Immune Response after Vaccination against Tick-Borne Encephalitis Virus (TBEV) in Horses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Experimental Design
2.3. Serological Testing
2.4. Statistical Analysis
3. Results
3.1. Clinical Findings
3.2. IgG Concentrations
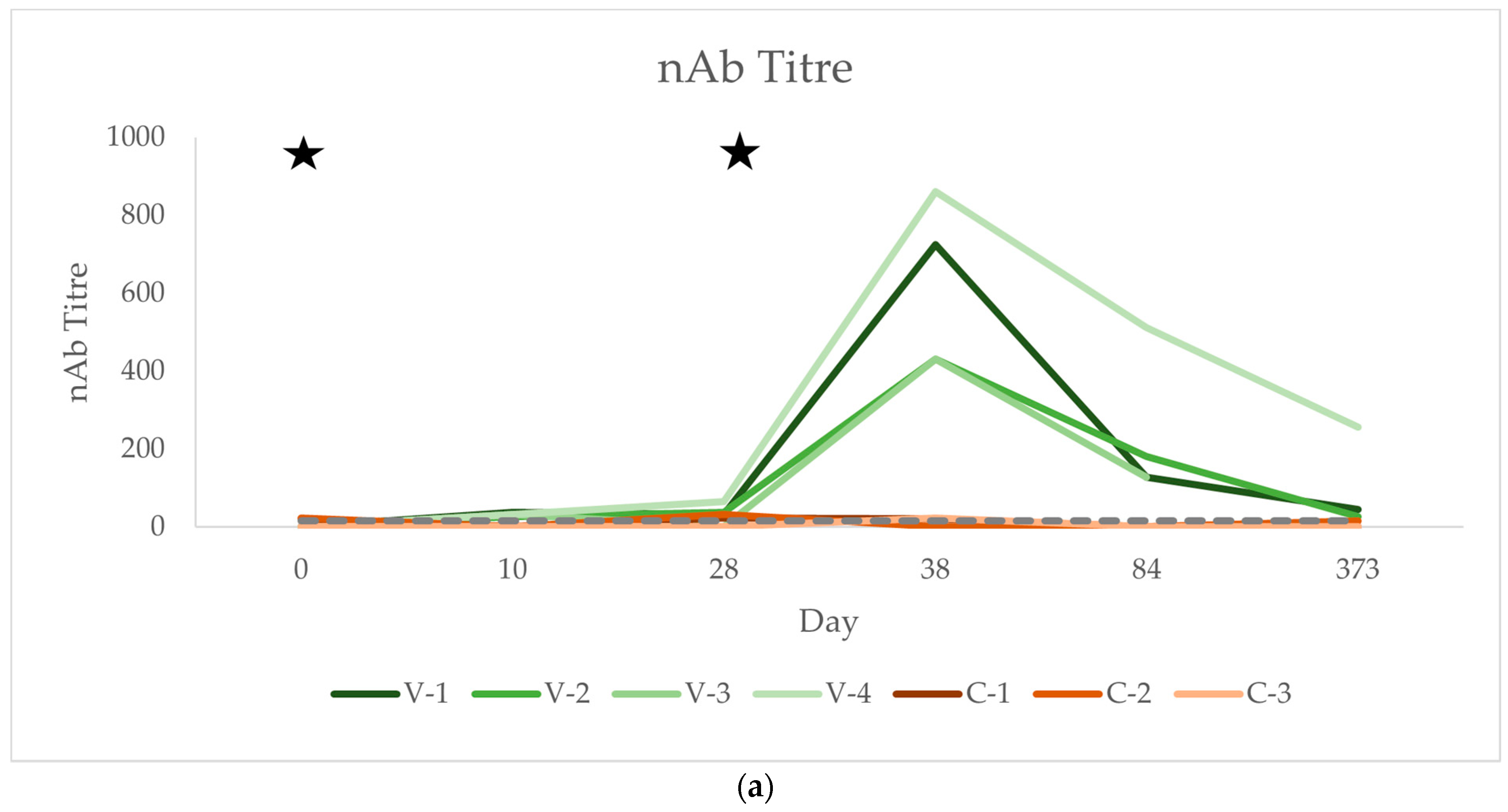
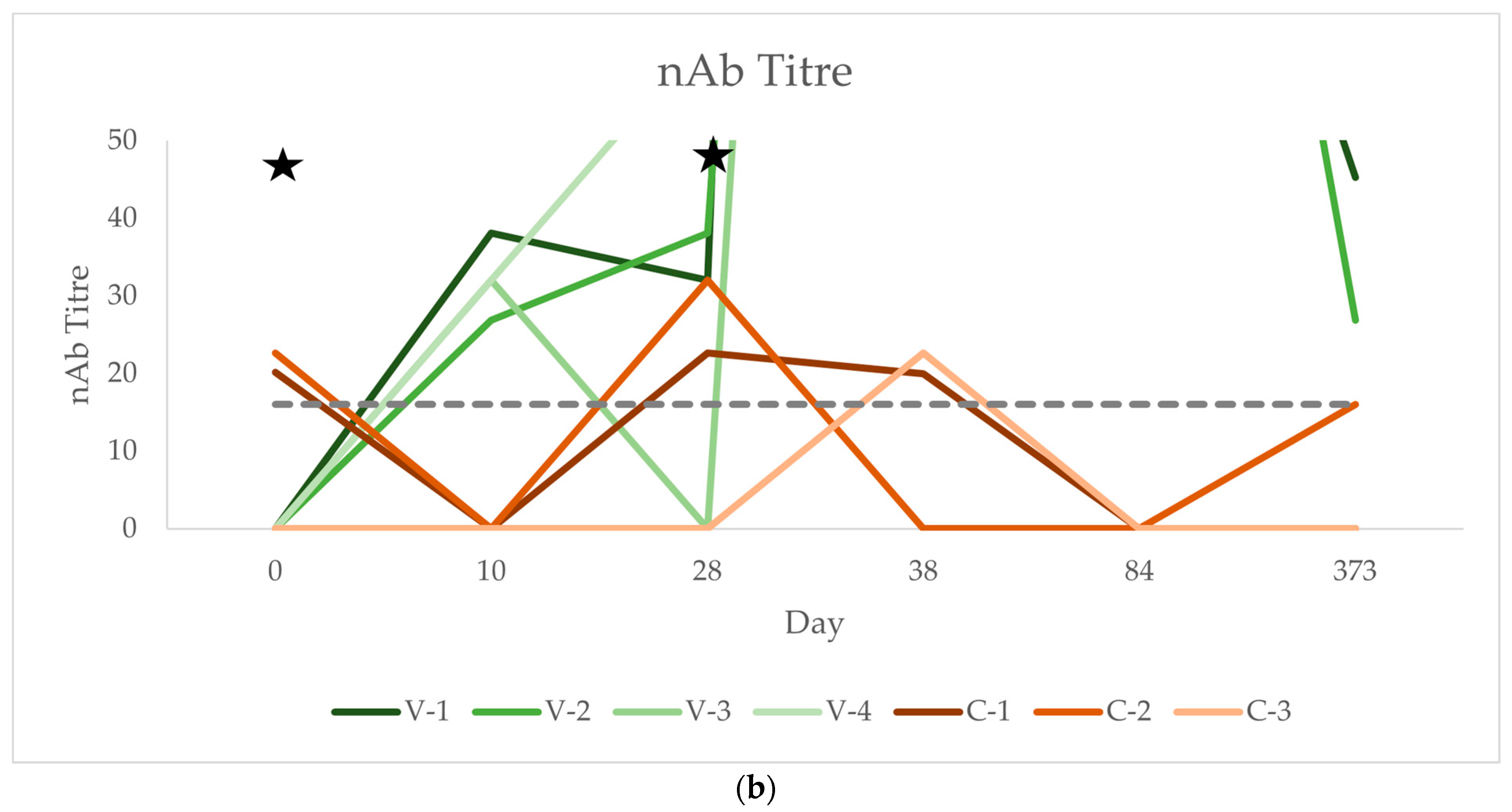
3.3. Neutralising Antibody Titres (nAb)
3.4. IgM Concentration
3.5. Inflammatory Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Horse | Breed | Age (Years) | IgG (VIEU/mL) | Group |
|---|---|---|---|---|
| V-1 | Swiss Warmblood | 5 | 18.18 | Vaccinated |
| V-2 | Trotter | 13 | 61.74 | Vaccinated |
| V-3 | Franche Montagne | 11 | 87.34 | Vaccinated |
| V-4 | Trotter | 17 | 250.44 | Vaccinated |
| C-1 | Franche Montagne | 12 | 69.72 | Control |
| C-2 | Trotter | 19 | 94.06 | Control |
| C-3 | Dutch Warmblood | 21 | 255.28 | Control |
| Day | 0 | 2 | 4 | 6 | 8 | 10 | 14 | 28 | 30 | 32 | 34 | 36 | 38 | 43 | 56 | 84 | 373 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | V-1 | 15.04 | 19.61 | 3.94 | 19.57 | 18.72 | 121.13 | 386.35 | 542.25 | 572.84 | 414.91 | 553.79 | 540.58 | 504.93 | 520.48 | 568.96 | 822.78 | 173.22 |
| V-2 | 62.75 | 68.96 | 67.18 | 61.80 | 76.47 | 138.77 | 164.08 | 173.67 | 127.94 | 181.10 | 464.84 | 602.68 | 701.16 | 664.33 | 570.12 | 522.32 | 98.87 | |
| V-3 | 122.14 | 95.48 | 88.96 | 130.89 | 67.49 | 88.87 | 59.18 | 82.78 | 173.30 | 141.73 | 213.45 | 370.38 | 546.77 | 496.07 | 487.72 | 467.21 | ||
| V-4 | 283.91 | 177.92 | 282.00 | 216.49 | 199.41 | 283.64 | 260.32 | 246.05 | 324.94 | 366.15 | 486.45 | 625.37 | 658.15 | 641.01 | 678.42 | 912.82 | 400.04 | |
| C-1 | 123.57 | 69.36 | 109.20 | 92.01 | 102.34 | 93.89 | 47.66 | 64.50 | 57.43 | 66.07 | 73.51 | 64.40 | 64.53 | 64.14 | 57.01 | 55.39 | 66.93 | |
| C-2 | 149.06 | 131.75 | 137.06 | 88.66 | 85.75 | 95.15 | 107.78 | 137.98 | 136.59 | 129.00 | 113.78 | 105.36 | 90.36 | 127.15 | 107.17 | 116.75 | 104.63 | |
| C-3 | 116.77 | 152.58 | 178.27 | 87.21 | 114.80 | 104.26 | 163.35 | 158.62 | 270.99 | 364.05 | 348.57 | 368.50 | 346.12 | 331.44 | 304.94 | 362.30 | 227.57 | |
| nAb | V-1 | <16 | 38.055 | 32.00 | 724.08 | 128.00 | 45.25 | |||||||||||
| V-2 | <16 | 26.91 | 38.05 | 430.54 | 181.02 | 26.91 | ||||||||||||
| V-3 | <16 | 32.00 | <16 | 430.54 | 128.00 | |||||||||||||
| V-4 | <16 | 32.00 | 64.00 | 861.08 | 512.00 | 256.00 | ||||||||||||
| C-1 | 20.16 | <16 | 22.63 | 20.00 | <16 | <16 | ||||||||||||
| C-2 | 22.63 | <16 | 32.00 | <16 | <16 | 16.00 | ||||||||||||
| C-3 | <16 | <16 | <16 | 22.63 | <16 | <16 | ||||||||||||
| IgM | V-1 | 1.25 | 3.84 | 1.31 | 0.63 | 1.20 | 25.53 | 1.84 | 2.09 | 8.19 | 3.71 | 2.98 | 1.92 | 3.30 | 1.92 | 1.17 | 4.44 | |
| V-2 | 1.81 | 1.69 | 1.90 | 1.29 | 1.14 | 2.03 | 2.13 | 2.26 | 1.72 | 1.67 | 1.37 | 1.26 | 1.47 | 0.85 | 1.67 | 2.29 | ||
| V-3 | 1.62 | 1.33 | 0.89 | 1.03 | 0.25 | 1.62 | 1.41 | 0.53 | 2.47 | 1.26 | 0.66 | 1.19 | 6.48 | 1.65 | 1.83 | 1.72 | ||
| V-4 | 1.01 | 0.84 | 1.37 | 1.06 | 1.16 | 2.36 | 1.67 | 1.16 | 2.24 | 0.96 | 1.14 | 1.17 | 0.96 | 1.90 | 0.37 | 2.15 | ||
| C-1 | 2.81 | 3.71 | 3.99 | 1.06 | 1.20 | 9.33 | 1.35 | 1.10 | 8.58 | 1.03 | 3.16 | 1.97 | 1.53 | 1.05 | 6.61 | 1.44 | ||
| C-2 | 11.48 | 1.25 | 2.17 | 1.08 | 0.99 | 1.71 | 2.28 | 1.25 | 3.75 | 1.28 | 1.14 | 0.89 | 1.56 | 1.28 | 1.65 | 3.14 | ||
| C-3 | 1.12 | 1.54 | 0.93 | 1.58 | 1.25 | 1.65 | 1.01 | 2.13 | 1.90 | 1.24 | 0.87 | 0.87 | 2.11 | 0.82 | 10.37 | 3.91 |
| Day | 0 | 2 | 4 | 28 | 30 | 32 | 84 | |
|---|---|---|---|---|---|---|---|---|
| Leucocyte count | V-1 | 10.8 | 10.0 | 10.6 | 9.3 | 10.7 | 9.4 | 11.5 |
| V-2 | 6.9 | 6.3 | 6.1 | 6.3 | 7.0 | 6.4 | 7.7 | |
| V-3 | 5.1 | 5.2 | 4.8 | 4.7 | 5.3 | 6.6 | ||
| V-4 | 6.5 | 6.3 | 5.5 | 5.7 | 7.2 | 6.9 | 5.7 | |
| C-1 | 5.6 | 5.5 | 5.3 | 4.7 | 5.4 | 4.9 | 6.4 | |
| C-2 | 7.2 | 7.0 | 7.3 | 6.5 | 8.6 | 7.6 | 7.0 | |
| C-3 | 8.7 | 9.2 | 7.0 | 6.2 | 6.7 | 7.1 | 9.6 | |
| SAA | V-1 | 0.6 | 0.0 | 3.0 | 0.0 | 0.0 | 1.0 | 0.0 |
| V-2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 5.8 | |
| V-3 | 2.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.5 | 0.0 | |
| V-4 | 0.0 | 5.4 | 4.0 | 0.0 | 3.1 | 1.3 | 0.0 | |
| C-1 | 4.7 | 0.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| C-2 | 0.4 | 0.0 | 2.0 | 0.0 | 3.5 | 0.0 | 0.0 | |
| C-3 | 0.0 | 11.0 | 16.8 | 452.0 | 462.3 | 158.9 | 0.0 | |
| Fibrinogen | V-1 | 2.0 | 2.1 | 2.0 | 2.4 | 2.3 | 2.5 | 3.0 |
| V-2 | 1.5 | 1.7 | 1.5 | 2.1 | 1.8 | 1.9 | 2.0 | |
| V-3 | 1.6 | 1.4 | 1.7 | 1.6 | 1.4 | 1.6 | 1.9 | |
| V-4 | 1.6 | 1.6 | 1.7 | 1.8 | 1.6 | 1.6 | 1.6 | |
| C-1 | 1.6 | 1.5 | 1.5 | 1.8 | 1.7 | 1.9 | 1.8 | |
| C-2 | 1.9 | 2.1 | 1.8 | 1.9 | 1.6 | 1.8 | 1.8 | |
| C-3 | 2.0 | 2.1 | 2.0 | 3.5 | 2.9 | 2.9 | 2.5 | |
| Globulin | V-1 | 32 | 30 | 33 | 28 | 29 | 29 | 33 |
| V-2 | 34 | 31 | 33 | 31 | 31 | 28 | 34 | |
| V-3 | 28 | 29 | 31 | 26 | 28 | 26 | 32 | |
| V-4 | 30 | 28 | 33 | 29 | 28 | 27 | 30 | |
| C-1 | 34 | 34 | 35 | 30 | 26 | 30 | 34 | |
| C-2 | 29 | 31 | 31 | 28 | 27 | 27 | 28 | |
| C-3 | 31 | 32 | 32 | 30 | 34 | 32 | 34 |
References
- Kubinski, M.; Beicht, J.; Gerlach, T.; Volz, A.; Sutter, G.; Rimmelzwaan, G.F. Tick-Borne Encephalitis Virus: A Quest for Better Vaccines against a Virus on the Rise. Vaccines 2020, 8, 451. [Google Scholar] [CrossRef] [PubMed]
- Michelitsch, A.; Wernike, K.; Klaus, C.; Dobler, G.; Beer, M. Exploring the Reservoir Hosts of Tick-Borne Encephalitis Virus. Viruses 2019, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Conze, T.M.; Bagó, Z.; Revilla-Fernández, S.; Schlegel, J.; Goehring, L.S.; Matiasek, K. Tick-Borne Encephalitis Virus (TBEV) Infection in Two Horses. Viruses 2021, 13, 1775. [Google Scholar] [CrossRef] [PubMed]
- Klaus, C.; Horugel, U.; Hoffmann, B.; Beer, M. Tick-borne encephalitis virus (TBEV) infection in horses: Clinical and laboratory findings and epidemiological investigations. Vet. Microbiol. 2013, 163, 368–372. [Google Scholar] [CrossRef]
- Fouché, N.; Oesch, S.; Ziegler, U.; Gerber, V. Clinical Presentation and Laboratory Diagnostic Work-Up of a Horse with Tick-Borne Encephalitis in Switzerland. Viruses 2021, 13, 1474. [Google Scholar] [CrossRef]
- Csank, T.; Drzewnioková, P.; Korytár, Ľ.; Major, P.; Gyuranecz, M.; Pistl, J.; Bakonyi, T. A Serosurvey of Flavivirus Infection in Horses and Birds in Slovakia. Vector Borne Zoonotic Dis. 2018, 18, 206–213. [Google Scholar] [CrossRef]
- de Heus, P.; Kolodziejek, J.; Hubálek, Z.; Dimmel, K.; Racher, V.; Nowotny, N.; Cavalleri, J.V. West Nile Virus and Tick-Borne Encephalitis Virus Are Endemic in Equids in Eastern Austria. Viruses 2021, 13, 1873. [Google Scholar] [CrossRef]
- Gothe, L.M.R.; Ganzenberg, S.; Ziegler, U.; Obiegala, A.; Lohmann, K.L.; Sieg, M.; Vahlenkamp, T.W.; Groschup, M.H.; Hörügel, U.; Pfeffer, M. Horses as Sentinels for the Circulation of Flaviviruses in Eastern-Central Germany. Viruses 2023, 15, 1108. [Google Scholar] [CrossRef] [PubMed]
- Albinsson, B.; Vene, S.; Rombo, L.; Blomberg, J.; Lundkvist, Å.; Rönnberg, B. Distinction between serological responses following tick-borne encephalitis virus (TBEV) infection vs vaccination, Sweden 2017. Euro Surveill. 2018, 23, 17-00838. [Google Scholar] [CrossRef]
- Rockstroh, A.; Moges, B.; Berneck, B.S.; Sattler, T.; Revilla-Fernández, S.; Schmoll, F.; Pacenti, M.; Sinigaglia, A.; Barzon, L.; Schmidt-Chanasit, J.; et al. Specific detection and differentiation of tick-borne encephalitis and West Nile virus induced IgG antibodies in humans and horses. Transbound. Emerg. Dis. 2019, 66, 1701–1708. [Google Scholar] [CrossRef]
- Cleton, N.B.; van Maanen, K.; Bergervoet, S.A.; Bon, N.; Beck, C.; Godeke, G.J.; Lecollinet, S.; Bowen, R.; Lelli, D.; Nowotny, N.; et al. A Serological Protein Microarray for Detection of Multiple Cross-Reactive Flavivirus Infections in Horses for Veterinary and Public Health Surveillance. Transbound. Emerg. Dis. 2017, 64, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Magouras, I.; Schoster, A.; Fouché, N.; Gerber, V.; Groschup, M.H.; Ziegler, U.; Fricker, R.; Griot, C.; Vögtlin, A. Neurological disease suspected to be caused by tick-borne encephalitis virus infection in 6 horses in Switzerland. J. Vet. Intern. Med. 2022, 36, 2254–2262. [Google Scholar] [CrossRef]
- Harabacz, I.; Bock, H.; Jüngst, C.; Klockmann, U.; Praus, M.; Weber, R. A randomized phase II study of a new tick-borne encephalitis vaccine using three different doses and two immunization regimens. Vaccine 1992, 10, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Schöndorf, I.; Beran, J.; Cizkova, D.; Lesna, V.; Banzhoff, A.; Zent, O. Tick-borne encephalitis (TBE) vaccination: Applying the most suitable vaccination schedule. Vaccine 2007, 25, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Klaus, C.; Beer, M.; Saier, R.; Schubert, H.; Bischoff, S.; Suss, J. Evaluation of serological tests for detecting tick-borne encephalitis virus (TBEV) antibodies in animals. Berl. Munch. Tierarztl. Wochenschr. 2011, 124, 443–449. [Google Scholar] [PubMed]
- Kohler, P.; Jonsdottir, H.R.; Risch, L.; Vernazza, P.; Ackermann-Gaumann, R.; Kahlert, C.R. No neutralizing effect of pre-existing tick-borne encephalitis virus antibodies against severe acute respiratory syndrome coronavirus-2: A prospective healthcare worker study. Sci. Rep. 2021, 11, 24198. [Google Scholar] [CrossRef]
- Ackermann-Gaumann, R.; Tritten, M.L.; Hassan, M.; Lienhard, R. Comparison of three commercial IgG and IgM ELISA kits for the detection of tick-borne encephalitis virus antibodies. Ticks Tick. Borne Dis. 2018, 9, 956–962. [Google Scholar] [CrossRef]
- Namekar, M.; Kumar, M.; O’Connell, M.; Nerurkar, V.R. Effect of serum heat-inactivation and dilution on detection of anti-WNV antibodies in mice by West Nile virus E-protein microsphere immunoassay. PLoS ONE 2012, 7, e45851. [Google Scholar] [CrossRef]
- Skipper, L.; Pusterla, N. Correlation Between Serum Amyloid A and Antibody Response to West Nile Virus Vaccine Antigen in Healthy Horses. J. Equine Vet. Sci. 2021, 106, 103755. [Google Scholar] [CrossRef]
- Ehrlich, H.J.; Pavlova, B.G.; Fritsch, S.; Poellabauer, E.M.; Loew-Baselli, A.; Obermann-Slupetzky, O.; Maritsch, F.; Cil, I.; Dorner, F.; Barrett, P.N. Randomized, phase II dose-finding studies of a modified tick-borne encephalitis vaccine: Evaluation of safety and immunogenicity. Vaccine 2003, 22, 217–223. [Google Scholar] [CrossRef]
- Loew-Baselli, A.; Konior, R.; Pavlova, B.G.; Fritsch, S.; Poellabauer, E.; Maritsch, F.; Harmacek, P.; Krammer, M.; Barrett, P.N.; Ehrlich, H.J.; et al. Safety and immunogenicity of the modified adult tick-borne encephalitis vaccine FSME-IMMUN: Results of two large phase 3 clinical studies. Vaccine 2006, 24, 5256–5263. [Google Scholar] [CrossRef] [PubMed]
- Duran, M.C.; Dumrath, C.A.C.; Bartmann, C.P.; Medina Torres, C.E.; Moschos, A.; Goehring, L.S. Serum Amyloid A (SAA) Concentration after Vaccination in Horses and Mules. J. Equine Vet. Sci. 2020, 92, 103165. [Google Scholar] [CrossRef] [PubMed]
- Balogh, Z.; Egyed, L.; Ferenczi, E.; Bán, E.; Szomor, K.N.; Takács, M.; Berencsi, G. Experimental infection of goats with tick-borne encephalitis virus and the possibilities to prevent virus transmission by raw goat milk. Intervirology 2012, 55, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Klaus, C.; Ziegler, U.; Hoffmann, D.; Press, F.; Fast, C.; Beer, M. Tick-borne encephalitis virus (TBEV) antibodies in animal sera—Occurrence in goat flocks in Germany, longevity and ability to recall immunological information after more than six years. BMC Vet. Res. 2019, 15, 399. [Google Scholar] [CrossRef]
- Kreil, T.R.; Maier, E.; Fraiss, S.; Attakpah, E.; Burger, I.; Mannhalter, J.W.; Eibl, M.M. Vaccination against tick-borne encephalitis virus, a flavivirus, prevents disease but not infection, although viremia is undetectable. Vaccine 1998, 16, 1083–1086. [Google Scholar] [CrossRef]
- Poellabauer, E.; Angermayr, R.; Behre, U.; Zhang, P.; Harper, L.; Schmitt, H.J.; Erber, W. Seropersistence and booster response following vaccination with FSME-IMMUN in children, adolescents, and young adults. Vaccine 2019, 37, 3241–3250. [Google Scholar] [CrossRef]
- Klaus, C.; Ziegler, U.; Kalthoff, D.; Hoffmann, B.; Beer, M. Tick-borne encephalitis virus (TBEV)—findings on cross reactivity and longevity of TBEV antibodies in animal sera. BMC Vet. Res. 2014, 10, 78. [Google Scholar] [CrossRef]
- Vene, S.; Haglund, M.; Lundkvist, A.; Lindquist, L.; Forsgren, M. Study of the serological response after vaccination against tick-borne encephalitis in Sweden. Vaccine 2007, 25, 366–372. [Google Scholar] [CrossRef]
- Zoulek, G.; Roggendorf, M.; Deinhardt, F.; Kunz, C. Different immune responses after intradermal and intramuscular administration of vaccine against tick-borne encephalitis virus. J. Med. Virol. 1986, 19, 55–61. [Google Scholar] [CrossRef]
- Monaco, F.; Purpari, G.; Di Gennaro, A.; Mira, F.; Di Marco, P.; Guercio, A.; Savini, G. Immunological response in horses following West Nile virus vaccination with inactivated or recombinant vaccine. Vet. Ital. 2019, 55, 73–79. [Google Scholar]
- Joó, K.; Bakonyi, T.; Szenci, O.; Sárdi, S.; Ferenczi, E.; Barna, M.; Malik, P.; Hubalek, Z.; Fehér, O.; Kutasi, O. Comparison of assays for the detection of West Nile virus antibodies in equine serum after natural infection or vaccination. Vet. Immunol. Immunopathol. 2017, 183, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Springer, A.; Glass, A.; Topp, A.K.; Strube, C. Zoonotic Tick-Borne Pathogens in Temperate and Cold Regions of Europe-A Review on the Prevalence in Domestic Animals. Front. Vet. Sci. 2020, 7, 604910. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.A.; Petersen, H.H.; Ersbøll, A.K.; Falk-Rønne, J.; Jacobsen, S. Vaccination elicits a prominent acute phase response in horses. Vet. J. 2012, 191, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Biondi, V.; Landi, A.; Pugliese, M.; Merola, G.; Passantino, A. Inflammatory Response and Electrocardiographic Examination in Horses Vaccinated against Equine Herpesvirus (Ehv-1). Animals 2022, 12, 778. [Google Scholar] [CrossRef]
- Arfuso, F.; Giudice, E.; Di Pietro, S.; Piccione, G.; Giannetto, C. Modulation of Serum Protein Electrophoretic Pattern and Leukocyte Population in Horses Vaccinated against West Nile Virus. Animals 2021, 11, 477. [Google Scholar] [CrossRef]



| Day | 0 | 2 | 4 | 6 | 8 | 10 | 14 | 28 | 30 | 32 | 34 | 36 | 38 | 43 | 56 | 84 | 373 | p Group | p Time | p Group × Time | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | Mean Vacc. | 120.96 | 90.49 | 110.52 | 107.19 | 90.52 | 158.11 | 217.48 | 261.19 | 299.76 | 275.97 | 429.63 | 534.75 | 602.75 | 580.47 | 576.31 | 681.28 | 224.04 | 0.58 | 0.37 | <0.001 |
| SD Vacc. | 101.44 | 57.35 | 103.81 | 74.58 | 66.60 | 74.65 | 120.69 | 172.28 | 173.72 | 116.68 | 129.05 | 99.85 | 79.78 | 73.18 | 67.76 | 190.21 | 128.10 | ||||
| Mean Control | 129.80 | 117.90 | 141.51 | 89.29 | 100.97 | 97.77 | 106.26 | 120.37 | 155.00 | 186.38 | 178.62 | 179.42 | 167.00 | 174.24 | 156.37 | 178.15 | 133.04 | ||||
| SD Control | 13.90 | 35.36 | 28.38 | 2.01 | 11.90 | 4.62 | 47.25 | 40.40 | 88.16 | 128.24 | 121.29 | 134.74 | 127.09 | 114.09 | 107.03 | 132.60 | 68.59 | ||||
| nAb | Mean Vacc. | 0.00 | 32.24 | 33.51 | 611.56 | 237.25 | 109.39 | 0.01 | 0.06 | <0.001 | |||||||||||
| SD Vacc. | 0.00 | 3.95 | 22.78 | 187.39 | 160.09 | 103.94 | |||||||||||||||
| Mean Control | 14.26 | 0.00 | 18.21 | 14.21 | 0.00 | 5.33 | |||||||||||||||
| SD Control | 10.14 | 0.00 | 13.43 | 10.10 | 0.00 | 7.54 | |||||||||||||||
| IgM | Mean Vacc. | 1.42 | 1.93 | 1.37 | 1.00 | 0.94 | 7.89 | 1.76 | 1.51 | 3.66 | 1.90 | 1.54 | 1.39 | 3.05 | 1.58 | 1.26 | 2.65 | 0.69 | 0.55 | 0.85 | |
| SD Vacc. | 0.31 | 1.15 | 0.36 | 0.24 | 0.40 | 10.19 | 0.26 | 0.70 | 2.63 | 1.07 | 0.87 | 0.31 | 2.16 | 0.44 | 0.57 | 1.06 | |||||
| Mean Control | 5.13 | 2.17 | 2.36 | 1.24 | 1.15 | 4.23 | 1.55 | 1.49 | 4.74 | 1.18 | 1.72 | 1.24 | 1.73 | 1.05 | 6.21 | 2.83 | |||||
| SD Control | 4.54 | 1.10 | 1.26 | 0.24 | 0.11 | 3.61 | 0.54 | 0.46 | 2.82 | 0.11 | 1.02 | 0.51 | 0.26 | 0.19 | 3.57 | 1.03 | |||||
| Leucocyte count | Mean Vacc. | 7.33 | 6.95 | 6.75 | 6.70 | 6.50 | 7.55 | 7.57 | 7.88 | 0.89 | 0.23 | 0.61 | |||||||||
| SD Vacc. | 2.11 | 1.82 | 2.27 | 1.99 | 1.71 | 1.96 | 1.31 | 2.21 | |||||||||||||
| Mean Control | 7.17 | 7.23 | 6.53 | 6.57 | 5.80 | 6.90 | 6.53 | 7.67 | |||||||||||||
| SD Control | 1.27 | 1.52 | 0.88 | 1.04 | 0.79 | 1.31 | 1.17 | 1.39 | |||||||||||||
| SAA | Mean Vac | 0.65 | 1.60 | 1.75 | 2.63 | 0.00 | 0.78 | 1.10 | 1.45 | 0.38 | 0.58 | 0.67 | |||||||||
| SD Vacc. | 0.82 | 2.23 | 1.79 | 1.57 | 0.00 | 1.34 | 0.34 | 2.51 | |||||||||||||
| Mean Control | 1.70 | 3.93 | 6.27 | 1.87 | 150.67 | 155.27 | 52.97 | 0.00 | |||||||||||||
| SD Control | 2.13 | 5.01 | 7.49 | 1.03 | 213.07 | 217.11 | 74.91 | 0.00 | |||||||||||||
| Fibrinogen | Mean Vacc. | 1.68 | 1.70 | 1.73 | 1.85 | 1.98 | 1.78 | 1.90 | 2.13 | 0.44 | 0.31 | 0.71 | |||||||||
| SD Vacc. | 0.19 | 0.25 | 0.18 | 0.38 | 0.30 | 0.33 | 0.37 | 0.53 | |||||||||||||
| Mean Control | 1.83 | 1.90 | 1.77 | 1.90 | 2.40 | 2.07 | 2.20 | 2.03 | |||||||||||||
| SD Control | 0.17 | 0.28 | 0.21 | 0.28 | 0.78 | 0.59 | 0.50 | 0.33 | |||||||||||||
| Globulin | Mean Vacc. | 31.00 | 29.50 | 32.50 | 28.50 | 29.00 | 27.50 | 32.25 | 0.34 | 0.69 | 0.55 | ||||||||||
| SD Vacc. | 2.24 | 1.12 | 0.87 | 1.80 | 1.22 | 1.12 | 1.48 | ||||||||||||||
| Mean Control | 31.33 | 32.33 | 32.67 | 29.33 | 29.00 | 29.67 | 32.00 | ||||||||||||||
| SD Control | 2.05 | 1.25 | 1.70 | 0.94 | 3.56 | 2.05 | 2.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kälin, D.; Becsek, A.; Stürmer, H.; Bachofen, C.; Siegrist, D.; Jonsdottir, H.R.; Schoster, A. Immune Response after Vaccination against Tick-Borne Encephalitis Virus (TBEV) in Horses. Vaccines 2024, 12, 1074. https://doi.org/10.3390/vaccines12091074
Kälin D, Becsek A, Stürmer H, Bachofen C, Siegrist D, Jonsdottir HR, Schoster A. Immune Response after Vaccination against Tick-Borne Encephalitis Virus (TBEV) in Horses. Vaccines. 2024; 12(9):1074. https://doi.org/10.3390/vaccines12091074
Chicago/Turabian StyleKälin, Dana, Angela Becsek, Helen Stürmer, Claudia Bachofen, Denise Siegrist, Hulda R. Jonsdottir, and Angelika Schoster. 2024. "Immune Response after Vaccination against Tick-Borne Encephalitis Virus (TBEV) in Horses" Vaccines 12, no. 9: 1074. https://doi.org/10.3390/vaccines12091074
APA StyleKälin, D., Becsek, A., Stürmer, H., Bachofen, C., Siegrist, D., Jonsdottir, H. R., & Schoster, A. (2024). Immune Response after Vaccination against Tick-Borne Encephalitis Virus (TBEV) in Horses. Vaccines, 12(9), 1074. https://doi.org/10.3390/vaccines12091074






