Exploring the Biological Activities of Ionic Liquids and Their Potential to Develop Novel Vaccine Adjuvants
Abstract
1. Introduction
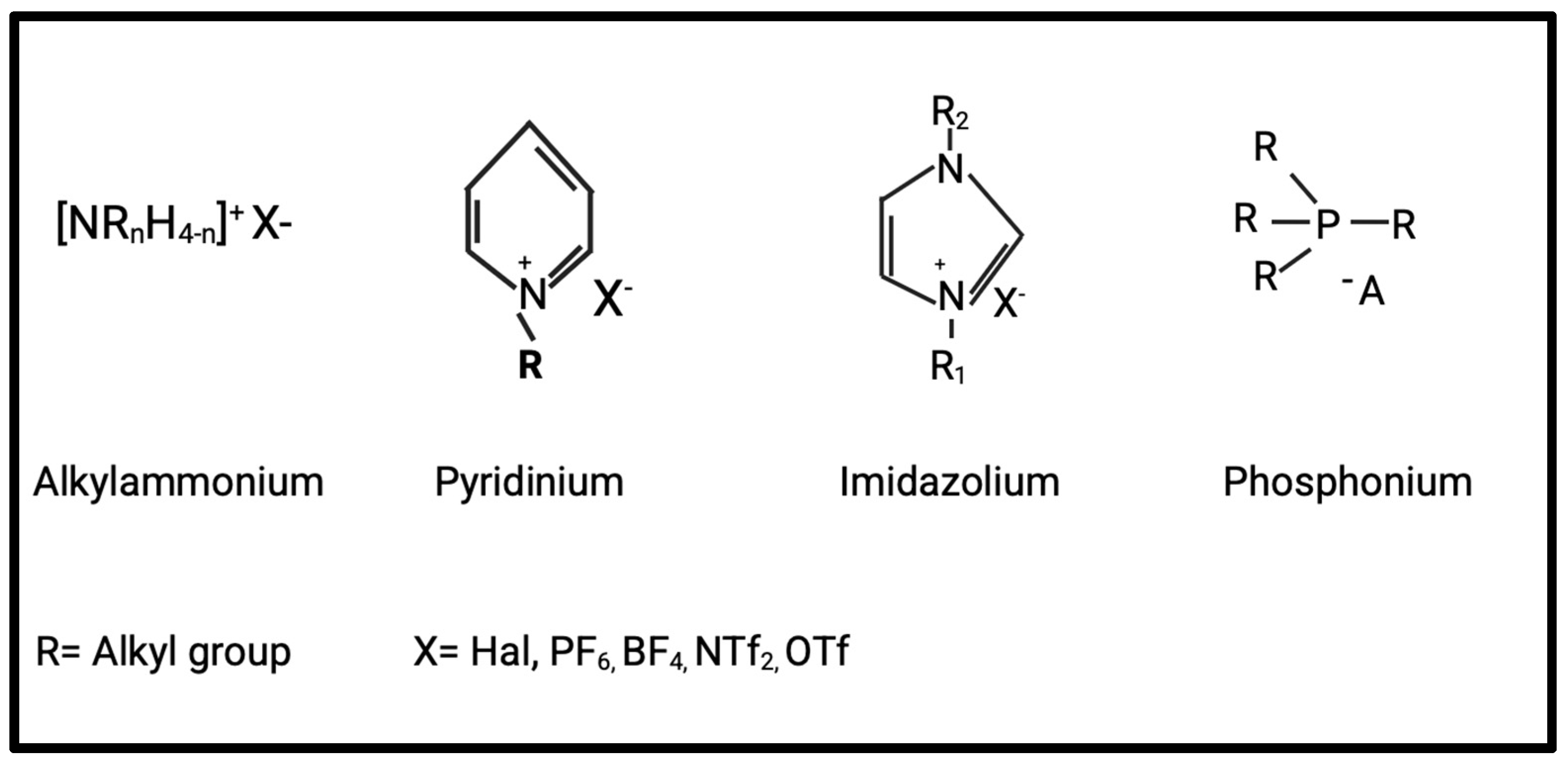
2. Perspectives and Challenges of Ionic Liquids
3. Biological Activities of Ionic Liquids
4. Toxicity and Fate of Ionic Liquids
5. Potential Immunogenic Targets of Ionic Liquids
6. Ionic Liquids as Vaccine Adjuvants
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R. Catalytic reactions in ionic liquids. Chem. Commun. 2001, 23, 2399–2407. [Google Scholar] [CrossRef]
- Meine, N.; Benedito, F.; Rinaldi, R. Thermal stability of ionic liquids assessed by potentiometric titration. Green Chem. 2010, 12, 1711. [Google Scholar] [CrossRef]
- Uddin, M.N.; Basak, D.; Hopefl, R.; Minofar, B. Potential Application of Ionic Liquids in Pharmaceutical Dosage Forms for Small Molecule Drug and Vaccine Delivery System. J. Pharm. Pharm. Sci. 2020, 23, 158–176. [Google Scholar] [CrossRef]
- Araki, S.; Wakabayashi, R.; Moniruzzaman, M.; Kamiya, N.; Goto, M. Ionic liquid-mediated transcutaneous protein delivery with solid-in-oil nanodispersions. Med. Chem. Commun. 2015, 6, 2124–2128. [Google Scholar] [CrossRef]
- Williams, H.D.; Ford, L.; Lim, S.; Han, S.; Baumann, J.; Sullivan, H.; Vodak, D.; Igonin, A.; Benameur, H.; Pouton, C.W.; et al. Transformation of Biopharmaceutical Classification System Class I and III Drugs Into Ionic Liquids and Lipophilic Salts for Enhanced Developability Using Lipid Formulations. J. Pharm. Sci. 2018, 107, 203–216. [Google Scholar] [CrossRef]
- Sahbaz, Y.; Nguyen, T.-H.; Ford, L.; McEvoy, C.L.; Williams, H.D.; Scammells, P.J.; Porter, C.J.H. Ionic Liquid Forms of Weakly Acidic Drugs in Oral Lipid Formulations: Preparation, Characterization, in Vitro Digestion, and in Vivo Absorption Studies. Mol. Pharm. 2017, 14, 3669–3683. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Byrne, N.; Rodoni, B.; Constable, F.; Varghese, S.; Davis, J.H. Enhanced stabilization of the Tobacco mosaic virus using protic ionic liquids. Phys. Chem. Chem. Phys. 2012, 14, 10119. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Kelley, S.P.; Gurau, G.; Rogers, R.D. Chemistry: Develop ionic liquid drugs. Nature 2015, 528, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Kouzi, S.S.; Harrod, H.; Uddin, M.N.; Shah, S.M. Potential Application of Ionic Liquid 1,4-Diazabicyclo-Octane for Enhanced Buccal Permeability of Diphenhydramine Hydrochloride in Oral Dissolving Polymeric Film. EC Pharmacol. Toxicol. 2023, 12, 1–16. [Google Scholar]
- Brunner, R.; Jensen-Jarolim, E.; Pali-Schöll, I. The ABC of clinical and experimental adjuvants—A brief overview. Immunol. Lett. 2010, 128, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Partidos, C.D.; Halmuthur, S.K.M.; Muller, S. An Overview of Novel Adjuvants Designed for Improving Vaccine Efficacy. Trends Pharmacol. Sci. 2017, 38, 771–793. [Google Scholar] [CrossRef]
- Xie, C.; Yao, R.; Xia, X. The advances of adjuvants in mRNA vaccines. npj Vaccines 2023, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, X.; Gao, C.; Ni, L.; Wang, X.; Zhang, W.; Ren, S. Catalytic hydrogenolysis of larix bark proanthocyanidins in ionic liquids produces UV blockers with potential for use in cosmetics. RSC Adv. 2021, 11, 30078–30087. [Google Scholar] [CrossRef]
- Zhuo, Y.; Cheng, H.-L.; Zhao, Y.-G.; Cui, H.-R. Ionic Liquids in Pharmaceutical and Biomedical Applications: A Review. Pharmaceutics 2024, 16, 151. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef]
- Qiao, Y.; Ma, W.; Theyssen, N.; Chen, C.; Hou, Z. Temperature-Responsive Ionic Liquids: Fundamental Behaviors and Catalytic Applications. Chem. Rev. 2017, 117, 6881–6928. [Google Scholar] [CrossRef]
- Baaqel, H.; Díaz, I.; Tulus, V.; Chachuat, B.; Guillén-Gosálbez, G.; Hallett, J.P. Role of life-cycle externalities in the valuation of protic ionic liquids—A case study in biomass pretreatment solvents. Green Chem. 2020, 22, 3132–3140. [Google Scholar] [CrossRef]
- Joseph, K. Navigating the Pros and Cons: Advantages and Disadvantages of Ionic Liquids. Polym. Sci. 2023, 8, 19. [Google Scholar] [CrossRef]
- Jain, A.; Shakya, A.K.; Prajapati, S.K.; Eldesoqui, M.; Mody, N.; Jain, S.K.; Naik, R.R.; Patil, U.K. An insight into pharmaceutical challenges with ionic liquids: Where do we stand in transdermal delivery? Front. Bioeng. Biotechnol. 2024, 12, 1454247. [Google Scholar] [CrossRef]
- Studzińska, S.; Kowalkowski, T.; Buszewski, B. Study of ionic liquid cations transport in soil. J. Hazard. Mater. 2009, 168, 1542–1547. [Google Scholar] [CrossRef]
- Matzke, M.; Stolte, S.; Arning, J.; Uebers, U.; Filser, J. Imidazolium based ionic liquids in soils: Effects of the side chain length on wheat (Triticum aestivum) and cress (Lepidium sativum) as affected by different clays and organic matter. Green Chem. 2008, 10, 584. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, L. Imidazolium ionic liquids as potential persistent pollutants in aqueous environments: Indirect photochemical degradation kinetics and mechanism. Environ. Res. 2022, 211, 113031. [Google Scholar] [CrossRef]
- Oskarsson, A.; Wright, M.C. Ionic Liquids: New Emerging Pollutants, Similarities with Perfluorinated Alkyl Substances (PFASs). Environ. Sci. Technol. 2019, 53, 10539–10541. [Google Scholar] [CrossRef]
- Probert, P.M.; Leitch, A.C.; Dunn, M.P.; Meyer, S.K.; Palmer, J.M.; Abdelghany, T.M.; Lakey, A.F.; Cooke, M.P.; Talbot, H.; Wills, C.; et al. Identification of a xenobiotic as a potential environmental trigger in primary biliary cholangitis. J. Hepatol. 2018, 69, 1123–1135. [Google Scholar] [CrossRef]
- Handy, S. (Ed.) Ionic Liquids Recycling for Reuse. In Ionic Liquids—Classes and Properties; InTech: Rijeka, Croatia, 2011; ISBN 978-953-307-634-8. [Google Scholar] [CrossRef]
- Yang, Q.; Dionysiou, D.D. Photolytic degradation of chlorinated phenols in room temperature ionic liquids. J. Photochem. Photobiol. A Chem. 2004, 165, 229–240. [Google Scholar] [CrossRef]
- Khodadoust, A.P.; Chandrasekaran, S.; Dionysiou, D.D. Preliminary Assessment of Imidazolium-Based Room-Temperature Ionic Liquids for Extraction of Organic Contaminants from Soils. Environ. Sci. Technol. 2006, 40, 2339–2345. [Google Scholar] [CrossRef]
- Volkov, A.V.; Korneeva, G.A.; Tereshchenko, G.F. Organic solvent nanofiltration: Prospects and application. Russ. Chem. Rev. 2008, 77, 983–993. [Google Scholar] [CrossRef]
- Wu, B.; Liu, W.; Zhang, Y.; Wang, H. Do We Understand the Recyclability of Ionic Liquids? Chem. A Eur. J. 2009, 15, 1804–1810. [Google Scholar] [CrossRef]
- Fujita, K.; MacFarlane, D.R.; Forsyth, M. Protein solubilising and stabilising ionic liquids. Chem. Commun. 2005, 38, 4804–4806. [Google Scholar] [CrossRef]
- Bose, S.; Armstrong, D.W.; Petrich, J.W. Enzyme-Catalyzed Hydrolysis of Cellulose in Ionic Liquids: A Green Approach Toward the Production of Biofuels. J. Phys. Chem. B 2010, 114, 8221–8227. [Google Scholar] [CrossRef]
- Kumar, A.; Bisht, M.; Venkatesu, P. Biocompatibility of ionic liquids towards protein stability: A comprehensive overview on the current understanding and their implications. Int. J. Biol. Macromol. 2017, 96, 611–651. [Google Scholar] [CrossRef] [PubMed]
- Buettner, C.S.; Cognigni, A.; Schröder, C.; Bica-Schröder, K. Surface-active ionic liquids: A review. J. Mol. Liq. 2022, 347, 118160. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kamma, H.; Das, R.; Ainavarapu, S.R.K. Ionic Liquid-Induced Modulation of Ubiquitin Stability: The Dominant Role of Hydrophobic Interactions. Langmuir 2025, 41, 5823–5837. [Google Scholar] [CrossRef]
- Wolski, P.; Blankenship, B.W.; Umar, A.; Cabrera, M.; Simmons, B.A.; Sale, K.L.; Achinivu, E.C. Factors that influence the activity of biomass-degrading enzymes in the presence of ionic liquids—A review. Front. Energy Res. 2023, 11, 1212719. [Google Scholar] [CrossRef]
- Haj Kacem, S.; Silva, R.; Rosatella, A.; Afonso, C.; Omar, S.; Galai, S. Screening of newly synthesized Ionic Liquids and Deep eutectic solvent for enhancing the activity and stability of fungal laccase: Empowering kinetic parameters and long-term conservation. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Nicholson, J.H.; Chagas De Avila, M.; Rodrigues De Melo, R.; Zanphorlin, L.M.; Brogan, A.P.S. Enhancing the reactivity of a P450 decarboxylase with ionic liquids. Green Chem. 2025, 27, 517–526. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, W.; Zhang, Y.; Nian, B.; Hu, Y. Functionalized Ionic Liquids-Modified Metal–Organic Framework Material Boosted the Enzymatic Performance of Lipase. Molecules 2024, 29, 2381. [Google Scholar] [CrossRef]
- Khavani, M.; Mehranfar, A.; Vahid, H. Application of amino acid ionic liquids for increasing the stability of DNA in long term storage. J. Biomol. Struct. Dyn. 2023, 41, 4383–4397. [Google Scholar] [CrossRef] [PubMed]
- Tulsiyan, K.D.; Panda, S.K.; Rana, M.K.; Biswal, H.S. Critical assessment of interactions between ct-DNA and choline-based magnetic ionic liquids: Evidences of compaction. Chem. Sci. 2024, 15, 5507–5515. [Google Scholar] [CrossRef]
- Vijayaraghavan, R.; Izgorodin, A.; Ganesh, V.; Surianarayanan, M.; MacFarlane, D.R. Long-Term Structural and Chemical Stability of DNA in Hydrated Ionic Liquids. Angew. Chem. Int. Ed. 2010, 49, 1631–1633. [Google Scholar] [CrossRef]
- Li, X.-Y.; Jing, C.-Q.; Lei, W.-L.; Li, J.; Wang, J.-J. Apoptosis caused by imidazolium-based ionic liquids in PC12 cells. Ecotoxicol. Environ. Saf. 2012, 83, 102–107. [Google Scholar] [CrossRef]
- Thamke, V.R.; Chaudhari, A.U.; Tapase, S.R.; Paul, D.; Kodam, K.M. In vitro toxicological evaluation of ionic liquids and development of effective bioremediation process for their removal. Environ. Pollut. 2019, 250, 567–577. [Google Scholar] [CrossRef]
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic Liquids in Pharmaceutical Applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, L.; Li, C.; Wu, S.; Xiao, Y. Preparation and antimicrobial activity of sulfopropyl chitosan in an ionic liquid aqueous solution. J. Appl. Polym. Sci. 2017, 134, 26. [Google Scholar] [CrossRef]
- Amde, M.; Liu, J.-F.; Pang, L. Environmental Application, Fate, Effects, and Concerns of Ionic Liquids: A Review. Environ. Sci. Technol. 2015, 49, 12611–12627. [Google Scholar] [CrossRef] [PubMed]
- Petkovic, M.; Ferguson, J.; Bohn, A.; Trindade, J.; Martins, I.; Carvalho, M.B.; Leitão, M.C.; Rodrigues, C.; Garcia, H.; Ferreira, R.; et al. Exploring fungal activity in the presence of ionic liquids. Green Chem. 2009, 11, 889. [Google Scholar] [CrossRef]
- Parajó, J.J.; Santiago-Alonso, A.; Vallet, P.; Teijeira, T.; Emeterio, R.S.; Villanueva, M.; Salgado, J. Comprehensive Analysis of the Acute Toxicity of Ionic Liquids Using Microtox® Bioassays. Appl. Sci. 2024, 14, 2480. [Google Scholar] [CrossRef]
- Yoo, B.; Jing, B.; Jones, S.E.; Lamberti, G.A.; Zhu, Y.; Shah, J.K.; Maginn, E.J. Molecular mechanisms of ionic liquid cytotoxicity probed by an integrated experimental and computational approach. Sci. Rep. 2016, 6, 19889. [Google Scholar] [CrossRef]
- Stock, F.; Hoffmann, J.; Ranke, J.; Störmann, R.; Ondruschka, B.; Jastorff, B. Effects of ionic liquids on the acetylcholinesterase—A structure–activity relationship consideration. Green Chem. 2004, 6, 286–290. [Google Scholar] [CrossRef]
- Vraneš, M.; Tot, A.; Jovanović-Šanta, S.; Karaman, M.; Dožić, S.; Tešanović, K.; Kojić, V.; Gadžurić, S. Toxicity reduction of imidazolium-based ionic liquids by the oxygenation of the alkyl substituent. RSC Adv. 2016, 6, 96289–96295. [Google Scholar] [CrossRef]
- Cho, C.-W.; Stolte, S.; Yun, Y.-S. Comprehensive approach for predicting toxicological effects of ionic liquids on several biological systems using unified descriptors. Sci. Rep. 2016, 6, 33403. [Google Scholar] [CrossRef]
- Arakelyan, L.A.; Arkhipova, D.M.; Seitkalieva, M.M.; Vavina, A.V.; Sahharova, L.T.; Kurbanalieva, S.K.; Posvyatenko, A.V.; Egorova, K.S.; Ananikov, V.P. A comprehensive dataset on cytotoxicity of ionic liquids. Sci. Data 2024, 11, 1379. [Google Scholar] [CrossRef]
- Sipes, I.G.; Knudsen, G.A.; Kuester, R.K. The Effects of Dose and Route on the Toxicokinetics and Disposition of 1-Butyl-3-methylimidazolium Chloride in Male F-344 Rats and Female B6C3F1 Mice. Drug Metab. Dispos. 2008, 36, 284–293. [Google Scholar] [CrossRef]
- Hattori, T.; Tagawa, H.; Inai, M.; Kan, T.; Kimura, S.; Itai, S.; Mitragotri, S.; Iwao, Y. Transdermal delivery of nobiletin using ionic liquids. Sci. Rep. 2019, 9, 20191. [Google Scholar] [CrossRef]
- Benedetto, A. Room-temperature ionic liquids meet bio-membranes: The state-of-the-art. Biophys. Rev. 2017, 9, 309–320. [Google Scholar] [CrossRef]
- Kumari, P.; Pillai, V.V.S.; Benedetto, A. Mechanisms of action of ionic liquids on living cells: The state of the art. Biophys. Rev. 2020, 12, 1187–1215. [Google Scholar] [CrossRef]
- Bakshi, K.; Mitra, S.; Sharma, V.K.; Jayadev, M.S.K.; Sakai, V.G.; Mukhopadhyay, R.; Gupta, A.; Ghosh, S.K. Imidazolium-based ionic liquids cause mammalian cell death due to modulated structures and dynamics of cellular membrane. Biochim. Biophys. Acta (BBA)—Biomembr. 2020, 1862, 183103. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Liu, H.; Dong, Y.; Chen, C.; Wang, Z.; Guo, J.; Du, S. Growth inhibition and oxidative stress caused by four ionic liquids in Scenedesmus obliquus: Role of cations and anions. Sci. Total Environ. 2019, 651, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Docherty, K.M.; Kulpa, C.F., Jr. Toxicity and antimicrobial activity of imidazolium and pyridinium ionic liquids. Green Chem. 2005, 7, 185. [Google Scholar] [CrossRef]
- Couling, D.J.; Bernot, R.J.; Docherty, K.M.; Dixon, J.K.; Maginn, E.J. Assessing the factors responsible for ionic liquid toxicity to aquatic organisms via quantitative structure–property relationship modeling. Green Chem. 2006, 8, 82–90. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, P.; Lei, M.; Tong, Y.; Zheng, L.; Zhang, R.; Ji, J.; Chen, Q.; Wu, Y. Investigation of the toxicity of the ionic liquid 1-butyl-3-methylimidazolium chloride to Saccharomyces cerevisiae AY93161 for lignocellulosic ethanol production. Pol. J. Chem. Technol. 2013, 15, 94–98. [Google Scholar] [CrossRef]
- Gouveia, W.; Jorge, T.F.; Martins, S.; Meireles, M.; Carolino, M.; Cruz, C.; Almeida, T.V.; Araújo, M.E.M. Toxicity of ionic liquids prepared from biomaterials. Chemosphere 2014, 104, 51–56. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Zhu, L.; Wang, J.; Wang, J.; Zhou, T. The acute toxic effects of 1-alkyl-3-methylimidazolium nitrate ionic liquids on Chlorella vulgaris and Daphnia magna. Environ. Pollut. 2017, 229, 887–895. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mikkola, J.-P. Use of Ionic Liquids in Protein and DNA Chemistry. Front. Chem. 2020, 8, 598662. [Google Scholar] [CrossRef]
- Klähn, M.; Lim, G.S.; Seduraman, A.; Wu, P. On the different roles of anions and cations in the solvation of enzymes in ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 1649–1662. [Google Scholar] [CrossRef]
- Dhiman, D.; Bisht, M.; Tavares, A.P.M.; Freire, M.G.; Venkatesu, P. Cholinium-Based Ionic Liquids as Efficient Media for Improving the Structural and Thermal Stability of Immunoglobulin G Antibodies. ACS Sustain. Chem. Eng. 2022, 10, 5404–5420. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Alquraini, A.; El Khoury, J. Scavenger receptors. Curr. Biol. 2020, 30, R790–R795. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Matsuzaki, T.; Fukuda, S.; Yoshioka, C.; Shimazaki, Y.; Takese, S.; Yamanaka, K.; Nakae, T.; Ishibashi, M.; Hamamoto, H.; et al. Ionic Liquid-Based Transcutaneous Peptide Antitumor Vaccine: Therapeutic Effect in a Mouse Tumor Model. AAPS J. 2023, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 Cells: Different Patterns of Lymphokine Secretion Lead to Different Functional Properties. Annu. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef]
- Lin, X.; Yang, Y.; Li, S.; Li, Z.; Sheng, Y.; Su, Z.; Zhang, S. Oil-in-ionic liquid nanoemulsion-based adjuvant simultaneously enhances the stability and immune responses of inactivated foot-and-mouth disease virus. Int. J. Pharm. 2022, 625, 122083. [Google Scholar] [CrossRef]
- Lin, X.; Yang, Y.; Li, S.; Song, Y.; Ma, G.; Su, Z.; Zhang, S. Unique stabilizing mechanism provided by biocompatible choline-based ionic liquids for inhibiting dissociation of inactivated foot-and-mouth disease virus particles. RSC Adv. 2019, 9, 13933–13939. [Google Scholar] [CrossRef]
- Islam, R.; Nabila, F.H.; Wakabayashi, R.; Kawaguchi, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic Liquid-Based Immunization Patch for the Transdermal Delivery of Antigens. Molecules 2024, 29, 2995. [Google Scholar] [CrossRef]
- Zhang, J. Ionic Liquid-Based Microemulsions. In Ionic Liquid-Based Surfactant Science, 1st ed.; Paul, B.K., Moulik, S.P., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 325–341. ISBN 978-1-118-83419-0. [Google Scholar] [CrossRef]
- Abujubara, H.; Bharmoria, P.; Alvarez, S.; Appiah, E.; Moth-Poulsen, K.; Sayin, V.; Tietze, A. Transmembrane peptide-loaded ionic liquid nanocarriers for targeting ErbB2-positive cancer. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Facciolà, A.; Visalli, G.; Laganà, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Perspectives. Vaccines 2022, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- USFDA. Common Ingredients in FDA-Approved Vaccines; USFDA: Silver Spring, MD, USA, 2024. [Google Scholar]
- CDC. Adjuvants and Vccines; CDC: Atlanta, GA, USA, 2024. [Google Scholar]
- HogenEsch, H.; O’Hagan, D.T.; Fox, C.B. Optimizing the utilization of aluminum adjuvants in vaccines: You might just get what you want. npj Vaccines 2018, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Vijayanand, S.; Menon, I.; Gomes, K.B.; Kale, A.; Bagwe, P.; Yacoub, S.; Uddin, M.N.; D’Souza, M.J. Adjuvanted-SARS-CoV-2 Spike Protein-Based Microparticulate Vaccine Delivered by Dissolving Microneedles Induces Humoral, Mucosal, and Cellular Immune Responses in Mice. Pharmaceuticals 2023, 16, 1131. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; O’Hagan, D.T. Recent advances in vaccine adjuvants. Pharm. Res. 2002, 19, 715–728. [Google Scholar] [CrossRef]
- Pashine, A.; Valiante, N.M.; Ulmer, J.B. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 2005, 11, S63–S68. [Google Scholar] [CrossRef]
- NIH. Vaccine Adjuvant Compendium. Available online: https://www.niaid.nih.gov/research/vaccine-adjuvant-compendium-vac (accessed on 23 February 2025).
- Harmsen, M.M.; Fijten, H.P.D.; Westra, D.F.; Dekker, A. Stabilizing effects of excipients on dissociation of intact (146S) foot-and-mouth disease virions into 12S particles during storage as oil-emulsion vaccine. Vaccine 2015, 33, 2477–2484. [Google Scholar] [CrossRef]
- Lin, X.; Su, Z.; Yang, Y.; Zhang, S. The potential of ionic liquids in biopharmaceutical engineering. Chin. J. Chem. Eng. 2021, 30, 236–243. [Google Scholar] [CrossRef]
- Goetz, M.J.; Park, K.S.; Joshi, M.; Gottlieb, A.P.; Dowling, D.J.; Mitragotri, S. An ionic liquid-based adjuvant for modulating cellular and humoral immune responses. J. Control. Release 2024, 376, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Moyer, T.J.; Kato, Y.; Abraham, W.; Chang, J.Y.H.; Kulp, D.W.; Watson, N.; Turner, H.L.; Menis, S.; Abbott, R.K.; Bhiman, J.N.; et al. Engineered immunogen binding to alum adjuvant enhances humoral immunity. Nat. Med. 2020, 26, 430–440. [Google Scholar] [CrossRef]
- Ukidve, A.; Cu, K.; Goetz, M.; Angsantikul, P.; Curreri, A.; Tanner, E.E.L.; Lahann, J.; Mitragotri, S. Ionic-Liquid-Based Safe Adjuvants. Adv. Mater. 2020, 32, 2002990. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of Action of Adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef]
- Lycke, N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Sheng, Y.; Zhang, X.; Li, Z.; Yang, Y.; Wu, J.; Su, Z.; Ma, G.; Zhang, S. Oil-in-ionic liquid nanoemulsion-based intranasal delivery system for influenza split-virus vaccine. J. Control. Release 2022, 346, 380–391. [Google Scholar] [CrossRef]
- Uddin, S.; Islam, M.d.R.; Md Moshikur, R.; Wakabayashi, R.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Transdermal Delivery of Antigenic Protein Using Ionic Liquid-Based Nanocarriers for Tumor Immunotherapy. ACS Appl. Bio Mater. 2022, 5, 2586–2597. [Google Scholar] [CrossRef]
| IONIC LIQUIDS | STRUCTURAL FORMULAS |
|---|---|
| ChoSorb—Choline, Sorbic Acid | CH3 | CH3-N+-CH2-CH2-OH | CH3 + CH3-CH=CH-CH=CH-COOH |
| ChoLa—Choline and lactic acid | CH3 | CH3-N+-CH2-CH2-OH | CH3 + OH | CH3-CH-COOH |
| ChoNic—Choline and Niacin | CH3 | CH3-N+-CH2-CH2-OH | CH3 + COO- | C5H4N |
| 1-dodecyl-3-methyl imidazolium bis(trifluoromethyl sulfonyl) amide | N / \ C12H25 CH3 \ / N | O + O O || || CF3-S-N-S-CF3 || || O O |
| EDMPC LIN-1,2-dimyristoyl-sn-glycero-3-ethyl-phosphatidylcholine, Linoleic acid | CH2-O-CO-(CH2)4CH=CHCH2CH=CH(CH2)7CH3 | CH-O-CO-(CH2)12-CH3 | CH2-O-P-O-CH2-CH3 | O− | CH2-CH2-N+(CH3)3 |
| [BMIM][Ac]-1-Butyl-3-methylimidazolium acetate | N / \ C4H9 CH3 \ / N + CH3-COO− |
| [Chol][Ac]—Choline acetate | CH3 | CH3-N+-CH2CH2OH | CH3 + CH3-COO- |
| [Chol][Prop]—Choline propionate | CH3 | CH3-N+-CH2CH2OH | CH3 + C2H5-COO− |
| NAME OF MARKETED ADJUVANT | PROPERTIES | LIMITATIONS | VACCINES | REFERENCES |
|---|---|---|---|---|
| Aluminium Hydroxide, Aluminium Phosphate |
| Possible allergic reactions and weak cellular immune response. | DTaP, Pneumococcal conjugate vaccine, HPV vaccines, haemophilus Influenza type b, hepatitis A, hepatitis B | [83,84,85,86] |
| Potassium Aluminum Sulphate |
| Replaced by aluminium hydroxide and aluminum phosphate due to poor reproducibility and poor Th1 responses. | Decavac-Td Vaccine, DT vaccine-Diptheria and tetanus vaccine. | |
| MF59 |
| Biased Th2 immune response and weak Th1 response. | Fluad | |
| AS01—Monophosphoryl Lipid A+ QS21 |
| Similar to MF59; shows weak Th1 response. | Shingrix | |
| AS04—Monophosphoryl Lipid A + aluminium salt |
| Weaker cellular immunity, especially CD8+ T cell responses. | HPV | |
| CpG 1018 |
| The addition of alum is necessary for enhancing their effect. | Heplisav-B | |
| Matrix M |
| Limited long-term data available. | Novavax adjuvanted-COVID-19 |
| IONIC LIQUID USED | ANTIGEN | SAFETY PROFILE | PROPERTIES OF IONIC LIQUID | REFERENCES |
|---|---|---|---|---|
| ChoSorb—Choline and Sorbic Acid | Ovalbumin, SARS-CoV-2 Spike protein | Used materials generally regarded as safe for IL synthesis. Low risk of toxicity due to addition of adjuvant. | Produced both cellular and humoral responses; effective in low doses. | [94] |
| ChoLa—Choline and lactic acid | Ovalbumin | Natural metabolites in human body classified as GRAS. Potential toxicity will be reported in future studies. | Potent immune response against the antigen; distributed antigen effectively. | [96] |
| ChoNic—Choline and Niacin | Influenza split virus antigen | In range of physiological salts—IC50 was 57.63 ± 5.63 mmol L−1. | Enhanced humoral response. The oil in IL used as injection adjuvant induced stronger humoral responses than MF59. | [99] |
| 1-dodecyl-3-methyl imidazolium bis(trifluoromethyl sulfonyl) amide | Ovalbumin as model antigen | Does not specifically mention toxicity, as ILs are green solvents which suggest a favorable safety profile. | Enhanced skin permeability along with increased OVA-specific serum IgG compared to control and non-IL formulation. | [6] |
| 1,2-dimyristoyl-sn-glycero-3-ethyl-phosphatidylcholine, Linoleic acid- EDMPC LIN | Ovalbumin as model antigen | Tested in animal model; no adverse effects observed. | Simulated OVA-specific tumor response suppressed tumor growth and improved survival rates. Increased cytotoxic T cells expressing CD8 antibodies in tumor microenvironment. | [100] |
| ChoNic—Choline and Niacin | Inactivated foot and mouth disease virus (iFMDV) | Has not been discussed specifically, as both compounds are naturally occurring metabolites. | Improved antigen dispersion and humoral responses when compared to unadjuvanted iFMDV. | [78] |
| ChoH2PO4 ChoCl ChoSO4 | Inactivated foot and mouth disease virus (iFMDV) | Has not been discussed specifically, considering that Choline is regarded as safe by the FDA. | ILs did not affect the immunogenicity of iFMDV antigens but have outperformed other stabilizers like BSA and sorbitol. | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkineni, S.; Rawas-Qalaji, M.; Kouzi, S.A.; Chbib, C.; Uddin, M.N. Exploring the Biological Activities of Ionic Liquids and Their Potential to Develop Novel Vaccine Adjuvants. Vaccines 2025, 13, 365. https://doi.org/10.3390/vaccines13040365
Akkineni S, Rawas-Qalaji M, Kouzi SA, Chbib C, Uddin MN. Exploring the Biological Activities of Ionic Liquids and Their Potential to Develop Novel Vaccine Adjuvants. Vaccines. 2025; 13(4):365. https://doi.org/10.3390/vaccines13040365
Chicago/Turabian StyleAkkineni, Snehitha, Mutasem Rawas-Qalaji, Samir A. Kouzi, Christiane Chbib, and Mohammad N. Uddin. 2025. "Exploring the Biological Activities of Ionic Liquids and Their Potential to Develop Novel Vaccine Adjuvants" Vaccines 13, no. 4: 365. https://doi.org/10.3390/vaccines13040365
APA StyleAkkineni, S., Rawas-Qalaji, M., Kouzi, S. A., Chbib, C., & Uddin, M. N. (2025). Exploring the Biological Activities of Ionic Liquids and Their Potential to Develop Novel Vaccine Adjuvants. Vaccines, 13(4), 365. https://doi.org/10.3390/vaccines13040365








