Immunogenicity Evaluation of Combination Respiratory Syncytial Virus and Varicella–Zoster Virus mRNA Vaccines in C57BL/6J Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of mRNA Vaccines
2.2. Animal Studies
2.3. Antibody Titer Detection
2.4. RSV Cytopathic Effect Neutralization Test (CPENT)
2.5. Isolation of Splenocytes
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Enzyme-Linked Immuno-Spot (ELISpot) Assay
2.8. Flow Cytometry
2.9. Statistical Analysis
3. Results
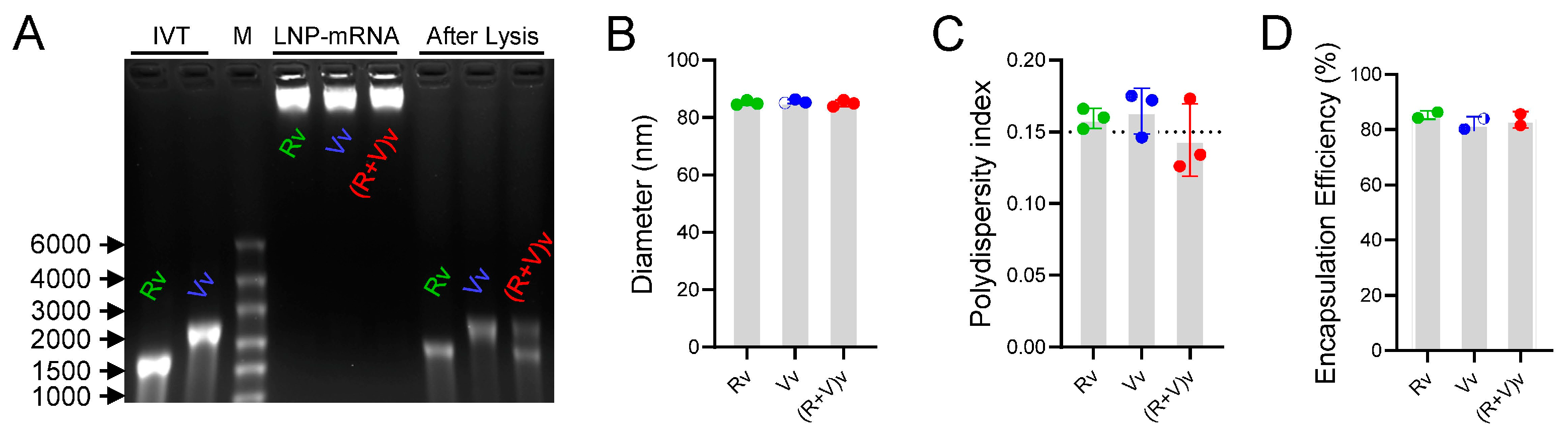
3.1. LNPs Efficiently Encapsulated mRNA Antigens with Homogeneous Particle Sizes
3.2. Combination Vaccine Groups Achieved Antibody Titer Levels Similar to Those of Single Vaccine Injection Groups
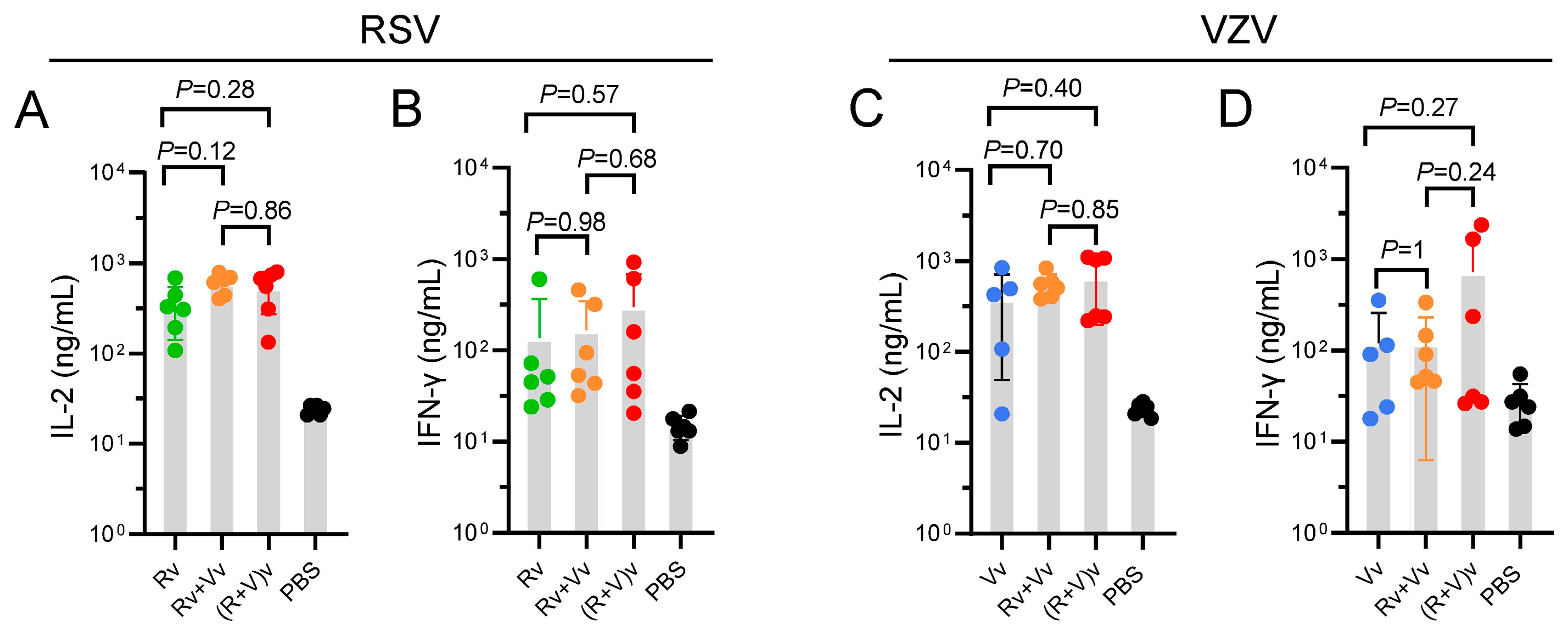
3.3. Groups in Which the Combination Vaccine Was Injected Presented Slightly Increased CMI
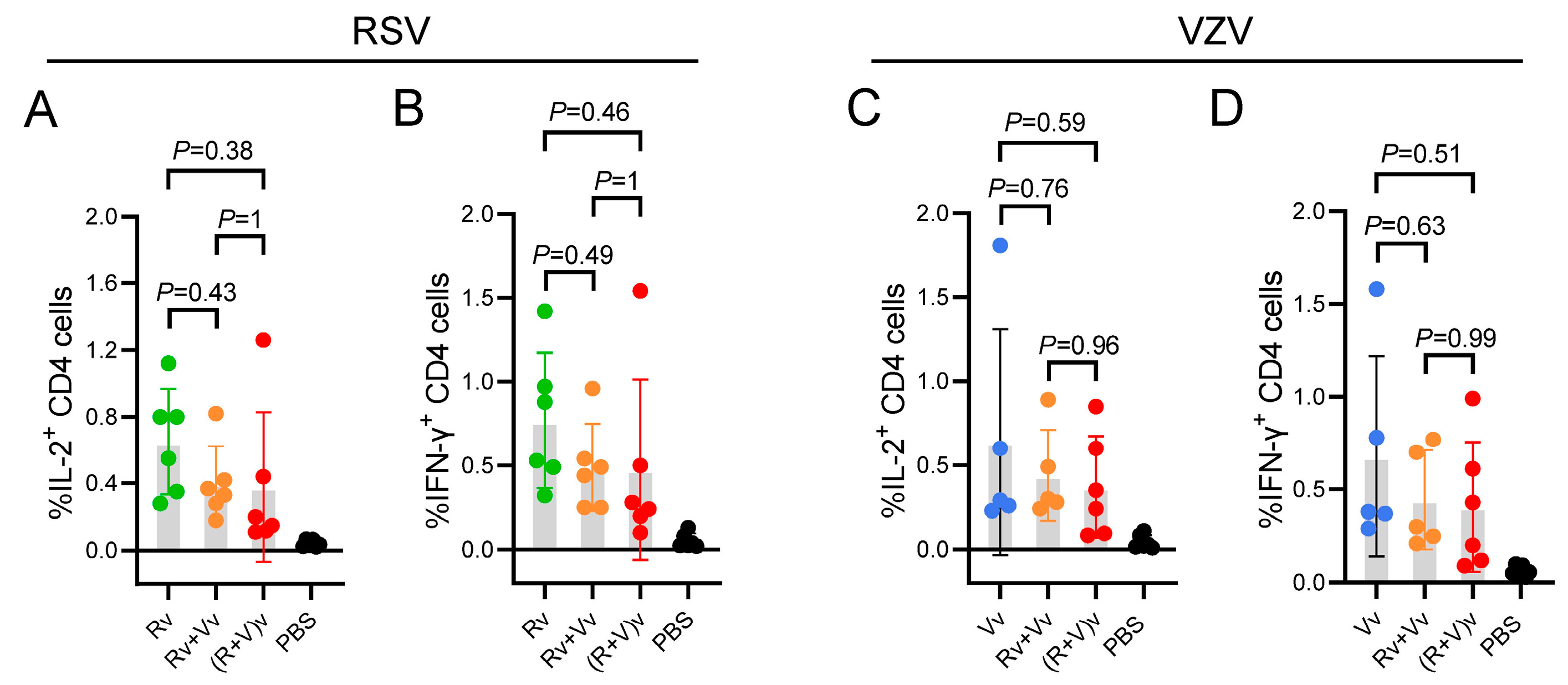
3.4. Compared with the LNP-mRNA Vaccine Group, the Combined LNP-mRNA Vaccine Group Presented Slightly Fewer CD4+ T-Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Li, H.; Li, Z.; Wang, Y. Vaccine and therapeutic agents against the respiratory syncytial virus: Resolved and unresolved issue. MedComm 2024, 5, e70016. [Google Scholar] [CrossRef] [PubMed]
- Haber, N. Respiratory syncytial virus infection in elderly adults. Med. Mal. Infect. 2018, 48, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, X.; Du, Z.; Zhang, L.; Wang, Y.; Wu, Y.; Lin, Y.; He, Y. Prevention of respiratory syncytial virus from 1991 to 2024: A systematic review and bibliometrics analysis. Transl. Pediatr. 2024, 13, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
- Rozenbaum, M.H.; Begier, E.; Kurosky, S.K.; Whelan, J.; Bem, D.; Pouwels, K.B.; Postma, M.; Bont, L. Incidence of Respiratory Syncytial Virus Infection in Older Adults: Limitations of Current Data. Infect. Dis. Ther. 2023, 12, 1487–1504. [Google Scholar] [CrossRef]
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.; Oxman, M.N.; et al. Varicella zoster virus infection. Nat. Rev. Dis. Primers 2015, 1, 15016. [Google Scholar] [CrossRef]
- Asada, H. VZV-specific cell-mediated immunity, but not humoral immunity, correlates inversely with the incidence of herpes zoster and the severity of skin symptoms and zoster-associated pain: The SHEZ study. Vaccine 2019, 37, 6776–6781. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Chlibek, R.; Bayas, J.M.; Collins, H.; de la Pinta, M.L.; Ledent, E.; Mols, J.F.; Heineman, T.C. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults ≥50 years of age. J. Infect. Dis. 2013, 208, 1953–1961. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Lal, H.; Kovac, M.; Chlibek, R.; Hwang, S.J.; Diez-Domingo, J.; Godeaux, O.; Levin, M.J.; McElhaney, J.E.; Puig-Barbera, J.; et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N. Engl. J. Med. 2016, 375, 1019–1032. [Google Scholar] [CrossRef]
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.B.; Yu, D. mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mol. Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef]
- Monslow, M.A.; Elbashir, S.; Sullivan, N.L.; Thiriot, D.S.; Ahl, P.; Smith, J.; Miller, E.; Cook, J.; Cosmi, S.; Thoryk, E.; et al. Immunogenicity generated by mRNA vaccine encoding VZV gE antigen is comparable to adjuvanted subunit vaccine and better than live attenuated vaccine in nonhuman primates. Vaccine 2020, 38, 5793–5802. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, T.; Zhao, W.; Shao, A.; Zhao, H.; Ma, W.; Gong, Y.; Zeng, X.; Weng, C.; Bu, L.; et al. Herpes zoster mRNA vaccine induces superior vaccine immunity over licensed vaccine in mice and rhesus macaques. Emerg. Microbes Infect. 2024, 13, 2309985. [Google Scholar] [CrossRef]
- FDA Approves First Respiratory Syncytial Virus (Rsv) Vaccine. 2023. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytial-virus-rsv-vaccine (accessed on 4 May 2023).
- US FDA Approves ABRYSVO, Pfizer’s Vaccine for the Prevention of Respiratory Syncytial Virus (RSV) in Older Adults. Available online: https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-abrysvotm-pfizers-vaccine-prevention (accessed on 2 June 2023).
- Chang, L.A.; Phung, E.; Crank, M.C.; Morabito, K.M.; Villafana, T.; Dubovsky, F.; Falloon, J.; Esser, M.T.; Lin, B.C.; Chen, G.L.; et al. A prefusion-stabilized RSV F subunit vaccine elicits B cell responses with greater breadth and potency than a postfusion F vaccine. Sci. Transl. Med. 2022, 14, eade0424. [Google Scholar] [CrossRef] [PubMed]
- Mejias, A.; Rodriguez-Fernandez, R.; Oliva, S.; Peeples, M.E.; Ramilo, O. The journey to a respiratory syncytial virus vaccine. Ann. Allergy Asthma Immunol. 2020, 125, 36–46. [Google Scholar] [CrossRef]
- Mo, C.; Schneeberger, E.E.; Arvin, A.M. Glycoprotein E of varicella-zoster virus enhances cell-cell contact in polarized epithelial cells. J. Virol. 2000, 74, 11377–11387. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, Y.; Luan, N.; Lin, K.; Liu, C. Effects of Varicella-Zoster Virus Glycoprotein E Carboxyl-Terminal Mutation on mRNA Vaccine Efficacy. Vaccines 2021, 9, 1440. [Google Scholar] [CrossRef]
- Moffat, J.; Mo, C.; Cheng, J.J.; Sommer, M.; Zerboni, L.; Stamatis, S.; Arvin, A.M. Functions of the C-terminal domain of varicella-zoster virus glycoprotein E in viral replication in vitro and skin and T-cell tropism in vivo. J. Virol. 2004, 78, 12406–12415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, H.; Lin, K.; Hu, J.; Luan, N.; Liu, C. Evaluation of the Immunological Efficacy of an LNP-mRNA Vaccine Prepared from Varicella Zoster Virus Glycoprotein gE with a Double-Mutated Carboxyl Terminus in Different Untranslated Regions in Mice. Vaccines 2023, 11, 1475. [Google Scholar] [CrossRef]
- Shaw Christine, and August Allison. Respiratory Syncytial Virus mRNA Vaccines Patent WO 2022/221336 A1, 20 October 2022.
- World Health Organization. Messenger RNA Encoding the Full-Length SARS-CoV-2 Spike Glycoprotein. 2020. Available online: https://web.archive.org/web/20210105162941/https://mednet-communities.net/inn/db/media/docs/11889.doc (accessed on 7 June 2021).
- Luan, N.; Cao, H.; Wang, Y.; Lin, K.; Liu, C. LNP-CpG ODN-adjuvanted varicella-zoster virus glycoprotein E induced comparable levels of immunity with Shingrix in VZV-primed mice. Virol. Sin. 2022, 37, 731–739. [Google Scholar] [CrossRef]
- Xu, S.; Yang, K.; Li, R.; Zhang, L. mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection. Int. J. Mol. Sci. 2020, 21, 6582. [Google Scholar] [CrossRef]
- Liu, B.; Cao, B.; Wang, C.; Han, B.; Sun, T.; Miao, Y.; Lu, Q.; Cui, F. Immunogenicity and Safety of Childhood Combination Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 472. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Q.; Vishwanath, S.; Carnell, G.W.; Chan, A.C.Y.; Heeney, J.L. Immune imprinting and next-generation coronavirus vaccines. Nat. Microbiol. 2023, 8, 1971–1985. [Google Scholar] [CrossRef] [PubMed]
- Kurosky, S.K.; Davis, K.L.; Krishnarajah, G. Effect of combination vaccines on completion and compliance of childhood vaccinations in the United States. Hum. Vaccin. Immunother. 2017, 13, 2494–2502. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Unger, S.A.; Walton, M.; Schwarze, J. The Human Immune Response to Respiratory Syncytial Virus Infection. Clin. Microbiol. Rev. 2017, 30, 481–502. [Google Scholar] [CrossRef]
- De Carli, M.; D’Elios, M.M.; Zancuoghi, G.; Romagnani, S.; Del Prete, G. Human Th1 and Th2 cells: Functional properties, regulation of development and role in autoimmunity. Autoimmunity 1994, 18, 301–308. [Google Scholar] [CrossRef]
- Chlibek, R.; Smetana, J.; Pauksens, K.; Rombo, L.; Van den Hoek, J.A.; Richardus, J.H.; Plassmann, G.; Schwarz, T.F.; Ledent, E.; Heineman, T.C. Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: A phase II, randomized, controlled study. Vaccine 2014, 32, 1745–1753. [Google Scholar] [CrossRef]
- Walsh, E.E.; Perez Marc, G.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef]
- Ison, M.G.; Papi, A.; Athan, E.; Feldman, R.G.; Langley, J.M.; Lee, D.G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; et al. Efficacy and Safety of Respiratory Syncytial Virus (RSV) Prefusion F Protein Vaccine (RSVPreF3 OA) in Older Adults Over 2 RSV Seasons. Clin. Infect. Dis. 2024, 78, 1732–1744. [Google Scholar] [CrossRef]
- Xia, X. Detailed Dissection and Critical Evaluation of the Pfizer/BioNTech and Moderna mRNA Vaccines. Vaccines 2021, 9, 734. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, N.; Huang, L.; Hu, J.; Zhang, H.; Gao, D.; Lei, Z.; Zhang, X.; Cao, H.; Liu, C. Immunogenicity Evaluation of Combination Respiratory Syncytial Virus and Varicella–Zoster Virus mRNA Vaccines in C57BL/6J Mice. Vaccines 2025, 13, 361. https://doi.org/10.3390/vaccines13040361
Luan N, Huang L, Hu J, Zhang H, Gao D, Lei Z, Zhang X, Cao H, Liu C. Immunogenicity Evaluation of Combination Respiratory Syncytial Virus and Varicella–Zoster Virus mRNA Vaccines in C57BL/6J Mice. Vaccines. 2025; 13(4):361. https://doi.org/10.3390/vaccines13040361
Chicago/Turabian StyleLuan, Ning, Luxia Huang, Jingping Hu, Haihao Zhang, Dandan Gao, Zhentao Lei, Xiaolong Zhang, Han Cao, and Cunbao Liu. 2025. "Immunogenicity Evaluation of Combination Respiratory Syncytial Virus and Varicella–Zoster Virus mRNA Vaccines in C57BL/6J Mice" Vaccines 13, no. 4: 361. https://doi.org/10.3390/vaccines13040361
APA StyleLuan, N., Huang, L., Hu, J., Zhang, H., Gao, D., Lei, Z., Zhang, X., Cao, H., & Liu, C. (2025). Immunogenicity Evaluation of Combination Respiratory Syncytial Virus and Varicella–Zoster Virus mRNA Vaccines in C57BL/6J Mice. Vaccines, 13(4), 361. https://doi.org/10.3390/vaccines13040361




