Objective Methods of Assessing Fluid Status to Optimize Volume Management in Kidney Disease and Hypertension: The Importance of Ultrasound
Abstract
:1. Introduction
2. Impact and Consequences of Volume Overload
3. Diagnosis of Volume Overload
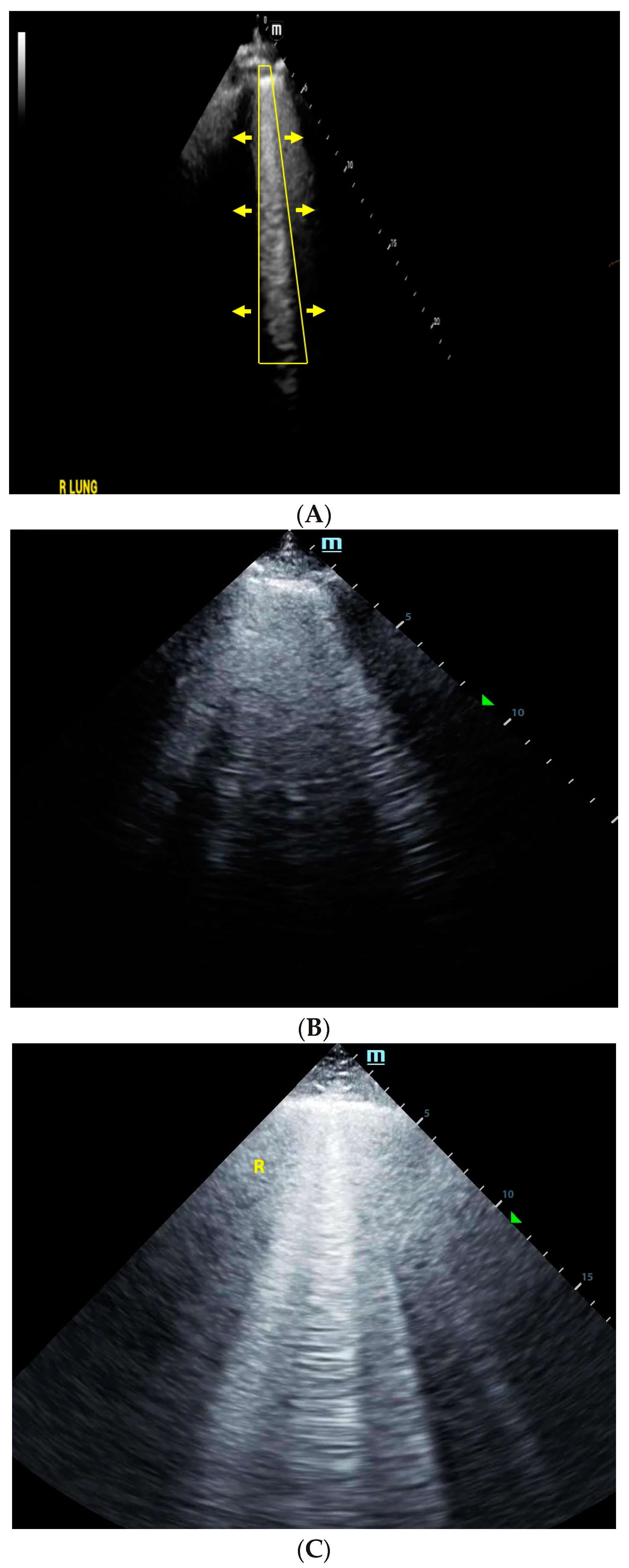
4. Lung Ultrasound
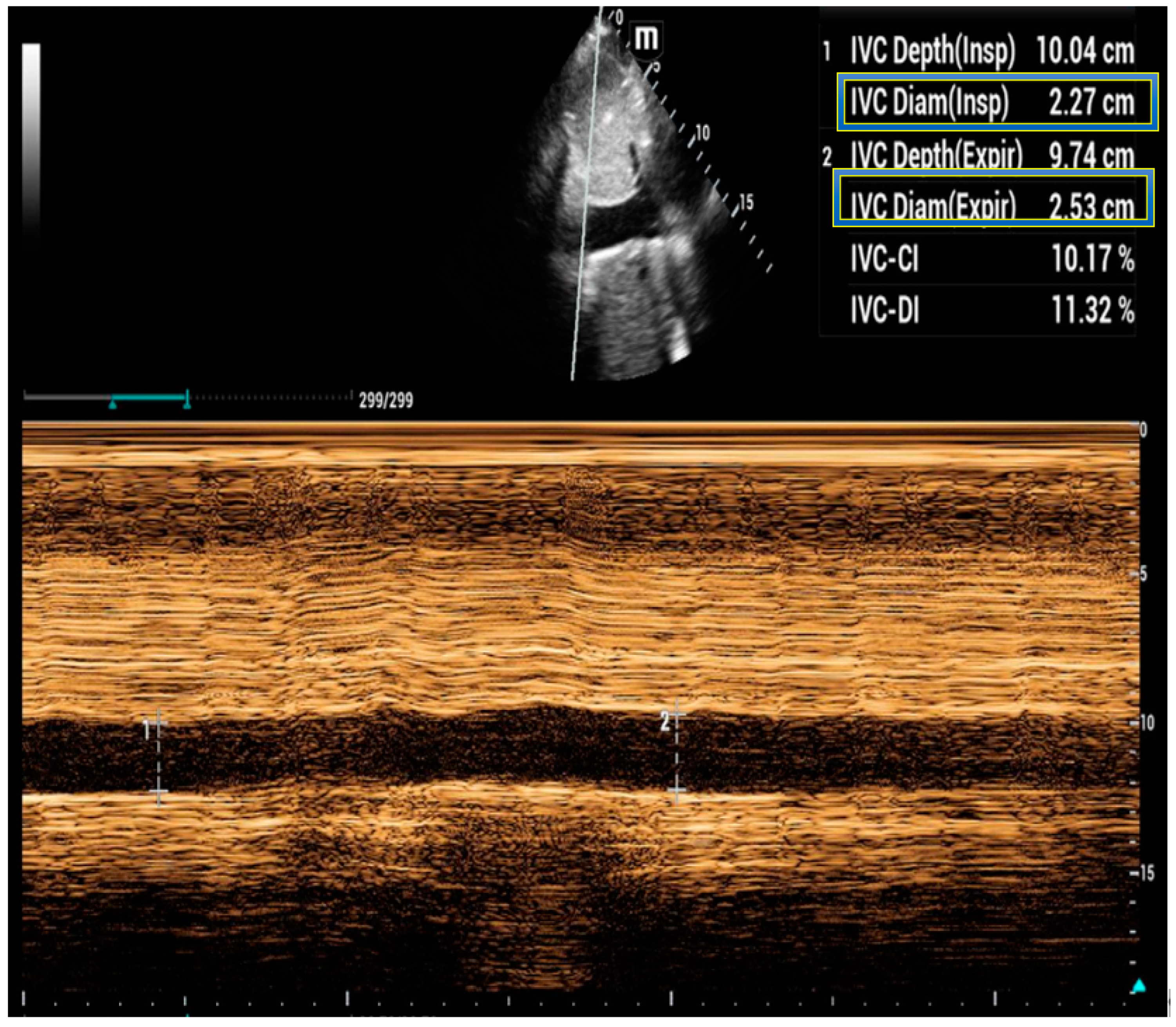
5. Inferior Vena Cava Ultrasound
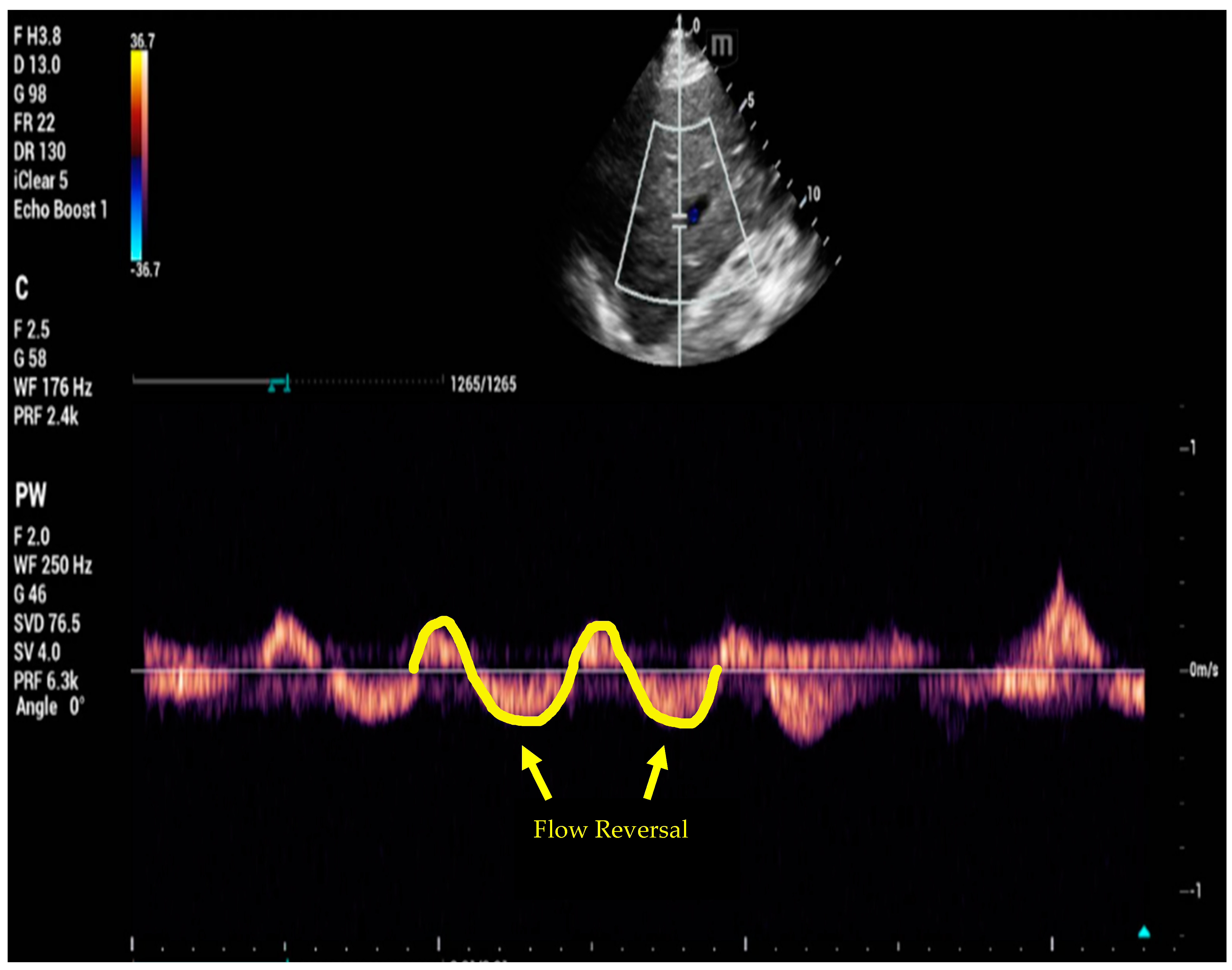
6. Venous Excess Ultrasound Score
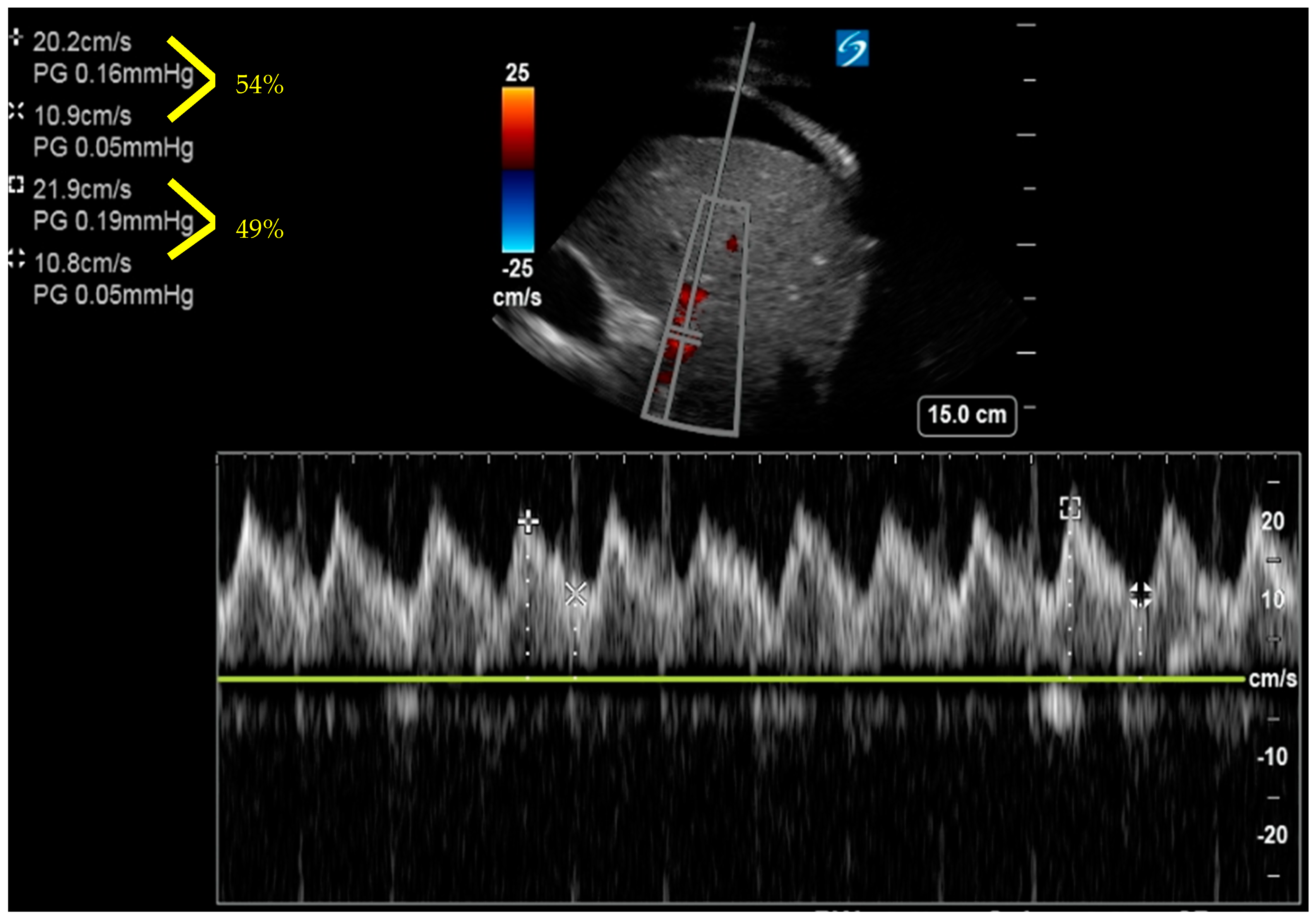
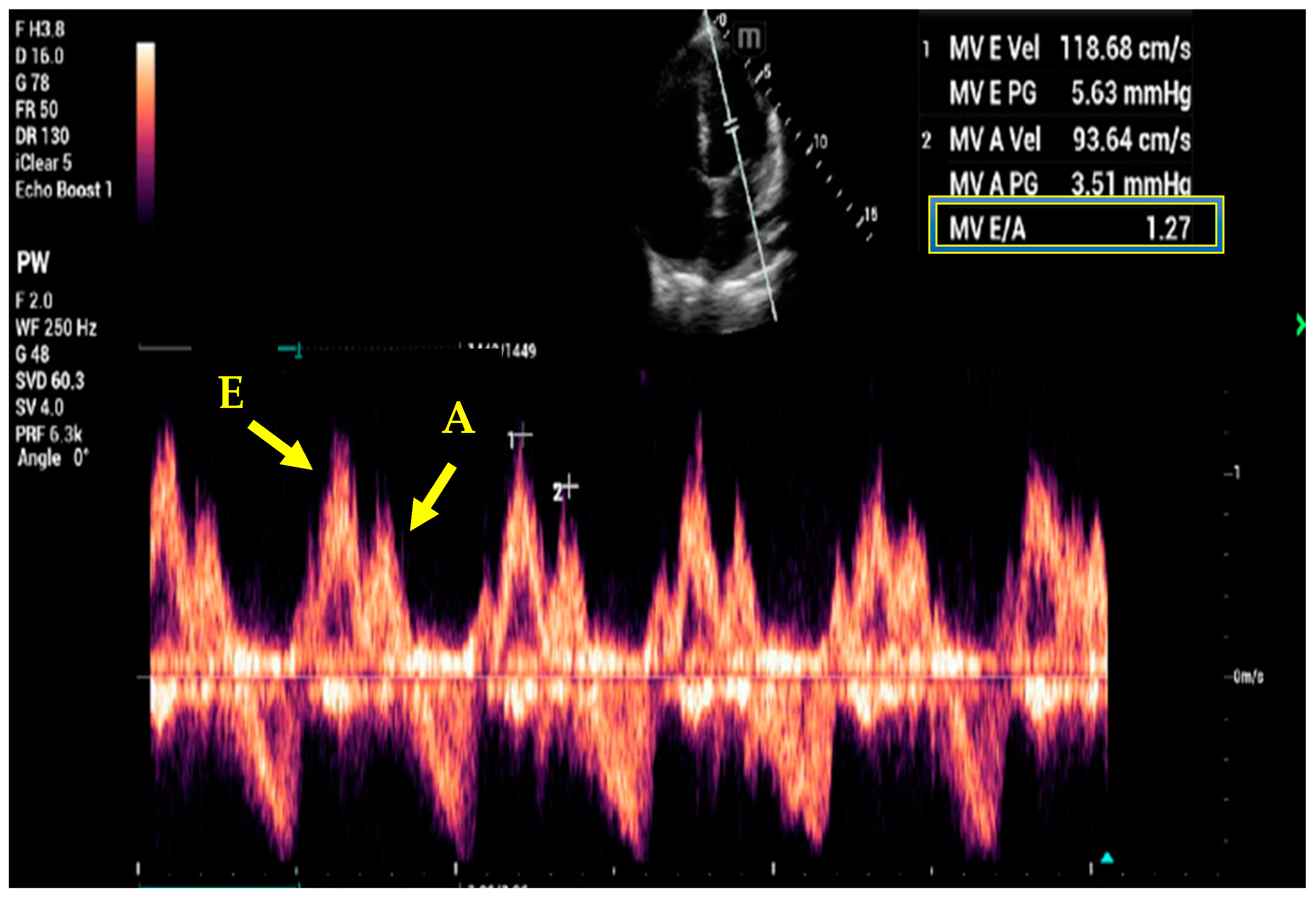
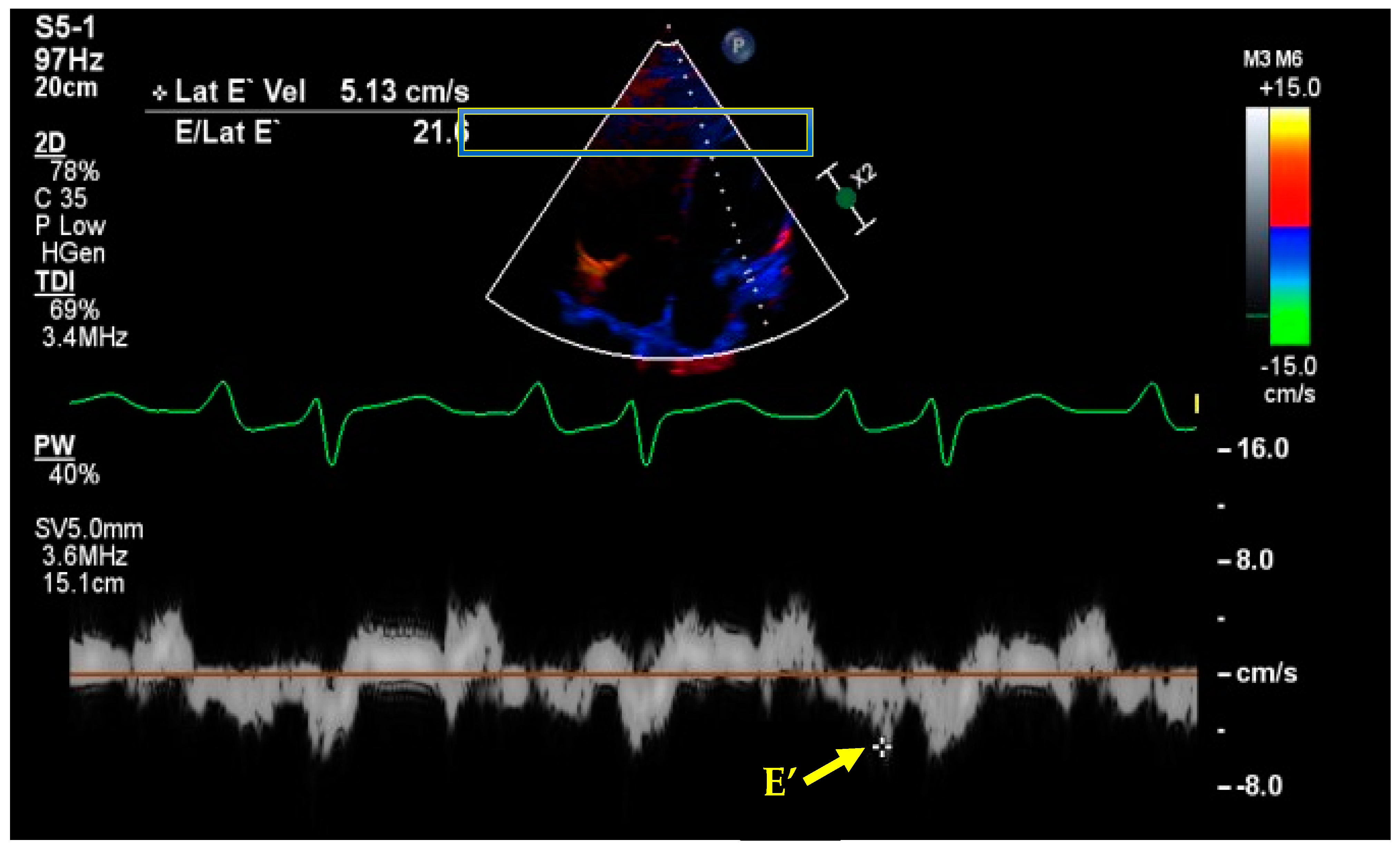
7. Basic and Advanced Echocardiography
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loutradis, C.; Sarafidis, P.A.; Ferro, C.J.; Zoccali, C. Volume overload in hemodialysis: Diagnosis, cardiovascular consequences, and management. Nephrol. Dial. Transpl. 2021, 36, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.E.; Ellison, D.H. Diuretics in States of Volume Overload: Core Curriculum 2022. Am. J. Kidney Dis. 2022, 80, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Messmer, A.S.; Zingg, C.; Muller, M.; Gerber, J.L.; Schefold, J.C.; Pfortmueller, C.A. Fluid Overload and Mortality in Adult Critical Care Patients-A Systematic Review and Meta-Analysis of Observational Studies. Crit. Care Med. 2020, 48, 1862–1870. [Google Scholar] [CrossRef]
- Saran, R.; Bragg-Gresham, J.L.; Rayner, H.C.; Goodkin, D.A.; Keen, M.L.; Van Dijk, P.C.; Kurokawa, K.; Piera, L.; Saito, A.; Fukuhara, S.; et al. Nonadherence in hemodialysis: Associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int. 2003, 64, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Shin, D.D.; Thomas, T.O.; Brandimarte, F.; Fonarow, G.C.; Abraham, W.T. Congestion is an important diagnostic and therapeutic target in heart failure. Rev. Cardiovasc. Med. 2006, 7 (Suppl. 1), S12–S24. [Google Scholar]
- Husain-Syed, F.; Grone, H.J.; Assmus, B.; Bauer, P.; Gall, H.; Seeger, W.; Ghofrani, A.; Ronco, C.; Birk, H.W. Congestive nephropathy: A neglected entity? Proposal for diagnostic criteria and future perspectives. ESC Heart Fail 2021, 8, 183–203. [Google Scholar] [CrossRef]
- Koratala, A.; Ronco, C.; Kazory, A. Diagnosis of Fluid Overload: From Conventional to Contemporary Concepts. Cardiorenal Med. 2022, 12, 141–154. [Google Scholar] [CrossRef]
- Sakka, S.G.; Klein, M.; Reinhart, K.; Meier-Hellmann, A. Prognostic value of extravascular lung water in critically ill patients. Chest 2002, 122, 2080–2086. [Google Scholar] [CrossRef]
- Veiga, D.; Luis, C.; Parente, D.; Fernandes, V.; Botelho, M.; Santos, P.; Abelha, F. Postoperative delirium in intensive care patients: Risk factors and outcome. Rev. Bras. Anestesiol. 2012, 62, 469–483. [Google Scholar] [CrossRef]
- Guazzi, M.; Gatto, P.; Giusti, G.; Pizzamiglio, F.; Previtali, I.; Vignati, C.; Arena, R. Pathophysiology of cardiorenal syndrome in decompensated heart failure: Role of lung-right heart-kidney interaction. Int. J. Cardiol. 2013, 169, 379–384. [Google Scholar] [CrossRef]
- Malbrain, M.L.; Marik, P.E.; Witters, I.; Cordemans, C.; Kirkpatrick, A.W.; Roberts, D.J.; Van Regenmortel, N. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: A systematic review with suggestions for clinical practice. Anaesthesiol. Intensive Ther. 2014, 46, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Child, D.L.; Cao, Z.; Seiberlich, L.E.; Brown, H.; Greenberg, J.; Swanson, A.; Sewall, M.R.; Robinson, S.B. The costs of fluid overload in the adult intensive care unit: Is a small-volume infusion model a proactive solution? Clin. Outcomes Res. 2015, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.H.; Carlbom, D.; Caldwell, E.; Leary, P.J.; Himmelfarb, J.; Hough, C.L. Volume Overload: Prevalence, Risk Factors, and Functional Outcome in Survivors of Septic Shock. Ann. Am. Thorac. Soc. 2015, 12, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Chiu, Y.W.; Tsai, J.C.; Kuo, H.T.; Hung, C.C.; Hwang, S.J.; Chen, T.H.; Kuo, M.C.; Chen, H.C. Association of fluid overload with cardiovascular morbidity and all-cause mortality in stages 4 and 5 CKD. Clin. J. Am. Soc. Nephrol. 2015, 10, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Tsai, J.C.; Chen, S.C.; Chiu, Y.W.; Hwang, S.J.; Hung, C.C.; Chen, T.H.; Kuo, M.C.; Chen, H.C. Association of fluid overload with kidney disease progression in advanced CKD: A prospective cohort study. Am. J. Kidney Dis. 2014, 63, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Woodward, C.W.; Lambert, J.; Ortiz-Soriano, V.; Li, Y.; Ruiz-Conejo, M.; Bissell, B.D.; Kelly, A.; Adams, P.; Yessayan, L.; Morris, P.E.; et al. Fluid Overload Associates With Major Adverse Kidney Events in Critically Ill Patients With Acute Kidney Injury Requiring Continuous Renal Replacement Therapy. Crit. Care Med. 2019, 47, e753–e760. [Google Scholar] [CrossRef]
- Brandstrup, B.; Tonnesen, H.; Beier-Holgersen, R.; Hjortso, E.; Ording, H.; Lindorff-Larsen, K.; Rasmussen, M.S.; Lanng, C.; Wallin, L.; Iversen, L.H.; et al. Effects of intravenous fluid restriction on postoperative complications: Comparison of two perioperative fluid regimens: A randomized assessor-blinded multicenter trial. Ann. Surg. 2003, 238, 641–648. [Google Scholar] [CrossRef]
- McArdle, G.T.; Price, G.; Lewis, A.; Hood, J.M.; McKinley, A.; Blair, P.H.; Harkin, D.W. Positive fluid balance is associated with complications after elective open infrarenal abdominal aortic aneurysm repair. Eur. J. Vasc. Endovasc. Surg. 2007, 34, 522–527. [Google Scholar] [CrossRef]
- Codes, L.; de Souza, Y.G.; D’Oliveira, R.A.C.; Bastos, J.L.A.; Bittencourt, P.L. Cumulative positive fluid balance is a risk factor for acute kidney injury and requirement for renal replacement therapy after liver transplantation. World J. Transpl. 2018, 8, 44–51. [Google Scholar] [CrossRef]
- Fudim, M.; Parikh, K.S.; Dunning, A.; DeVore, A.D.; Mentz, R.J.; Schulte, P.J.; Armstrong, P.W.; Ezekowitz, J.A.; Tang, W.H.W.; McMurray, J.J.V.; et al. Relation of Volume Overload to Clinical Outcomes in Acute Heart Failure (From ASCEND-HF). Am. J. Cardiol. 2018, 122, 1506–1512. [Google Scholar] [CrossRef]
- Lucas, C.; Johnson, W.; Hamilton, M.A.; Fonarow, G.C.; Woo, M.A.; Flavell, C.M.; Creaser, J.A.; Stevenson, L.W. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am. Heart J. 2000, 140, 840–847. [Google Scholar] [CrossRef]
- Wang, C.S.; FitzGerald, J.M.; Schulzer, M.; Mak, E.; Ayas, N.T. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005, 294, 1944–1956. [Google Scholar] [CrossRef]
- Badgett, R.G.; Lucey, C.R.; Mulrow, C.D. Can the clinical examination diagnose left-sided heart failure in adults? JAMA 1997, 277, 1712–1719. [Google Scholar] [CrossRef]
- Torino, C.; Gargani, L.; Sicari, R.; Letachowicz, K.; Ekart, R.; Fliser, D.; Covic, A.; Siamopoulos, K.; Stavroulopoulos, A.; Massy, Z.A.; et al. The Agreement between Auscultation and Lung Ultrasound in Hemodialysis Patients: The LUST Study. Clin. J. Am. Soc. Nephrol. 2016, 11, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Mangione, S.; Nieman, L.Z.; Gracely, E.; Kaye, D. The teaching and practice of cardiac auscultation during internal medicine and cardiology training. A nationwide survey. Ann. Intern. Med. 1993, 119, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.C.; Matchar, D.B.; Samsa, G.P.; Mulrow, C.D.; Waugh, R.A.; Feussner, J.R. Accuracy and reliability of apical S3 gallop detection. J. Gen. Intern. Med. 1995, 10, 455–457. [Google Scholar] [CrossRef]
- Daniels, L.B.; Maisel, A.S. Natriuretic peptides. J. Am. Coll. Cardiol. 2007, 50, 2357–2368. [Google Scholar] [CrossRef]
- Liquori, M.E.; Christenson, R.H.; Collinson, P.O.; Defilippi, C.R. Cardiac biomarkers in heart failure. Clin. Biochem. 2014, 47, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.S.; Krishnaswamy, P.; Nowak, R.M.; McCord, J.; Hollander, J.E.; Duc, P.; Omland, T.; Storrow, A.B.; Abraham, W.T.; Wu, A.H.; et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N. Engl. J. Med. 2002, 347, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.K.; Harrison, A.; Krishnaswamy, P.; Kazanegra, R.; Clopton, P.; Maisel, A. Utility of a rapid B-natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea. J. Am. Coll. Cardiol. 2002, 39, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L., Jr.; Camargo, C.A.; Anwaruddin, S.; Baggish, A.L.; Chen, A.A.; Krauser, D.G.; Tung, R.; Cameron, R.; Nagurney, J.T.; Chae, C.U.; et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am. J. Cardiol. 2005, 95, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Koratala, A.; Kazory, A. Natriuretic Peptides as Biomarkers for Congestive States: The Cardiorenal Divergence. Dis. Markers 2017, 2017, 1454986. [Google Scholar] [CrossRef]
- Schaub, J.A.; Coca, S.G.; Moledina, D.G.; Gentry, M.; Testani, J.M.; Parikh, C.R. Amino-Terminal Pro-B-Type Natriuretic Peptide for Diagnosis and Prognosis in Patients With Renal Dysfunction: A Systematic Review and Meta-Analysis. JACC Heart Fail 2015, 3, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Costello-Boerrigter, L.C.; Boerrigter, G.; Redfield, M.M.; Rodeheffer, R.J.; Urban, L.H.; Mahoney, D.W.; Jacobsen, S.J.; Heublein, D.M.; Burnett, J.C., Jr. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: Determinants and detection of left ventricular dysfunction. J. Am. Coll. Cardiol. 2006, 47, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.B.; Clopton, P.; Bhalla, V.; Krishnaswamy, P.; Nowak, R.M.; McCord, J.; Hollander, J.E.; Duc, P.; Omland, T.; Storrow, A.B.; et al. How obesity affects the cut-points for B-type natriuretic peptide in the diagnosis of acute heart failure. Results from the Breathing Not Properly Multinational Study. Am. Heart J. 2006, 151, 999–1005. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Omote, K.; Reddy, Y.N.V.; Sorimachi, H.; Obokata, M.; Borlaug, B.A. Heart failure with preserved ejection fraction in patients with normal natriuretic peptide levels is associated with increased morbidity and mortality. Eur. Heart J. 2022, 43, 1941–1951. [Google Scholar] [CrossRef]
- Shah, S.J. BNP: Biomarker Not Perfect in heart failure with preserved ejection fraction. Eur. Heart J. 2022, 43, 1952–1954. [Google Scholar] [CrossRef]
- Collins, S.P.; Lindsell, C.J.; Storrow, A.B.; Abraham, W.T. Adhere Scientific Advisory Committee I, Study G: Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann. Emerg. Med. 2006, 47, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Anne, L. Fuhlbrigge AMKC: Diagnostic procedures in Respiratory disease. In Harrison’s Principles of Internal Medicine, 19th ed.; Braunmald, E.F.A., Kasper, D., Hauser, S., Longo, D., Jameson, L., Eds.; McGraw-Hill: New York, NY, USA, 2015; Volume 1, pp. 1663–1664. [Google Scholar]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef]
- Rouby, J.J.; Arbelot, C.; Gao, Y.; Zhang, M.; Lv, J.; An, Y.; Chunyao, W.; Bin, D.; Valente Barbas, C.S.; Dexheimer Neto, F.L.; et al. Training for Lung Ultrasound Score Measurement in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2018, 198, 398–401. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Meziere, G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest 2008, 134, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Meziere, G.; Biderman, P.; Gepner, A.; Barre, O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am. J. Respir. Crit. Care Med. 1997, 156, 1640–1646. [Google Scholar] [CrossRef]
- Agricola, E.; Bove, T.; Oppizzi, M.; Marino, G.; Zangrillo, A.; Margonato, A.; Picano, E. “Ultrasound comet-tail images”: A marker of pulmonary edema: A comparative study with wedge pressure and extravascular lung water. Chest 2005, 127, 1690–1695. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Meziere, G.A.; Lagoueyte, J.F.; Biderman, P.; Goldstein, I.; Gepner, A. A-lines and B-lines: Lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest 2009, 136, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Noble, V.E.; Murray, A.F.; Capp, R.; Sylvia-Reardon, M.H.; Steele, D.J.R.; Liteplo, A. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest 2009, 135, 1433–1439. [Google Scholar] [CrossRef]
- Volpicelli, G.; Skurzak, S.; Boero, E.; Carpinteri, G.; Tengattini, M.; Stefanone, V.; Luberto, L.; Anile, A.; Cerutti, E.; Radeschi, G.; et al. Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology 2014, 121, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Enghard, P.; Rademacher, S.; Nee, J.; Hasper, D.; Engert, U.; Jorres, A.; Kruse, J.M. Simplified lung ultrasound protocol shows excellent prediction of extravascular lung water in ventilated intensive care patients. Crit. Care 2015, 19, 36. [Google Scholar] [CrossRef]
- Pivetta, E.; Goffi, A.; Lupia, E.; Tizzani, M.; Porrino, G.; Ferreri, E.; Volpicelli, G.; Balzaretti, P.; Banderali, A.; Iacobucci, A.; et al. Lung Ultrasound-Implemented Diagnosis of Acute Decompensated Heart Failure in the ED: A SIMEU Multicenter Study. Chest 2015, 148, 202–210. [Google Scholar] [CrossRef]
- Laursen, C.B.; Sloth, E.; Lassen, A.T.; Christensen, R.; Lambrechtsen, J.; Madsen, P.H.; Henriksen, D.P.; Davidsen, J.R.; Rasmussen, F. Point-of-care ultrasonography in patients admitted with respiratory symptoms: A single-blind, randomised controlled trial. Lancet Respir. Med. 2014, 2, 638–646. [Google Scholar] [CrossRef]
- Brennan, J.M.; Blair, J.E.; Goonewardena, S.; Ronan, A.; Shah, D.; Vasaiwala, S.; Kirkpatrick, J.N.; Spencer, K.T. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J. Am. Soc. Echocardiogr. 2007, 20, 857–861. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 16, 233–271. [Google Scholar]
- Nakao, S.; Come, P.C.; McKay, R.G.; Ransil, B.J. Effects of positional changes on inferior vena caval size and dynamics and correlations with right-sided cardiac pressure. Am. J. Cardiol. 1987, 59, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713; quiz 686–688. [Google Scholar]
- La Via, L.; Astuto, M.; Dezio, V.; Muscara, L.; Palella, S.; Zawadka, M.; Vignon, P.; Sanfilippo, F. Agreement between subcostal and transhepatic longitudinal imaging of the inferior vena cava for the evaluation of fluid responsiveness: A systematic review. J. Crit. Care 2022, 71, 154108. [Google Scholar] [CrossRef]
- Sanfilippo, F.; La Via, L.; Dezio, V.; Santonocito, C.; Amelio, P.; Genoese, G.; Astuto, M.; Noto, A. Assessment of the inferior vena cava collapsibility from subcostal and trans-hepatic imaging using both M-mode or artificial intelligence: A prospective study on healthy volunteers. Intensive Care Med. Exp. 2023, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; La Via, L.; Dezio, V.; Amelio, P.; Genoese, G.; Franchi, F.; Messina, A.; Robba, C.; Noto, A. Inferior vena cava distensibility from subcostal and trans-hepatic imaging using both M-mode or artificial intelligence: A prospective study on mechanically ventilated patients. Intensive Care Med. Exp. 2023, 11, 40. [Google Scholar] [CrossRef]
- Wallace, D.J.; Allison, M.; Stone, M.B. Inferior vena cava percentage collapse during respiration is affected by the sampling location: An ultrasound study in healthy volunteers. Acad. Emerg. Med. 2010, 17, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Feissel, M.; Michard, F.; Faller, J.P.; Teboul, J.L. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004, 30, 1834–1837. [Google Scholar] [CrossRef]
- Barbier, C.; Loubieres, Y.; Schmit, C.; Hayon, J.; Ricome, J.L.; Jardin, F.; Vieillard-Baron, A. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004, 30, 1740–1746. [Google Scholar] [CrossRef]
- Besli, F.; Kecebas, M.; Caliskan, S.; Dereli, S.; Baran, I.; Turker, Y. The utility of inferior vena cava diameter and the degree of inspiratory collapse in patients with systolic heart failure. Am. J. Emerg. Med. 2015, 33, 653–657. [Google Scholar] [CrossRef]
- Pellicori, P.; Carubelli, V.; Zhang, J.; Castiello, T.; Sherwi, N.; Clark, A.L.; Cleland, J.G. IVC diameter in patients with chronic heart failure: Relationships and prognostic significance. JACC Cardiovasc. Imaging 2013, 6, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Khandwalla, R.M.; Birkeland, K.T.; Zimmer, R.; Henry, T.D.; Nazarian, R.; Sudan, M.; Mirocha, J.; Cha, J.; Kedan, I. Usefulness of Serial Measurements of Inferior Vena Cava Diameter by Vscan(TM) to Identify Patients With Heart Failure at High Risk of Hospitalization. Am. J. Cardiol. 2017, 119, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Cubo-Romano, P.; Torres-Macho, J.; Soni, N.J.; Reyes, L.F.; Rodriguez-Almodovar, A.; Fernandez-Alonso, J.M.; Gonzalez-Davia, R.; Casas-Rojo, J.M.; Restrepo, M.I.; de Casasola, G.G. Admission inferior vena cava measurements are associated with mortality after hospitalization for acute decompensated heart failure. J. Hosp. Med. 2016, 11, 778–784. [Google Scholar] [CrossRef]
- Simonson, J.S.; Schiller, N.B. Sonospirometry: A new method for noninvasive estimation of mean right atrial pressure based on two-dimensional echographic measurements of the inferior vena cava during measured inspiration. J. Am. Coll. Cardiol. 1988, 11, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Goldhammer, E.; Mesnick, N.; Abinader, E.G.; Sagiv, M. Dilated inferior vena cava: A common echocardiographic finding in highly trained elite athletes. J. Am. Soc. Echocardiogr. 1999, 12, 988–993. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying systemic congestion with Point-Of-Care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound J. 2020, 12, 16. [Google Scholar] [CrossRef]
- Longino, A.; Martin, K.; Leyba, K.; Siegel, G.; Gill, E.; Douglas, I.S.; Burke, J. Correlation between the VExUS score and right atrial pressure: A pilot prospective observational study. Crit. Care 2023, 27, 205. [Google Scholar] [CrossRef]
- Spiegel, R.; Teeter, W.; Sullivan, S.; Tupchong, K.; Mohammed, N.; Sutherland, M.; Leibner, E.; Rola, P.; Galvagno, S.M.; Jr Murthi, S.B. The use of venous Doppler to predict adverse kidney events in a general ICU cohort. Crit. Care 2020, 24, 615. [Google Scholar] [CrossRef]
- Andrei, S.; Bahr, P.A.; Nguyen, M.; Bouhemad, B.; Guinot, P.G. Prevalence of systemic venous congestion assessed by Venous Excess Ultrasound Grading System (VExUS) and association with acute kidney injury in a general ICU cohort: A prospective multicentric study. Crit. Care 2023, 27, 224. [Google Scholar] [CrossRef]
- Argaiz, E.R.; Rola, P.; Gamba, G. Dynamic Changes in Portal Vein Flow during Decongestion in Patients with Heart Failure and Cardio-Renal Syndrome: A POCUS Case Series. Cardiorenal Med. 2021, 11, 59–66. [Google Scholar] [CrossRef]
- Rola, P.; Miralles-Aguiar, F.; Argaiz, E.; Beaubien-Souligny, W.; Haycock, K.; Karimov, T.; Dinh, V.A.; Spiegel, R. Clinical applications of the venous excess ultrasound (VExUS) score: Conceptual review and case series. Ultrasound J. 2021, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Albaroudi, B.; Haddad, M.; Albaroudi, O.; Abdel-Rahman, M.E.; Jarman, R.; Harris, T. Assessing left ventricular systolic function by emergency physician using point of care echocardiography compared to expert: Systematic review and meta-analysis. Eur. J. Emerg. Med. 2022, 29, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Kim, J.S.; Yang, J.W.; Yoo, J.S.; Choi, S.O.; Han, B.G. Association between E/e ratio and fluid overload in patients with predialysis chronic kidney disease. PLoS ONE 2017, 12, e0184764. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Middleton, K.J.; Kopelen, H.A.; Zoghbi, W.A.; Quinones, M.A. Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J. Am. Coll. Cardiol. 1997, 30, 1527–1533. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Lanspa, M.J.; Gutsche, A.R.; Wilson, E.L.; Olsen, T.D.; Hirshberg, E.L.; Knox, D.B.; Brown, S.M.; Grissom, C.K. Application of a simplified definition of diastolic function in severe sepsis and septic shock. Crit. Care 2016, 20, 243. [Google Scholar] [CrossRef]
- La Via, L.; Dezio, V.; Santonocito, C.; Astuto, M.; Morelli, A.; Huang, S.; Vieillard-Baron, A.; Sanfilippo, F. Full and simplified assessment of left ventricular diastolic function in covid-19 patients admitted to ICU: Feasibility, incidence, and association with mortality. Echocardiography 2022, 39, 1391–1400. [Google Scholar] [CrossRef]
- Denault, A.Y.; Langevin, S.; Lessard, M.R.; Courval, J.F.; Desjardins, G. Transthoracic echocardiographic evaluation of the heart and great vessels. Can. J. Anaesth. 2018, 65, 449–472. [Google Scholar] [CrossRef]
- Arbo, J.E.; Maslove, D.M.; Beraud, A.S. Bedside assessment of right atrial pressure in critically ill septic patients using tissue Doppler ultrasonography. J. Crit. Care 2013, 28, e1111–e1115. [Google Scholar] [CrossRef]
- Simioniuc, A.; Carluccio, E.; Ghio, S.; Rossi, A.; Biagioli, P.; Reboldi, G.; Galeotti, G.G.; Lu, F.; Zara, C.; Whalley, G.; et al. Echo and natriuretic peptide guided therapy improves outcome and reduces worsening renal function in systolic heart failure: An observational study of 1137 outpatients. Int. J. Cardiol. 2016, 224, 416–423. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, S.; Green, A.; Ashokumar, S.; Hoke, A.; Rachoin, J.-S. Objective Methods of Assessing Fluid Status to Optimize Volume Management in Kidney Disease and Hypertension: The Importance of Ultrasound. J. Clin. Med. 2023, 12, 6368. https://doi.org/10.3390/jcm12196368
Patel S, Green A, Ashokumar S, Hoke A, Rachoin J-S. Objective Methods of Assessing Fluid Status to Optimize Volume Management in Kidney Disease and Hypertension: The Importance of Ultrasound. Journal of Clinical Medicine. 2023; 12(19):6368. https://doi.org/10.3390/jcm12196368
Chicago/Turabian StylePatel, Sharad, Adam Green, Sandhya Ashokumar, Andrew Hoke, and Jean-Sebastien Rachoin. 2023. "Objective Methods of Assessing Fluid Status to Optimize Volume Management in Kidney Disease and Hypertension: The Importance of Ultrasound" Journal of Clinical Medicine 12, no. 19: 6368. https://doi.org/10.3390/jcm12196368
APA StylePatel, S., Green, A., Ashokumar, S., Hoke, A., & Rachoin, J.-S. (2023). Objective Methods of Assessing Fluid Status to Optimize Volume Management in Kidney Disease and Hypertension: The Importance of Ultrasound. Journal of Clinical Medicine, 12(19), 6368. https://doi.org/10.3390/jcm12196368




