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin. J. Am. Soc. Nephrol. 2011, 6, 1192–1197. [Google Scholar] [CrossRef]
- Scharpe, J.; Evenepoel, P.; Maes, B.; Bammens, B.; Claes, K.; Osterhaus, A.D.; Vanrenterghem, Y.; Peetermans, W.E. Influenza vaccination is efficacious and safe in renal transplant recipients. Am. J. Transplant. 2008, 8, 332–337. [Google Scholar] [CrossRef]
- Connolly, J.; Douglas, J.; Kumar, R.; Middleton, D.; McEvoy, J.; Nelson, S.; McGeown, M.G. Letter: Influenza virus vaccination and renal transplant rejection. Br. Med. J. 1974, 1, 638. [Google Scholar] [CrossRef]
- Cainelli, F.; Vento, S. Infections and solid organ transplant rejection: A cause-and-effect relationship? Lancet Infect. Dis. 2002, 2, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Candon, S.; Thervet, E.; Lebon, P.; Suberbielle, C.; Zuber, J.; Lima, C.; Charron, D.; Legendre, C.; Chatenoud, L. Humoral and cellular immune responses after influenza vaccination in kidney transplant recipients. Am. J. Transplant. 2009, 9, 2346–2354. [Google Scholar] [CrossRef]
- Mulley, W.R.; Dendle, C.; Ling, J.E.H.; Knight, S.R. Does vaccination in solid-organ transplant recipients result in adverse immunologic sequelae? A systematic review and meta-analysis. J. Heart Lung Transplant. 2018, 37, 844–852. [Google Scholar] [CrossRef]
- European Medicines Agency: Assessment report for Prevenar 13. Available online: https://www.ema.europa.eu/en/documents/assessment-report/prevenar-13-epar-public-assessment-report_en.pdf (accessed on 4 July 2024).
- Paul-Ehrlich-Institut. Fachinformation PNEUMOVAX® 23. Available online: https://www.pei.de/SharedDocs/Downloads/DE/newsroom/meldungen/fachinformation-pneumovax.pdf?__blob=publicationFile&v=4 (accessed on 9 July 2024).
- Lindemann, M.; Heinemann, F.M.; Horn, P.A.; Witzke, O. Vaccination against Streptococcus pneumoniae does not induce antibodies against HLA or MICA in clinically stable kidney transplant recipients. Hum. Immunol. 2013, 74, 1267–1270. [Google Scholar] [CrossRef]
- Heinemann, F.M. HLA Genotyping and Antibody Characterization Using the Luminex Multiplex Technology. Transfus. Med. Hemother. 2009, 36, 273–278. [Google Scholar] [CrossRef]
- Heinemann, F.M.; Roth, I.; Rebmann, V.; Arnold, M.L.; Witzke, O.; Wilde, B.; Spriewald, B.M.; Grosse-Wilde, H. Immunoglobulin isotype-specific characterization of anti-human leukocyte antigen antibodies eluted from explanted renal allografts. Hum. Immunol. 2007, 68, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, M.; Lenz, V.; Nyadu, B.; Heinemann, F.M.; Heinold, A.; Guberina, H.; Eisenberger, U.; Lachmann, N.; Schonemann, C.; Kribben, A.; et al. Effect of ABO incompatibility on T-cell flow cytometry cross-match results prior to living donor kidney transplantation. Cytom. B Clin. Cytom. 2018, 94, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, M.; Oesterreich, S.; Wilde, B.; Eisenberger, U.; Muelling, N.; Horn, P.A.; Heinemann, F.M.; Witzke, O. Sex-Specific Differences in HLA Antibodies after Pneumococcal Vaccination in Kidney Transplant Recipients. Vaccines 2019, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Everly, M.J.; Rebellato, L.M.; Haisch, C.E.; Ozawa, M.; Parker, K.; Briley, K.P.; Catrou, P.G.; Bolin, P.; Kendrick, W.T.; Kendrick, S.A.; et al. Incidence and impact of de Novo donor-specific alloantibody in primary renal allografts. Transplantation 2013, 95, 410–417. [Google Scholar] [CrossRef]
- Wiebe, C.; Gibson, I.W.; Blydt-Hansen, T.D.; Karpinski, M.; Ho, J.; Storsley, L.J.; Goldberg, A.; Birk, P.E.; Rush, D.N.; Nickerson, P.W. Evolution and Clinical Pathologic Correlations of De Novo Donor-Specific HLA Antibody Post Kidney Transplant. Am. J. Transplant. 2012, 12, 1157–1167. [Google Scholar] [CrossRef]
- Smith, J.; Skeans, M.; Horslen, S.; Edwards, E.; Harper, A.; Snyder, J.; Israni, A.; Kasiske, B. Organ procurement and transplantation network (OPTN) and scientific registry of transplant recipients (SRTR). OPTN/SRTR 2012 annual data report. Am. J. Transplant. 2014, 14, 97–111. [Google Scholar] [CrossRef]
- European Medicines Agency: Prevenar 20. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/prevenar-20-previously-apexxnar (accessed on 9 July 2024).
- Robert-Koch-Institut. Empfehlungen der Ständigen Impfkommission beim Robert Koch-Institut 2024. Epid. Bull. 2024, 4, 1–30. [Google Scholar]
- Pfizer. Fachinformation Prevenar 20®. Available online: https://figi.pfizer.de/sites/default/files/FI-24324.pdf (accessed on 9 July 2024).
- Nishida, H.; Takai, S.; Ito, H.; Fukuhara, H.; Nawano, T.; Narisawa, T.; Kanno, H.; Yagi, M.; Yamagishi, A.; Sakurai, T.; et al. Anti-human leukocyte antigen and anti-ABO antibodies after SARS-CoV-2 mRNA vaccination in kidney transplant recipients. Clin. Transplant. 2023, 37, e14952. [Google Scholar] [CrossRef]
- McCune, T.R.; Bray, R.A.; Baran, D.A.; Toepp, A.J.; Forte, S.J.; Gilgannon, L.T.; Williams, T.; Chen, S.; Sadr, H.; Gebel, H.M.; et al. Development of donor specific antibodies after SARS-CoV-2 vaccination in kidney and heart transplant recipients. Transpl. Immunol. 2022, 75, 101722. [Google Scholar] [CrossRef]
- Brakemeier, S.; Schweiger, B.; Lachmann, N.; Glander, P.; Schonemann, C.; Diekmann, F.; Neumayer, H.H.; Budde, K. Immune response to an adjuvanted influenza A H1N1 vaccine (Pandemrix(R)) in renal transplant recipients. Nephrol. Dial. Transplant. 2012, 27, 423–428. [Google Scholar] [CrossRef]
- Melenhorst, J.J.; Leen, A.M.; Bollard, C.M.; Quigley, M.F.; Price, D.A.; Rooney, C.M.; Brenner, M.K.; Barrett, A.J.; Heslop, H.E. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood 2010, 116, 4700–4702. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, N.A.; Nguyen, T.H.O.; Tait, B.D.; Kotsimbos, T.C. Quantitative and functional diversity of cross-reactive EBV-specific CD8+ T cells in a longitudinal study cohort of lung transplant recipients. Transplantation 2010, 90, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Inkinen, K.; Lahesmaa, R.; Brandt, A.; Katajamaa, M.; Halme, L.; Höckerstedt, K.; Lautenschlager, I. DNA Microarray-Based Gene Expression Profiles of Cytomegalovirus Infection and Acute Rejection in Liver Transplants. Transplant. Proc. 2005, 37, 1227–1229. [Google Scholar] [CrossRef] [PubMed]
- Dzabic, M.; Rahbar, A.; Yaiw, K.-C.; Naghibi, M.; Religa, P.; Fellström, B.; Larsson, E.; Söderberg-Nauclér, C. Intragraft Cytomegalovirus Protein Expression Is Associated With Reduced Renal Allograft Survival. Clin. Infect. Dis. 2011, 53, 969–976. [Google Scholar] [CrossRef]
- Lopez, C.; Simmons, R.L.; Mauer, S.M.; Najarian, J.S.; Good, R.A. Association of renal allograft rejection with virus infections. Am. J. Med. 1974, 56, 280–289. [Google Scholar] [CrossRef]
- Lachmann, N.; Terasaki, P.I.; Budde, K.; Liefeldt, L.; Kahl, A.; Reinke, P.; Pratschke, J.; Rudolph, B.; Schmidt, D.; Salama, A.; et al. Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation 2009, 87, 1505–1513. [Google Scholar] [CrossRef]
- Ozawa, M.; Terasaki, P.I.; Lee, J.H.; Castro, R.; Alberu, J.; Alonso, C.; Alvarez, I.; Toledo, R.; Alvez, H.; Monterio, M.; et al. 14th International HLA and Immunogenetics Workshop: Report on the Prospective Chronic Rejection Project. Tissue Antigens 2007, 69 (Suppl. S1), 174–179. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Heinemann, F.M.; Grosse-Wilde, H.; Sireci, G.; Wang, Z.; Lavingia, B.; Stastny, P. Detection of anti-MICA antibodies in patients awaiting kidney transplantation, during the post-transplant course, and in eluates from rejected kidney allografts by Luminex flow cytometry. Hum. Immunol. 2006, 67, 230–237. [Google Scholar] [CrossRef]
- Baranwal, A.K.; Mehra, N.K. Major Histocompatibility Complex Class I Chain-Related A (MICA) Molecules: Relevance in Solid Organ Transplantation. Front. Immunol. 2017, 8, 182. [Google Scholar] [CrossRef]
- Terasaki, P.I.; Ozawa, M.; Castro, R. Four-year follow-up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am. J. Transplant. 2007, 7, 408–415. [Google Scholar] [CrossRef]
- Ming, Y.; Peng, B.; Guo, X.; Luo, W.; Shao, M.; Cheng, K.; Luo, Q.; Zou, Y. Posttransplant-Alloantibodies Against MICA Antigens Associated With Decreased Long-Term Allograft Survival of Kidney Transplant Recipients. Transplant. Proc. 2022, 54, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Travers, P.; Walport, M. Janeway’s Immunobiology, 7th ed.; Garland Science: New York, NY, USA; London, UK, 2008. [Google Scholar]
- Lindemann, M.; Heinemann, F.M.; Horn, P.A.; Witzke, O. Immunity to pneumococcal antigens in kidney transplant recipients. Transplantation 2010, 90, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, M.; Witzke, O.; Lütkes, P.; Fiedler, M.; Kreuzfelder, E.; Philipp, T.; Roggendorf, M.; Grosse-Wilde, H. ELISpot assay as a sensitive tool to detect cellular immunity following influenza vaccination in kidney transplant recipients. Clin. Immunol. 2006, 120, 342–348. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Median (Range) or Number (No.) |
|---|---|
| Median age (range), years 1 | 59 (33–77) |
| Patient sex (female/male) | 10/31 |
| Median interval TX-vaccination (range), months | 38 (3–338) |
| Mean eGFR (range), mL/min/1.73 m2 | |
| Pre-vaccination | 48 (17–109) |
| Month 1 post-vaccination | 48 (15–109) |
| Month 6 post-vaccination | 50 (17–106) |
| Month 7 post-vaccination | 48 (15–105) |
| Month 12 post-vaccination | 47 (16–102) |
| Immunosuppression, no. 1 | |
| Cyclosporine A | 5 |
| Tacrolimus | 30 |
| Mycofenolate mofetil | 27 |
| mTOR inhibitors | 9 |
| Corticosteroids | 38 |
| Kidney transplantation, no. | |
| 1st | 37 |
| 2nd | 4 |
| ID | Sex | Age | HLA Ab ** | Cum. Ab Score HLA Class I | Cum. Ab Score HLA Class II | eGFR | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class I | Class II | 0 | 1 | 6 | 7 | 12 | 0 | 1 | 6 | 7 | 12 | 0 | 1 | 6 | 7 | 12 | |||
| 1 | F | 60 | −/+ | −/− | 96 | 96 | 96 | 92 | 96 | 32 | 32 | 32 | 11 | 26 | 68 | 61 | 69 | 69 | 63 |
| 2 | F | 53 | +/+ | −/− | 82 | 74 | 82 | 85 | 88 | 11 | 11 | 22 | 15 | 19 | 36 | 38 | 36 | 38 | 29 |
| 3 | M | 62 | +/+ | +/+ | 36 | 53 | 64 | 24 | 46 | 40 | 40 | 40 | 40 | 40 | 29 | 28 | 31 | 31 | 31 |
| 4 | F | 57 | +/+ | +/+ | 12 | 24 | 12 | 12 | 30 | 11 | 20 | 5 | 5 | 20 | 37 | NT | NT | 36 | 31 |
| 5 | M | 47 | +/+ | +/+ | 96 | 96 | 96 | 96 | 96 | 5 | 26 | 22 | 26 | 22 | 106 | 91 | 106 | 91 | 96 |
| 6 | M | 59 | −/− | −/− | 12 | 12 | 92 | 96 | 45 | 5 | 5 | 40 | 40 | 14 | 72 | NT | 81 | 76 | 84 |
| 7 | M | 68 | −/− | −/− | 12 | 12 | 12 | 12 | 12 | 29 | 17 | 5 | 25 | 40 | 25 | 25 | 20 | 20 | 18 |
| 8 | F | 76 | −/− | −/− | 12 | 12 | 12 | 12 | 12 | 5 | 14 | 25 | 17 | 29 | 76 | NT | 53 | 69 | 67 |
| 9 | M | 50 | −/− | −/− | 12 | 21 | 12 | 12 | 12 | 21 | 36 | 15 | 21 | 36 | 40 | 54 | 58 | 43 | 50 |
| 10 | M | 59 | −/− | −/− | 33 | 32 | 35 | 45 | 54 | 5 | 5 | 8 | 8 | 15 | 60 | 59 | 56 | 47 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindemann, M.; van de Sand, L.; Mülling, N.; Völk, K.L.; Aufderhorst, U.W.; Wilde, B.; Horn, P.A.; Kribben, A.; Krawczyk, A.; Witzke, O.; et al. Sequential Vaccination Against Streptococcus pneumoniae Appears as Immunologically Safe in Clinically Stable Kidney Transplant Recipients. Vaccines 2024, 12, 1244. https://doi.org/10.3390/vaccines12111244
Lindemann M, van de Sand L, Mülling N, Völk KL, Aufderhorst UW, Wilde B, Horn PA, Kribben A, Krawczyk A, Witzke O, et al. Sequential Vaccination Against Streptococcus pneumoniae Appears as Immunologically Safe in Clinically Stable Kidney Transplant Recipients. Vaccines. 2024; 12(11):1244. https://doi.org/10.3390/vaccines12111244
Chicago/Turabian StyleLindemann, Monika, Lukas van de Sand, Nils Mülling, Kim L. Völk, Ulrich W. Aufderhorst, Benjamin Wilde, Peter A. Horn, Andreas Kribben, Adalbert Krawczyk, Oliver Witzke, and et al. 2024. "Sequential Vaccination Against Streptococcus pneumoniae Appears as Immunologically Safe in Clinically Stable Kidney Transplant Recipients" Vaccines 12, no. 11: 1244. https://doi.org/10.3390/vaccines12111244
APA StyleLindemann, M., van de Sand, L., Mülling, N., Völk, K. L., Aufderhorst, U. W., Wilde, B., Horn, P. A., Kribben, A., Krawczyk, A., Witzke, O., & Heinemann, F. M. (2024). Sequential Vaccination Against Streptococcus pneumoniae Appears as Immunologically Safe in Clinically Stable Kidney Transplant Recipients. Vaccines, 12(11), 1244. https://doi.org/10.3390/vaccines12111244







