Influence of Maternal and Neonatal Factors on Transplacental Passive Immunity after Vaccination against COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Clinical and Sociodemographic Characteristics of the Study Population
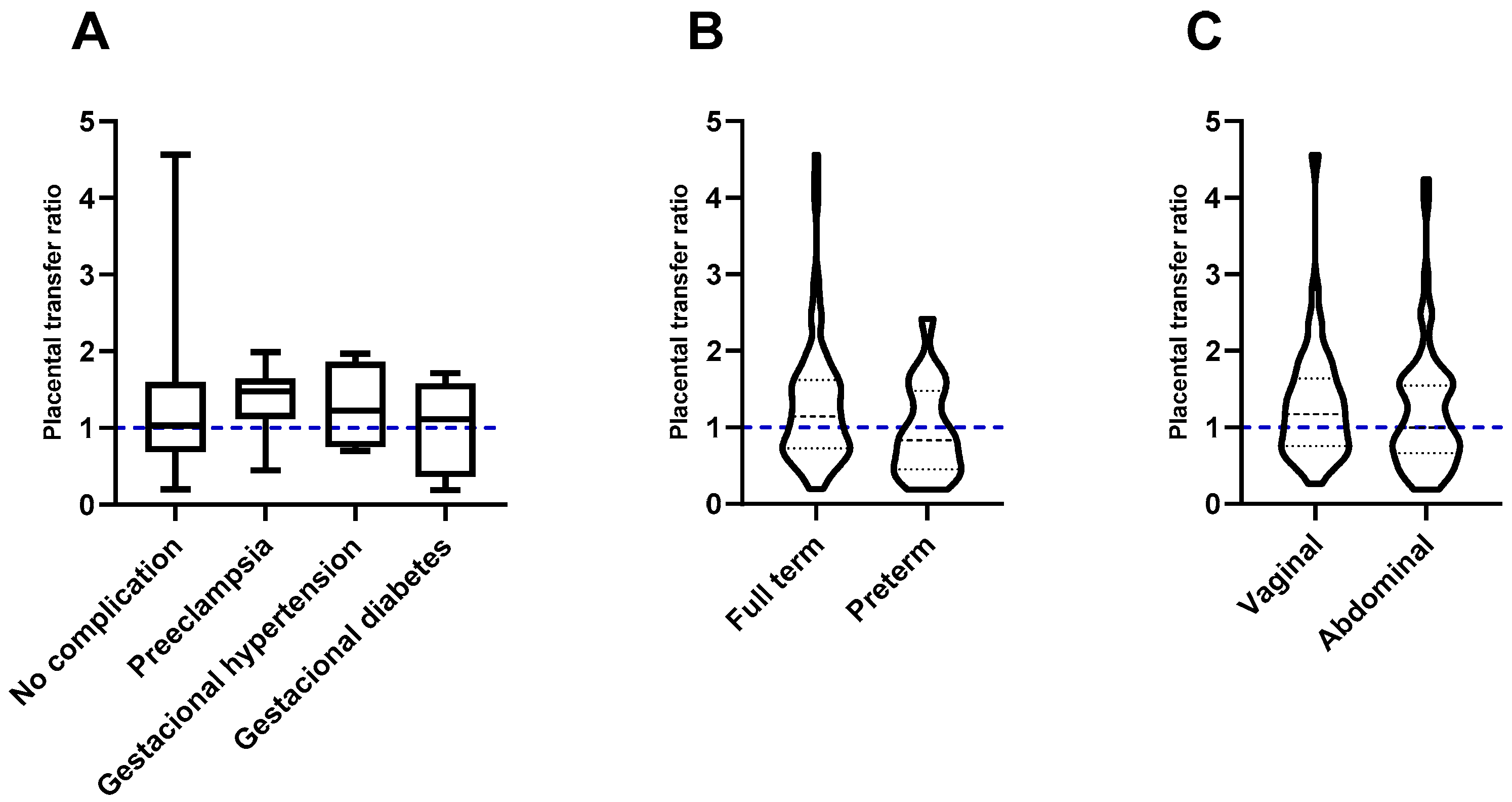
3.2. Influence of Maternal Factors on Transplacental Passive Immunity after Vaccination against COVID-19
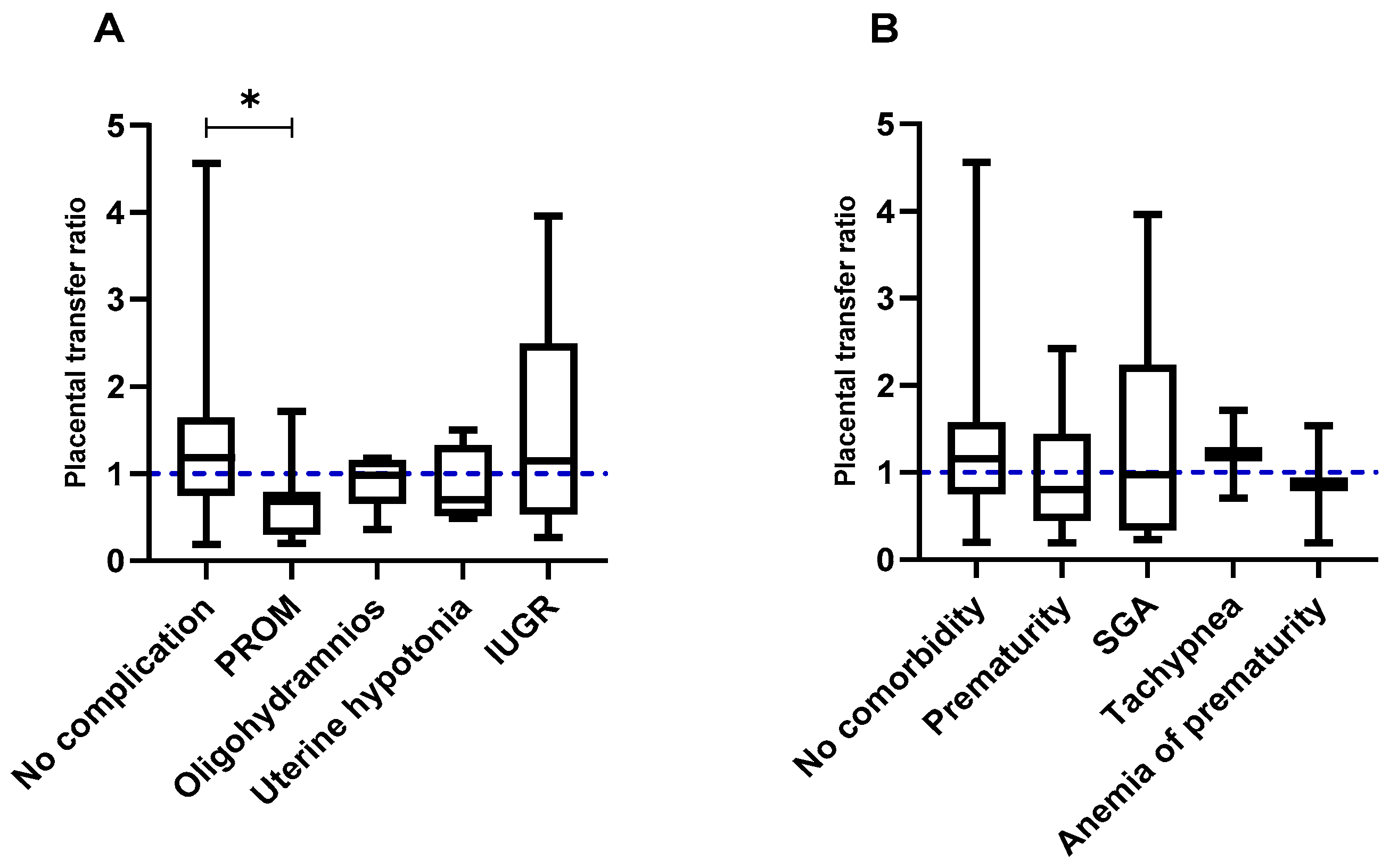
3.3. Effect of Factors Associated with Pregnancy and Childbirth on the Transplacental Passive Immunity after Vaccination Against COVID-19
3.4. Impact of Fetal and Neonatal Factors on the Transplacental Passive Immunity after Vaccination against COVID-19
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popescu, D.E.; Cîtu, C.; Jura, A.M.C.; Lungu, N.; Navolan, D.; Craina, M.; Semenescu, A.; Gorun, F.; Jura, M.A.; Belengeanu, V.; et al. The Benefits of Vaccination against SARS-CoV-2 during Pregnancy in Favor of the Mother/Newborn Dyad. Vaccines 2022, 10, 848. [Google Scholar] [CrossRef] [PubMed]
- Sebghati, M.; Khalil, A. Uptake of Vaccination in Pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 76, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Amaral, E.; Money, D.; Jamieson, D.; Pasupathy, D.; Aronoff, D.; Jacobsson, B.; Lizcano, E.I.O. Vaccination during Pregnancy: A Golden Opportunity to Embrace. Int. J. Gynecol. Obstet. 2023, 163, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Mackin, D.W.; Walker, S.P. The Historical Aspects of Vaccination in Pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 76, 13. [Google Scholar] [CrossRef] [PubMed]
- Marchand, G.; Masoud, A.T.; Grover, S.; King, A.; Brazil, G.; Ulibarri, H.; Parise, J.; Arroyo, A.; Coriell, C.; Goetz, S.; et al. Maternal and Neonatal Outcomes of COVID-19 Vaccination during Pregnancy: A Systematic Review and Meta-Analysis. NPJ Vaccines 2023, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Munoz, F.M.; Posavad, C.M.; Richardson, B.A.; Badell, M.L.; Bunge, K.E.; Mulligan, M.J.; Parameswaran, L.; Kelly, C.W.; Olson-Chen, C.; Novak, R.M.; et al. COVID-19 Booster Vaccination during Pregnancy Enhances Maternal Binding and Neutralizing Antibody Responses and Transplacental Antibody Transfer to the Newborn. Vaccine 2023, 41, 5296–5303. [Google Scholar] [CrossRef] [PubMed]
- Rottenstreich, A.; Zarbiv, G.; Oiknine-Djian, E.; Zigron, R.; Wolf, D.G.; Porat, S. Efficient Maternofetal Transplacental Transfer of Anti- SARS-CoV-2 Spike Antibodies after Antenatal SARS-CoV-2 BNT162b2 MRNA Vaccination. Clin. Infect. Dis. 2021, 73, 1909–1912. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.E.; Blanton, M.B.; Doratt, B.M.; Malherbe, D.C.; Rincon, M.; True, H.; Mcdonald, T.; Beauregard, C.; Adatorwovor, R.; Messaoudi, I. SARS-CoV-2 Vaccine Booster Elicits Robust Prolonged Maternal Antibody 1 Responses and Passive Transfer via the Placenta and Breastmilk. bioRxiv 2022. [Google Scholar] [CrossRef]
- Nir, O.; Schwartz, A.; Toussia-Cohen, S.; Leibovitch, L.; Strauss, T.; Asraf, K.; Doolman, R.; Sharabi, S.; Cohen, C.; Lustig, Y.; et al. Maternal-Neonatal Transfer of SARS-CoV-2 Immunoglobulin G Antibodies among Parturient Women Treated with BNT162b2 Messenger RNA Vaccine during Pregnancy. Am. J. Obstet. Gynecol. MFM 2021, 4, 100492. [Google Scholar] [CrossRef]
- Rottenstreich, A.; Zarbiv, G.; Oiknine-Djian, E.; Vorontsov, O.; Zigron, R.; Kleinstern, G.; Wolf, D.G.; Porat, S. Timing of SARS-CoV-2 Vaccination during the Third Trimester of Pregnancy and Transplacental Antibody Transfer: A Prospective Cohort Study. Clin. Microbiol. Infect. 2022, 28, 419. [Google Scholar] [CrossRef]
- Rottenstreich, A.; Zarbiv, G.; Oiknine-Djian, E.; Vorontsov, O.; Zigron, R.; Kleinstern, G.; Porat, S.; Wolf, D.G. Kinetics of Maternally-Derived Anti- SARS-CoV-2 Antibodies in Infants in Relation to the Timing of Antenatal Vaccination. Clin. Infect. Dis. 2022, 76, e274–e279. [Google Scholar] [CrossRef] [PubMed]
- Beharier, O.; Mayo, R.P.; Raz, T.; Sacks, K.N.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R.; et al. Efficient Maternal to Neonatal Transfer of Antibodies against SARS-CoV-2 and BNT162b2 MRNA COVID-19 Vaccine. J. Clin. Investig. 2021, 131, e150319. [Google Scholar] [CrossRef] [PubMed]
- Mithal, L.B.; Otero, S.; Shanes, E.D.; Goldstein, J.A.; Miller, E.S. Cord Blood Antibodies Following Maternal Coronavirus Disease 2019 Vaccination during Pregnancy. Am. J. Obstet. Gynecol. 2021, 225, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Zdanowski, W.; Waśniewskii, T. Evaluation of SARS-CoV-2 Spike Protein Antibody Titers in Cord Blood after COVID-19 Vaccination during Pregnancy in Polish Healthcare Workers: Preliminary Results. Vaccines 2021, 9, 675. [Google Scholar] [CrossRef] [PubMed]
- Prahl, M.; Golan, Y.; Cassidy, A.G.; Matsui, Y.; Li, L.; Alvarenga, B.; Chen, H.; Jigmeddagva, U.; Lin, C.Y.; Gonzalez, V.J.; et al. Evaluation of Transplacental Transfer of MRNA Vaccine Products and Functional Antibodies during Pregnancy and Infancy. Nat. Commun. 2022, 13, 4422. [Google Scholar] [CrossRef]
- Martínez-Quezada, R.; Miguel-Rodríguez, C.E.; Ramírez-Lozada, T.; Valencia-Ledezma, O.E.; Acosta-Altamirano, G. Placental Transfer Efficiency of Neutralizing Antibodies on SARS-CoV-2 Vaccination before and after Pregnancy in Mexican Women. Int. J. Mol. Sci. 2024, 25, 1516. [Google Scholar] [CrossRef] [PubMed]
- Atwell, J.E.; Lutz, C.S.; Sparrow, E.G.; Feikin, D.R. Biological Factors That May Impair Transplacental Transfer of RSV Antibodies: Implications for Maternal Immunization Policy and Research Priorities for Low- and Middle-Income Countries. Vaccine 2022, 40, 4361. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.R.; Holder, B.; Jones, C.E. Factors Affecting the FcRn-Mediated Transplacental Transfer of Antibodies and Implications for Vaccination in Pregnancy. Front. Immunol. 2017, 8, 292560. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, J.P.; Westerbeek, E.A.M.; Van der Klis, F.R.M.; Berbers, G.A.M.; Van Elburg, R.M. Transplacental Transport of IgG Antibodies to Preterm Infants: A Review of the Literature. Early Hum. Dev. 2011, 87, 67–72. [Google Scholar] [CrossRef]
- Atyeo, C.; Pullen, K.M.; Bordt, E.A.; Fischinger, S.; Burke, J.; Michell, A.; Slein, M.D.; Loos, C.; Shook, L.L.; Boatin, A.A.; et al. Compromised SARS-CoV-2-Specific Placental Antibody Transfer. Cell 2021, 184, 628–642. [Google Scholar] [CrossRef]
- Edlow, A.G.; Li, J.Z.; Collier, A.R.Y.; Atyeo, C.; James, K.E.; Boatin, A.A.; Gray, K.J.; Bordt, E.A.; Shook, L.L.; Yonker, L.M.; et al. Assessment of Maternal and Neonatal SARS-CoV-2 Viral Load, Transplacental Antibody Transfer, and Placental Pathology in Pregnancies during the COVID-19 Pandemic. JAMA Netw. Open 2020, 3, e2030455. [Google Scholar] [CrossRef]
- Atyeo, C.G.; Shook, L.L.; Brigida, S.; De Guzman, R.M.; Demidkin, S.; Muir, C.; Akinwunmi, B.; Baez, A.M.; Sheehan, M.L.; McSweeney, E.; et al. Maternal Immune Response and Placental Antibody Transfer after COVID-19 Vaccination across Trimester and Platforms. Nat. Commun. 2022, 13, 3571. [Google Scholar] [CrossRef] [PubMed]
- Doroudchi, M.; Samsami Dehaghani, A.; Emad, K.; Ghaderi, A. Placental Transfer of Rubella-Specific IgG in Fullterm and Preterm Newborns. Int. J. Gynecol. Obstet. 2003, 81, 157–162. [Google Scholar] [CrossRef]
- França, E.L.; Calderon, I.D.M.P.; Vieira, E.L.; Morceli, G.; Honorio-França, A.C. Transfer of Maternal Immunity to Newborns of Diabetic Mothers. Clin. Dev. Immunol. 2012, 2012, 928187. [Google Scholar] [CrossRef]
- Stach, S.C.L.; Brizot, M.L.; Liao, A.W.; Palmeira, P.; Francisco, R.P.V.; Carneiro-Sampaio, M.M.S.; Zugaib, M. Placental Transfer of IgG Antibodies Specific to Klebsiella and Pseudomonas LPS and to Group B Streptococcus in Twin Pregnancies. Scand. J. Immunol. 2015, 81, 135–141. [Google Scholar] [CrossRef]
- de Souza, E.G.; Hara, C.C.P.; Fagundes, D.L.G.; de Queiroz, A.A.; Morceli, G.; Calderon, I.M.P.; França, E.L.; Honorio-França, A.C. Maternal-Foetal Diabetes Modifies Neonatal Fc Receptor Expression on Human Leucocytes. Scand. J. Immunol. 2016, 84, 237–244. [Google Scholar] [CrossRef]
- Alcorta-Nuñez, F.; Pérez-Ibave, D.C.; Burciaga-Flores, C.H.; Garza, M.Á.; González-Escamilla, M.; Rodríguez-Niño, P.; González-Guerrero, J.F.; Alcorta-Garza, A.; Vidal-Gutiérrez, O.; Ramírez-Correa, G.A.; et al. SARS-CoV-2 Neutralizing Antibodies in Mexican Population: A Five Vaccine Comparison. Diagnostics 2023, 13, 1194. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.Y.; Newman, K.L.; Englund, J.A.; Cho, S.; Bull, C.; Lacombe, K.; Carlin, K.; Bulkow, L.R.; Rudolph, K.; Debyle, C.; et al. Transplacental Respiratory Syncytial Virus and Influenza Virus Antibody Transfer in Alaska Native and Seattle Mother-Infant Pairs. J. Pediatr. Infect. Dis. Soc. 2021, 10, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Nyiro, J.U.; Bukusi, E.; Mwaengo, D.; Nyaguara, A.; Nyawanda, B.; Otieno, N.; Bigogo, G.; Murunga, N.; Widdowson, M.A.; Verani, J.R.; et al. Efficiency of Transplacental Transfer of Respiratory Syncytial Virus (RSV) Specific Antibodies among Pregnant Women in Kenya. Wellcome Open Res. 2022, 7, 43. [Google Scholar] [CrossRef]
- Okoko, B.J.; Wesumperuma, L.H.; Hart, A.C. Materno-Foetal Transfer of H. Influenzae and Pneumococcal Antibodies Is Influenced by Prematurity and Low Birth Weight: Implications for Conjugate Vaccine Trials. Vaccine 2001, 20, 647–650. [Google Scholar] [CrossRef]
- Van Den Berg, J.P.; Westerbeek, E.A.M.; Berbers, G.A.M.; Van Gageldonk, P.G.M.; Van Der Klis, F.R.M.; Van Elburg, R.M. Transplacental Transport of IgG Antibodies Specific for Pertussis, Diphtheria, Tetanus, Haemophilus Influenzae Type b, and Neisseria Meningitidis Serogroup C Is Lower in Preterm Compared with Term Infants. Pediatr. Infect. Dis. J. 2010, 29, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, J.P.; Westerbeek, E.A.M.; Smits, G.P.; Van Der Klis, F.R.M.; Berbers, G.A.M.; Van Elburg, R.M. Lower Transplacental Antibody Transport for Measles, Mumps, Rubella and Varicella Zoster in Very Preterm Infants. PLoS ONE 2014, 9, e94714. [Google Scholar] [CrossRef] [PubMed]
- Kachikis, A.; Pike, M.; Eckert, L.O.; Roberts, E.; Frank, Y.; Young, A.L.; Goecker, E.; Gravett, M.G.; Greninger, A.L.; Englund, J.A. Timing of Maternal COVID-19 Vaccine and Antibody Concentrations in Infants Born Preterm. JAMA Netw. Open 2024, 7, E2352387. [Google Scholar] [CrossRef] [PubMed]

| N (%) | |
|---|---|
| Maternal Data | |
| Maternal age | |
| Adolescent age (≤19 years) | 29 (27.88) |
| Optimal age (20–34 years) | 58 (55.77) |
| Advanced age (≥35 years) | 17 (16.34) |
| BMI | |
| Normal | 67 (64.42) |
| Overweight | 11 (10.58) |
| Grade I obesity | 10 (9.61) |
| Grade II obesity | 10 (9.61) |
| Morbid obesity | 6 (5.77) |
| Maternal comorbidities | |
| No comorbidity | 65 (62.5) |
| With comorbidity | 39 (37.5) |
| Overweight or obesity | 37 (35.57) |
| Hypothyroidism | 5 (4.8) |
| Diabetes mellitus type 2 (T2DM) | 3 (2.88) |
| High blood pressure (hypertension) | 3 (2.88) |
| Anemia | 5 (4.8) |
| Pregnancy and Birth Data | |
| Complications in pregnancy | |
| No complications | 84 (80.2) |
| With complications | 20 (19.2) |
| Gestational hypertension | 4 (3.84) |
| Pre-eclampsia | 8 (7.7) |
| Gestational diabetes | 8 (7.7) |
| Duration of pregnancy | |
| Full term | 86 (82.7) |
| Preterm | 18 (17.3) |
| Type of birth | |
| Abdominal | 63 (60.58) |
| Vaginal | 41 (39.42) |
| Fetal Data | |
| Fetal complications | |
| No complications | 79 (73.83) |
| With complications | 28 (26.17) |
| Premature rupture of membranes (PROM) | 9 (8.41) |
| Oligohydramnios | 6 (5.6) |
| Uterine hypotonia | 4 (3.73) |
| Intrauterine growth restriction (IUGR) | 9 (8.41) |
| Neonatal Data | |
| Gender | |
| Male | 51 (47.66) |
| Female | 56 (52.34) |
| Neonatal comorbidities | |
| No comorbidities | 74 (69.16) |
| With comorbidities | 33 (30.84) |
| Prematurity | 18 (16.82) |
| Very premature (28–32 weeks) | 3 (2.8) |
| Moderately premature (32–34 weeks) | 5 (4.67) |
| Late preterm (34–37 weeks) | 10 (9.34) |
| Low weight for gestational age (SGA) | 10 (9.34) |
| Tachypnea | 3 (2.8) |
| Anemia of prematurity | 2 (1.87) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Quezada, R.; Valencia-Ledezma, O.E.; Ramírez-Lozada, T.; Miguel-Rodríguez, C.E.; Fernández-Hernández, J.C.; Acosta-Altamirano, G. Influence of Maternal and Neonatal Factors on Transplacental Passive Immunity after Vaccination against COVID-19. Vaccines 2024, 12, 860. https://doi.org/10.3390/vaccines12080860
Martínez-Quezada R, Valencia-Ledezma OE, Ramírez-Lozada T, Miguel-Rodríguez CE, Fernández-Hernández JC, Acosta-Altamirano G. Influence of Maternal and Neonatal Factors on Transplacental Passive Immunity after Vaccination against COVID-19. Vaccines. 2024; 12(8):860. https://doi.org/10.3390/vaccines12080860
Chicago/Turabian StyleMartínez-Quezada, Rebeca, Omar Esteban Valencia-Ledezma, Tito Ramírez-Lozada, Carlos Emilio Miguel-Rodríguez, Juan Carlos Fernández-Hernández, and Gustavo Acosta-Altamirano. 2024. "Influence of Maternal and Neonatal Factors on Transplacental Passive Immunity after Vaccination against COVID-19" Vaccines 12, no. 8: 860. https://doi.org/10.3390/vaccines12080860






