Frequency, Characteristics, and Predictive Factors of Adverse Drug Events in an Adult Emergency Department according to Age: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
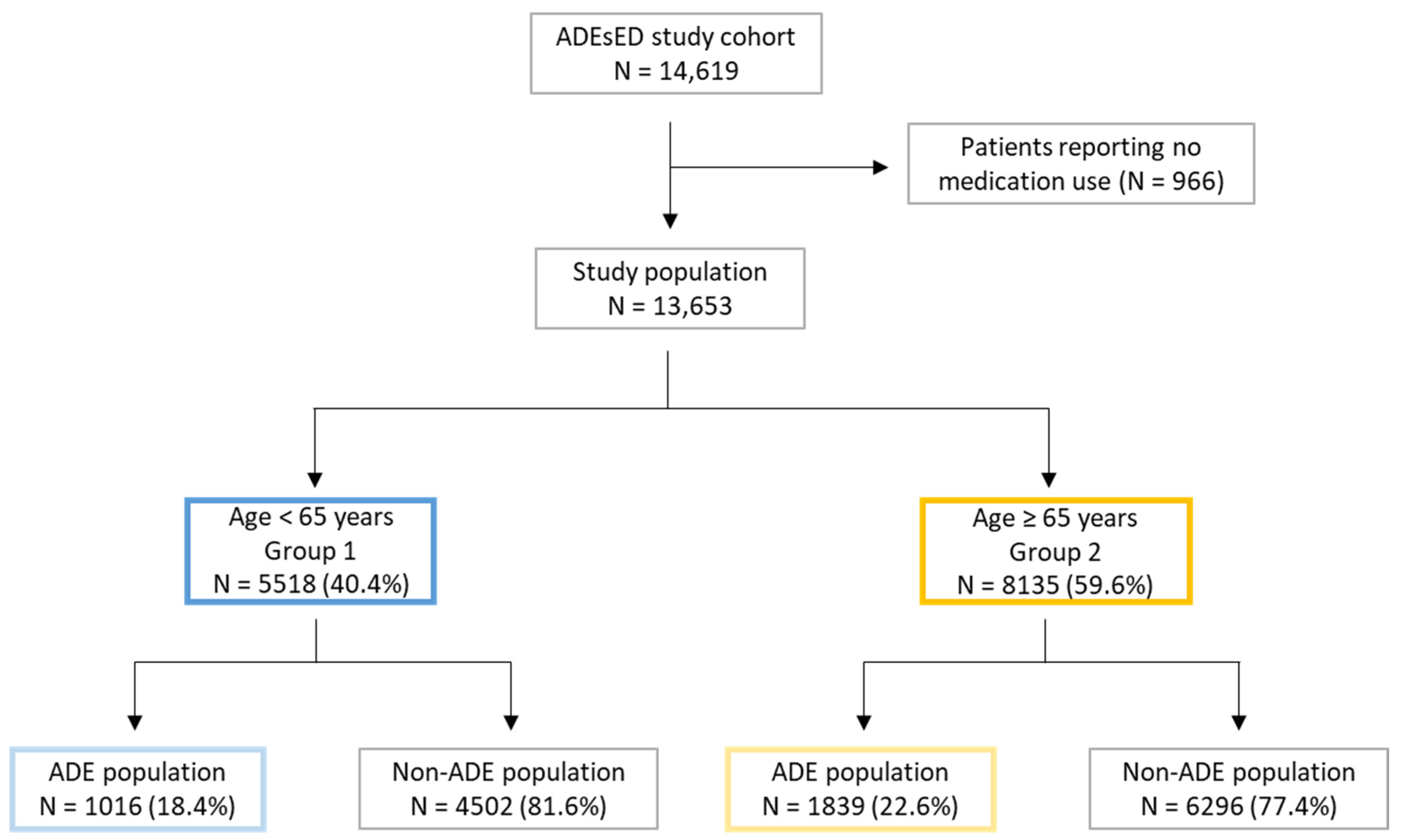
2.2. Study Population
2.3. Intervention and Measurements
2.4. Analysis
3. Results
3.1. Frequency of ADEs
3.2. Comparison of General, Clinical and Therapeutic Characteristics of ADE Patients
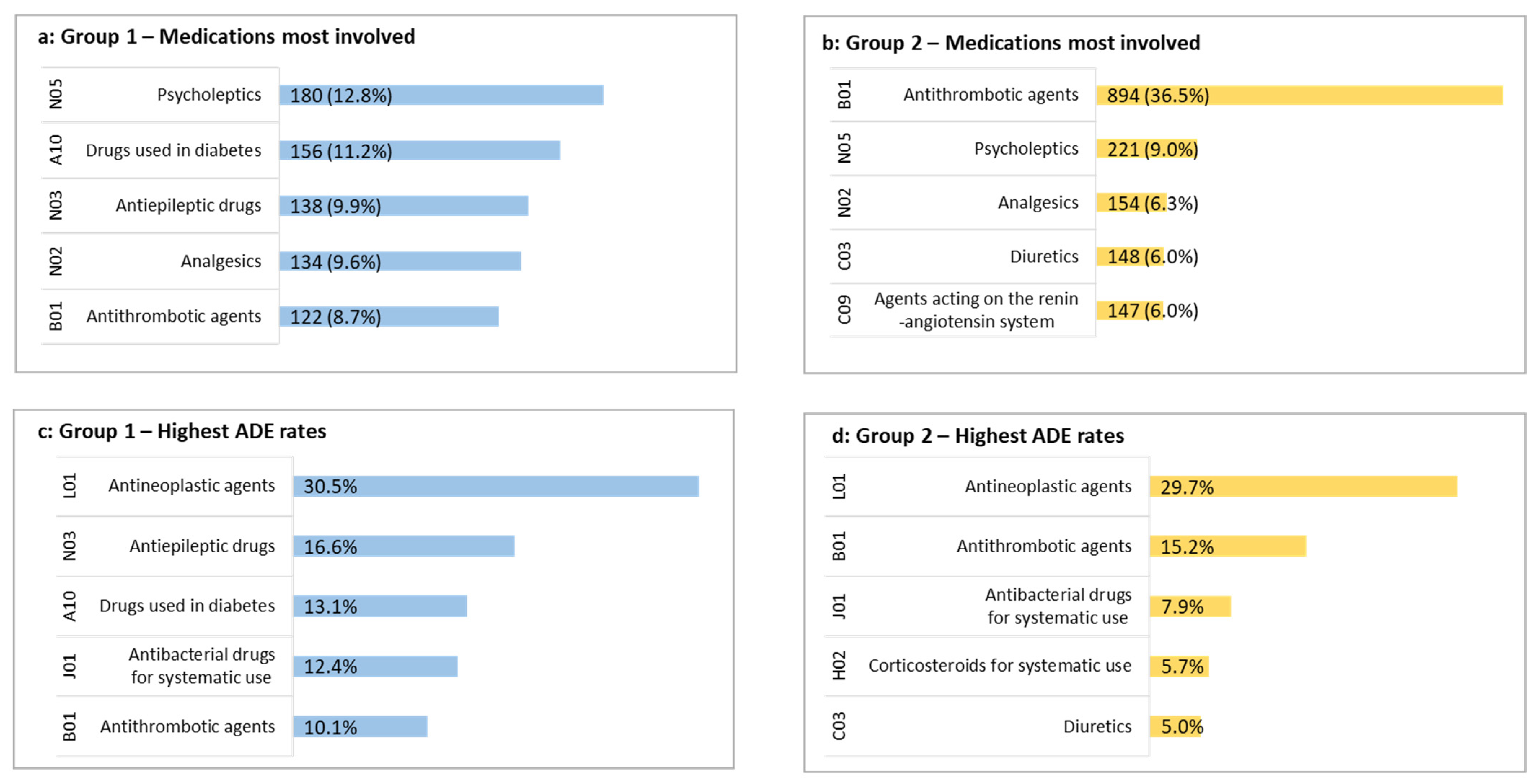
3.3. Characteristics of ADEs including Medication Involved
3.4. Predictive Factors of ADEs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayalew, M.B.; Tegegn, H.G.; Abdela, O.A. Drug related hospital admissions; A systematic review of the recent literatures. Bull. Emerg. Trauma 2019, 7, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Al Hamid, A.; Ghaleb, M.; Aljadhey, H.; Aslanpour, Z. A systematic review of hospitalization resulting from medicine-related problems in adult patients. Br. J. Clin. Pharmacol. 2014, 78, 202–217. [Google Scholar] [CrossRef]
- Patel, P.; Zed, P.J. Drug-related visits to the emergency department: How big is the problem? Pharmacotherapy 2002, 22, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Jatau, A.I.; Shitu, Z.; Khalid, G.M.; Yunusa, I.; Awaisu, A. Understanding adverse drug-related emergency department visits: Development of a conceptual model through a systematic review. Adv. Drug Saf. 2019, 10. [Google Scholar] [CrossRef]
- Hafner, J.W., Jr.; Belknap, S.M.; Squillante, M.D.; Bucheit, K.A. Adverse drug events in emergency department patients. Ann. Emerg. Med. 2002, 39, 258–267. [Google Scholar] [CrossRef]
- Queneau, P.; Bannwarth, B.; Carpentier, F.; Guliana, J.M.; Bouget, J.; Trombert, B.; Leverve, X.; Lapostolle, F.; Borron, S.W.; Adnet, F. Emergency department visits caused by adverse drug events: Results of a French survey. Drug Saf. 2007, 30, 81–88. [Google Scholar] [CrossRef]
- Zed, P.J.; Abu-Laban, R.B.; Balen, R.M.; Loewen, P.S.; Hohl, C.M.; Brubacher, J.R.; Wilbur, K.; Wiens, M.O.; Samoy, L.J.; Lacaria, K.; et al. Incidence, severity and preventability of medication-related visits to the emergency department: A prospective study. CMAJ Can. Med. Assoc. J. 2008, 178, 1563–1569. [Google Scholar] [CrossRef]
- Trifirò, G.; Calogero, G.; Ippolito, F.M.; Cosentino, M.; Giuliani, R.; Conforti, A.; Venegoni, M.; Mazzaglia, G.; Caputi, A.P. Adverse drug events in emergency department population: A prospective Italian study. Pharm. Drug Saf 2005, 14, 333–340. [Google Scholar] [CrossRef]
- Hohl, C.M.; Nosyk, B.; Kuramoto, L.; Zed, P.J.; Brubacher, J.R.; Abu-Laban, R.B.; Sheps, S.B.; Sobolev, B. Outcomes of emergency department patients presenting with adverse drug events. Ann. Emerg. Med. 2011, 58, 270–279.e274. [Google Scholar] [CrossRef]
- Classen, D.C.; Pestotnik, S.L.; Evans, R.S.; Lloyd, J.F.; Burke, J.P. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1997, 277, 301–306. [Google Scholar] [CrossRef]
- Insani, W.N.; Whittlesea, C.; Alwafi, H.; Man, K.K.C.; Chapman, S.; Wei, L. Prevalence of adverse drug reactions in the primary care setting: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0252161. [Google Scholar] [CrossRef] [PubMed]
- Falconer, N.; Barras, M.; Cottrell, N. Systematic review of predictive risk models for adverse drug events in hospitalized patients. Br. J. Clin. Pharmacol. 2018, 84, 846–864. [Google Scholar] [CrossRef] [PubMed]
- Baena, M.I.; Faus, M.J.; Fajardo, P.C.; Luque, F.M.; Sierra, F.; Martinez-Olmos, J.; Cabrera, A.; Fernandez-Llimos, F.; Martinez-Martinez, F.; Jiménez, J.; et al. Medicine-related problems resulting in emergency department visits. Eur. J. Clin. Pharm. 2006, 62, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Assiri, G.A.; Shebl, N.A.; Mahmoud, M.A.; Aloudah, N.; Grant, E.; Aljadhey, H.; Sheikh, A. What is the epidemiology of medication errors, error-related adverse events and risk factors for errors in adults managed in community care contexts? A systematic review of the international literature. BMJ Open 2018, 8, e019101. [Google Scholar] [CrossRef]
- Angamo, M.T.; Chalmers, L.; Curtain, C.M.; Bereznicki, L.R. Adverse-drug-reaction-related hospitalisations in developed and developing countries: A review of prevalence and contributing factors. Drug Saf. 2016, 39, 847–857. [Google Scholar] [CrossRef]
- Budnitz, D.S.; Pollock, D.A.; Mendelsohn, A.B.; Weidenbach, K.N.; McDonald, A.K.; Annest, J.L. Emergency department visits for outpatient adverse drug events: Demonstration for a national surveillance system. Ann. Emerg. Med. 2005, 45, 197–206. [Google Scholar] [CrossRef]
- Budnitz, D.S.; Pollock, D.A.; Weidenbach, K.N.; Mendelsohn, A.B.; Schroeder, T.J.; Annest, J.L. National surveillance of emergency department visits for outpatient adverse drug events. JAMA 2006, 296, 1858–1866. [Google Scholar] [CrossRef]
- Jhung, M.A.; Budnitz, D.S.; Mendelsohn, A.B.; Weidenbach, K.N.; Nelson, T.D.; Pollock, D.A. Evaluation and overview of the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance Project (NEISS-CADES). Med. Care 2007, 45, S96–S102. [Google Scholar] [CrossRef]
- Perrone, V.; Conti, V.; Venegoni, M.; Scotto, S.; Degli Esposti, L.; Sangiorgi, D.; Prestini, L.; Radice, S.; Clementi, E.; Vighi, G. Seriousness, preventability, and burden impact of reported adverse drug reactions in Lombardy emergency departments: A retrospective 2-year characterization. Clin. Outcomes Res. CEOR 2014, 6, 505–514. [Google Scholar] [CrossRef]
- Lombardi, N.; Crescioli, G.; Bettiol, A.; Tuccori, M.; Capuano, A.; Bonaiuti, R.; Mugelli, A.; Venegoni, M.; Vighi, G.D.; Vannacci, A. Italian emergency department visits and hospitalizations for outpatients’ adverse drug events: 12-year active pharmacovigilance surveillance (the MEREAFaPS study). Front. Pharmacol. 2020, 11, 412. [Google Scholar] [CrossRef]
- Karpov, A.; Parcero, C.; Mok, C.P.; Panditha, C.; Yu, E.; Dempster, L.; Hohl, C.M. Performance of trigger tools in identifying adverse drug events in emergency department patients: A validation study. Br. J. Clin. Pharmacol. 2016, 82, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- WHO. Medication Without Harm—Global Patient Safety Challenge on Medication Safety; World Health Organization: Geneva, Switzerland, 2017. Available online: https://www.who.int/initiatives/medication-without-harm (accessed on 13 December 2021).
- Zazzara, M.B.; Palmer, K.; Vetrano, D.L.; Carfì, A.; Graziano, O. Adverse drug reactions in older adults: A narrative review of the literature. Eur. Geriatr. Med. 2021, 12, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Motter, F.R.; Fritzen, J.S.; Hilmer, S.N.; Paniz, É.V.; Paniz, V.M.V. Potentially inappropriate medication in the elderly: A systematic review of validated explicit criteria. Eur. J. Clin. Pharm. 2018, 74, 679–700. [Google Scholar] [CrossRef]
- Alhawassi, T.M.; Krass, I.; Bajorek, B.V.; Pont, L.G. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin. Interv. Aging 2014, 9, 2079–2086. [Google Scholar] [CrossRef]
- Linkens, A.; Milosevic, V.; van der Kuy, P.H.M.; Damen-Hendriks, V.H.; Mestres Gonzalvo, C.; Hurkens, K. Medication-related hospital admissions and readmissions in older patients: An overview of literature. Int. J. Clin. Pharm. 2020, 42, 1243–1251. [Google Scholar] [CrossRef]
- Laureau, M.; Vuillot, O.; Gourhant, V.; Perier, D.; Pinzani, V.; Lohan, L.; Faucanie, M.; Macioce, V.; Marin, G.; Giraud, I.; et al. Adverse drug events detected by clinical pharmacists in an emergency department: A prospective monocentric observational study. J. Patient Saf. 2021, 17, e1040–e1049. [Google Scholar] [CrossRef] [PubMed]
- Lohan, L.; Marin, G.; Faucanie, M.; Laureau, M.; Macioce, V.; Perier, D.; Pinzani, V.; Giraud, I.; Castet-Nicolas, A.; Jalabert, A.; et al. Impact of medication characteristics and adverse drug events on hospital admission after an emergency department visit: Prospective cohort study. Int. J. Clin. Pract. 2021, 75, e14224. [Google Scholar] [CrossRef] [PubMed]
- van den Bemt, P.M.; van der Schrieck-de Loos, E.M.; van der Linden, C.; Theeuwes, A.M.; Pol, A.G.; Dutch CBO WHO High 5s Study Group. Effect of medication reconciliation on unintentional medication discrepancies in acute hospital admissions of elderly adults: A multicenter study. J. Am. Geriatr. Soc. 2013, 61, 1262–1268. [Google Scholar] [CrossRef]
- Arimone, Y.; Bidault, I.; Dutertre, J.P.; Gerardin, M.; Guy, C.; Haramburu, F.; Hillaire-Buys, D.; Meglio, C.; Penfornis, C.; Theophile, H.; et al. Updating the French method for the causality assessment of adverse drug reactions. Therapie 2013, 68, 69–76. [Google Scholar] [CrossRef]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharm. 1981, 30, 239–245. [Google Scholar] [CrossRef]
- Nebeker, J.R.; Barach, P.; Samore, M.H. Clarifying adverse drug events: A clinician’s guide to terminology, documentation, and reporting. Ann. Intern. Med. 2004, 140, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Hohl, C.M.; Dankoff, J.; Colacone, A.; Afilalo, M. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann. Emerg. Med. 2001, 38, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Asseray, N.; Ballereau, F.; Trombert-Paviot, B.; Bouget, J.; Foucher, N.; Renaud, B.; Roulet, L.; Kierzek, G.; Armand-Perroux, A.; Potel, G.; et al. Frequency and severity of adverse drug reactions due to self-medication: A cross-sectional multicentre survey in emergency departments. Drug Saf. 2013, 36, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Trotti, A.; Colevas, A.D.; Setser, A.; Rusch, V.; Jaques, D.; Budach, V.; Langer, C.; Murphy, B.; Cumberlin, R.; Coleman, C.N.; et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin. Radiat. Oncol. 2003, 13, 176–181. [Google Scholar] [CrossRef]
- WHO. Collaborating Centre for Drug Statistics Methodology. Complete ATC index. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 12 June 2021).
- Laroche, M.L.; Bouthier, F.; Merle, L.; Charmes, J.P. Médicaments potentiellement inappropriés aux personnes âgées: Intérêt d’une liste adaptée à la pratique médicale française. Rev. Médecine Interne 2009, 30, 592–601. [Google Scholar] [CrossRef]
- The American Geriatrics Society Beers Criteria® Update Expert Panel. American geriatrics society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef]
- Renom-Guiteras, A.; Meyer, G.; Thürmann, P.A. The EU(7)-PIM list: A list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur. J. Clin. Pharm. 2015, 71, 861–875. [Google Scholar] [CrossRef]
- Boustani, M.C.; Munger, S.; Maidment, I.; Fox, C. Impact of anticholinergics on the aging brain: A review and practical application. Aging Health. 2008, 4, 311–320. [Google Scholar] [CrossRef]
- Carnahan, R.M.; Lund, B.C.; Perry, P.J.; Pollock, B.G.; Culp, K.R. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: Associations with serum anticholinergic activity. J. Clin. Pharmacol. 2006, 46, 1481–1486. [Google Scholar] [CrossRef]
- Rudolph, J.L.; Salow, M.J.; Angelini, M.C.; McGlinchey, R.E. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch. Intern. Med. 2008, 168, 508–513. [Google Scholar] [CrossRef]
- Taboulet, P.; Moreira, V.; Haas, L.; Porcher, R.; Braganca, A.; Fontaine, J.P.; Poncet, M.C. Triage with the French emergency nurses classification in hospital scale: Reliability and validity. Eur. J. Emerg. Med. 2009, 16, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Hohl, C.M.; Yu, E.; Hunte, G.S.; Brubacher, J.R.; Hosseini, F.; Argent, C.P.; Chan, W.W.; Wiens, M.O.; Sheps, S.B.; Singer, J. Clinical decision rules to improve the detection of adverse drug events in emergency department patients. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2012, 19, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Hohl, C.M.; Zed, P.J.; Brubacher, J.R.; Abu-Laban, R.B.; Loewen, P.S.; Purssell, R.A. Do emergency physicians attribute drug-related emergency department visits to medication-related problems? Ann. Emerg. Med. 2010, 55, 493–502.e494. [Google Scholar] [CrossRef] [PubMed]
- Roulet, L.; Asseray, N.; Dary, M.; Chiffoleau, A.; Potel, G.; Ballereau, F. Implementing a clinical pharmacy survey of adverse drug events in a French emergency department. Int. J. Clin. Pharm. 2012, 34, 902–910. [Google Scholar] [CrossRef]
- Roulet, L.; Ballereau, F.; Hardouin, J.B.; Chiffoleau, A.; Moret, L.; Potel, G.; Asseray, N. Assessment of adverse drug event recognition by emergency physicians in a French teaching hospital. Emerg. Med. J. EMJ 2013, 30, 63–67. [Google Scholar] [CrossRef]
- Malhotra, S.; Karan, R.S.; Pandhi, P.; Jain, S. Drug related medical emergencies in the elderly: Role of adverse drug reactions and non-compliance. Postgrad. Med. J. 2001, 77, 703–707. [Google Scholar] [CrossRef][Green Version]
- Hohl, C.M.; Robitaille, C.; Lord, V.; Dankoff, J.; Colacone, A.; Pham, L.; Berard, A.; Pepin, J.; Afilalo, M. Emergency physician recognition of adverse drug-related events in elder patients presenting to an emergency department. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2005, 12, 197–205. [Google Scholar] [CrossRef]
- Tipping, B.; Kalula, S.; Badri, M. The burden and risk factors for adverse drug events in older patients--a prospective cross-sectional study. S. Afr. Med. J. 2006, 96, 1255–1259. [Google Scholar]
- Budnitz, D.S.; Lovegrove, M.C.; Shehab, N.; Richards, C.L. Emergency hospitalizations for adverse drug events in older Americans. N. Engl. J. Med. 2011, 365, 2002–2012. [Google Scholar] [CrossRef]
- Shehab, N.; Lovegrove, M.C.; Geller, A.I.; Rose, K.O.; Weidle, N.J.; Budnitz, D.S. US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA 2016, 316, 2115–2125. [Google Scholar] [CrossRef]
- Chen, Y.C.; Huang, H.H.; Fan, J.S.; Chen, M.H.; Hsu, T.F.; Yen, D.H.; Huang, M.S.; Wang, C.Y.; Huang, C.I.; Lee, C.H. Comparing characteristics of adverse drug events between older and younger adults presenting to a Taiwan emergency department. Medicine 2015, 94, e547. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.D.; Hutchison, L.C.; Li, C.; Painter, J.T.; Martin, B.C. Predictive validity of the beers and screening tool of older persons’ potentially inappropriate prescriptions (STOPP) criteria to detect adverse drug events, hospitalizations, and emergency department visits in the United States. J. Am. Geriatr. Soc. 2016, 64, 22–30. [Google Scholar] [CrossRef]
- Mazer, M.; Deroos, F.; Hollander, J.E.; McCusker, C.; Peacock, N.; Perrone, J. Medication history taking in emergency department triage is inaccurate and incomplete. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2011, 18, 102–104. [Google Scholar] [CrossRef]
- Caglar, S.; Henneman, P.L.; Blank, F.S.; Smithline, H.A.; Henneman, E.A. Emergency department medication lists are not accurate. J. Emerg. Med. 2011, 40, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulos, S.; Brown, C.A.; Felix, T.; Grampp, G.; Getz, K.A. A Survey of adverse event reporting practices among US healthcare professionals. Drug Saf. 2016, 39, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Howe, C.L. A review of the Office of Inspector General’s reports on adverse event identification and reporting. J. Healthc. Risk Manag. J. Am. Soc. Healthc. Risk Manag. 2011, 30, 48–54. [Google Scholar] [CrossRef] [PubMed]
| Study Population (n = 13,653) | Group 1 | Group 2 | Comparison of ADE Populations (Group 1 vs. Group 2) p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| ADE Population (n = 1016) | Non-ADE Population (n = 4502) | p-Value | ADE Population (n = 1839) | Non-ADE Population (n = 6296) | p-Value | |||
| Sociodemographic data | ||||||||
| Age (n = 13,653) | 66.1 (±14.1) | 43.8 (±14.1) | 43.8 (±14.1) | 0.93 | 81.9 (±16.9) | 81.0 (14.2) | <0.01 | |
| Gender (n = 13,653)—Female | 7135(52.26) | 488 (48.0) | 2301 (51.1) | 0.08 | 943 (51.3) | 3403 (54.1) | 0.04 | 0.1 |
| Lifestyle (n = 13,620) | <0.01 | 0.13 | <0.01 | |||||
| At home | 12,120 (89.0) | 940 (93.4) | 4383 (97.7) | 1515 (82.5) | 5282 (83.9) | |||
| In institution | 1500 (11.0) | 66 (6.6) | 102 (2.3) | 322 (17.5) | 1010 (16.1) | |||
| Admission data | ||||||||
| ED unit of inclusion (n = 13,635) | <0.01 | <0.01 | <0.01 | |||||
| Emergency critical care unit | 455 (3.3) | 42 (4.2) | 76 (1.7) | 120 (6.5) | 217 (3.4) | |||
| Observation emergency unit | 11,983 (87.9) | 861 (85.1) | 4233 (94.2) | 1409 (76.7) | 5480 (87.1) | |||
| Short-stay hospitalization unit | 1197 (8.8) | 109 (10.8) | 184 (4.1) | 309 (16.8) | 595 (9.5) | |||
| FRENCH Triage Scale (n = 13,528) | <0.01 | <0.01 | <0.01 | |||||
| Level 1 | 372 (2.7) | 32 (3.2) | 59 (1.3) | 108 (5.9) | 173 (2.8) | |||
| Level 2 | 1873 (13.8) | 134 (13.3) | 607 (13.7) | 245 (13.4) | 887 (14.2) | |||
| Level 3 | 7278 (53.8) | 452 (44.8) | 2246 (50.6) | 1019 (55.6) | 3561 (57.0) | |||
| Level 4 | 2668 (19.7) | 258 (25.5) | 1021 (23.0) | 289 (15.8) | 1100 (17.6) | |||
| Level 5 | 1337 (9.9) | 134 (13.3) | 508 (11.4) | 172 (9.4) | 523 (8.4) | |||
| Main reason for ED visit (n = 13,637) | ||||||||
| Bleeding | 621 (4.6) | 54 (5.3) | 108 (2.4) | <0.01 | 310 (16.9) | 149 (2.4) | <0.01 | <0.01 |
| Cardiovascular | 1584 (11.6) | 70 (6.9) | 665 (14.8) | <0.01 | 104 (5.7) | 745 (11.8) | <0.01 | 0.19 |
| Fall | 1020 (7.5) | 11 (1.1) | 58 (1.3) | 0.59 | 238 (12.9) | 713 (11.3) | 0.06 | <0.01 |
| Hepatic/gastrointestinal | 2756 (20.2) | 210 (20.7) | 1499 (33.3) | <0.01 | 185 (10.1) | 862 (13.7) | <0.01 | <0.01 |
| Malaise and fatigue | 2417 (17.7) | 143 (14.1) | 612 (13.6) | 0.69 | 353 (19.2) | 1309 (20.8) | 0.13 | <0.01 |
| Neurologic | 942 (6.9) | 167 (16.5) | 278 (6.2) | <0.01 | 156 (8.5) | 341 (5.4) | <0.01 | <0.01 |
| Respiratory | 1776 (13.0) | 58 (5.7) | 404 (9.0) | <0.01 | 177 (9.6) | 1137 (18.1) | <0.01 | <0.01 |
| Rheumatologic | 514 (3.8) | 30 (3.0) | 252 (5.6) | <0.01 | 22 (1.2) | 210 (3.3) | <0.01 | <0.01 |
| Trauma | 475 (3.5) | 21 (2.1) | 143 (3.2) | 0.06 | 56 (3.0) | 255 (4.1) | 0.05 | 0.12 |
| Others | 1532 (11.2) | 251 (24.7) | 493 (11.0) | 238 (12.9) | 566 (9.0) | <0.01 | <0.01 | |
| Outcome data | ||||||||
| Disposition (n = 13,600) | <0.01 | <0.01 | <0.01 | |||||
| Discharge | 7842 (57.7) | 680 (67.1) | 3241 (72.4) | 785 (42.7) | 3136 (50.0) | |||
| Hospitalization | 5692 (41.9) | 333 (32.8) | 1232 (27.5) | 1021 (55.6) | 3106 (49.5) | |||
| Death | 66 (0.5) | 1 (0.1) | 1 (0.0) | 31 (1.7) | 33 (0.5) | |||
| Clinical-biological data | ||||||||
| Obesity (BMI > 30 kg/m2) (n = 11,342) | 1577 (13.9) | 103 (13.8) | 601 (14.7) | 0.54 | 167 (12.6) | 707 (13.6) | 0.34 | 0.43 |
| Treated comorbidities (n = 13,653) | ||||||||
| Diabetes | 2554 (18.7) | 175 (17.2) | 455 (10.1) | <0.01 | 457 (24.9) | 1467 (23.3) | 0.17 | <0.01 |
| Cardiovascular disorder | 8386 (61.4) | 342 (33.7) | 1371 (30.5) | 0.05 | 1588 (86.4) | 5085 (80.8) | <0.01 | <0.01 |
| Active cancer | 287 (2.1) | 49 (4.8) | 78 (1.7) | <0.01 | 57 (3.1) | 103 (1.6) | <0.01 | 0.02 |
| Mental or behavioural disorder | 5995 (43.9) | 404 (39.8) | 1398 (31.1) | <0.01 | 978 (53.2) | 3215 (51.1) | 0.11 | <0.01 |
| Chronic respiratory disease | 1806 (13.2) | 77 (7.6) | 506 (11.2) | <0.01 | 238 (12.9) | 985 (15.6) | <0.01 | <0.01 |
| Kidney and hepatic function | ||||||||
| GFR (ml/min/1.73 m2) (n = 11,159) | 72.9 (±30.5) | 92.2 (±44.2) | 93.6 (±23.7) | 0.79 | 57.9 (±26.4) | 62.0 (±24.6) | <0.01 | <0.01 |
| Kidney failure (GFR < 60) (n = 11,825) | 3768 (31.9) | 101 (13.0) | 287 (7.9) | <0.01 | 859 (51.1) | 2521 (44.0) | <0.01 | <0.01 |
| Increased AST and/or ALT (n = 6758) | 1801 (26.6) | 153 (31.1) | 614 (27.0) | 0.06 | 200 (22.1) | 834 (27.0) | <0.01 | <0.01 |
| Electrolyte disorders | ||||||||
| Dysnatraemia (n = 12,172) | 2330 (19.1) | 148 (18.6) | 478 (12.5) | <0.01 | 462 (27.0) | 1242 (21.2) | <0.01 | <0.01 |
| Dyskalaemia (n = 11,603) | 2981 (25.7) | 180 (23.4) | 628 (17.2) | <0.01 | 590 (36.2) | 1583 (28.5) | <0.01 | <0.01 |
| Blood cell count disturbances | ||||||||
| Anaemia (n = 12,171) | 4115 (33.8) | 228 (28.8) | 720 (18.8) | <0.01 | 903 (52.4) | 2264 (38.8) | <0.01 | <0.01 |
| Leucocytosis (n = 12,104) | 4101 (33.9) | 270 (34.2) | 1200 (31.6) | 0.15 | 615 (35.9) | 2016 (34.8) | 0.38 | 0.4 |
| Thrombocytopenia (n = 12,117) | 1200 (9.9) | 89 (11.3) | 303 (8.0) | <0.01 | 204 (11.9) | 604 (10.4) | 0.08 | 0.66 |
| Study Population (n = 13,653) | Group 1 | Group 2 | Comparison of ADE Population (Group 1 vs. Group 2) p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| ADE Population (n = 1016) | Non-ADE Population (n = 4502) | p-Value | ADE Population (n = 1839) | Non-ADE Population (n = 6296) | p-Value | |||
| Number of treatments (n = 13,653) | 6.5 (±4.0) | 5.1 (±3.8) | 4.4 (±3.5) | <0.01 | 8.4 (±3.7) | 7.7 (±3.8) | <0.01 | <0.01 |
| Treatment management | ||||||||
| Independent management of medications (n = 13,609) | 9616 (70.7) | 892 (87.9) | 4158 (93.0) | <0.01 | 997 (54.2) | 3569 (56.8) | 0.05 | <0.01 |
| Self-medication (n = 13,653) | 3808 (27.9) | 248 (24.4) | 2078 (46.2) | <0.01 | 242 (13.2) | 1240 (19.7) | <0.01 | <0.01 |
| Compliance with treatment (n = 13,639) | 8176 (59.9) | 386 (38.1) | 2430 (54.0) | <0.01 | 1088 (59.2) | 4272 (67.9) | <0.01 | <0.01 |
| Treatment omission (n = 13,639) | 1162 (8.5) | 186 (18.3) | 432 (9.6) | <0.01 | 132 (7.2) | 412 (6.6) | 0.35 | <0.01 |
| Self-modification of treatment duration (n = 13,639) | 638 (4.7) | 132 (13.0) | 231 (5.1) | <0.01 | 93 (5.1) | 182 (2.9) | <0.01 | <0.01 |
| Self-modification of treatment dose (n = 13,639) | 760 (5.6) | 136 (13.4) | 274 (6.1) | <0.01 | 109 (5.9) | 241 (3.8) | <0.01 | <0.01 |
| Specific drug classes (n = 13,653) | ||||||||
| A02. Drugs for acid-related disorders | 5008 (36.7) | 242 (23.8) | 1104 (24.5) | 0.64 | 813 (44.2) | 2849 (45.3) | 0.43 | <0.01 |
| A10. Drugs used in diabetes | 2554 (18.7) | 175 (17.2) | 455 (10.1) | <0.01 | 457 (24.9) | 1467 (23.3) | 0.17 | <0.01 |
| B01. Antithrombotic agents | 6110 (44.8) | 208 (20.5) | 794 (17.6) | 0.03 | 1384 (75.3) | 3724 (59.1) | <0.01 | <0.01 |
| C03. Diuretics | 3056 (22.4) | 105 (10.3) | 263 (5.8) | <0.01 | 716 (38.9) | 1972 (31.3) | <0.01 | <0.01 |
| C07. Β-blocking agents | 3451 (25.3) | 146 (14.4) | 511 (11.4) | <0.01 | 731 (39.7) | 2063 (32.8) | <0.01 | <0.01 |
| C09. Agents acting on the renin-angiotensin system | 4640 (34.0) | 179 (17.6) | 705 (15.7) | 0.12 | 902 (49.0) | 2854 (45.3) | <0.01 | <0.01 |
| C10. Lipid-modifying agents | 3464 (25.4) | 136 (13.4) | 610 (13.5) | 0.89 | 629 (34.2) | 2089 (33.2) | 0.41 | <0.01 |
| H02. Corticosteroids for systemic use | 982 (7.2) | 92 (9.1) | 314 (7.0) | 0.02 | 142 (7.7) | 434 (6.9) | 0.22 | 0.21 |
| J01. Antibacterial drugs for systemic use | 1645 (12.0) | 145 (14.3) | 489 (10.9) | <0.01 | 252 (13.7) | 759 (12.1) | 0.06 | 0.67 |
| L01. Antineoplastic agents | 287 (2.1) | 49 (4.8) | 78 (1.7) | <0.01 | 57 (3.1) | 103 (1.6) | <0.01 | 0.02 |
| L04. Immunosuppressants | 217 (1.6) | 29 (2.9) | 97 (2.2) | 0.18 | 25 (1.4) | 66 (1.0) | 0.26 | <0.01 |
| M01. Anti-inflammatory and antirheumatic products | 1493 (10.9) | 146 (14.4) | 729 (16.2) | 0.15 | 139 (7.6) | 479 (7.6) | 0.94 | <0.01 |
| N02. Analgesics | 6655 (48.7) | 379 (37.3) | 2352 (52.2) | <0.01 | 840 (45.7) | 3084 (49.0) | <0.01 | <0.01 |
| N04. Anti-Parkinson drugs | 583 (4.3) | 34 (3.3) | 77 (1.7) | <0.01 | 108 (5.8) | 364 (5.8) | 0.88 | <0.01 |
| N05. Psycholeptics | 4746 (34.8) | 363 (35.7) | 1170 (26.0) | <0.01 | 767 (41.7) | 2446 (38.9) | 0.03 | <0.01 |
| N06. Psychoanaleptics | 3372 (24.7) | 185 (18.2) | 718 (15.9) | 0.08 | 572 (31.1) | 1897 (30.1) | 0.42 | <0.01 |
| Inappropriate medications (n = 13,653) | ||||||||
| According to Beers Criteria | <0.01 | <0.01 | <0.01 | |||||
| Always | 3795 (27.8) | 282 (27.8) | 912 (20.3) | 639 (34.7) | 1962 (31.2) | |||
| Conditionally | 5479 (40.1) | 384 (37.8) | 1548 (34.4) | 844 (45.9) | 2703 (42.9) | |||
| According to Laroche’s list | <0.01 | 0.03 | <0.01 | |||||
| Always | 3303 (24.2) | 298 (29.3) | 1016 (22.6) | 454 (24.7) | 1535 (24.4) | |||
| Conditionally | 2203 (16.1) | 89 (8.8) | 346 (7.7) | 437 (23.8) | 1331 (21.1) | |||
| According to PIM-EU7 list | <0.01 | <0.01 | <0.01 | |||||
| Always | 6331 (46.4) | 448 (44.1) | 1573 (34.9) | 1081 (58.8) | 3229 (51.3) | |||
| Conditionally | 3058 (22.4) | 166 (16.3) | 917 (20.4) | 409 (22.2) | 1566 (24.9) | |||
| According to at least one | ||||||||
| Always | 7745 (56.7) | 535 (52.7) | 1935 (43.0) | <0.01 | 1305 (71.0) | 3970 (63.1) | <0.01 | <0.01 |
| Always or conditionally | 10,407 (76.2) | 731 (71.9) | 2857 (63.5) | <0.01 | 1616 (87.9) | 5203 (82.6) | <0.01 | <0.01 |
| Anticholinergic agents (n = 13,653) | ||||||||
| According to ARS | 2628 (19.2) | 212 (20.9) | 720 (16.0) | <0.01 | 391 (21.3) | 1305 (20.7) | 0.62 | 0.8 |
| According to ADS | 7570 (55.4) | 590 (58.1) | 1817 (40.4) | <0.01 | 1321 (71.8) | 3842 (61.0) | <0.01 | <0.01 |
| According to ACB | 6816 (49.9) | 495 (48.7) | 1546 (34.3) | <0.01 | 1239 (67.4) | 3536 (56.2) | <0.01 | <0.01 |
| Group 1 (n = 1016) | Group 2 (n = 1839) | p-Value | |
|---|---|---|---|
| ADE Symptoms (n = 2855) | |||
| Bleeding | 93 (9.2) | 648 (35.2) | <0.01 |
| Cardiovascular disorders | 78 (7.7) | 189 (10.3) | 0.02 |
| Fall | 6 (0.6) | 56 (3.0) | <0.01 |
| Gastrointestinal disorders | 174 (17.1) | 121 (6.6) | <0.01 |
| Hematology and coagulation test abnormalities | 38 (3.7) | 165 (9.0) | <0.01 |
| Infections | 56 (5.5) | 36 (2.0) | <0.01 |
| Malaise and fatigue | 44 (4.3) | 56 (3.0) | 0.07 |
| Metabolic disorders | 129 (12.7) | 242 (13.2) | 0.72 |
| Neurologic disorders | 203 (20.0) | 181 (9.8) | <0.01 |
| Psychiatric disorders | 31 (3.1) | 14 (0.8) | <0.01 |
| Respiratory disorders | 24 (2.4) | 35 (1.9) | 0.41 |
| Skin disorders | 77 (7.6) | 31 (1.7) | <0.01 |
| Urologic or renal disorders | 24 (2.4) | 40 (2.2) | 0.75 |
| Others | 39 (3.8) | 25 (1.4) | <0.01 |
| ADE Categories (n = 2847) | |||
| Direct ADR | 478 (47.2) | 801 (43.7) | 0.07 |
| Participating ADR | 193 (19.1) | 816 (44.5) | <0.01 |
| Noncompliance with drug prescription | 342 (33.8) | 217 (11.8) | <0.01 |
| ADE Severity (n = 2855) | |||
| Spontaneous regression | 75 (7.4) | 110 (6.0) | 0.15 |
| Regression after symptomatic treatment | 590 (58.1) | 722 (39.3) | <0.01 |
| Hospitalization with no life threat | 261 (25.7) | 816 (44.4) | <0.01 |
| Hospitalization with life-threatening risk | 47 (4.6) | 111 (6.0) | 0.11 |
| Death | 1 (0.1) | 31 (1.7) | <0.01 |
| Undetermined | 42 (4.1) | 49 (2.7) | 0.03 |
| Group 1 | Group 2 | |||||
|---|---|---|---|---|---|---|
| Variables | Odds Ratio | CI 95% | p-Value | Odds Ratio | CI 95% | p-Value |
| Admission data | ||||||
| ED unit of inclusion | ||||||
| Emergency critical care or short-stay hospitalization unit (vs. Observation emergency unit) | 2.31 | 1.80–2.96 | <0.01 | 1.78 | 1.52–2.08 | <0.01 |
| FRENCH Triage Scale (vs. other levels) | ||||||
| Level 1 | 1.53 | 1.13–2.06 | <0.01 | |||
| Main reason for ED visit (vs. other reasons) | ||||||
| Respiratory | 0.43 | 0.35–0.52 | <0.01 | |||
| Hepatic/gastrointestinal | 0.79 | 0.65–0.96 | 0.02 | |||
| Cardiovascular | 0.4 | 0.33–0.52 | <0.01 | |||
| Neurologic | 2.7 | 2.10–3.47 | <0.01 | 1.48 | 1.18–1.86 | <0.01 |
| Bleeding | 2.48 | 1.71–3.60 | <0.01 | 6.43 | 5.12–8.07 | <0.01 |
| Rheumatologic | 0.27 | 0.15–0.49 | <0.01 | |||
| Clinical-biological data | ||||||
| Kidney failure (GFR <60 vs. ≥60 mL/min/1.73 m2) | 1.44 | 1.11–1.88 | <0.01 | |||
| Dysnatraemia (vs. normal) | 1.27 | 1.10–2.49 | <0.01 | |||
| Dyskalaemia (vs. normal) | 1.2 | 1.15–1.48 | <0.01 | |||
| Therapeutic data | ||||||
| Presence of specific drug classes (vs. other medication types) | ||||||
| A10. Drugs used in diabetes | 1.76 | 1.40–2.20 | <0.01 | |||
| B01. Antithrombotic agents | 1.89 | 1.64–2.17 | <0.01 | |||
| C07. Β-blocking agents | 1.2 | 1.06–1.37 | <0.01 | |||
| L01. Antineoplastic agents | 4.34 | 2.88–6.56 | <0.01 | 1.97 | 1.34–2.91 | <0.01 |
| N02. Analgesics | 0.55 | 0.46–0.65 | <0.01 | 0.83 | 0.74–0.94 | <0.01 |
| Presence of always inappropriate medications according to at least one list (yes vs. under condition and no) | 1.27 | 1.10–1.45 | <0.01 | |||
| Presence of anticholinergic medications according to ADS (vs. absence) | 1.92 | 1.63–2.29 | <0.01 | 1.46 | 1.27–1.68 | <0.01 |
| Compliance with treatment (yes vs. no) | 0.55 | 0.46–0.65 | <0.01 | 0.74 | 0.65–0.84 | <0.01 |
| Self-modification of treatment duration (yes vs. no) | 1.98 | 1.51–2.61 | <0.01 | 1.73 | 1.29–2.31 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohan, L.; Marin, G.; Faucanie, M.; Laureau, M.; Perier, D.; Pinzani, V.; Giraud, I.; Villiet, M.; Sebbane, M.; Sultan, A.; et al. Frequency, Characteristics, and Predictive Factors of Adverse Drug Events in an Adult Emergency Department according to Age: A Cross-Sectional Study. J. Clin. Med. 2022, 11, 5731. https://doi.org/10.3390/jcm11195731
Lohan L, Marin G, Faucanie M, Laureau M, Perier D, Pinzani V, Giraud I, Villiet M, Sebbane M, Sultan A, et al. Frequency, Characteristics, and Predictive Factors of Adverse Drug Events in an Adult Emergency Department according to Age: A Cross-Sectional Study. Journal of Clinical Medicine. 2022; 11(19):5731. https://doi.org/10.3390/jcm11195731
Chicago/Turabian StyleLohan, Laura, Grégory Marin, Marie Faucanie, Marion Laureau, Damien Perier, Véronique Pinzani, Isabelle Giraud, Maxime Villiet, Mustapha Sebbane, Ariane Sultan, and et al. 2022. "Frequency, Characteristics, and Predictive Factors of Adverse Drug Events in an Adult Emergency Department according to Age: A Cross-Sectional Study" Journal of Clinical Medicine 11, no. 19: 5731. https://doi.org/10.3390/jcm11195731
APA StyleLohan, L., Marin, G., Faucanie, M., Laureau, M., Perier, D., Pinzani, V., Giraud, I., Villiet, M., Sebbane, M., Sultan, A., & Breuker, C. (2022). Frequency, Characteristics, and Predictive Factors of Adverse Drug Events in an Adult Emergency Department according to Age: A Cross-Sectional Study. Journal of Clinical Medicine, 11(19), 5731. https://doi.org/10.3390/jcm11195731






