Association of Obesity and Severe Asthma in Adults
Abstract
1. Introduction
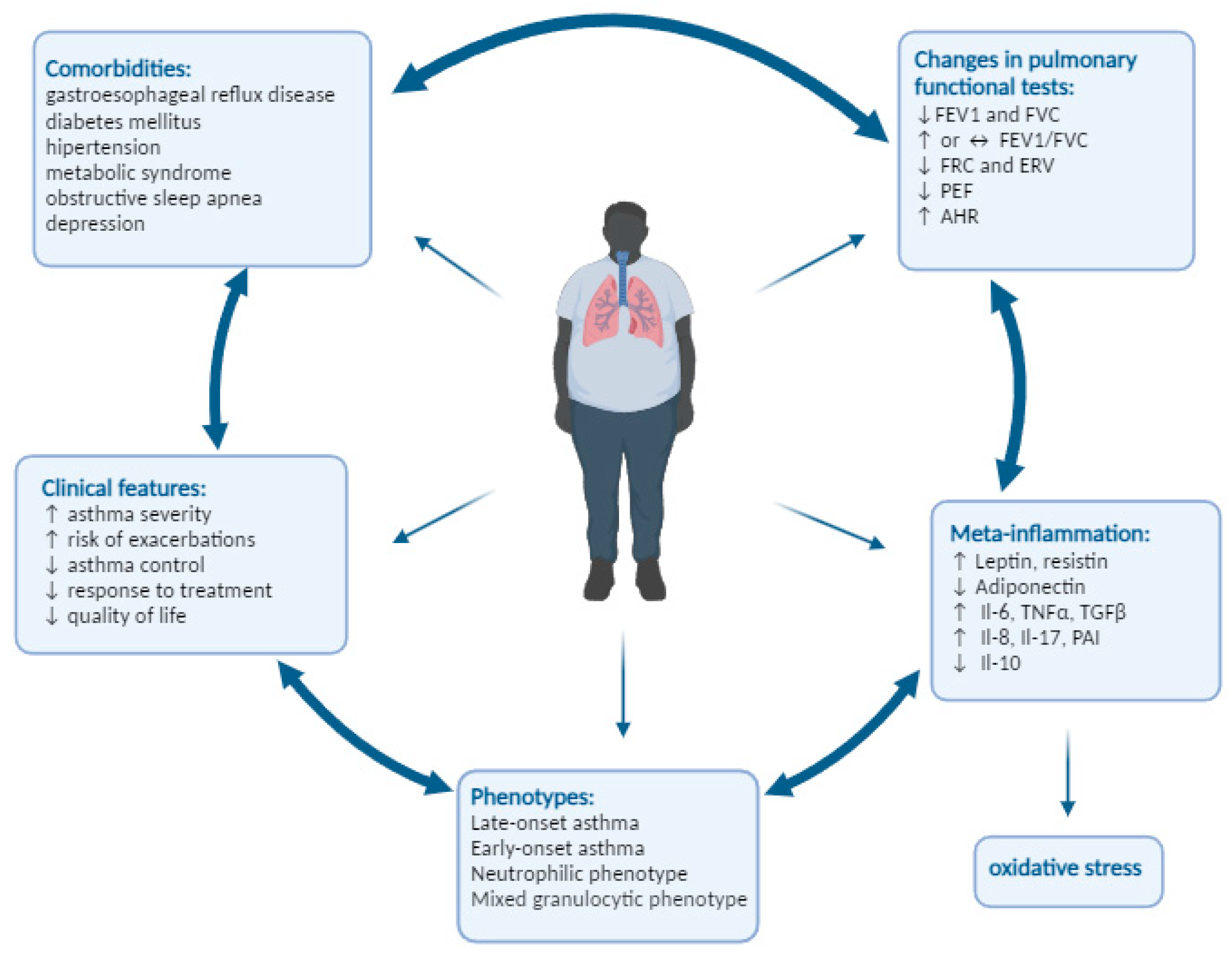
2. The Classifications Connected with Asthma and Obesity
3. Obesity-Related Changes in Pulmonary Functional Tests
4. Obesity and Inflammation
5. Clinical Characteristics of Asthma in Obesity
5.1. Endotypes and Phenotypes
5.1.1. Late-Onset Asthma (LOA)
5.1.2. Early-Onset Asthma (EOA)
5.1.3. Neutrophilic Phenotype of Severe Asthma
5.1.4. Mixed Granulocytic Asthma
5.2. Comorbidities
5.2.1. Chronic Rhinosinusitis and Nasal Polypus
5.2.2. Obstructive Sleep Apnea (OSA)
5.2.3. Gastroesophageal Reflux Disease (GERD)
5.2.4. Diabetes Mellitus Type 2 and Metabolic Syndrome
6. Influence of Obesity on Asthma Management
6.1. Montelukast
6.2. ICS and LABA/LAMA
6.3. Biological Treatment
7. Other Approaches to Severe Asthma Management
7.1. Macrolide Antibiotics
7.2. Roflumilast
7.3. Bronchial Thermoplasty
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| ACD | asthma control days |
| ACT | Asthma Control Test |
| ACQ | Asthma Control Questionnaire |
| AdipoR1 | adiponectin receptor 1 |
| AdipoR2 | adiponectin receptor 2 |
| AER | annualized rate of exacerbations |
| AMPK | 5’AMP-activated protein kinase |
| anti-IL-4Rα | interleukin-4 receptor subunit α |
| ASM | airway smooth muscle |
| ATDCs | adipose tissue-derived dendritic cells |
| AQLQ | Asthma Quality of Life Questionnaire |
| AHR | airway hyperresponsivity |
| BAL | bronchoalveolar lavage |
| BMI | body mass index |
| BT | bronchial thermoplasty |
| cAMP | cyclic adenosine monophosphate |
| CD4 | glycoprotein cluster of differentiation 4 |
| CPAP | continuous positive airway pressure |
| CRS | chronic rhinosinusitis |
| CRTH2 | chemoattractant-receptor homologous molecule |
| CXCR2 | chemokine 8 receptor-2 |
| DM | diabetes mellitus |
| DMt2 | diabetes mellitus type 2 |
| ED | emergency department |
| EOA | early-onset asthma |
| ERV | expiratory reserve volume |
| FeNO | fractional exhaled nitric oxide |
| FEV1 | forced expiratory volume in one second |
| FP/SAL | fluticasone propionate/salmeterol |
| FRC | functional residual capacity |
| FVC | forced vital capacity |
| GCS | glucocorticosteroid |
| GERD | gastroesophageal reflux disease |
| GINA | Global Initiative for Asthma |
| GLP-1 | glucagon-like peptide-1 |
| GLP-1R | glucagon-like peptide-1 receptor |
| GLUT | glucose transport proteins |
| GR-α | glucocorticoid-alpha receptor |
| GR-β | glucocorticoid-beta receptor |
| Hb1AC | glycated hemoglobin |
| HDL | high-density lipoprotein |
| HRCT | high-resolution computer tomography |
| ICS | inhaled corticosteroid |
| ICU | intensive unit care |
| IgE | immunoglobulin E |
| IL | interleukin |
| LABA | long-acting beta-2 agonists |
| LAMA | long-acting antimuscarinic |
| LOA | late-onset asthma |
| M1, M2 | macrophage subpopulations 1 and 2 |
| MAP | mitogen-activated protein |
| MCP-1 | monocyte chemoattractant protein-1 |
| MetS | metabolic syndrome |
| MUC5B | mucin 5B protein |
| NIH | National Institutes of Health |
| NK | natural killer cell |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | inflammasome of nucleotide oligomerization domain-like receptor protein 3 |
| OCS | oral corticosteroid |
| OR | odds ratio |
| OSA | obstructive sleep apnea |
| PAI-1 | plasminogen activator inhibitor-1 |
| PBMC | peripheral blood mononuclear cells |
| PEF | peak expiratory flow |
| PGA | paucigranulocytic asthma |
| PPI | proton pump inhibitor |
| ROS | reactive oxygen species |
| RV | residual volume |
| SABA | short-acting beta-agonists |
| sHDL | serum high-density lipoprotein |
| SPT | skin prick test |
| ST2 receptor | interleukin 1 receptor-like 2 |
| Tc | cytotoxic T cell |
| TG | triglyceride |
| TGF-β1 | transforming growth factor β |
| Th | T-helper cell |
| TLC | total lung capacity |
| TLR | toll-like receptor |
| TNF-α | tumor necrosis factor α |
| tPA | tissue plasminogen activator (tPA) and urokinase (uPA) |
| Treg | T regulatory cell |
| TSLP | thymic stromal lymphopoietin |
| uPA | urokinase plasminogen activator |
| VC | vital capacity |
| WHO | World Health Organization |
References
- Global Initiative for Asthma. Global Strategy for Asthma Managment and Prevention. 2024. Updated May 2024. Available online: https://ginasthma.org/ (accessed on 7 May 2024).
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Hansen, S.; von Bülow, A.; Sandin, P.; Ernstsson, O.; Janson, C.; Lehtimäki, L.; Kankaanranta, H.; Ulrik, C.; Aarli, B.B.; Fues Wahl, H.; et al. Prevalence and Management of Severe Asthma in the Nordic Countries: Findings from the NORDSTAR Cohort. ERJ Open Res. 2023, 9, 00687-2022. [Google Scholar] [CrossRef] [PubMed]
- von Bülow, A.; Kriegbaum, M.; Backer, V.; Porsbjerg, C. The Prevalence of Severe Asthma and Low Asthma Control Among Danish Adults. J. Allergy Clin. Immunol. Pract. 2014, 2, 759–767.e2. [Google Scholar] [CrossRef]
- Hekking, P.-P.W.; Wener, R.R.; Amelink, M.; Zwinderman, A.H.; Bouvy, M.L.; Bel, E.H. The Prevalence of Severe Refractory Asthma. J. Allergy Clin. Immunol. 2015, 135, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Ställberg, B.; Lisspers, K.; Telg, G.; Johansson, G.; Thuresson, M.; Janson, C. Prevalence and Management of Severe Asthma in Primary Care: An Observational Cohort Study in Sweden (PACEHR). Respir. Res. 2018, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Sadatsafavi, M.; Lynd, L.; Marra, C.; Carleton, B.; Tan, W.C.; Sullivan, S.; FitzGerald, J.M. Direct Health Care Costs Associated with Asthma in British Columbia. Can. Respir. J. 2010, 17, 74–80. [Google Scholar] [CrossRef]
- Dixon, A.E.; Que, L.G. Obesity and Asthma. Semin. Respir. Crit. Care Med. 2022, 43, 662–674. [Google Scholar] [CrossRef]
- Barros, R.; Moreira, P.; Padrão, P.; Teixeira, V.H.; Carvalho, P.; Delgado, L.; Moreira, A. Obesity Increases the Prevalence and the Incidence of Asthma and Worsens Asthma Severity. Clin. Nutr. 2017, 36, 1068–1074. [Google Scholar] [CrossRef]
- Luthe, S.K.; Hirayama, A.; Goto, T.; Faridi, M.K.; Camargo, C.A.; Hasegawa, K. Association Between Obesity and Acute Severity Among Patients Hospitalized for Asthma Exacerbation. J. Allergy Clin. Immunol. Pract. 2018, 6, 1936–1941.e4. [Google Scholar] [CrossRef]
- Farzan, S.; Coyle, T.; Coscia, G.; Rebaza, A.; Santiago, M. Clinical Characteristics and Management Strategies for Adult Obese Asthma Patients. J. Asthma Allergy 2022, 15, 673–689. [Google Scholar] [CrossRef]
- Tashiro, H.; Shore, S.A. Obesity and Severe Asthma. Allergol. Int. 2019, 68, 135–142. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 March 2024).
- Wang, Y. Epidemiology of Childhood Obesity—Methodological Aspects and Guidelines: What Is New? Int. J. Obes. 2004, 28, S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.A.; Khavjou, O.A.; Thompson, H.; Trogdon, J.G.; Pan, L.; Sherry, B.; Dietz, W. Obesity and Severe Obesity Forecasts Through 2030. Am. J. Prev. Med. 2012, 42, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Koebnick, C.; Fischer, H.; Daley, M.F.; Ferrara, A.; Horberg, M.A.; Waitzfelder, B.; Young, D.R.; Gould, M.K. Interacting Effects of Obesity, Race, Ethnicity and Sex on the Incidence and Control of Adult-Onset Asthma. Allergy Asthma Clin. Immunol. 2016, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Beuther, D.A.; Sutherland, E.R. Overweight, Obesity, and Incident Asthma: A Meta-Analysis of Prospective Epidemiologic Studies. Am. J. Respir. Crit. Care Med. 2007, 175, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Stream, A.R.; Sutherland, E.R. Obesity and Asthma Disease Phenotypes. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Holguin, F.; Bleecker, E.R.; Busse, W.W.; Calhoun, W.J.; Castro, M.; Erzurum, S.C.; Fitzpatrick, A.M.; Gaston, B.; Israel, E.; Jarjour, N.N.; et al. Obesity and Asthma: An Association Modified by Age of Asthma Onset. J. Allergy Clin. Immunol. 2011, 127, 1486–1493.e2. [Google Scholar] [CrossRef]
- Chipps, B.E.; Zeiger, R.S.; Borish, L.; Wenzel, S.E.; Yegin, A.; Hayden, M.L.; Miller, D.P.; Bleecker, E.R.; Simons, F.E.R.; Szefler, S.J.; et al. Key Findings and Clinical Implications from The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) Study. J. Allergy Clin. Immunol. 2012, 130, 332–342.e10. [Google Scholar] [CrossRef]
- Biring, M.S.; Lewis, M.I.; Liu, J.T.; Mohsenifar, Z. Pulmonary Physiologic Changes of Morbid Obesity. Am. J. Med. Sci. 1999, 318, 293–297. [Google Scholar] [CrossRef]
- Jones, R.L.; Nzekwu, M.-M.U. The Effects of Body Mass Index on Lung Volumes. Chest 2006, 130, 827–833. [Google Scholar] [CrossRef]
- Watson, R.A.; Pride, N.B.; Thomas, E.L.; Fitzpatrick, J.; Durighel, G.; McCarthy, J.; Morin, S.X.; Ind, P.W.; Bell, J.D. Reduction of Total Lung Capacity in Obese Men: Comparison of Total Intrathoracic and Gas Volumes. J. Appl. Physiol. 2010, 108, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Mehari, A.; Afreen, S.; Ngwa, J.; Setse, R.; Thomas, A.N.; Poddar, V.; Davis, W.; Polk, O.D.; Hassan, S.; Thomas, A.V. Obesity and Pulmonary Function in African Americans. PLoS ONE 2015, 10, e0140610. [Google Scholar] [CrossRef]
- Pelosi, P.; Croci, M.; Ravagnan, I.; Tredici, S.; Pedoto, A.; Lissoni, A.; Gattinoni, L. The Effects of Body Mass on Lung Volumes, Respiratory Mechanics, and Gas Exchange During General Anesthesia. Anesth. Analg. 1998, 87, 654–660. [Google Scholar] [CrossRef]
- Zhou, L.N.; Wang, Q.; Gu, C.J.; Li, N.; Zhou, J.P.; Sun, X.W.; Zhou, J.; Li, Q.Y. Sex Differences in the Effects of Obesity on Lung Volume. Am. J. Med. Sci. 2017, 353, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Hedenstierna, G.; Santesson, J.; Norlander, O. Airway Closure and Distribution of Inspired Gas in the Extremely Obese, Breathing Spontaneously and During Anaesthesia with Intermittent Positive Pressure Ventilation. Acta Anaesthesiol. Scand. 1976, 20, 334–342. [Google Scholar] [CrossRef]
- Zhang, R.-H.; Zhou, J.-B.; Cai, Y.-H.; Shu, L.-P.; Yang, J.; Wei, W.; Lecube, A. Non-Linear Association of Anthropometric Measurements and Pulmonary Function. Sci. Rep. 2021, 11, 14596. [Google Scholar] [CrossRef] [PubMed]
- Brazzale, D.J.; Pretto, J.J.; Schachter, L.M. Optimizing Respiratory Function Assessments to Elucidate the Impact of Obesity on Respiratory Health. Respirology 2015, 20, 715–721. [Google Scholar] [CrossRef]
- Litonjua, A.A. Association of Body Mass Index with the Development of Methacholine Airway Hyperresponsiveness in Men: The Normative Aging Study. Thorax 2002, 57, 581–585. [Google Scholar] [CrossRef]
- Badier, M.; Guillot, C.; Delpierre, S. Increased Asymptomatic Airway Hyper-Responsiveness in Obese Individuals. J. Asthma 2013, 50, 573–578. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Ford, G.T. Obesity and Airway Hyper-Responsiveness. Can. J. Respir. Crit. Care Sleep Med. 2019, 3, 112–116. [Google Scholar] [CrossRef]
- Burgess, J.A.; Matheson, M.C.; Diao, F.; Johns, D.P.; Erbas, B.; Lowe, A.J.; Gurrin, L.C.; Lodge, C.J.; Thomas, P.S.; Morrison, S.; et al. Bronchial Hyperresponsiveness and Obesity in Middle Age: Insights from an Australian Cohort. Eur. Respir. J. 2017, 50, 1602181. [Google Scholar] [CrossRef]
- Chinn, S. Relation of Bronchial Responsiveness to Body Mass Index in the ECRHS. Thorax 2002, 57, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Sideleva, O.; Suratt, B.T.; Black, K.E.; Tharp, W.G.; Pratley, R.E.; Forgione, P.; Dienz, O.; Irvin, C.G.; Dixon, A.E. Obesity and Asthma. Am. J. Respir. Crit. Care Med. 2012, 186, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Schachter, L.M. Obesity Is a Risk for Asthma and Wheeze but Not Airway Hyperresponsiveness. Thorax 2001, 56, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Bustos, P.; Amigo, H.; Oyarzún, M.; Rona, R.J. Is There a Causal Relation between Obesity and Asthma? Evidence from Chile. Int. J. Obes. 2005, 29, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Salome, C.M.; Munoz, P.A.; Berend, N.; Thorpe, C.W.; Schachter, L.M.; King, G.G. Effect of Obesity on Breathlessness and Airway Responsiveness to Methacholine in Non-Asthmatic Subjects. Int. J. Obes. 2008, 32, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Nicolacakis, K.; Skowronski, M.E.; Coreno, A.J.; West, E.; Nader, N.Z.; Smith, R.L.; McFadden, E.R. Observations on the Physiological Interactions between Obesity and Asthma. J. Appl. Physiol. 2008, 105, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Meiliana, A.; Dewi, N.M.; Wijaya, A. Adipose Tissue, Inflammation (Meta-Inflammation) and Obesity Management. Indones. Biomed. J. 2015, 7, 129. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Ye, J. Emerging Role of Adipose Tissue Hypoxia in Obesity and Insulin Resistance. Int. J. Obes. 2009, 33, 54–66. [Google Scholar] [CrossRef]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The Role of Leptin and Ghrelin in the Regulation of Food Intake and Body Weight in Humans: A Review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mal, K.; Razaq, M.K.; Magsi, M.; Memon, M.K.; Memon, S.; Afroz, M.N.; Siddiqui, H.F.; Rizwan, A. Association of Leptin With Obesity and Insulin Resistance. Cureus 2020, 12, 12178. [Google Scholar] [CrossRef] [PubMed]
- Loffreda, S.; Yang, S.Q.; Lin, H.Z.; Karp, C.L.; Brengman, M.L.; Wang, D.J.; Klein, A.S.; Bulkley, G.B.; Bao, C.; Noble, P.W.; et al. Leptin Regulates Proinflammatory Immune Responses. FASEB J. 1998, 12, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Sartipy, P.; Loskutoff, D.J. Monocyte Chemoattractant Protein 1 in Obesity and Insulin Resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Bantulà, M.; Roca-Ferrer, J.; Arismendi, E.; Picado, C. Asthma and Obesity: Two Diseases on the Rise and Bridged by Inflammation. J. Clin. Med. 2021, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Gollapudi, S.; Su, H.; Gupta, S. Leptin Activates Human B Cells to Secrete TNF-α, IL-6, and IL-10 via JAK2/STAT3 and P38MAPK/ERK1/2 Signaling Pathway. J. Clin. Immunol. 2011, 31, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Caldefie-Chezet, F.; Poulin, A.; Tridon, A.; Sion, B.; Vasson, M.P. Leptin: A Potential Regulator of Polymorphonuclear Neutrophil Bactericidal Action? J. Leukoc. Biol. 2001, 69, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S.; Herbin, O.; Ait-Oufella, H.; Verreth, W.; Gourdy, P.; Barateau, V.; Merval, R.; Esposito, B.; Clément, K.; Holvoet, P.; et al. Defective Leptin/Leptin Receptor Signaling Improves Regulatory T Cell Immune Response and Protects Mice from Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Tan, Y.; Hu, C.; Liu, X.; He, R. Leptin Is Oversecreted by Respiratory Syncytial Virus-Infected Bronchial Epithelial Cells and Regulates Th2 and Th17 Cell Differentiation. Int. Arch. Allergy Immunol. 2015, 167, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Pace, E.; Chanez, P.; Gras, D.; Vachier, I.; Chiappara, G.; La Guardia, M.; Gerbino, S.; Profita, M.; Gjomarkaj, M. Leptin and Leptin Receptor Expression in Asthma. J. Allergy Clin. Immunol. 2009, 124, 230–237.e4. [Google Scholar] [CrossRef]
- Tsaroucha, A.; Daniil, Z.; Malli, F.; Georgoulias, P.; Minas, M.; Kostikas, K.; Bargiota, A.; Zintzaras, E.; Gourgoulianis, K.I. Leptin, Adiponectin, and Ghrelin Levels in Female Patients with Asthma during Stable and Exacerbation Periods. J. Asthma 2013, 50, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Kubota, N. The Physiological and Pathophysiological Role of Adiponectin and Adiponectin Receptors in the Peripheral Tissues and CNS. FEBS Lett. 2008, 582, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-S.; Lee, W.-J.; Funahashi, T.; Tanaka, S.; Matsuzawa, Y.; Chao, C.-L.; Chen, C.-L.; Tai, T.-Y.; Chuang, L.-M. Weight Reduction Increases Plasma Levels of an Adipose-Derived Anti-Inflammatory Protein, Adiponectin. J. Clin. Endocrinol. Metab. 2001, 86, 3815–3819. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.; Wolf, D.; Rumpold, H.; Enrich, B.; Tilg, H. Adiponectin Induces the Anti-Inflammatory Cytokines IL-10 and IL-1RA in Human Leukocytes. Biochem. Biophys. Res. Commun. 2004, 323, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Haugen, F.; Drevon, C.A. Activation of Nuclear Factor-ΚB by High Molecular Weight and Globular Adiponectin. Endocrinology 2007, 148, 5478–5486. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Parker, J.L.; Ouchi, N.; Higuchi, A.; Vita, J.A.; Gokce, N.; Pedersen, A.A.; Kalthoff, C.; Tullin, S.; Sams, A.; et al. Adiponectin Promotes Macrophage Polarization toward an Anti-Inflammatory Phenotype. J. Biol. Chem. 2010, 285, 6153–6160. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Shore, S.A. Adiponectin, Leptin, and Resistin in Asthma: Basic Mechanisms through Population Studies. J. Allergy 2013, 2013, 785835. [Google Scholar] [CrossRef] [PubMed]
- Shore, S.A. Obesity, Airway Hyperresponsiveness, and Inflammation. J. Appl. Physiol. 2010, 108, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Kattan, M.; Kumar, R.; Bloomberg, G.R.; Mitchell, H.E.; Calatroni, A.; Gergen, P.J.; Kercsmar, C.M.; Visness, C.M.; Matsui, E.C.; Steinbach, S.F.; et al. Asthma Control, Adiposity, and Adipokines among Inner-City Adolescents. J. Allergy Clin. Immunol. 2010, 125, 584–592. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The Hormone Resistin Links Obesity to Diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Sheng, C.H.; Di, J.; Jin, Y.; Zhang, Y.C.; Wu, M.; Sun, Y.; Zhang, G.Z. Resistin Is Expressed in Human Hepatocytes and Induces Insulin Resistance. Endocrine 2008, 33, 135–143. [Google Scholar] [CrossRef]
- Silswal, N.; Singh, A.K.; Aruna, B.; Mukhopadhyay, S.; Ghosh, S.; Ehtesham, N.Z. Human Resistin Stimulates the Pro-Inflammatory Cytokines TNF-α and IL-12 in Macrophages by NF-ΚB-Dependent Pathway. Biochem. Biophys. Res. Commun. 2005, 334, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Patel, L.; Buckels, A.C.; Kinghorn, I.J.; Murdock, P.R.; Holbrook, J.D.; Plumpton, C.; Macphee, C.H.; Smith, S.A. Resistin Is Expressed in Human Macrophages and Directly Regulated by PPARγ Activators. Biochem. Biophys. Res. Commun. 2003, 300, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.; Kim, Y.-D.; Na, H.G.; Bae, C.H.; Song, S.-Y.; Choi, Y.S. Resistin Upregulates MUC5AC/B Mucin Gene Expression in Human Airway Epithelial Cells. Biochem. Biophys. Res. Commun. 2018, 499, 655–661. [Google Scholar] [CrossRef]
- Ballantyne, D.; Scott, H.; MacDonald-Wicks, L.; Gibson, P.G.; Wood, L.G. Resistin Is a Predictor of Asthma Risk and Resistin:Adiponectin Ratio Is a Negative Predictor of Lung Function in Asthma. Clin. Exp. Allergy 2016, 46, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Meng, Q.; Wu, H.; Eid, G.; Zhang, G.; Zhang, X.; Yang, S.; Huang, K.; Lee, T.H.; Corrigan, C.J.; et al. Resistin-like Molecule- Is a Human Airway Remodelling Mediator. Eur. Respir. J. 2012, 39, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-α: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J. Cell Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef]
- Chen, X.-H.; Zhao, Y.-P.; Xue, M.; Ji, C.-B.; Gao, C.-L.; Zhu, J.-G.; Qin, D.-N.; Kou, C.-Z.; Qin, X.-H.; Tong, M.-L.; et al. TNF-α Induces Mitochondrial Dysfunction in 3T3-L1 Adipocytes. Mol Cell Endocrinol 2010, 328, 63–69. [Google Scholar] [CrossRef]
- Alessi, M.C.; Bastelica, D.; Morange, P.; Berthet, B.; Leduc, I.; Verdier, M.; Geel, O.; Juhan-Vague, I. Plasminogen Activator Inhibitor 1, Transforming Growth Factor-Beta1, and BMI Are Closely Associated in Human Adipose Tissue during Morbid Obesity. Diabetes 2000, 49, 1374–1380. [Google Scholar] [CrossRef]
- Thomas, P.S.; Yates, D.H.; Barnes, P.J. Tumor Necrosis Factor-Alpha Increases Airway Responsiveness and Sputum Neutrophilia in Normal Human Subjects. Am. J. Respir. Crit. Care Med. 1995, 152, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.A.; Hargadon, B.; Shelley, M.; Parker, D.; Shaw, D.E.; Green, R.H.; Bradding, P.; Brightling, C.E.; Wardlaw, A.J.; Pavord, I.D. Evidence of a Role of Tumor Necrosis Factor α in Refractory Asthma. N. Engl. J. Med. 2006, 354, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, U.D.; Lee, J.-H.; Dagur, P.K.; Yadav, H.; Shen, M.; Chen, W.; Kulkarni, A.B.; McCoy, J.P.; Finkel, T.; Cypess, A.M.; et al. TGF-β Receptor 1 Regulates Progenitors That Promote Browning of White Fat. Mol. Metab. 2018, 16, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Pulleyn, L.J.; Newton, R.; Adcock, I.M.; Barnes, P.J. TGFβ1 Allele Association with Asthma Severity. Hum. Genet. 2001, 109, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Lee, S.H.; Kato, A.; Takabayashi, T.; Kulka, M.; Shin, S.C.; Schleimer, R.P. Cross-Talk between Human Mast Cells and Bronchial Epithelial Cells in Plasminogen Activator Inhibitor-1 Production via Transforming Growth Factor-Β1. Am. J. Respir. Cell Mol. Biol. 2015, 52, 88–95. [Google Scholar] [CrossRef]
- D’Alessandro, V.F.; Takeshita, A.; Yasuma, T.; Toda, M.; D’Alessandro-Gabazza, C.N.; Okano, Y.; Tharavecharak, S.; Inoue, C.; Nishihama, K.; Fujimoto, H.; et al. Transforming Growth Factorβ1 Overexpression Is Associated with Insulin Resistance and Rapidly Progressive Kidney Fibrosis under Diabetic Conditions. Int. J. Mol. Sci. 2022, 23, 14265. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Koziol-White, C.; Panettieri, R.; Jude, J. TGF-β: The Missing Link in Obesity-Associated Airway Diseases? Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100016. [Google Scholar] [CrossRef]
- Ojiaku, C.A.; Cao, G.; Zhu, W.; Yoo, E.J.; Shumyatcher, M.; Himes, B.E.; An, S.S.; Panettieri, R.A. TGF-Β1 Evokes Human Airway Smooth Muscle Cell Shortening and Hyperresponsiveness via Smad3. Am. J. Respir. Cell Mol. Biol. 2018, 58, 575–584. [Google Scholar] [CrossRef]
- Ma, Z.; Paek, D.; Oh, C.K. Plasminogen Activator Inhibitor-1 and Asthma: Role in the Pathogenesis and Molecular Regulation. Clin. Exp. Allergy 2009, 39, 1136–1144. [Google Scholar] [CrossRef]
- Al-Alawi, M.; Hassan, T.; Chotirmall, S.H. Transforming Growth Factor β and Severe Asthma: A Perfect Storm. Respir. Med. 2014, 108, 1409–1423. [Google Scholar] [CrossRef]
- Binder, B.R.; Christ, G.; Gruber, F.; Grubic, N.; Hufnagl, P.; Krebs, M.; Mihaly, J.; Prager, G.W. Plasminogen Activator Inhibitor 1: Physiological and Pathophysiological Roles. Physiology 2002, 17, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Pahor, M.; Incalzi, R.A. REVIEW: Plasminogen Activator Inhibitor-1 (PAI-1): A Key Factor Linking Fibrinolysis and Age-Related Subclinical and Clinical Conditions. Cardiovasc. Ther. 2010, 28, e72–e91. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Park, J.Y.; Yu, R. Relationship of Obesity and Visceral Adiposity with Serum Concentrations of CRP, TNF-α and IL-6. Diabetes Res. Clin. Pract. 2005, 69, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, R.; Palsdottir, V.; Collander, J.; Anesten, F.; Vogel, H.; Langlet, F.; Jaschke, A.; Schürmann, A.; Prévot, V.; Shao, R.; et al. Glucagon-like Peptide 1 Receptor Induced Suppression of Food Intake, and Body Weight Is Mediated by Central IL-1 and IL-6. Proc. Natl. Acad. Sci. USA 2013, 110, 16199–16204. [Google Scholar] [CrossRef] [PubMed]
- Halwani, R.; Sultana, A.; Vazquez-Tello, A.; Jamhawi, A.; Al-Masri, A.A.; Al-Muhsen, S. Th-17 Regulatory Cytokines IL-21, IL-23, and IL-6 Enhance Neutrophil Production of IL-17 Cytokines during Asthma. J. Asthma 2017, 54, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tian, J.; Tian, X.; Tang, X.; Rui, K.; Tong, J.; Lu, L.; Xu, H.; Wang, S. Adipose Tissue Dendritic Cells Enhances Inflammation by Prompting the Generation of Th17 Cells. PLoS ONE 2014, 9, e92450. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.C.; McGrath, K.W.; Hawkins, G.A.; Hastie, A.T.; Levy, B.D.; Israel, E.; Phillips, B.R.; Mauger, D.T.; Comhair, S.A.; Erzurum, S.C.; et al. Plasma Interleukin-6 Concentrations, Metabolic Dysfunction, and Asthma Severity: A Cross-Sectional Analysis of Two Cohorts. Lancet Respir. Med. 2016, 4, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Jevnikar, Z.; Östling, J.; Ax, E.; Calvén, J.; Thörn, K.; Israelsson, E.; Öberg, L.; Singhania, A.; Lau, L.C.K.; Wilson, S.J.; et al. Epithelial IL-6 Trans-Signaling Defines a New Asthma Phenotype with Increased Airway Inflammation. J. Allergy Clin. Immunol. 2019, 143, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Sumarac-Dumanovic, M.; Stevanovic, D.; Ljubic, A.; Jorga, J.; Simic, M.; Stamenkovic-Pejkovic, D.; Starcevic, V.; Trajkovic, V.; Micic, D. Increased Activity of Interleukin-23/Interleukin-17 Proinflammatory Axis in Obese Women. Int. J. Obes. 2009, 33, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; You, Z. Expression of Interleukin-17RC Protein in Normal Human Tissues. Int. Arch. Med. 2008, 1, 19. [Google Scholar] [CrossRef]
- Moseley, T.A.; Haudenschild, D.R.; Rose, L.; Reddi, A.H. Interleukin-17 Family and IL-17 Receptors. Cytokine Growth Factor Rev. 2003, 14, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Onishi, R.M.; Gaffen, S.L. Interleukin-17 and Its Target Genes: Mechanisms of Interleukin-17 Function in Disease. Immunology 2010, 129, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Gurney, A.L. IL-17: Prototype Member of an Emerging Cytokine Family. J. Leukoc. Biol. 2002, 71, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hynes, G.M.; Hinks, T.S.C. The Role of Interleukin-17 in Asthma: A Protective Response? ERJ Open Res. 2020, 6, 00364–2019. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Qin, L.; Shi, Y.-Y.; Feng, J.-T.; Zheng, Y.-L.; Wan, Y.-F.; Xu, C.-Q.; Yang, X.-M.; Hu, C.-P. IL-17 Protein Levels in Both Induced Sputum and Plasma Are Increased in Stable but Not Acute Asthma Individuals with Obesity. Respir. Med. 2016, 121, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Chang, M.M.-J.; Velichko, S.; Thai, P.; Hung, L.-Y.; Huang, F.; Phuong, N.; Chen, Y.; Wu, R. NF-ΚB Mediates IL-1β– and IL-17A–Induced MUC5B Expression in Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2011, 45, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. The History of Fever, Leukocytic Pyrogen and Interleukin-1. Temperature 2015, 2, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Parisi, M.M.; Grun, L.K.; Lavandoski, P.; Alves, L.B.; Bristot, I.J.; Mattiello, R.; Mottin, C.C.; Klamt, F.; Jones, M.H.; Padoin, A.V.; et al. Immunosenescence Induced by Plasma from Individuals with Obesity Caused Cell Signaling Dysfunction and Inflammation. Obesity 2017, 25, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Madi, M.; Ding, C.; Fok, M.; Steele, T.; Ford, C.; Hunter, L.; Bing, C. Interleukin-1β Mediates Macrophage-Induced Impairment of Insulin Signaling in Human Primary Adipocytes. Am. J. Physiol.-Endocrinol. Metab. 2014, 307, E289–E304. [Google Scholar] [CrossRef]
- Rosenwasser, L.J. New Immunopharmacologic Approaches to Asthma: Role of Cytokine Antagonism. J. Allergy Clin. Immunol. 2000, 105, S586–S592. [Google Scholar] [CrossRef]
- Wood, L.G.; Li, Q.; Scott, H.A.; Rutting, S.; Berthon, B.S.; Gibson, P.G.; Hansbro, P.M.; Williams, E.; Horvat, J.; Simpson, J.L.; et al. Saturated Fatty Acids, Obesity, and the Nucleotide Oligomerization Domain–like Receptor Protein 3 (NLRP3) Inflammasome in Asthmatic Patients. J. Allergy Clin. Immunol. 2019, 143, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Whelan, R. Role and Regulation of Interleukin-1 Molecules in pro-Asthmatic Sensitised Airway Smooth Muscle. Eur. Respir. J. 2004, 24, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Osei, E.T.; Brandsma, C.-A.; Timens, W.; Heijink, I.H.; Hackett, T.-L. Current Perspectives on the Role of Interleukin-1 Signalling in the Pathogenesis of Asthma and COPD. Eur. Respir. J. 2020, 55, 1900563. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.Y.; Pinkerton, J.W.; Essilfie, A.T.; Robertson, A.A.B.; Baines, K.J.; Brown, A.C.; Mayall, J.R.; Ali, M.K.; Starkey, M.R.; Hansbro, N.G.; et al. Role for NLRP3 Inflammasome–Mediated, IL-1β–Dependent Responses in Severe, Steroid-Resistant Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Straczkowski, M.; Dzienis-Straczkowska, S.; Stêpieñ, A.; Kowalska, I.; Szelachowska, M.; Kinalska, I. Plasma Interleukin-8 Concentrations Are Increased in Obese Subjects and Related to Fat Mass and Tumor Necrosis Factor-α System. J. Clin. Endocrinol. Metab. 2002, 87, 4602–4606. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, C.; Asamizu, S.; Ishiki, M.; Iwata, M.; Usui, I.; Yamazaki, K.; Tobe, K.; Kobayashi, M.; Urakaze, M. Inhibitory Effect of IL-8 on Insulin Action in Human Adipocytes via MAP Kinase Pathway. J. Inflamm. 2009, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.M. The Neutrophil-Activating Peptide 1/Interleukin 8, a Novel Neutrophil Chemotactic Cytokine. Arch. Immunol. Ther. Exp. 1992, 40, 23–31. [Google Scholar]
- Dobreva, I.; Waeber, G.; James, R.W.; Widmann, C. Interleukin-8 Secretion by Fibroblasts Induced by Low Density Lipoproteins Is P38 MAPK-Dependent and Leads to Cell Spreading and Wound Closure. J. Biol. Chem. 2006, 281, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.E.; Polverini, P.J.; Kunkel, S.L.; Harlow, L.A.; DiPietro, L.A.; Elner, V.M.; Elner, S.G.; Strieter, R.M. Interleukin-8 as a Macrophage-Derived Mediator of Angiogenesis. Science 1992, 258, 1798–1801. [Google Scholar] [CrossRef]
- Qazi, B.S.; Tang, K.; Qazi, A. Recent Advances in Underlying Pathologies Provide Insight into Interleukin-8 Expression-Mediated Inflammation and Angiogenesis. Int. J. Inflam. 2011, 2011, 908468. [Google Scholar] [CrossRef]
- Nocker, R.E.T.; Schoonbrood, D.F.M.; van de Graaf, E.A.; Hack, E.; Lutter, R.; Jansen, H.M.; Out, T.A. Lnterleukin-8 in Airway Inflammation in Patients with Asthma and Chronic Obstructive Pulmonary Disease. Int. Arch. Allergy Immunol. 1996, 109, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; Vittori, E.; Hollemborg, J.; Mattoli, S. Expression of the Potent Inflammatory Cytokines, Granulocyte-Macrophage-Colony-Stimulating Factor and Interleukin-6 and Interleukin-8, in Bronchial Epithelial Cells of Patients with Asthma. J. Allergy Clin. Immunol. 1992, 89, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Shute, J.K.; Vrugt, B.; Lindley, I.J.; Holgate, S.T.; Bron, A.; Aalbers, R.; Djukanović, R. Free and Complexed Interleukin-8 in Blood and Bronchial Mucosa in Asthma. Am. J. Respir. Crit. Care Med. 1997, 155, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Kurashima, K.; Mukaida, N.; Fujimura, M.; Schröder, J.-M.; Matsuda, T.; Matsushima, K. Increase of Chemokine Levels in Sputum Precedes Exacerbation of Acute Asthma Attacks. J. Leukoc. Biol. 1996, 59, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M.; Fasshauer, M.; Tönjes, A.; Kratzsch, J.; Schön, M.; Paschke, R. Association of Interleukin-6, C-Reactive Protein, Interleukin-10 and Adiponectin Plasma Concentrations with Measures of Obesity, Insulin Sensitivity and Glucose Metabolism. Exp. Clin. Endocrinol. Diabetes 2005, 113, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Pyo, S. Interleukin-10 Suppresses Adipogenesis via Wnt5a Signaling Pathway in 3T3-L1 Preadipocytes. Biochem. Biophys. Res. Commun. 2019, 509, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Yilma, A.N.; Singh, S.R.; Fairley, S.J.; Taha, M.A.; Dennis, V.A. The Anti-Inflammatory Cytokine, Interleukin-10, Inhibits Inflammatory Mediators in Human Epithelial Cells and Mouse Macrophages Exposed to Live and UV-Inactivated Chlamydia trachomatis. Mediat. Inflamm. 2012, 2012, 520174. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, S.; Hasegawa, Y.; Kanehira, Y.; Yamamoto, K.; Fujimoto, K.; Satoh, K.; Okamura, K. Interleukin-10 Level in Sputum Is Reduced in Bronchial Asthma, COPD and in Smokers. Eur. Respir. J. 1999, 14, 309–314. [Google Scholar] [CrossRef]
- Wenzel, S.E.; Schwartz, L.B.; Langmack, E.L.; Halliday, J.L.; Trudeau, J.B.; Gibbs, R.L.; Chu, H.W. Evidence That Severe Asthma Can Be Divided Pathologically into Two Inflammatory Subtypes with Distinct Physiologic and Clinical Characteristics. Am. J. Respir. Crit. Care Med. 1999, 160, 1001–1008. [Google Scholar] [CrossRef]
- Haldar, P.; Pavord, I.D.; Shaw, D.E.; Berry, M.A.; Thomas, M.; Brightling, C.E.; Wardlaw, A.J.; Green, R.H. Cluster Analysis and Clinical Asthma Phenotypes. Am. J. Respir. Crit. Care Med. 2008, 178, 218–224. [Google Scholar] [CrossRef]
- Moore, W.C.; Meyers, D.A.; Wenzel, S.E.; Teague, W.G.; Li, H.; Li, X.; D’Agostino, R.; Castro, M.; Curran-Everett, D.; Fitzpatrick, A.M.; et al. Identification of Asthma Phenotypes Using Cluster Analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010, 181, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Syabbalo, N. Neutrophilic Asthma: A Complex Phenotype of Severe Asthma. J. Lung Pulm. Respir. Res. 2020, 7, 18–24. [Google Scholar] [CrossRef]
- Nair, P.; Aziz-Ur-Rehman, A.; Radford, K. Therapeutic Implications of ‘Neutrophilic Asthma’. Curr. Opin. Pulm. Med. 2015, 21, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F. Neutrophilic Asthma: A Distinct Target for Treatment? Lancet Respir. Med. 2016, 4, 765–767. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Kolls, J.K. Neutrophilic Inflammation in Asthma and Association with Disease Severity. Trends Immunol. 2017, 38, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L.; Scott, R.; Boyle, M.J.; Gibson, P.G. Inflammatory Subtypes in Asthma: Assessment and Identification Using Induced Sputum. Respirology 2006, 11, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Bruijnzeel, P.L.B.; Uddin, M.; Koenderman, L. Targeting Neutrophilic Inflammation in Severe Neutrophilic Asthma: Can We Target the Disease-Relevant Neutrophil Phenotype? J. Leukoc. Biol. 2015, 98, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.A.; Wood, L.G.; Gibson, P.G. Role of Obesity in Asthma: Mechanisms and Management Strategies. Curr. Allergy Asthma Rep. 2017, 17, 53. [Google Scholar] [CrossRef]
- Miranda, C.; Busacker, A.; Balzar, S.; Trudeau, J.; Wenzel, S.E. Distinguishing Severe Asthma Phenotypes: Role of Age at Onset and Eosinophilic Inflammation. J. Allergy Clin. Immunol. 2004, 113, 101–108. [Google Scholar] [CrossRef]
- Shaw, D.E.; Berry, M.A.; Hargadon, B.; McKenna, S.; Shelley, M.J.; Green, R.H.; Brightling, C.E.; Wardlaw, A.J.; Pavord, I.D. Association Between Neutrophilic Airway Inflammation and Airflow Limitation in Adults With Asthma. Chest 2007, 132, 1871–1875. [Google Scholar] [CrossRef]
- Little, S.A.; MacLeod, K.J.; Chalmers, G.W.; Love, J.G.; McSharry, C.; Thomson, N.C. Association of Forced Expiratory Volume with Disease Duration and Sputum Neutrophils in Chronic Asthma. Am. J. Med. 2002, 112, 446–452. [Google Scholar] [CrossRef]
- Moore, W.C.; Hastie, A.T.; Li, X.; Li, H.; Busse, W.W.; Jarjour, N.N.; Wenzel, S.E.; Peters, S.P.; Meyers, D.A.; Bleecker, E.R. Sputum Neutrophil Counts Are Associated with More Severe Asthma Phenotypes Using Cluster Analysis. J. Allergy Clin. Immunol. 2014, 133, 1557–1563.e5. [Google Scholar] [CrossRef]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS Guidelines on Definition, Evaluation and Treatment of Severe Asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef]
- Pavord, I.D.; Brightling, C.E.; Woltmann, G.; Wardlaw, A.J. Non-Eosinophilic Cor Ticosteroid Unresponsive Asthma. The Lancet 1999, 353, 2213–2214. [Google Scholar] [CrossRef] [PubMed]
- Green, R.H. Analysis of Induced Sputum in Adults with Asthma: Identification of Subgroup with Isolated Sputum Neutrophilia and Poor Response to Inhaled Corticosteroids. Thorax 2002, 57, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.J.; Cicutto, L.C.; Smith, H.R.; Ballard, R.D.; Szefler, S.J. Airways Inflammation in Nocturnal Asthma. Am. Rev. Respir. Dis. 1991, 143, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Haldar, P.; Pavord, I.D. Noneosinophilic Asthma: A Distinct Clinical and Pathologic Phenotype. J. Allergy Clin. Immunol. 2007, 119, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V.; Kim, K.W.; Liu, J.; Boushey, H.A. Prominent Neutrophilic Inflammation in Sputum from Subjects with Asthma Exacerbation. J. Allergy Clin. Immunol. 1995, 95, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Simpson, J.L.; Hansbro, P.M.; Gibson, P.G. Potentially Pathogenic Bacteria Cultured from the Sputum of Stable Asthmatics Are Associated with Increased 8-Isoprostane and Airway Neutrophilia. Free Radic. Res. 2010, 44, 146–154. [Google Scholar] [CrossRef]
- Rogliani, P.; Sforza, M.; Calzetta, L. The Impact of Comorbidities on Severe Asthma. Curr. Opin. Pulm. Med. 2020, 26, 47–55. [Google Scholar] [CrossRef]
- Gaffin, J.M.; Castro, M.; Bacharier, L.B.; Fuhlbrigge, A.L. The Role of Comorbidities in Difficult-to-Control Asthma in Adults and Children. J. Allergy Clin. Immunol. Pract. 2022, 10, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Tomisa, G.; Horváth, A.; Sánta, B.; Keglevich, A.; Tamási, L. Epidemiology of Comorbidities and Their Association with Asthma Control. Allergy Asthma Clin. Immunol. 2021, 17, 95. [Google Scholar] [CrossRef]
- Paiva Ferreira, L.K.D.; Paiva Ferreira, L.A.M.; Monteiro, T.M.; Bezerra, G.C.; Bernardo, L.R.; Piuvezam, M.R. Combined Allergic Rhinitis and Asthma Syndrome (CARAS). Int. Immunopharmacol. 2019, 74, 105718. [Google Scholar] [CrossRef] [PubMed]
- Massoth, L.; Anderson, C.; McKinney, K.A. Asthma and Chronic Rhinosinusitis: Diagnosis and Medical Management. Med. Sci. 2019, 7, 53. [Google Scholar] [CrossRef]
- Maesano, A. Epidemiological Evidence of the Occurrence of Rhinitis and Sinusitis in Asthmatics. Allergy 1999, 54, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L. Neutrophilic Asthma Is Characterised by Increased Rhinosinusitis with Sleep Disturbance and GERD. Asian Pac. J. Allergy Immunol. 2013, 32, 64–74. [Google Scholar] [CrossRef]
- Benjamin, M.R.; Stevens, W.W.; Li, N.; Bose, S.; Grammer, L.C.; Kern, R.C.; Tan, B.K.; Conley, D.B.; Smith, S.S.; Welch, K.C.; et al. Clinical Characteristics of Patients with Chronic Rhinosinusitis without Nasal Polyps in an Academic Setting. J. Allergy Clin. Immunol. Pract. 2019, 7, 1010–1016. [Google Scholar] [CrossRef]
- Lee, T.-J.; Fu, C.-H.; Wang, C.-H.; Huang, C.-C.; Huang, C.-C.; Chang, P.-H.; Chen, Y.-W.; Wu, C.-C.; Wu, C.-L.; Kuo, H.-P. Impact of Chronic Rhinosinusitis on Severe Asthma Patients. PLoS ONE 2017, 12, e0171047. [Google Scholar] [CrossRef]
- Patel, G.B.; Peters, A.T. The Role of Biologics in Chronic Rhinosinusitis With Nasal Polyps. Ear. Nose Throat. J. 2021, 100, 44–47. [Google Scholar] [CrossRef]
- Young, T.; Peppard, P.E.; Gottlieb, D.J. Epidemiology of Obstructive Sleep Apnea: A Population Health Perspective. Am. J. Respir. Crit. Care Med. 2002, 165, 1217–1239. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, Y.; Chen, R.; Zeng, X.; Zhang, S.; Su, X.; Luo, Y.; Tang, Y.; Li, S.; Zhuang, Z.; et al. The Relationship between Obstructive Sleep Apnea and Asthma Severity and Vice Versa: A Systematic Review and Meta-Analysis. Eur. J. Med. Res. 2023, 28, 139. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Broytman, O.; Curran-Everett, D.; Sorkness, R.L.; Crisafi, G.; Bleecker, E.R.; Erzurum, S.; Gaston, B.M.; Wenzel, S.E.; Jarjour, N.N. Obstructive Sleep Apnea Risk, Asthma Burden, and Lower Airway Inflammation in Adults in the Severe Asthma Research Program (SARP) II. J. Allergy Clin. Immunol. Pract. 2015, 3, 566–575.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, K.; Hu, K.; Yang, J.; Li, Z.; Nie, M.; Dong, Y.; Huang, H.; Chen, J. Impact of Obstructive Sleep Apnea on Severe Asthma Exacerbations. Sleep Med. 2016, 26, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Karamanlı, H.; Özol, D.; Ugur, K.S.; Yıldırım, Z.; Armutçu, F.; Bozkurt, B.; Yigitoglu, R. Influence of CPAP Treatment on Airway and Systemic Inflammation in OSAS Patients. Sleep Breath. 2014, 18, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L. Role of Sleep Apnea and Gastroesophageal Reflux in Severe Asthma. Immunol. Allergy Clin. N. Am. 2016, 36, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.L.; Schmidt, A.; Bundgaard, A. Pulmonary Function and Acid Application in the Esophagus. Chest 1986, 90, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xu, X.-H.; Chen, Q.; Liang, S.-W.; Lv, H.-J.; Qiu, Z.-M. Gastro-Esophageal Reflux Induced Cough with Airway Hyperresponsiveness. Int. J. Clin. Exp. Med. 2014, 7, 728–735. [Google Scholar] [PubMed]
- Cazzola, M.; Rogliani, P.; Calzetta, L.; Matera, M.G. Bronchodilators in Subjects with Asthma-Related Comorbidities. Respir. Med. 2019, 151, 43–48. [Google Scholar] [CrossRef]
- Kopsaftis, Z.; Yap, H.S.; Tin, K.S.; Hnin, K.; Carson-Chahhoud, K.V. Pharmacological and Surgical Interventions for the Treatment of Gastro-Oesophageal Reflux in Adults and Children with Asthma. Cochrane Database Syst. Rev. 2021, 2021. [Google Scholar] [CrossRef]
- Huang, P.L. A Comprehensive Definition for Metabolic Syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Karamzad, N.; Izadi, N.; Sanaie, S.; Ahmadian, E.; Eftekhari, A.; Sullman, M.J.M.; Safiri, S. Asthma and Metabolic Syndrome: A Comprehensive Systematic Review and Meta-Analysis of Observational Studies. J. Cardiovasc. Thorac. Res. 2020, 12, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Emami-Ardestani, M.; Sajadi, G. Relationship between Metabolic Syndrome Components and Severity of Asthma in Outpatients Referring to Alzahra Hospital Clinic. Tanaffos 2021, 20, 327–331. [Google Scholar] [PubMed]
- Forno, E.; Zhang, P.; Nouraie, M.; Courcoulas, A.; Mitchell, J.E.; Wolfe, B.M.; Strain, G.; Khandelwal, S.; Holguin, F. The Impact of Bariatric Surgery on Asthma Control Differs among Obese Individuals with Reported Prior or Current Asthma, with or without Metabolic Syndrome. PLoS ONE 2019, 14, e0214730. [Google Scholar] [CrossRef] [PubMed]
- Naing, C.; Ni, H. Statins for Asthma. Cochrane Database Syst. Rev. 2020, 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Gowdy, K.M.; Fessler, M.B. Emerging Roles for Cholesterol and Lipoproteins in Lung Disease. Pulm. Pharmacol. Ther. 2013, 26, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Sunata, K.; Kabata, H.; Kuno, T.; Takagi, H.; So, M.; Masaki, K.; Fukunaga, K. The Effect of Statins for Asthma. A Systematic Review and Meta-Analysis. J. Asthma 2022, 59, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.T.; Koh, W.-P.; Odegaard, A.O.; Gross, M.D.; Yuan, J.-M.; Pereira, M.A. Asthma and the Risk of Type 2 Diabetes in the Singapore Chinese Health Study. Diabetes Res. Clin. Pract. 2013, 99, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Jacoby, D.B.; Fryer, A.D. Hyperinsulinemia Potentiates Airway Responsiveness to Parasympathetic Nerve Stimulation in Obese Rats. Am. J. Respir. Cell Mol. Biol. 2014, 51, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, J.; Patterson, C.C.; Menzies-Gow, A.; Niven, R.M.; Mansur, A.H.; Bucknall, C.; Chaudhuri, R.; Price, D.; Brightling, C.E.; Heaney, L.G. Comorbidity in Severe Asthma Requiring Systemic Corticosteroid Therapy: Cross-Sectional Data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax 2016, 71, 339–346. [Google Scholar] [CrossRef]
- Wu, T.D.; Brigham, E.P.; Keet, C.A.; Brown, T.T.; Hansel, N.N.; McCormack, M.C. Association Between Prediabetes/Diabetes and Asthma Exacerbations in a Claims-Based Obese Asthma Cohort. J. Allergy Clin. Immunol. Pract. 2019, 7, 1868–1873.e5. [Google Scholar] [CrossRef]
- Yang, G.; Han, Y.-Y.; Forno, E.; Yan, Q.; Rosser, F.; Chen, W.; Celedón, J.C. Glycated Hemoglobin A1c, Lung Function, and Hospitalizations Among Adults with Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 3409–3415.e1. [Google Scholar] [CrossRef] [PubMed]
- Wytrychowski, K.; Obojski, A.; Hans-Wytrychowska, A. The Influence of Insulin Therapy on the Course of Acute Exacerbation of Bronchial Asthma. Pathophysiol. Respir. 2016, 2015, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Koskela, H.O.; Salonen, P.H.; Romppanen, J.; Niskanen, L. A History of Diabetes but Not Hyperglycaemia during Exacerbation of Obstructive Lung Disease Has Impact on Long-Term Mortality: A Prospective, Observational Cohort Study. BMJ Open 2015, 5, e006794. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Erickson, S.R.; Wu, C. Metformin Use and Asthma Outcomes among Patients with Concurrent Asthma and Diabetes. Respirology 2016, 21, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Foer, D.; Beeler, P.E.; Cui, J.; Karlson, E.W.; Bates, D.W.; Cahill, K.N. Asthma Exacerbations in Patients with Type 2 Diabetes and Asthma on Glucagon-like Peptide-1 Receptor Agonists. Am. J. Respir. Crit. Care Med. 2021, 203, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Mat, A.; Hogan, A.E.; Kent, B.D.; Eigenheer, S.; Corrigan, M.A.; O’Shea, D.; Butler, M.W. Preliminary Asthma-Related Outcomes Following Glucagon-like Peptide 1 Agonist Therapy. QJM Int. J. Med. 2017, 110, 853–854. [Google Scholar] [CrossRef]
- Hur, J.; Kang, J.Y.; Kim, Y.K.; Lee, S.Y.; Lee, H.Y. Glucagon-like Peptide 1 Receptor (GLP-1R) Agonist Relieved Asthmatic Airway Inflammation via Suppression of NLRP3 Inflammasome Activation in Obese Asthma Mice Model. Pulm. Pharmacol. Ther. 2021, 67, 102003. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Calzetta, L.; Capuani, B.; Facciolo, F.; Cazzola, M.; Lauro, D.; Matera, M.G. Glucagon-Like Peptide 1 Receptor: A Novel Pharmacological Target for Treating Human Bronchial Hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 2016, 55, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Peters-Golden, M. Influence of Body Mass Index on the Response to Asthma Controller Agents. Eur. Respir. J. 2006, 27, 495–503. [Google Scholar] [CrossRef]
- Mancuso, P.; Canetti, C.; Gottschalk, A.; Tithof, P.K.; Peters-Golden, M. Leptin Augments Alveolar Macrophage Leukotriene Synthesis by Increasing Phospholipase Activity and Enhancing Group IVC IPLA2(CPLA2γ) Protein Expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L497–L502. [Google Scholar] [CrossRef]
- Giouleka, P.; Papatheodorou, G.; Lyberopoulos, P.; Karakatsani, A.; Alchanatis, M.; Roussos, C.; Papiris, S.; Loukides, S. Body Mass Index Is Associated with Leukotriene Inflammation in Asthmatics. Eur. J. Clin. Invest. 2011, 41, 30–38. [Google Scholar] [CrossRef]
- Camargo, C.A.; Boulet, L.-P.; Sutherland, E.R.; Busse, W.W.; Yancey, S.W.; Emmett, A.H.; Ortega, H.G.; Ferro, T.J. Body Mass Index and Response to Asthma Therapy: Fluticasone Propionate/Salmeterol versus Montelukast. J. Asthma 2010, 47, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.; Mannino, D.; Brown, C.; Crocker, D.; Twum-Baah, N.; Holguin, F. Body Mass Index and Asthma Severity in the National Asthma Survey. Thorax 2008, 63, 14–20. [Google Scholar] [CrossRef]
- Tashiro, H.; Takahashi, K.; Sadamatsu, H.; Kurihara, Y.; Haraguchi, T.; Tajiri, R.; Takamori, A.; Kimura, S.; Sueoka-Aragane, N. Biomarkers for Overweight in Adult-Onset Asthma. J. Asthma Allergy 2020, 13, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, E.R.; Lehman, E.B.; Teodorescu, M.; Wechsler, M.E. Body Mass Index and Phenotype in Subjects with Mild-to-Moderate Persistent Asthma. J. Allergy Clin. Immunol. 2009, 123, 1328–1334.e1. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Paggiaro, P.; Buhl, R.; Bernstein, J.A.; Haddon, J.; Unseld, A.; Engel, M.; Casale, T.B.; Dixon, A.E. Tiotropium Reduces Airflow Obstruction in Asthma Patients, Independent of Body Mass Index. J. Allergy Clin. Immunol. Pract. 2019, 7, 2425–2428.e7. [Google Scholar] [CrossRef]
- McGrath, K.W.; Icitovic, N.; Boushey, H.A.; Lazarus, S.C.; Sutherland, E.R.; Chinchilli, V.M.; Fahy, J.V. A Large Subgroup of Mild-to-Moderate Asthma Is Persistently Noneosinophilic. Am. J. Respir. Crit. Care Med. 2012, 185, 612–619. [Google Scholar] [CrossRef]
- Peerboom, S.; Graff, S.; Seidel, L.; Paulus, V.; Henket, M.; Sanchez, C.; Guissard, F.; Moermans, C.; Louis, R.; Schleich, F. Predictors of a Good Response to Inhaled Corticosteroids in Obesity-Associated Asthma. Biochem. Pharmacol. 2020, 179, 113994. [Google Scholar] [CrossRef]
- Vazquez-Tello, A.; Halwani, R.; Hamid, Q.; Al-Muhsen, S. Glucocorticoid Receptor-Beta Up-Regulation and Steroid Resistance Induction by IL-17 and IL-23 Cytokine Stimulation in Peripheral Mononuclear Cells. J. Clin. Immunol. 2013, 33, 466–478. [Google Scholar] [CrossRef]
- Al Heialy, S.; Gaudet, M.; Ramakrishnan, R.K.; Mogas, A.; Salameh, L.; Mahboub, B.; Hamid, Q. Contribution of IL-17 in Steroid Hyporesponsiveness in Obese Asthmatics Through Dysregulation of Glucocorticoid Receptors α and β. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Sutherland, E.R.; Goleva, E.; Strand, M.; Beuther, D.A.; Leung, D.Y.M. Body Mass and Glucocorticoid Response in Asthma. Am. J. Respir. Crit. Care Med. 2008, 178, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Komakula, S.; Khatri, S.; Mermis, J.; Savill, S.; Haque, S.; Rojas, M.; Brown, L.; Teague, G.W.; Holguin, F. Body Mass Index Is Associated with Reduced Exhaled Nitric Oxide and Higher Exhaled 8-Isoprostanes in Asthmatics. Respir. Res. 2007, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Lugogo, N.; Green, C.L.; Agada, N.; Zhang, S.; Meghdadpour, S.; Zhou, R.; Yang, S.; Anstrom, K.J.; Israel, E.; Martin, R.; et al. Obesity’s Effect on Asthma Extends to Diagnostic Criteria. J. Allergy Clin. Immunol. 2018, 141, 1096–1104. [Google Scholar] [CrossRef]
- Gu, C.; Upchurch, K.; Mamaril-Davis, J.; Wiest, M.; Lanier, B.; Millard, M.; Turner, J.; Joo, H.; Oh, S. Obesity Influences the Outcomes of Anti-IgE (Omalizumab) Therapy of Asthma. Clin. Exp. Allergy 2020, 50, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Sposato, B.; Scalese, M.; Milanese, M.; Masieri, S.; Cavaliere, C.; Latorre, M.; Scichilone, N.; Matucci, A.; Vultaggio, A.; Ricci, A.; et al. Factors Reducing Omalizumab Response in Severe Asthma. Eur. J. Int. Med. 2018, 52, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.J.; Vieira, M.; Coutinho, D.; Ladeira, I.; Pascoal, I.; Ferreira, J.; da Silva, J.M.; Carvalho, A.; Lima, R. Severe Asthma in Obese Patients: Improvement of Lung Function after Treatment with Omalizumab. Pulmonology 2019, 25, 15–20. [Google Scholar] [CrossRef] [PubMed]
- European Medicine Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xolair (accessed on 19 December 2023).
- Hashimoto, S.; Kroes, J.A.; Eger, K.A.; Mau Asam, P.F.; Hofstee, H.B.; Bendien, S.A.; Braunstahl, G.J.; Broeders, M.E.A.C.; Imming, L.M.; Langeveld, B.; et al. Real-World Effectiveness of Reslizumab in Patients With Severe Eosinophilic Asthma–First Initiators and Switchers. J. Allergy Clin. Immunol. Pract. 2022, 10, 2099–2108.e6. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.; Li, H.; Suruki, R.; Albers, F.; Gordon, D.; Yancey, S. Cluster Analysis and Characterization of Response to Mepolizumab. A Step Closer to Personalized Medicine for Patients with Severe Asthma. Ann. Am. Thorac. Soc. 2014, 11, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.G.; Prazma, C.M.; Chupp, G.L.; Bradford, E.S.; Forshag, M.; Mallett, S.A.; Yancey, S.W.; Smith, S.G.; Bel, E.H. Mepolizumab Improves Clinical Outcomes in Patients with Severe Asthma and Comorbid Conditions. Respir. Res. 2021, 22, 171. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Menzies-Gow, A.; Zangrilli, J.G.; Hirsch, I.; Metcalfe, P.; Newbold, P.; Goldman, M. Predictors of Enhanced Response with Benralizumab for Patients with Severe Asthma: Pooled Analysis of the SIROCCO and CALIMA Studies. Lancet Respir. Med. 2018, 6, 51–64. [Google Scholar] [CrossRef]
- Kuruvilla, M.; Patrawala, M.; Levy, J.M.; Shih, J.; Lee, F.E. Association of Antieosinophil Therapy with Decreased Body Mass Index in Patients with Severe Asthma. Ann. Allergy Asthma Immunol. 2019, 122, 649–650. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Paggiaro, P.; Muñoz, X.; Casale, T.B.; Castro, M.; Canonica, G.W.; Douglass, J.A.; Tohda, Y.; Daizadeh, N.; Ortiz, B.; et al. Impact of Baseline Patient Characteristics on Dupilumab Efficacy in Type 2 Asthma. Eur. Respir. J. 2021, 58, 2004605. [Google Scholar] [CrossRef] [PubMed]
- Gauvreau, G.M.; Sehmi, R.; Ambrose, C.S.; Griffiths, J.M. Thymic Stromal Lymphopoietin: Its Role and Potential as a Therapeutic Target in Asthma. Expert Opin. Ther. Targets 2020, 24, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Verma, M.; Michalec, L.; Liu, W.; Sripada, A.; Rollins, D.; Good, J.; Ito, Y.; Chu, H.; Gorska, M.M.; et al. Steroid Resistance of Airway Type 2 Innate Lymphoid Cells from Patients with Severe Asthma: The Role of Thymic Stromal Lymphopoietin. J. Allergy Clin. Immunol. 2018, 141, 257–268.e6. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Parnes, J.R.; Wang, L.; Mo, M.; Roseti, S.L.; Griffiths, J.M.; van der Merwe, R. Tezepelumab in Adults with Uncontrolled Asthma. N. Engl. J. Med. 2017, 377, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, Å.; Bowen, K.; et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N. Engl. J. Med. 2021, 384, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.G.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; Peters, M.J.; Marks, G.B.; Baraket, M.; et al. Effect of Azithromycin on Asthma Exacerbations and Quality of Life in Adults with Persistent Uncontrolled Asthma (AMAZES): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2017, 390, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Brusselle, G.G.; VanderStichele, C.; Jordens, P.; Deman, R.; Slabbynck, H.; Ringoet, V.; Verleden, G.; Demedts, I.K.; Verhamme, K.; Delporte, A.; et al. Azithromycin for Prevention of Exacerbations in Severe Asthma (AZISAST): A Multicentre Randomised Double-Blind Placebo-Controlled Trial. Thorax 2013, 68, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L.; Powell, H.; Boyle, M.J.; Scott, R.J.; Gibson, P.G. Clarithromycin Targets Neutrophilic Airway Inflammation in Refractory Asthma. Am. J. Respir. Crit. Care Med. 2008, 177, 148–155. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; Boulet, L.-P.; Schmid-Wirlitsch, C.; Côté, J.; Duong, M.; Killian, K.J.; Milot, J.; Deschesnes, F.; Strinich, T.; Watson, R.M.; et al. Roflumilast Attenuates Allergen-Induced Inflammation in Mild Asthmatic Subjects. Respir. Res. 2011, 12, 140. [Google Scholar] [CrossRef]
- Bateman, E.D.; Goehring, U.-M.; Richard, F.; Watz, H. Roflumilast Combined with Montelukast versus Montelukast Alone as Add-on Treatment in Patients with Moderate-to-Severe Asthma. J. Allergy Clin. Immunol. 2016, 138, 142–149.e8. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Gaga, M.; Zervas, E.; Alagha, K.; Hargreave, F.E.; O’Byrne, P.M.; Stryszak, P.; Gann, L.; Sadeh, J.; Chanez, P. Safety and Efficacy of a CXCR 2 Antagonist in Patients with Severe Asthma and Sputum Neutrophils: A Randomized, Placebo-controlled Clinical Trial. Clin. Exp. Allergy 2012, 42, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, A.; Sattar, S.B.A.; Rafay Khan Niazi, M.; Muhammad, M.; Jahangir, A.; Sahra, S.; Sharif, M.A.; Anwar, M.Y.; Chalhoub, M. Efficacy and Safety of Fevipiprant in Asthma: A Review and Meta-Analysis. Cureus 2022, 14, e24641. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.H.; Lugogo, N.L.; Castro, M.; Hanania, N.A.; Ludwig-Sengpiel, A.; Saralaya, D.; Dobek, R.; Ojanguren, I.; Vyshnyvetskyy, I.; Bruey, J.-M.; et al. Results of a Phase 2b Trial With GB001, a Prostaglandin D2 Receptor 2 Antagonist, in Moderate to Severe Eosinophilic Asthma. Chest 2022, 162, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E.; Barnes, P.J.; Bleecker, E.R.; Bousquet, J.; Busse, W.; Dahlén, S.-E.; Holgate, S.T.; Meyers, D.A.; Rabe, K.F.; Antczak, A.; et al. A Randomized, Double-Blind, Placebo-Controlled Study of Tumor Necrosis Factor-α Blockade in Severe Persistent Asthma. Am. J. Respir. Crit. Care Med. 2009, 179, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T.; Noonan, M.; Chanez, P.; Busse, W.; Dupont, L.; Pavord, I.; Hakulinen, A.; Paolozzi, L.; Wajdula, J.; Zang, C.; et al. Efficacy and Safety of Etanercept in Moderate-to-Severe Asthma: A Randomised, Controlled Trial. Eur. Respir. J. 2011, 37, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Holgate, S.; Kerwin, E.; Chon, Y.; Feng, J.; Lin, J.; Lin, S.-L. Randomized, Double-Blind, Placebo-Controlled Study of Brodalumab, a Human Anti–IL-17 Receptor Monoclonal Antibody, in Moderate to Severe Asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 1294–1302. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals. National Library of Medicine. National Center of Biotechnology Information. Available online: https://clinicaltrials.gov/study/NCT01478360?tab=results#part (accessed on 20 November 2015).
- Brightling, C.E.; Nair, P.; Cousins, D.J.; Louis, R.; Singh, D. Risankizumab in Severe Asthma—A Phase 2a, Placebo-Controlled Trial. N. Engl. J. Med. 2021, 385, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Kelsen, S.G.; Agache, I.O.; Soong, W.; Israel, E.; Chupp, G.L.; Cheung, D.S.; Theess, W.; Yang, X.; Staton, T.L.; Choy, D.F.; et al. Astegolimab (Anti-ST2) Efficacy and Safety in Adults with Severe Asthma: A Randomized Clinical Trial. J. Allergy Clin. Immunol. 2021, 148, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Ruddy, M.K.; Pavord, I.D.; Israel, E.; Rabe, K.F.; Ford, L.B.; Maspero, J.F.; Abdulai, R.M.; Hu, C.-C.; Martincova, R.; et al. Efficacy and Safety of Itepekimab in Patients with Moderate-to-Severe Asthma. N. Engl. J. Med. 2021, 385, 1656–1668. [Google Scholar] [CrossRef]
- Panettieri, R.A.; Sjöbring, U.; Péterffy, A.; Wessman, P.; Bowen, K.; Piper, E.; Colice, G.; Brightling, C.E. Tralokinumab for Severe, Uncontrolled Asthma (STRATOS 1 and STRATOS 2): Two Randomised, Double-Blind, Placebo-Controlled, Phase 3 Clinical Trials. Lancet Respir. Med. 2018, 6, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; Korenblat, P.; Chapman, K.R.; Bateman, E.D.; Kopecky, P.; Paggiaro, P.; Yokoyama, A.; Olsson, J.; Gray, S.; Holweg, C.T.J.; et al. Efficacy and Safety of Lebrikizumab in Patients with Uncontrolled Asthma (LAVOLTA I and LAVOLTA II): Replicate, Phase 3, Randomised, Double-Blind, Placebo-Controlled Trials. Lancet Respir. Med. 2016, 4, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, S.; Low, S.; Weigle, B.; Dutcher, D.; Jerath, K.; Menzenski, M.; Frego, L.; Truncali, K.; Gupta, P.; Kroe-Barrett, R.; et al. Design and Characterization of Zweimab and Doppelmab, High Affinity Dual Antagonistic Anti-TSLP/IL13 Bispecific Antibodies. Biochem. Biophys. Res. Commun. 2018, 504, 19–24. [Google Scholar] [CrossRef]
- Pavord, I.D.; Cox, G.; Thomson, N.C.; Rubin, A.S.; Corris, P.A.; Niven, R.M.; Chung, K.F.; Laviolette, M. Safety and Efficacy of Bronchial Thermoplasty in Symptomatic, Severe Asthma. Am. J. Respir. Crit. Care Med. 2007, 176, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Rubin, A.S.; Laviolette, M.; Fiterman, J.; De Andrade Lima, M.; Shah, P.L.; Fiss, E.; Olivenstein, R.; Thomson, N.C.; Niven, R.M.; et al. Effectiveness and Safety of Bronchial Thermoplasty in the Treatment of Severe Asthma. Am. J. Respir. Crit. Care Med. 2010, 181, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Chupp, G.; Kline, J.N.; Khatri, S.B.; McEvoy, C.; Silvestri, G.A.; Shifren, A.; Castro, M.; Bansal, S.; McClelland, M.; Dransfield, M.; et al. Bronchial Thermoplasty in Patients With Severe Asthma at 5 Years. Chest 2022, 161, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Thomson, N.C.; Niven, R.M.; Corris, P.A.; Chung, K.F.; Cox, G.; Armstrong, B.; Shargill, N.S.; Laviolette, M. Safety of Bronchial Thermoplasty in Patients with Severe Refractory Asthma. Ann. Allergy Asthma Immunol. 2013, 111, 402–407. [Google Scholar] [CrossRef]
- Thomson, N.C.; Rubin, A.S.; Niven, R.M.; Corris, P.A.; Siersted, H.C.; Olivenstein, R.; Pavord, I.D.; McCormack, D.; Laviolette, M.; Shargill, N.S.; et al. Long-Term (5 Year) Safety of Bronchial Thermoplasty: Asthma Intervention Research (AIR) Trial. BMC Pulm. Med. 2011, 11, 8. [Google Scholar] [CrossRef]
- Chaudhuri, R.; Rubin, A.; Sumino, K.; Lapa e Silva, J.R.; Niven, R.; Siddiqui, S.; Klooster, K.; McEvoy, C.; Shah, P.L.; Simoff, M.; et al. Safety and Effectiveness of Bronchial Thermoplasty after 10 Years in Patients with Persistent Asthma (BT10+): A Follow-up of Three Randomised Controlled Trials. Lancet Respir. Med. 2021, 9, 457–466. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Wada, H.; Rossios, C.; Takagi, D.; Higaki, M.; Mikura, S.; Goto, H.; Barnes, P.J.; Ito, K. A Novel Macrolide Solithromycin Exerts Superior Anti-Inflammatory Effect via NF- κ B Inhibition. J. Pharmacol. Exp. Ther. 2013, 345, 76–84. [Google Scholar] [CrossRef]
- Hodge, S.; Hodge, G.; Brozyna, S.; Jersmann, H.; Holmes, M.; Reynolds, P.N. Azithromycin Increases Phagocytosis of Apoptotic Bronchial Epithelial Cells by Alveolar Macrophages. Eur. Respir. J. 2006, 28, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Niessen, N.M.; Gibson, P.G.; Baines, K.J.; Barker, D.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; et al. Sputum TNF Markers Are Increased in Neutrophilic and Severe Asthma and Are Reduced by Azithromycin Treatment. Allergy 2021, 76, 2090–2101. [Google Scholar] [CrossRef] [PubMed]
- Kawamatawong, T. Phosphodiesterase-4 Inhibitors for Non-COPD Respiratory Diseases. Front. Pharmacol. 2021, 12, 518345. [Google Scholar] [CrossRef] [PubMed]
- Bonta, P.I.; Chanez, P.; Annema, J.T.; Shah, P.L.; Niven, R. Bronchial Thermoplasty in Severe Asthma: Best Practice Recommendations from an Expert Panel. Respiration 2018, 95, 289–300. [Google Scholar] [CrossRef] [PubMed]
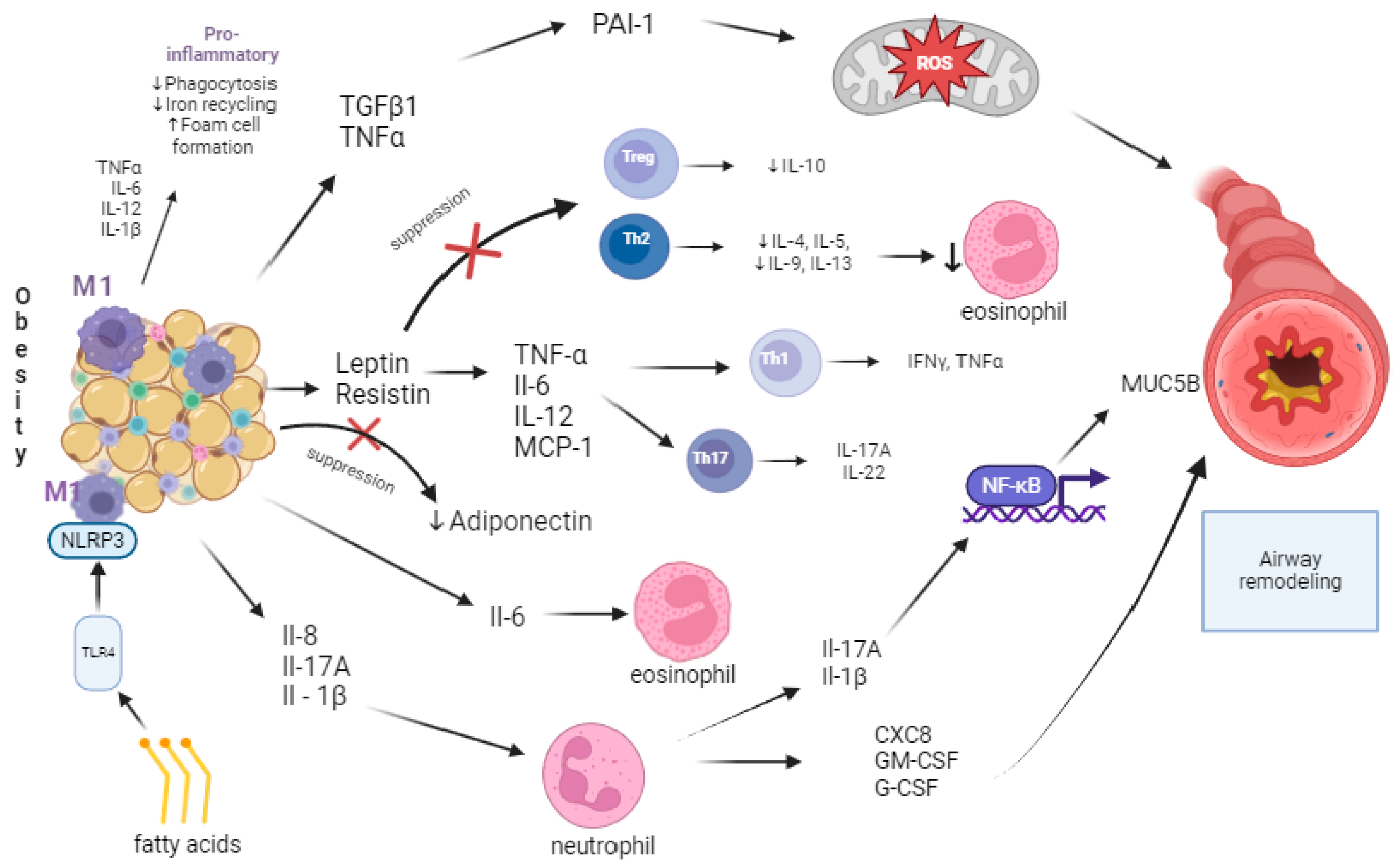
| Adipokine | Systemic Effect | Effect on Asthma |
|---|---|---|
| Leptin | ||
| Adiponectin |
| |
| Resistin | ||
| TNF-α |
| |
| TGF-β1 | ||
| PAI-1 |
|
|
| IL-6 |
|
|
| IL-17A |
|
|
| IL-1β |
| |
| IL-8 |
|
|
| IL-10 |
|
| Late-Onset Asthma Phenotype | Early-Onset Asthma Phenotype | Neutrophilic Phenotype | Mixed Granylocytic Phenotype |
|---|---|---|---|
| Older women Less Th2 inflammation Less atopy Low baseline lung function Frequent exacerbations More complicated treatment regimens: high doses of ICS, more frequent use of OCS Frequent sinus disease, GERD, hypertension | Young men in primary care, young women in secondary care Atopy High eosinophilic inflammation Greater airway obstruction and AHR Persistent treatment with OCS More frequent hospitalization due to exacerbations | Most common phenotype in severe asthma Older woman Longer asthma duration Less atopy Neutrophilic inflammation Chronic atypical bacterial inflammation Persistent remodeling airway obstruction Steroid resistance Recurrent nocturnal attacks, frequent non-infectious exacerbations | Older women Longer asthma duration Mixed inflammation: eosinophilic and neutrophilic Less atopy Greater airway obstruction Frequent exacerbations More complicated treatment regimens: high doses of ICS, more frequent use of OCS |
| Therapy | Author | Study Characterictic | Main Findings |
|---|---|---|---|
| Montelukast | Peters-Golden et al. [181] | Post hoc analysis of 4 randomized double-blind, placebo-controlled studies of 3073 moderate asthmatic adults | Response to montelukast, as measured by ACD, was stable across all BMI categories |
| Montelukast vs. fluticasone propionate/salmeterol | Camargo et al. [184] | Retrospective meta-analysis of 4 clinical trials | Higher FEV1 and better asthma control were observed in the fluticasone propionate/salmeterol arm than montelukast across the BMI range |
| ICS | Mc Grath et al. [189] | Post hoc analysis of 9 clinical trials of 995 subjects with persistent asthma | Improvement in airway obstruction was observed only in the group of patients with eosinophilic asthma after 2 weeks of intensive combined anti-inflammatory therapy with prednisone, inhaled budesonide, and zafirlukast |
| ICS + LABA | Camargo et al. [184] Taylor et al. [185] Sutherland et al. [187] | Retrospective meta-analysis of 4 clinical trials Retrospective analysis from the National Asthma Survey of 3095 patients with asthma Retrospective analysis from Asthma Clinical Research Network studies of 1265 patients with mild-to-moderate persistent asthma | Obesity was associated with poorer response to ICS and ICS + LABA in terms of FEV1 and FEV1/FVC ratio and less improvement in FeNO levels after ICS. Obese asthmatics required longer ICS + LABA treatment to reach peak FEV1 compared to their lean counterparts. |
| ICS + LABA + LAMA | Khurana et al. [188] | Post hoc analysis from 5 phase III clinical trials of tiotropium soft mist inhaler in patients with differing severities of asthma (912 of 3476 had severe asthma) | The addition of tiotropium to ICS+LABA therapy improved peak and trough FEV1 across all BMI categories in both moderate and severe asthma patients. |
| Omalizumab | GuC et al. [196] | Comparative study (obese vs. non-obese) of 45 patients with moderate-to-severe uncontrolled asthma | Improved asthma control, as measured by the Asthma Control Test (ACT), in all BMI groups |
| Omalizumab | Sposato et al. [197] | Real-life retrospective study of 340 patients with severe asthma | Reduced response of omalizumab to FEV1, FVC, ACT, and FeNO in obesity was noticed |
| Omalizumab | Oliveira et al. [198] | Prospective study of 32 subjects with severe asthma | Significant improvement in asthma control, lung function, reduction in the daily dose of budesonide, and statistically significant weight loss |
| Reslizumab | Hashimoto et al. [200] | Observational real-world study of 134 adults with severe eosinophilic asthma | Study of patients with severe eosinophilic asthma, 30.5% of whom were obese, a minority of patients did not improve after the use of reslizumab, the remaining patients had a reduction in asthma exacerbations, the use of OCS, and rescue medications, and an improvement in lung function. |
| Mepolizumab | Ortega et al. [201] | Supervised cluster analysis of data from DREAM study of 616 patients with severe asthma | Diminution in exacerbations, significantly greater than non-obese counterparts in other clusters |
| Mepolizumab | Gibson et al. [202] | Post hoc analysis of data from DREAM, MENSA, SIRIUS, and MUSCA studies (1878 patients with severe asthma) | Reduction in exacerbation rates and improvements in lung function, asthma control, and quality of life, notwithstanding comorbidities, including obesity |
| Benralizumab | FitzGerald et al. [203] | Analysis of the results from the randomized, double-blind, placebo-controlled SIROCCO and CALIMA studies (2295 patients with severe, uncontrolled asthma) | Reduced annual exacerbations in patients with a BMI > 35 kg/m2 |
| Reslizumab vs. mepolizumab vs. benralizumab | Kuruvilla et al. [204] | Retrospective analysis of 58 adults with asthma who were receiving anti-IL-5 therapy | Weight loss was demonstrated after 6 months of all treatments, more pronounced in people with initial BMI > 30 kg/m2 |
| Dupilumab | Busse et al. [205] | Randomized, double-blind, placebo-controlled study of 1584 patients with persistent asthma | Reduced the annual rate of asthma exacerbations, regardless of BMI |
| Tezepelumab | Corren et al. [208] Menzies-Gow et al. [209] | Randomized, double-blind, placebo-controlled trial of 584 subjects with moderate-to-severe asthma and multicenter, randomized, double-blind, placebo-controlled trial of 1061 patients with severe, uncontrolled asthma (12 to 80 years of age) | Reduced the annual rate of exacerbations, blood eosinophils, FeNO, and IgE, and improved FEV1 and quality of life regardless of baseline blood eosinophil level. No data on the effect on obesity-related asthma |
| Therapy | Mode of Action | Study Characteristic | Findings | Author and References |
|---|---|---|---|---|
| Azithromycin | macrolide antibiotic | randomized, double-blind, placebo-controlled study of 420 patients with symptomatic asthma (213 in the azithromycin group and 207 in the placebo group) | reduced exacerbation rates and improved quality of life in patients with both eosinophilic and non-eosinophilic asthma | Gibson et al. [210] |
| macrolide antibiotic | randomized, double-blind, placebo-controlled trial in subjects with severe asthma (55 in the azithromycin group and 54 in the placebo group) | reduced the risk of exacerbations and lower respiratory tract infections in severe non-eosinophilic asthma, improved the quality of life in eosinophilic and non-eosinophilic asthma | Brusselle et al. [211] | |
| Clarythromycin | macrolide antibiotic | randomized, placebo-controlled trial of 45 subjects with severe refractory asthma | reduced neutrophilic inflammation and improved quality of life scores | Simpson et al. [212] |
| Roflumilast | type 4 cAMP phosphodiesterase inhibitor | double-blind, placebo-controlled, crossover study of 25 subjects with mild allergic asthma | reduced eosinophilic and neutrophilic allergen-induced inflammation | Gauvreau et al. [213] |
| Roflumilast with montelukast | type 4 cAMP phosphodiesterase inhibitor and leukotriene receptor antagonist | randomized, double-blind, placebo-controlled, multiple-dose, two-sequence, crossover study of 64 patients with uncontrolled mild-to-moderate asthma | improved lung function and symptom control in moderate-to-severe asthma | Bateman et al. [214] |
| SCH527123 | selective CXCR2 receptor antagonist | randomized, double-blind, parallel study of 34 patients with severe asthma | reduced the rate of mild exacerbations and the sputum neutrophil count | Nair et al. [215] |
| Fevipiprant | non-steroidal prostaglandin D2 receptor antagonist | a systemic review of five articles, including seven randomized trials | increased in FEV1 pre- and post-bronchodilator, did not improve in ACQ score or reduce the number of exacerbations | Jahangir et al. [216] |
| GB001 | selective CRTH2 antagonist | randomized, double-blind, placebo-controlled, dose-ranging, parallel-group, multicenter study of 480 patients with moderate-to-severe asthma with a blood eosinophil count ≥ 250 cells/μL | did not result in a statistically significant improvement in the annual frequency of exacerbations; extended the time to first deterioration; resulted in elevated liver function tests, leading to discontinuation of the treatment | Moss et al. [217] |
| Golimumab | a human anti-TNF-α antibody | multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of 309 patients with severe and uncontrolled asthma | failed to improve FEV1 or reduce exacerbations; resulted in an unfavorable side effect profile, due to which its use was discontinued | Wenzel et al. [218] |
| Etanercept | recombinant TNF-α receptor | randomized, parallel, double-blind, placebo-controlled study of 131 subjects with moderate-to-severe persistent asthma | did not improve pred. FEV1, morning PEF, AHR, symptom control, or quality of life, and did not reduce the frequency of exacerbations | Holgate et al. [219] |
| Brodalumab | human monoclonal antibody against the IL-17 receptor | randomized, double-blind, placebo-controlled study of 302 subjects with inadequately controlled moderate-to-severe asthma | did not improve asthma control and FEV1 in patients with moderate-to-severe uncontrolled asthma who were taking regular inhaled corticosteroids | Busse et al. [220] |
| Secukinumab | anti-Il-17 antibody | a randomized, double-blind, placebo-controlled study of 46 patients with asthma inadequately controlled with ICS-LABA | did not improve asthma control | Novartis Pharmaceuticals [221] |
| Risankizumab | monoclonal antibody against IL-23p19 | multicenter, randomized, double-blind, placebo-controlled, parallel-group trial of 204 patients with severe asthma | resulted in a shorter time to the first exacerbation | Brightling et al. [222] |
| Astegolimab | monoclonal antibody against the ST2 receptor | randomized, double-blind, placebo-controlled, dose-ranging study of 502 adults with severe asthma | reduced annual exacerbation rate, increased time to first exacerbation, improved quality of life and lung function | Kelsen et al. [223] |
| Ipetekimab | monoclonal antibody against IL-33 | randomized, double-blind, placebo-controlled study of 296 adults with moderate-to-severe asthma | reduced mean blood eosinophil count, and improved lung function and quality of life | Wechsler et al. [224] |
| Tralokinumab | human monoclonal antibody against IL-13 | randomized, double-blind, parallel-group, placebo-controlled study of participants aged 12–75 years with severe asthma | did not significantly reduce the annual rate of asthma exacerbations (AER) in the all-subject population; improved AER in the high FeNO group | Panettieri et al. [225] |
| Lebrikizumab | human monoclonal antibody against IL-13 | randomized, double-blind, placebo-controlled study of 1081 patients with uncontrolled asthma | an increase in the time to the first exacerbation and a decrease in FeNO were observed in LAVOLTA I and LAVOLTA II; no statistically significant reduction in the frequency of asthma exacerbations was observed. | Hanania et al. [226] |
| Zweimab and doppelmab | bispecific antibody | study design of monovalent bispecific antibody format, called Zweimab, and a bivalent bispecific antibody, Doppelmab | inhibited Th2-high and Th2-low inflammation due to their targeting of TSLP and IL-13 | Venkataramani et al. [227] |
| Bronchial Thermoplasty | method of reducing ASM hypertrophy | RISA: randomized study of 32 subjects with symptomatic, severe asthma AIR: randomized study of 112 patients with moderate or severe asthma AIR2: randomized, double-blind, sham-controlled study of 297 patients with severe asthma BT10+: follow-up study of 192 participants who were previously enrolled in the AIR, RISA, and AIR2 trials and who had 10 or more years of follow-up since bronchial thermoplasty treatment | Studies showed significant improvements in ACQ and AQLQ and reductions in the use of rescue medications and severe exacerbations; clinical observations regarding lung function were conflicting Assessment of the effects and safety of BT ≥10 years after BT showed a similar incidence of severe exacerbations at the BT10+ visit and one year after the procedure; quality of life and lung function scores at one, five, and ten years after BT were similar. | Pavord et al. [228] Castro et al. [229] Chupp et al. [230] Pavord et al. [231] Thomson et al. [232] Chaudhuri et al. [233] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olejnik, A.E.; Kuźnar-Kamińska, B. Association of Obesity and Severe Asthma in Adults. J. Clin. Med. 2024, 13, 3474. https://doi.org/10.3390/jcm13123474
Olejnik AE, Kuźnar-Kamińska B. Association of Obesity and Severe Asthma in Adults. Journal of Clinical Medicine. 2024; 13(12):3474. https://doi.org/10.3390/jcm13123474
Chicago/Turabian StyleOlejnik, Aneta Elżbieta, and Barbara Kuźnar-Kamińska. 2024. "Association of Obesity and Severe Asthma in Adults" Journal of Clinical Medicine 13, no. 12: 3474. https://doi.org/10.3390/jcm13123474
APA StyleOlejnik, A. E., & Kuźnar-Kamińska, B. (2024). Association of Obesity and Severe Asthma in Adults. Journal of Clinical Medicine, 13(12), 3474. https://doi.org/10.3390/jcm13123474








