The Effectiveness of COVID-19 Vaccines During the Pre-Omicron and Omicron Periods: A Retrospective Test-Negative Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting, Study Population and Data
2.2. Study Design
2.3. Definition of Cases and Controls
2.4. Vaccination Status
2.5. Study Sample
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Sample
3.2. Vaccine Effectiveness on Hospitalizations
3.3. Vaccine Effectiveness on ICU Admissions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Callaway, E. COVID ‘variant soup’ is making winter surges hard to predict. Nature 2022, 611, 213–214. [Google Scholar] [CrossRef]
- Gili, R.; Burioni, R. SARS-CoV-2 before and after Omicron: Two different viruses and two different diseases? J. Transl. Med. 2023, 21, 251. [Google Scholar] [CrossRef]
- Menni, C.; May, A.; Polidori, L.; Louca, P.; Wolf, J.; Capdevila, J.; Hu, C.; Ourselin, S.; Steves, C.J.; Valdes, A.M.; et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: A prospective community study from the ZOE COVID Study. Lancet Infect. Dis. 2022, 22, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Patalon, T.; Saciuk, Y.; Peretz, A.; Perez, G.; Lurie, Y.; Maor, Y.; Gazit, S. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat. Commun. 2022, 13, 3203. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Hachmann, N.P.; Miller, J.; Collier, A.Y.; Ventura, J.D.; Yu, J.; Rowe, M.; Bondzie, E.A.; Powers, O.; Surve, N.; Hall, K.; et al. Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5. N. Engl. J. Med. 2022, 387, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.-W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Ferdinands, J.M.; Rao, S.; Dixon, B.E.; Mitchell, P.K.; DeSilva, M.B.; Irving, S.A.; Lewis, N.; Natarajan, K.; Stenehjem, E.; Grannis, S.J.; et al. Waning of vaccine effectiveness against moderate and severe COVID-19 among adults in the US from the VISION network: Test negative, case-control study. BMJ 2022, 379, e072141. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. Efficacy of a Fourth Dose of COVID-19 mRNA Vaccine against Omicron. N. Engl. J. Med. 2022, 386, 1377–1380. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Bertollini, R. National Study Group for COVID-19 Vaccination. Waning mRNA-1273 Vaccine Effectiveness against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2022, 386, 1091–1093. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef] [PubMed]
- Kirsebom, F.C.M.; Andrews, N.; Stowe, J.; Toffa, S.; Sachdeva, R.; Gallagher, E.; Groves, N.; O’Connell, A.-M.; Chand, M.; Ramsay, M.; et al. COVID-19 vaccine effectiveness against the omicron (BA.2) variant in England. Lancet Infect. Dis. 2022, 22, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.F.; Ackerson, B.K.; Bruxvoort, K.J.; Sy, L.S.; Tubert, J.E.; Lee, G.S.; Ku, J.H.; Florea, A.; Luo, Y.; Qiu, S.; et al. Effectiveness of mRNA-1273 vaccination against SARS-CoV-2 omicron subvariants BA.1, BA.2, BA.2.12.1, BA.4, and BA.5. Nat. Commun. 2023, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Hong, V.X.; Patel, M.M.; Kahn, R.; Lipsitch, M.; Tartof, S.Y. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California. Nat. Med. 2022, 28, 1933–1943. [Google Scholar] [CrossRef]
- Tang, F.; Hammel, I.S.; Andrew, M.K.; Ruiz, J.G. COVID-19 mRNA vaccine effectiveness against hospitalisation and death in veterans according to frailty status during the SARS-CoV-2 delta (B.1.617.2) variant surge in the USA: A retrospective cohort study. Lancet Healthy Longev. 2022, 3, e589–e598. [Google Scholar] [CrossRef]
- Ministero della Salute. Circolare n. 21209 del 8 Aprile 2022. Indicazioni sulla Somministrazione della Seconda Dose di Richiamo (Second Booster) Nell’Ambito della Campagna di Vaccinazione Anti SARS-CoV-2/COVID-19. Available online: https://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=86755 (accessed on 10 October 2024).
- European Medicines Agency. EMA and ECDC Statement on Updating COVID-19 Vaccines to Target New SARS-CoV-2 Virus Variants. Available online: https://www.ema.europa.eu/en/news/ema-ecdc-statement-updating-COVID-19-vaccines-target-new-sars-cov-2-virus-variants (accessed on 10 October 2024).
- Callaway, E. Mix-and-match COVID vaccines ace the effectiveness test. Nature 2021. online ahead of print. [Google Scholar] [CrossRef]
- Odone, A.; Vigezzi, G.P.; Baldanti, F. Implications of COVID-19 vaccine effectiveness waning for public health. Lancet Infect. Dis. 2022, 22, 918–919. [Google Scholar] [CrossRef]
- Thompson, M.G.; Stenehjem, E.; Grannis, S.; Ball, S.W.; Naleway, A.L.; Ong, T.C.; DeSilva, M.B.; Natarajan, K.; Bozio, C.H.; Lewis, N.; et al. Effectiveness of COVID-19 Vaccines in Ambulatory and Inpatient Care Settings. N. Engl. J. Med. 2021, 385, 1355–1371. [Google Scholar] [CrossRef]
- Dean, N.E.; Hogan, J.W.; Schnitzer, M.E. COVID-19 Vaccine Effectiveness and the Test-Negative Design. N. Engl. J. Med. 2021, 385, 1431–1433. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. Press Release No 03/2022 COVID-19: ISS Flash Survey. On January 3 Omicron-Positive Samples are 81%. Available online: https://www.iss.it/cov19-cosa-fa-iss-varianti/-/asset_publisher/yJS4xO2fauqM/content/id/5940827 (accessed on 10 October 2024).
- Fondazione GIMBE. Comunicato Stampa del 20 Ottobre 2020. Available online: https://www.gimbe.org/pagine/341/it/comunicatistampa?pagina=55 (accessed on 10 October 2024).
- Månsson, R.; Joffe, M.M.; Sun, W.; Hennessy, S. On the estimation and use of propensity scores in case-control and case-cohort studies. Am. J. Epidemiol. 2007, 166, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.L.; Nelson, J.C. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013, 31, 2165–2168. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, D.F.; Ridgeway, G.; Morral, A.R. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol. Methods 2004, 9, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Adams, K.; Rhoads, J.P.; Surie, D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Talbot, H.K.; Casey, J.D.; Zepeski, A.; Shapiro, N.I.; et al. Vaccine effectiveness of primary series and booster doses against COVID-19 associated hospital admissions in the United States: Living test negative design study. BMJ 2022, 379, e072065. [Google Scholar] [CrossRef]
- Grewal, R.; Kitchen, S.A.; Nguyen, L.; Buchan, S.A.; Wilson, S.E.; Costa, A.P.; Kwong, J.C. Effectiveness of a fourth dose of COVID-19 mRNA vaccine against the omicron variant among long term care residents in Ontario, Canada: Test negative design study. BMJ 2022, 378, e071502. [Google Scholar] [CrossRef]
- Lin, D.-Y.; Xu, Y.; Gu, Y.; Zeng, D.; Wheeler, B.; Young, H.; Sunny, S.K.; Moore, Z. Effectiveness of Bivalent Boosters against Severe Omicron Infection. N. Engl. J. Med. 2023, 388, 764–766. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Effectiveness of a fourth dose of mRNA COVID-19 vaccine against all-cause mortality in long-term care facility residents and in the oldest old: A nationwide, retrospective cohort study in Sweden. Lancet Reg. Health Eur. 2022, 21, 100466. [Google Scholar] [CrossRef]
- Tan, C.Y.; Chiew, C.J.; Lee, V.J.; Ong, B.; Lye, D.C.; Tan, K.B. Effectiveness of a Fourth Dose of COVID-19 mRNA Vaccine Against Omicron Variant Among Elderly People in Singapore. Ann. Intern. Med. 2022, 175, 1622–1623. [Google Scholar] [CrossRef]
- Johnson, A.G.; Linde, L.; Ali, A.R.; DeSantis, A.; Shi, M.; Adam, C.; Armstrong, B.; Armstrong, B.; Asbell, M.; Auche, S.; et al. COVID-19 Incidence and Mortality Among Unvaccinated and Vaccinated Persons Aged ≥12 Years by Receipt of Bivalent Booster Doses and Time Since Vaccination—24 U.S. Jurisdictions, October 3, 2021–December 24, 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 145–152. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, K.; Viboud, C.; Simonsen, L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006, 24, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Tanner, A.R.; Dorey, R.B.; Brendish, N.J.; Clark, T.W. Influenza vaccination: Protecting the most vulnerable. Eur. Respir. Rev. 2021, 30, 200258. [Google Scholar] [CrossRef]
- Killerby, M.E.; Link-Gelles, R.; Haight, S.C.; Schrodt, C.A.; England, L.; Gomes, D.J.; Shamout, M.; Pettrone, K.; O’Laughlin, K.; Kimball, A.; et al. Characteristics Associated with Hospitalization Among Patients with COVID-19—Metropolitan Atlanta, Georgia, March–April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 790–794. [Google Scholar] [CrossRef]
- Kim, L.; Garg, S.; O’halloran, A.; Whitaker, M.; Pham, H.; Anderson, E.J.; Armistead, I.; Bennett, N.M.; Billing, L.; Como-Sabetti, K.; et al. Risk Factors for Intensive Care Unit Admission and In-hospital Mortality Among Hospitalized Adults Identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin. Infect. Dis. 2021, 72, e206–e214. [Google Scholar] [CrossRef]
- Wingert, A.; Pillay, J.; Gates, M.; Guitard, S.; Rahman, S.; Beck, A.; Vandermeer, B.; Hartling, L. Risk factors for severity of COVID-19: A rapid review to inform vaccine prioritisation in Canada. BMJ Open 2021, 11, e044684. [Google Scholar] [CrossRef]
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; De Lorenzo, G.; Furnon, W.; Cantoni, D.; Scott, S.; Logan, N.; Ashraf, S.; et al. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nat. Microbiol. 2022, 7, 1161–1179. [Google Scholar] [CrossRef]
- Arbel, R.; Peretz, A.; Sergienko, R.; Friger, M.; Beckenstein, T.; Duskin-Bitan, H.; Yaron, S.; Hammerman, A.; Bilenko, N.; Netzer, D. Effectiveness of a bivalent mRNA vaccine booster dose to prevent severe COVID-19 outcomes: A retrospective cohort study. Lancet Infect. Dis. 2023, 23, 914–921. [Google Scholar] [CrossRef]
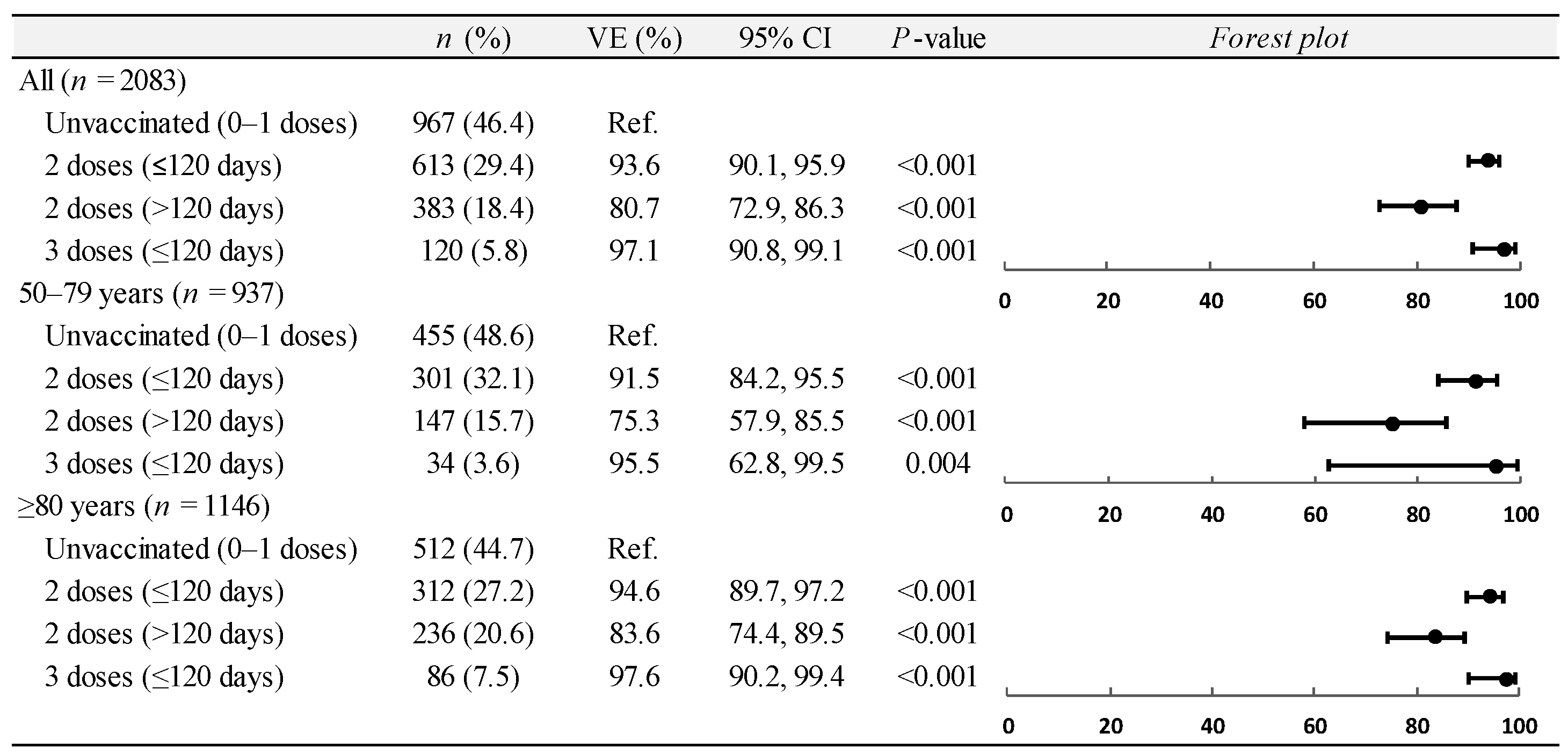
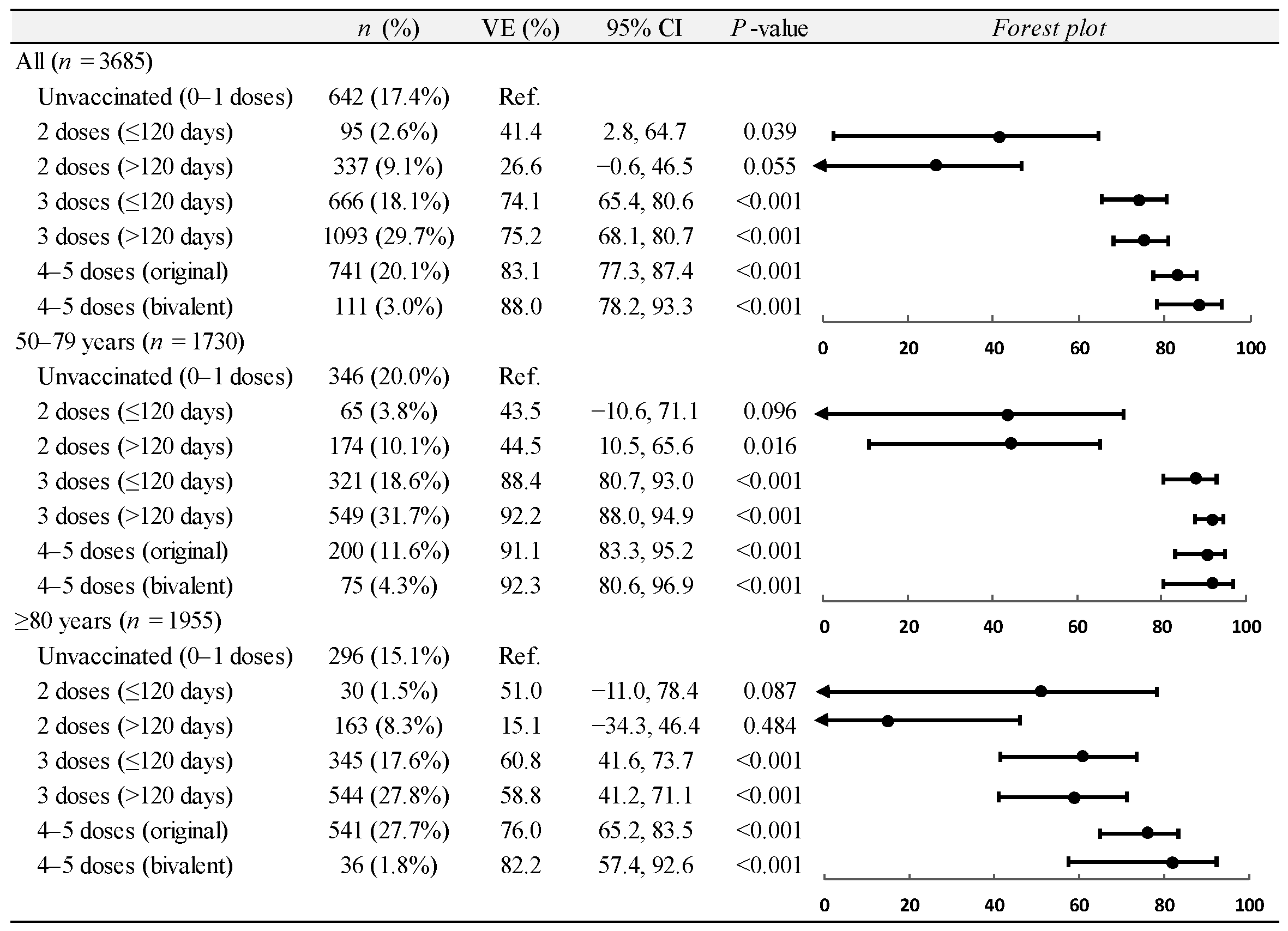
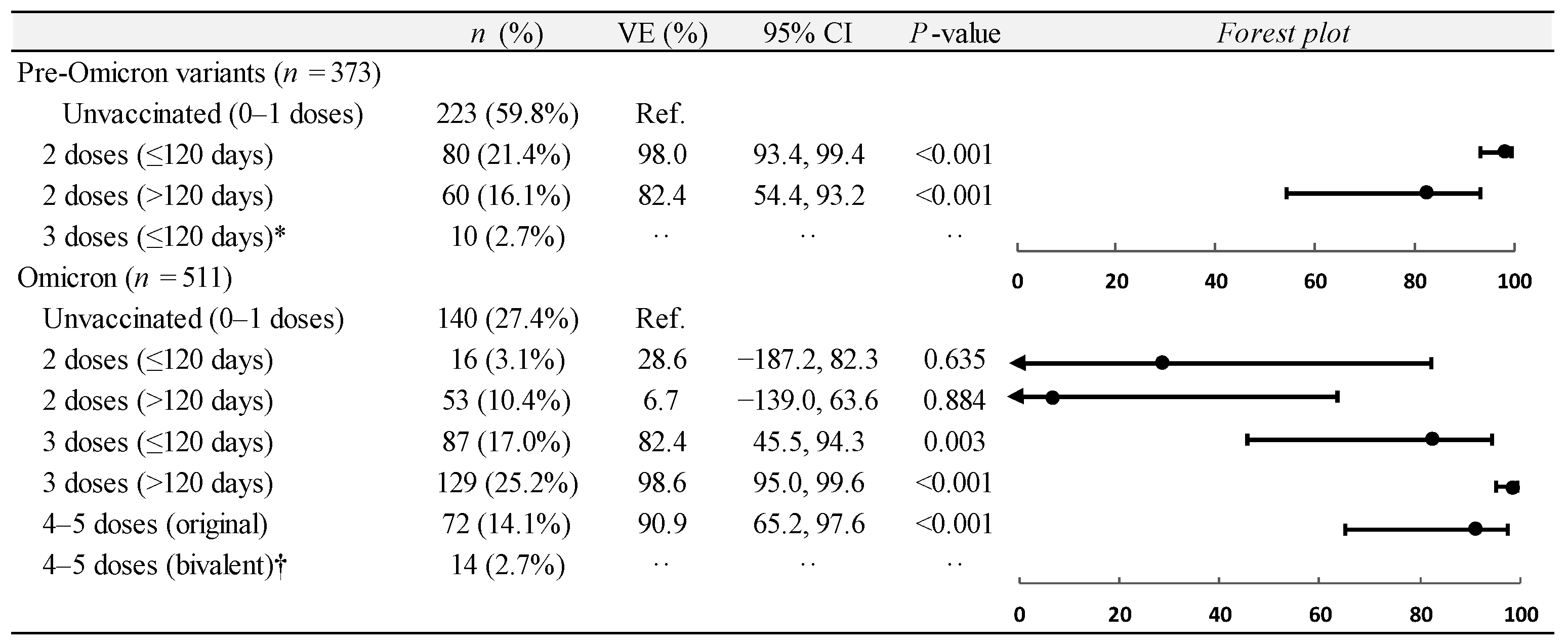
| All | Positives | Negatives | cOR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| (n = 2083) | (n = 539) | (n = 1544) | |||
| Sex | |||||
| Male | 1126 (54.1%) | 277 (51.4%) | 849 (55.0%) | Ref. | |
| Female | 957 (45.9%) | 262 (48.6%) | 695 (45.0%) | 1.155 (0.949, 1.406) | 0.149 |
| Age (y), mean ± SD | 78.4 ± 10.6 | 77.7 ± 11.0 | 78.7 ± 10.5 | 0.992 (0.983, 1.001) | 0.082 |
| Age group (y) | |||||
| 50–79 | 937 (45.0%) | 237 (44.0%) | 700 (45.3%) | Ref. | |
| ≥80 | 1146 (55.0%) | 302 (56.0%) | 844 (54.7%) | 1.057 (0.868, 1.287) | 0.583 |
| COVID-19 vaccination status | |||||
| Unvaccinated (0–1 doses) | 967 (46.4%) | 454 (84.2%) | 513 (33.2%) | Ref. | |
| 2 doses (≤120 days) | 613 (29.4%) | 30 (5.6%) | 583 (37.8%) | 0.058 (0.039, 0.086) | <0.001 |
| 2 doses (>120 days) | 383 (18.4%) | 52 (9.6%) | 331 (21.4%) | 0.178 (0.129, 0.244) | <0.001 |
| 3 doses (≤120 days) | 120 (5.8%) | 3 (0.6%) | 117 (7.6%) | 0.029 (0.009, 0.092) | <0.001 |
| Charlson Comorbidity Index | |||||
| 1–2 | 93 (4.5%) | 40 (7.4%) | 53 (3.4%) | Ref. | |
| 3–4 | 708 (34.0%) | 241 (44.7%) | 467 (30.2%) | 0.684 (0.441, 1.061) | 0.090 |
| ≥5 | 1282 (61.5%) | 258 (47.9%) | 1024 (66.3%) | 0.334 (0.217, 0.515) | <0.001 |
| ICU admission | |||||
| No | 1710 (82.1%) | 357 (66.2%) | 1353 (87.6%) | Ref. | |
| Yes | 373 (17.9%) | 182 (33.8%) | 191 (12.4%) | 3.611 (2.857, 4.564) | <0.001 |
| All | Positives | Negatives | cOR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| (n = 3685) | (n = 1313) | (n = 2372) | |||
| Sex | |||||
| Male | 1904 (51.7%) | 703 (53.5%) | 1201 (50.6%) | Ref. | |
| Female | 1781 (48.3%) | 610 (46.5%) | 1171 (49.4%) | 0.890 (0.778, 1.019) | 0.091 |
| Age (y), mean ± SD | 78.2 ± 11.0 | 78.6 ± 11.1 | 78.0 ± 11.0 | 1.006 (0.999, 1.012) | 0.081 |
| Age group (y) | |||||
| 50–79 | 1730 (46.9%) | 593 (45.2%) | 1137 (47.9%) | Ref. | |
| ≥80 | 1955 (53.1%) | 720 (54.8%) | 1235 (52.1%) | 1.118 (0.976, 1.280) | 0.107 |
| COVID-19 vaccination status | |||||
| Unvaccinated (0–1 doses) | 642 (17.4%) | 388 (29.6%) | 254 (10.7%) | Ref. | |
| 2 doses (≤120 days) | 95 (2.6%) | 44 (3.4%) | 51 (2.2%) | 0.565 (0.366, 0.871) | 0.010 |
| 2 doses (>120 days) | 337 (9.1%) | 174 (13.3%) | 163 (6.9%) | 0.699 (0.536, 0.912) | 0.008 |
| 3 doses (≤120 days) | 666 (18.1%) | 180 (13.7%) | 486 (20.5%) | 0.242 (0.192, 0.306) | <0.001 |
| 3 doses (>120 days) | 1093 (29.7%) | 321 (24.4%) | 772 (32.5%) | 0.272 (0.222, 0.334) | <0.001 |
| 4–5 doses (original) | 741 (20.1%) | 188 (14.3%) | 553 (23.3%) | 0.223 (0.177, 0.280) | <0.001 |
| 4–5 doses (bivalent) | 111 (3.0%) | 18 (1.4%) | 93 (3.9%) | 0.127 (0.075, 0.215) | <0.001 |
| Charlson Comorbidity Index | |||||
| 1–2 | 184 (5.0%) | 76 (5.8%) | 108 (4.6%) | Ref. | |
| 3–4 | 1399 (38.0%) | 638 (48.6%) | 761 (32.1%) | 1.191 (0.872, 1.627) | 0.271 |
| ≥5 | 2102 (57.0%) | 599 (45.6%) | 1503 (63.4%) | 0.566 (0.416, 0.771) | <0.001 |
| ICU admission | |||||
| No | 3174 (86.1%) | 1113 (84.8%) | 2061 (86.9%) | Ref. | |
| Yes | 511 (13.9%) | 200 (15.2%) | 311 (13.1%) | 1.191 (0.983, 1.443) | 0.075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brambilla, R.; Gili, R.; Vigna Taglianti, F.; Lenzi, J.; Riccò, M.; Burioni, R.; Scarvaglieri, M.; Rocco, R.; Buttafuoco, V.; Cristaudo, R.M.T.A.; et al. The Effectiveness of COVID-19 Vaccines During the Pre-Omicron and Omicron Periods: A Retrospective Test-Negative Case–Control Study. Vaccines 2024, 12, 1245. https://doi.org/10.3390/vaccines12111245
Brambilla R, Gili R, Vigna Taglianti F, Lenzi J, Riccò M, Burioni R, Scarvaglieri M, Rocco R, Buttafuoco V, Cristaudo RMTA, et al. The Effectiveness of COVID-19 Vaccines During the Pre-Omicron and Omicron Periods: A Retrospective Test-Negative Case–Control Study. Vaccines. 2024; 12(11):1245. https://doi.org/10.3390/vaccines12111245
Chicago/Turabian StyleBrambilla, Romeo, Renata Gili, Federica Vigna Taglianti, Jacopo Lenzi, Matteo Riccò, Roberto Burioni, Mariaelisabetta Scarvaglieri, Rachele Rocco, Vittorina Buttafuoco, Rosa Maria Teresa Antonia Cristaudo, and et al. 2024. "The Effectiveness of COVID-19 Vaccines During the Pre-Omicron and Omicron Periods: A Retrospective Test-Negative Case–Control Study" Vaccines 12, no. 11: 1245. https://doi.org/10.3390/vaccines12111245
APA StyleBrambilla, R., Gili, R., Vigna Taglianti, F., Lenzi, J., Riccò, M., Burioni, R., Scarvaglieri, M., Rocco, R., Buttafuoco, V., Cristaudo, R. M. T. A., & Gori, D. (2024). The Effectiveness of COVID-19 Vaccines During the Pre-Omicron and Omicron Periods: A Retrospective Test-Negative Case–Control Study. Vaccines, 12(11), 1245. https://doi.org/10.3390/vaccines12111245








