Serum Pancreatic Stone Protein Reference Values in Healthy Pregnant Women: A Prospective Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Generation of Reference Values for Pregnant Women
2.3. Sample Collection and Processing
2.4. Data Analysis
2.5. Ethics Approval and Registration
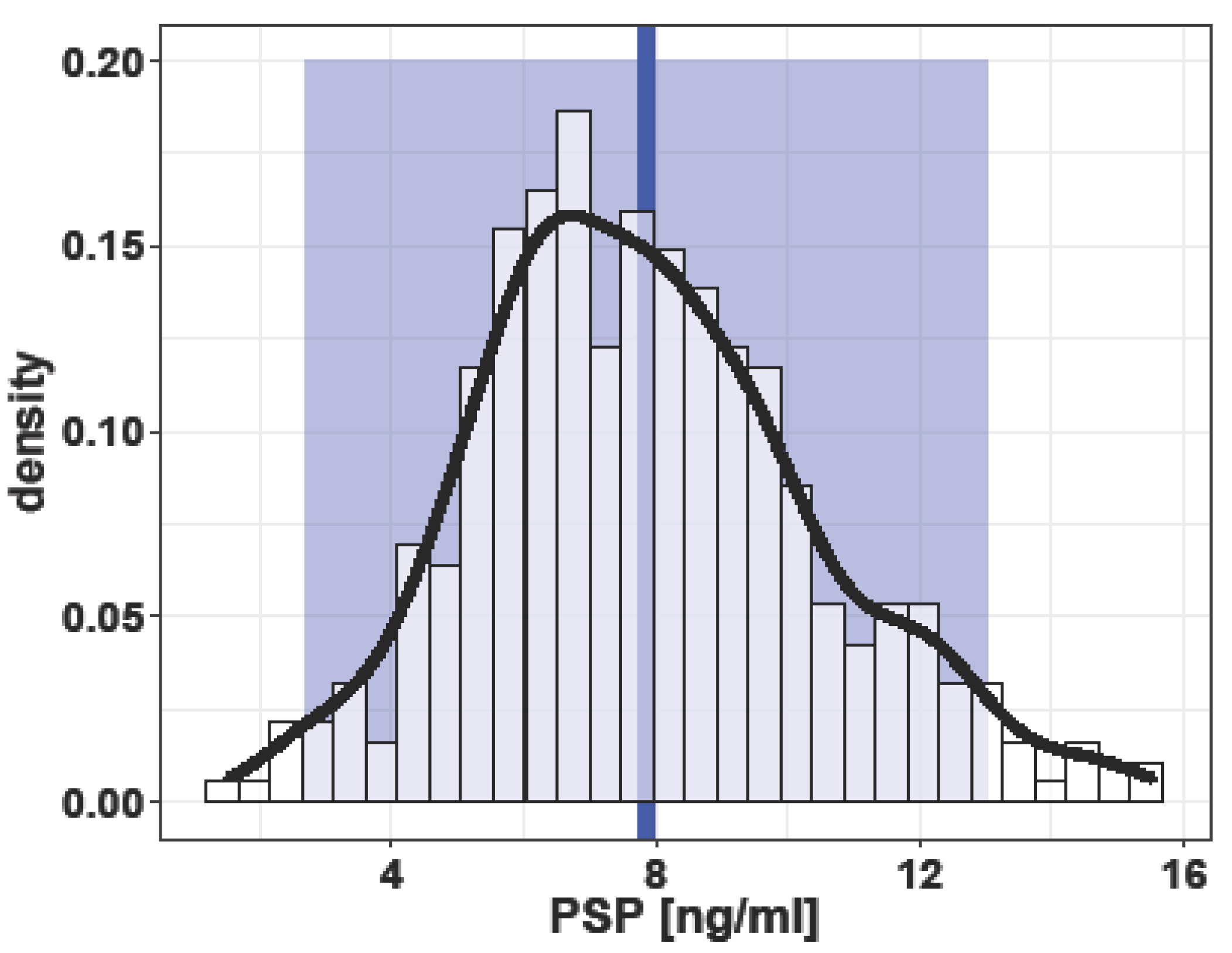
3. Results
3.1. Singleton Pregnancies
3.2. Multiple Pregnancies
4. Discussion
4.1. Main Findings
4.2. Interpretation
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIS | amniotic inflammatory syndrome |
| BMI | Body mass index |
| CRP | C-reactive protein |
| ELISA | Enzyme Linked Immunosorbent Assay |
| PE | preeclampsia |
| IL-6 | interleukin 6 |
| IQR | Interquartile range |
| PCT | procalcitonin |
| PSP | Pancreatic stone protein |
| SD | Standard deviation |
| SNCTP | Swiss National Clinical Trials Portal |
References
- Keel, M.; Härter, L.; Reding, T.; Sun, L.K.; Hersberger, M.; Seifert, B.; Bimmler, D.; Graf, R. Pancreatic stone protein is highly increased during posttraumatic sepsis and activates neutrophil granulocytes. Crit. Care Med. 2009, 37, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.J.; Niggemann, P.; Buehler, P.K.; Lehner, F.; Schweizer, R.; Rittirsch, D.; Fuchs, N.; Waldner, M.; Steiger, P.; Giovanoli, P.; et al. Pancreatic Stone Protein Predicts Sepsis in Severely Burned Patients Irrespective of Trauma Severity: A Monocentric Observational Study. Ann. Surg. 2020, 276, e1179–e1186. [Google Scholar] [CrossRef] [PubMed]
- Scherr, A.; Graf, R.; Bain, M.; Christ-Crain, M.; Müller, B.; Tamm, M.; Stolz, D. Pancreatic stone protein predicts positive sputum bacteriology in exacerbations of COPD. Chest 2013, 143, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Llewelyn, M.J.; Berger, M.; Gregory, M.; Ramaiah, R.; Taylor, A.L.; Curdt, I.; Lajaunias, F.; Graf, R.; Blincko, S.J.; Drage, S.; et al. Sepsis biomarkers in unselected patients on admission to intensive or high-dependency care. Crit. Care 2013, 17, R60. [Google Scholar] [CrossRef] [PubMed]
- García de Guadiana-Romualdo, L.; Albaladejo-Otón, M.D.; Berger, M.; Jiménez-Santos, E.; Jiménez-Sánchez, R.; Esteban-Torrella, P.; Rebollo-Acebes, S.; Hernando-Holgado, A.; Ortín-Freire, A.; Trujillo-Santos, J. Prognostic performance of pancreatic stone protein in critically ill patients with sepsis. Biomark. Med. 2019, 13, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Gukasjan, R.; Raptis, D.A.; Schulz, H.U.; Halangk, W.; Graf, R. Pancreatic stone protein predicts outcome in patients with peritonitis in the ICU. Crit. Care Med. 2013, 41, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Eggimann, P.; Que, Y.A.; Rebeaud, F. Measurement of pancreatic stone protein in the identification and management of sepsis. Biomark. Med. 2019, 13, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Pugin, J.; Daix, T.; Pagani, J.L.; Morri, D.; Giacomucci, A.; Dequin, P.F.; Guitton, C.; Que, Y.A.; Zani, G.; Brealey, D.; et al. Serial measurement of pancreatic stone protein for the early detection of sepsis in intensive care unit patients: A prospective multicentric study. Crit. Care 2021, 25, 151. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.J.; Giannoni, E.; Wellmann, S.; Stocker, M.; Ammann, R.A.; Graf, R. Normal values for pancreatic stone protein in different age groups. BMC Anesthesiol. 2015, 15, 168. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.J.; Graf, R.; Woerner, A.; Fontana, M.; Zimmermann-Baer, U.; Glauser, D.; Giannoni, E.; Roger, T.; Müller, C.; Nelle, M.; et al. Pancreatic stone protein as a novel marker for neonatal sepsis. Intensive Care Med. 2013, 39, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Dong, B.; Reding, T.; Peng, Y.; Lin, H.; Zhi, M.; Han, M.; Graf, R.; Li, L. Association of Serum PSP/REG Ialpha with Renal Function in Pregnant Women. Biomed. Res. Int. 2019, 2019, 6970890. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, L.; Raptis, D.; Li, X.; Li, F.; Chen, B.; He, J.; Graf, R.; Sun, Z. Pancreatic stone protein/regenerating protein (PSP/reg): A novel secreted protein up-regulated in type 2 diabetes mellitus. Endocrine 2015, 48, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Prazak, J.; Irincheeva, I.; Llewelyn, M.J.; Stolz, D.; García de Guadiana Romualdo, L.; Graf, R.; Reding, T.; Klein, H.J.; Eggimann, P.; Que, Y.A. Accuracy of pancreatic stone protein for the diagnosis of infection in hospitalized adults: A systematic review and individual patient level meta-analysis. Crit. Care 2021, 25, 182. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, A.P.; Read, J.S.; Jamieson, D.J. Pregnancy and infection. N. Engl. J. Med. 2014, 370, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jia, D.; Graf, R.; Yang, J. Elevated serum level of pancreatic stone protein/regenerating protein (PSP/reg) is observed in diabetic kidney disease. Oncotarget 2017, 8, 38145–38151. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.C.; Pickinpaugh, J. Physiologic changes in pregnancy. Surg. Clin. N. Am. 2008, 88, 391–401, vii. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Lafayette, R.A. Renal physiology of pregnancy. Adv. Chronic. Kidney Dis. 2013, 20, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Sobajima, H.; Niwa, T.; Shikano, M.; Naruse, S.; Kitagawa, M.; Nakae, Y.; Ishiguro, H.; Kondo, T.; Hayakawa, T. Urinary excretion of pancreatic stone protein in diabetic nephropathy. Intern. Med. 1998, 37, 500–503. [Google Scholar] [CrossRef] [PubMed]



| Singleton Pregnancy (N = 390) | Multiple Pregnancy (N = 47) | p-Value | Total (N = 440) | |
|---|---|---|---|---|
| PSP | ||||
| Mean ± SD [ng/m] | 7.86 ± 2.59 | 9.17 ± 3.06 | 0.001 | 8.26 ± 4.13 |
| Maternal Age | ||||
| Mean ± SD [years] | 32.9 ± 5.29 | 35.1 ± 4.17 | 0.001 | 33.1 ± 5.22 |
| Parity | ||||
| Mean ± SD | 2.12 ± 1.34 | 2.32 ± 1.32 | 0.33 | 2.14 ± 1.34 |
| BMI | ||||
| Mean ± SD [kg/m2] | 23.8 ± 5.19 | 24.2 ± 4.87 | 0.59 | 23.8 ± 5.15 |
| Ethnicity [N (%)] | 0.42 | |||
| Afro-Caribbean | 17 (4.4%) | 2 (4.3%) | 19 (4.3%) | |
| Asian | 23 (5.9%) | 1 (2.1%) | 24 (5.5%) | |
| Caucasian | 283 (72.1%) | 40 (85.1%) | 323 (73.4%) | |
| Mediterranean | 38 (9.7%) | 4 (8.5%) | 42 (9.5%) | |
| Mixed | 7 (1.8%) | 0 (0.0%) | 7 (1.6%) | |
| Oriental | 24 (6.2%) | 0 (0.0%) | 24 (5.5%) |
| Comorbidities | Singleton Pregancy [N (%)] | PSP Values Mean ± SD [ng/m] | p-Value |
|---|---|---|---|
| Thyroid disorders | |||
| Hypothyroidism | 18 (4.6%) | 7.10 ± 2.51 | 0.43 |
| Hyperthyroidism | 3 (0.8%) | 10.45 ± 3.41 | 0.20 |
| no | 369 (94.6%) | 7.87 ± 2.57 | |
| Gestational diabetes | |||
| Diet | 64 (16.3%) | 6.76 ± 1.91 | 0.99 |
| Insulin therapy | 8 (2.1%) | 7.84 ± 2.78 | 0.44 |
| no | 318 (81.5%) | 7.89 ± 2.56 | |
| Cardiac disease | |||
| yes | 13 (3.3%) | 8.38 ± 1.81 | 0.45 |
| no | 377 (96.7%) | 7.84 ± 2.61 | |
| Rheumatologic disease | |||
| yes | 8 (2.1%) | 7.31 ± 2.06 | 0.55 |
| no | 382 | 7.87 ± 2.60 | |
| Hematologic disease | |||
| yes | 13 (3.3%) | 8.10 ± 2.40 | 0.73 |
| no | 377 (96.7%) | 7.85 ± 2.59 | |
| Nicotine Abuse | |||
| yes | 13 (3.3%) | 8.10 ± 2.40 | 0.73 |
| no | 377 (96.7%) | 7.85 ± 2.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vonzun, L.; Brun, R.; Gadient-Limani, N.; Schneider, M.A.; Reding, T.; Graf, R.; Limani, P.; Ochsenbein-Kölble, N. Serum Pancreatic Stone Protein Reference Values in Healthy Pregnant Women: A Prospective Cohort Study. J. Clin. Med. 2023, 12, 3200. https://doi.org/10.3390/jcm12093200
Vonzun L, Brun R, Gadient-Limani N, Schneider MA, Reding T, Graf R, Limani P, Ochsenbein-Kölble N. Serum Pancreatic Stone Protein Reference Values in Healthy Pregnant Women: A Prospective Cohort Study. Journal of Clinical Medicine. 2023; 12(9):3200. https://doi.org/10.3390/jcm12093200
Chicago/Turabian StyleVonzun, Ladina, Romana Brun, Nora Gadient-Limani, Marcel André Schneider, Theresia Reding, Rolf Graf, Perparim Limani, and Nicole Ochsenbein-Kölble. 2023. "Serum Pancreatic Stone Protein Reference Values in Healthy Pregnant Women: A Prospective Cohort Study" Journal of Clinical Medicine 12, no. 9: 3200. https://doi.org/10.3390/jcm12093200






