Characteristics and Outcomes for Recipients of NVX-CoV2373: A Real-World Retrospective Study in Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Recipients
2.2. Study Objectives
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Recipient Characteristics
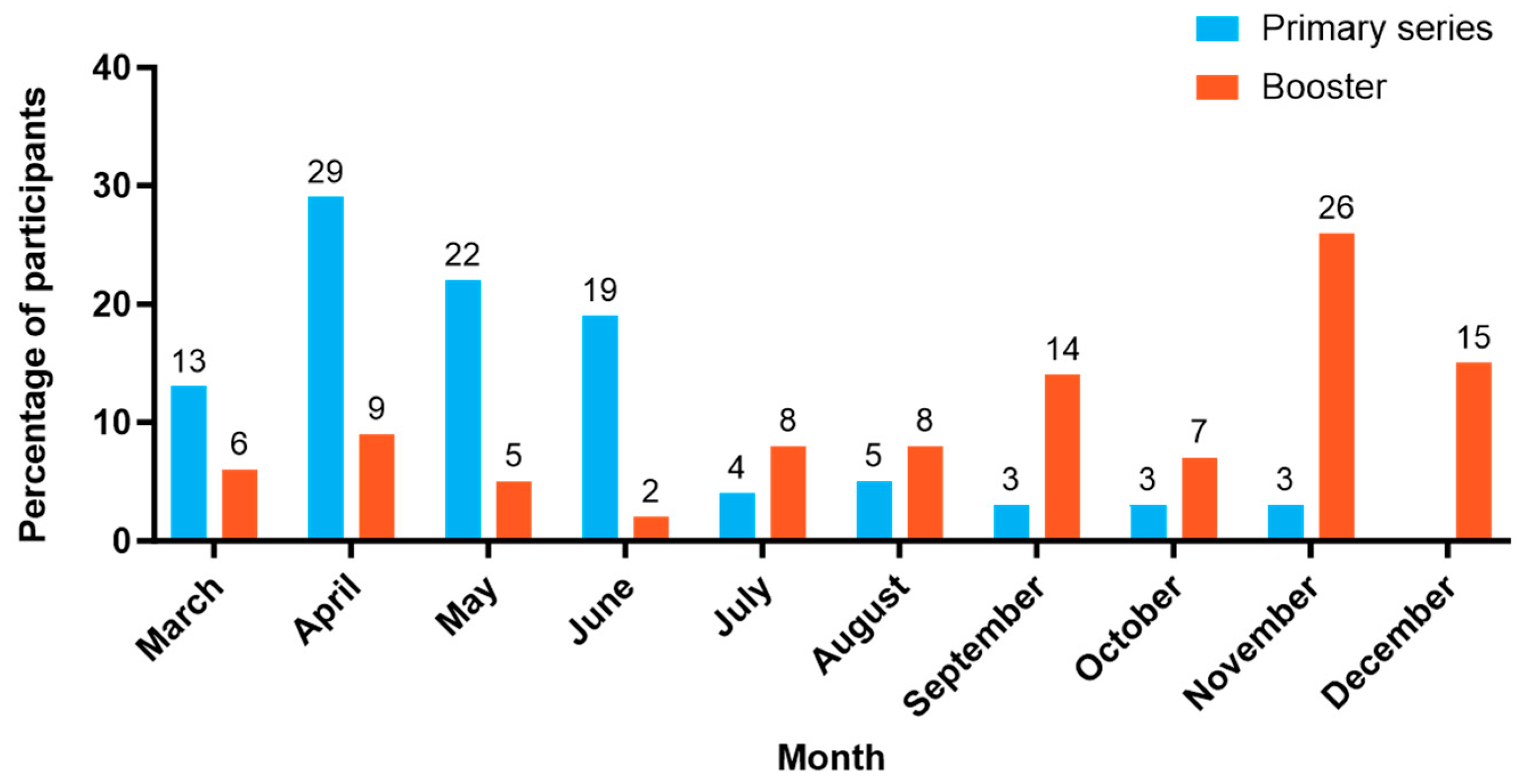
3.2. Tolerability-Related Outcomes
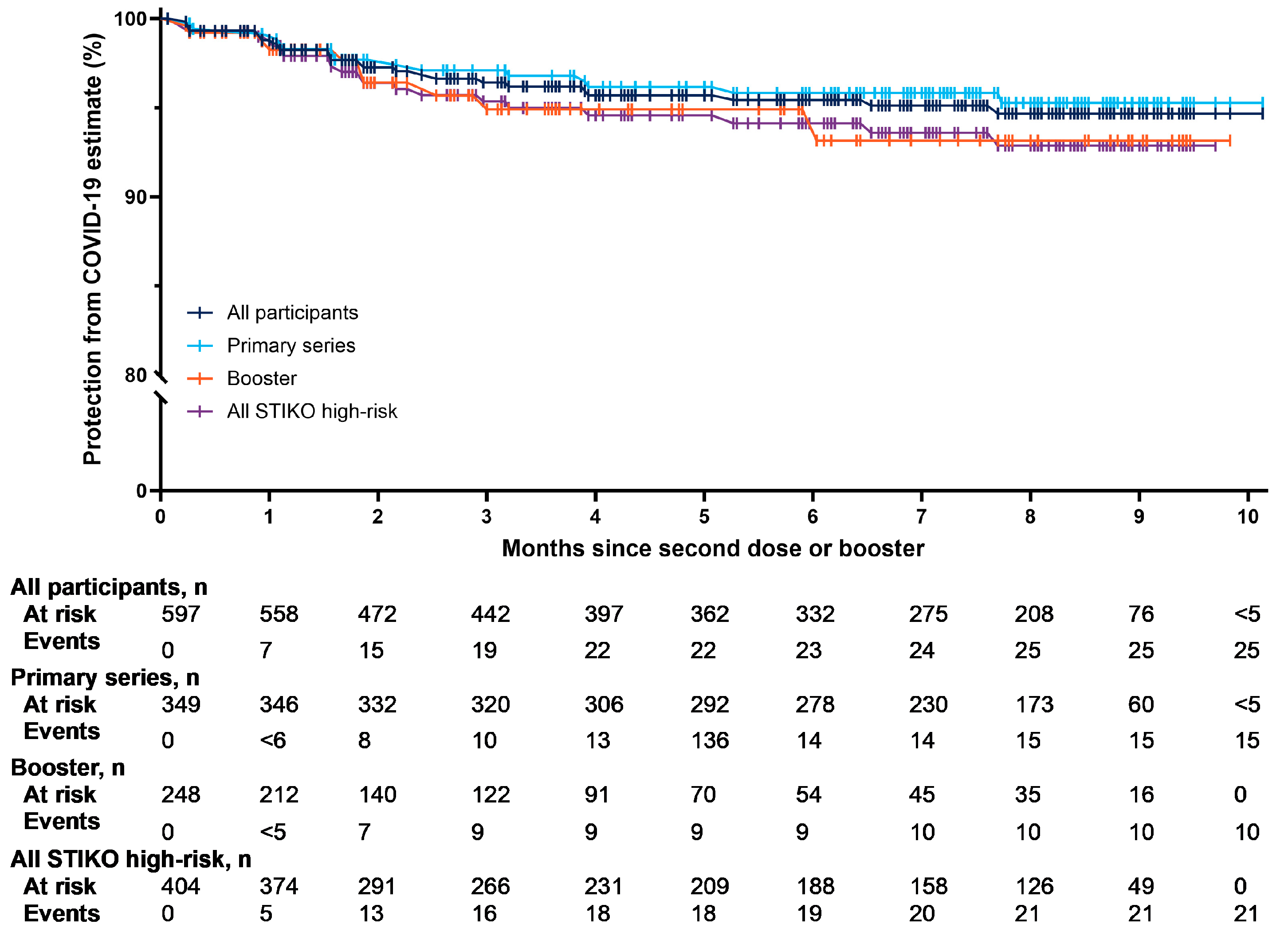
3.3. Protection from COVID-19
4. Discussion
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; Consortium, C.-G.U.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Anez, G.; Dunkle, L.M.; Gay, C.L.; Kotloff, K.L.; Adelglass, J.M.; Essink, B.; Campbell, J.D.; Cloney-Clark, S.; Zhu, M.; Plested, J.S.; et al. Safety, Immunogenicity, and Efficacy of the NVX-CoV2373 COVID-19 Vaccine in Adolescents: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e239135. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Anez, G.; Adelglass, J.M.; Barrat Hernandez, A.Q.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F.; et al. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.A.; Galloway, J.; et al. Safety and Efficacy of the NVX-CoV2373 Coronavirus Disease 2019 Vaccine at Completion of the Placebo-Controlled Phase of a Randomized Controlled Trial. Clin. Infect. Dis. 2023, 76, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Alves, K.; Plested, J.S.; Galbiati, S.; Chau, G.; Cloney-Clark, S.; Zhu, M.; Kalkeri, R.; Patel, N.; Smith, K.; Marcheschi, A.; et al. Immunogenicity and safety of a fourth homologous dose of NVX-CoV2373. Vaccine 2023, 41, 4280–4286. [Google Scholar] [CrossRef] [PubMed]
- Mallory, R.M.; Formica, N.; Pfeiffer, S.; Wilkinson, B.; Marcheschi, A.; Albert, G.; McFall, H.; Robinson, M.; Plested, J.S.; Zhu, M.; et al. Safety and immunogenicity following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): A secondary analysis of a randomised, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2022, 22, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Nuvaxovid. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/nuvaxovid#authorisation-details-section (accessed on 4 December 2023).
- Robert Koch Institute. Decision on the Implementation of the COVID-19 Vaccination into the General Recommendations of the STIKO 2023: STIKO Recommendation on COVID-19 Vaccination. Available online: https://www.rki.de/EN/Content/infections/Vaccination/recommandations/implementation_covid-19_vaccination.pdf?__blob=publicationFile (accessed on 2 November 2023).
- Robert Koch Institute. Aktualisierung der COVID-19-Impfempfehlung Epidemiologisches Bulletin 8/2023. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2023/Ausgaben/08_23.pdf?__blob=publicationFile (accessed on 2 November 2023).
- Rathmann, W.; Bongaerts, B.; Carius, H.J.; Kruppert, S.; Kostev, K. Basic characteristics and representativeness of the German Disease Analyzer database. Int. J. Clin. Pharmacol. Ther. 2018, 56, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kang, M.; Zhao, N.; Zhuang, Y.; Li, S.; Song, T. Comprehensive narrative review of real-world COVID-19 vaccines: Viewpoints and opportunities. Med. Rev. 2022, 2, 169–196. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.M.; Beyhaghi, H.; Orenstein, W.A. With established safe and effective use, protein vaccines offer another choice against COVID-19. Vaccine 2022, 40, 6567–6569. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.M.; Kalkeri, R.; Vadivale, M.; Suntronwong, N.; Toback, S.; Poovorawan, Y. Pivoting to protein: The immunogenicity and safety of protein-based NVX-CoV2373 as a heterologous booster for inactivated and viral vector COVID-19 vaccines. Expert Rev. Vaccines 2023, 22, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, S.; Huppertz, G.; Mueller, K.; Hitzenbichler, F.; Hardmann, L.; Pemmerl, S.; Hollnberger, H.; Sieber, W.; Wettstein, M.; Seeliger, S.; et al. Health Care Workers’ Sick Leave due to COVID-19 Vaccination in Context With SARS-CoV-2 Infection and Quarantine-A Multicenter Cross-Sectional Survey. Open Forum Infect. Dis. 2022, 9, ofac203. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, A.; Javan-Noughabi, J.; Tabatabaee, S.S.; Najafpoor, A.A.; Rezapour, A. The lost productivity cost of absenteeism due to COVID-19 in health care workers in Iran: A case study in the hospitals of Mashhad University of Medical Sciences. BMC Health Serv. Res. 2021, 21, 1169. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | All Recipients (n = 597) | Primary Series (n = 349) | Booster (n = 248) |
|---|---|---|---|
| Age, median years (IQR) | 58 (44–73) | 51 (39–63) | 71 (56–81) |
| Gender, % | |||
| Male | 46 | 42 | 51 |
| Female | 54 | 58 | 49 |
| Unknown | NA | NA | 0 |
| Median follow-up, months (IQR) | 7 (3–8) | 8 (7–9) | 3 (2–6) |
| Treating physician, % | |||
| General practitioner | 81 | 81 | 82 |
| Specialist | 19 | 19 | 18 |
| Geographical region, % | |||
| East Germany | 56 | 39 | 81 |
| West Germany | 44 | 61 | 19 |
| STIKO high-risk group, % | 68 | 58 | 81 |
| Age ≥ 60 years | 46 | 29 | 69 |
| Age ≥ 18 years + comorbidity | 53 | 45 | 65 |
| Care-facility resident | 3 | NA | NA |
| Characteristic | Age 12–35 (n = 78) | Age 36–59 (n = 245) | Age ≥ 60 (n = 274) |
|---|---|---|---|
| Age, median (IQR) years | 28 (23–33) | 49 (43–56) | 74 (67–82) |
| Gender, % | |||
| Female | 53 | 58 | 51 |
| Male | 47 | 42 | 49 |
| Unknown | 0 | 0 | NA |
| Median follow-up, months (IQR) | 8 (6–9) | 7 (6–8) | 4 (2–8) |
| Treating physician, % | |||
| General practitioner | 72 | 73 | 91 |
| Specialist | 28 | 27 | 9 |
| Geographical region, % | |||
| East Germany | 40 | 50 | 67 |
| West Germany | 60 | 50 | 33 |
| STIKO high-risk group, % | 24 | 45 | 100 |
| Age ≥ 60 years | – | – | 100 |
| Age ≥ 18 years + comorbidity | 24 | 45 | 68 |
| Care-facility resident | 0 | 0 | 7 |
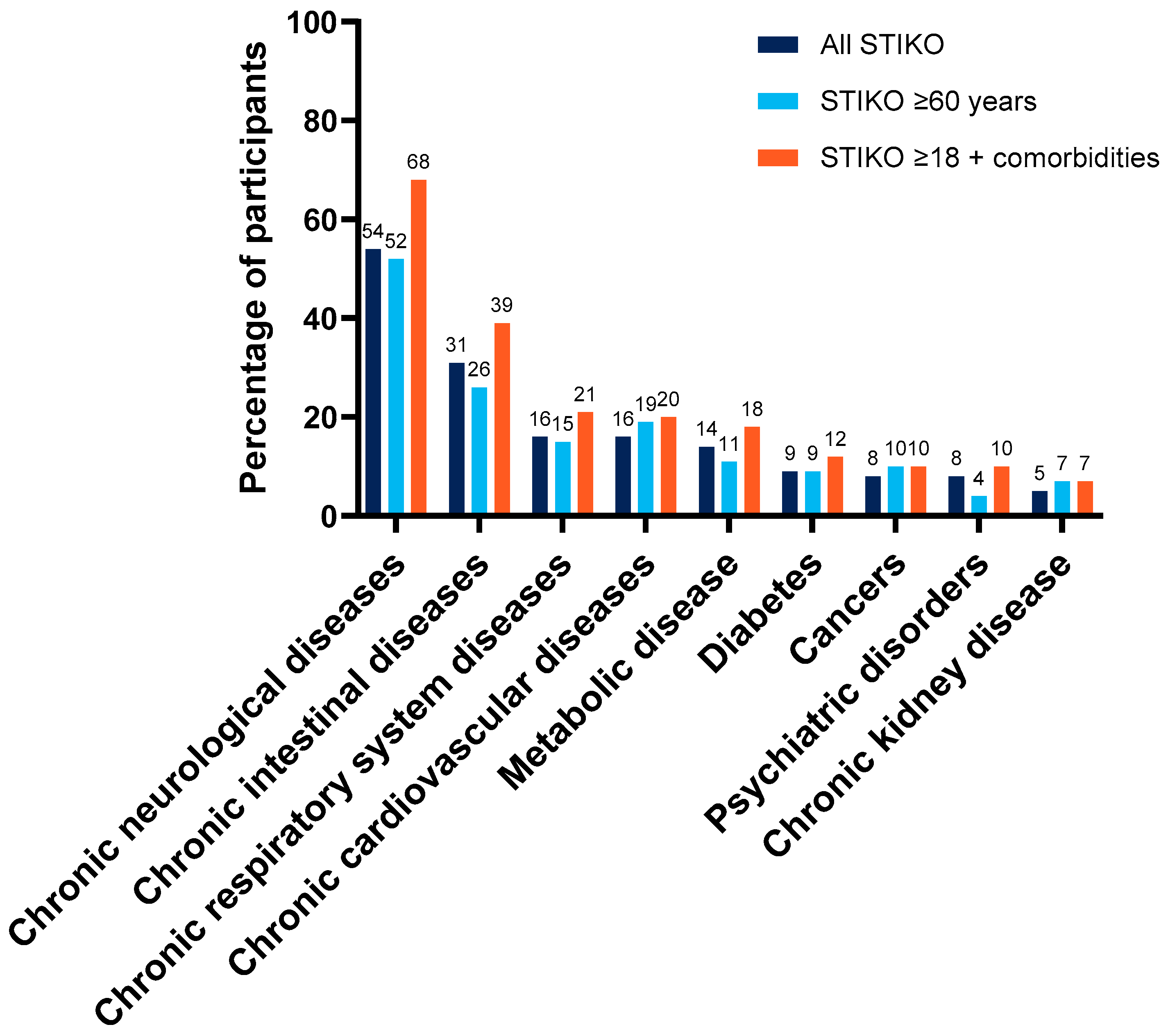
| Baseline Comorbidity a, % | All Recipients (n = 597) | Primary Series (n = 349) | Booster (n = 248) |
|---|---|---|---|
| ≥1 STIKO high-risk comorbidity | 53 | 45 | 65 |
| ≥2 STIKO high-risk comorbidity | 30 | 23 | 40 |
| ≥3 STIKO high-risk comorbidity | 17 | 13 | 23 |
| Chronic neurological disease | 36 | 29 | 47 |
| Chronic intestinal disease | 21 | 19 | 24 |
| Chronic respiratory disease | 11 | 9 | 13 |
| Chronic cardiovascular disease | 11 | 6 | 17 |
| Metabolic disease (including obesity and diabetes mellitus) | 10 | 9 | 10 |
| Diabetes | 6 | 6 | 4 |
| Psychiatric disorder | 5 | 6 | 4 |
| Cancer | 5 | 2 | 10 |
| Chronic kidney disease | 4 | 2 | 5 |
| Dementia or intellectual disability | 2 | NA | NA |
| Chronic liver disease (including cirrhosis) | 1 | NA | NA |
| Autoimmune disease (including rheumatologic diseases) | NA | NA | NA |
| Congenital/acquired immunodeficiency or immunosuppression b | NA | NA | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutikova, L.; Brash, J.T.; Helme, K.; Brewster, J.; Brand, M.; Adam, A.; Seager, S.; Kostev, K.; Schelling, J. Characteristics and Outcomes for Recipients of NVX-CoV2373: A Real-World Retrospective Study in Germany. Vaccines 2024, 12, 387. https://doi.org/10.3390/vaccines12040387
Kutikova L, Brash JT, Helme K, Brewster J, Brand M, Adam A, Seager S, Kostev K, Schelling J. Characteristics and Outcomes for Recipients of NVX-CoV2373: A Real-World Retrospective Study in Germany. Vaccines. 2024; 12(4):387. https://doi.org/10.3390/vaccines12040387
Chicago/Turabian StyleKutikova, Lucie, James T. Brash, Kawitha Helme, Jack Brewster, Milou Brand, Atif Adam, Sarah Seager, Karel Kostev, and Jörg Schelling. 2024. "Characteristics and Outcomes for Recipients of NVX-CoV2373: A Real-World Retrospective Study in Germany" Vaccines 12, no. 4: 387. https://doi.org/10.3390/vaccines12040387
APA StyleKutikova, L., Brash, J. T., Helme, K., Brewster, J., Brand, M., Adam, A., Seager, S., Kostev, K., & Schelling, J. (2024). Characteristics and Outcomes for Recipients of NVX-CoV2373: A Real-World Retrospective Study in Germany. Vaccines, 12(4), 387. https://doi.org/10.3390/vaccines12040387







