Unraveling the Enigma of Moderate Aortic Stenosis: Challenges and Future Prospects
Abstract
1. Introduction
2. Epidemiology
Risk Factor and Comorbidities
3. Diagnosis
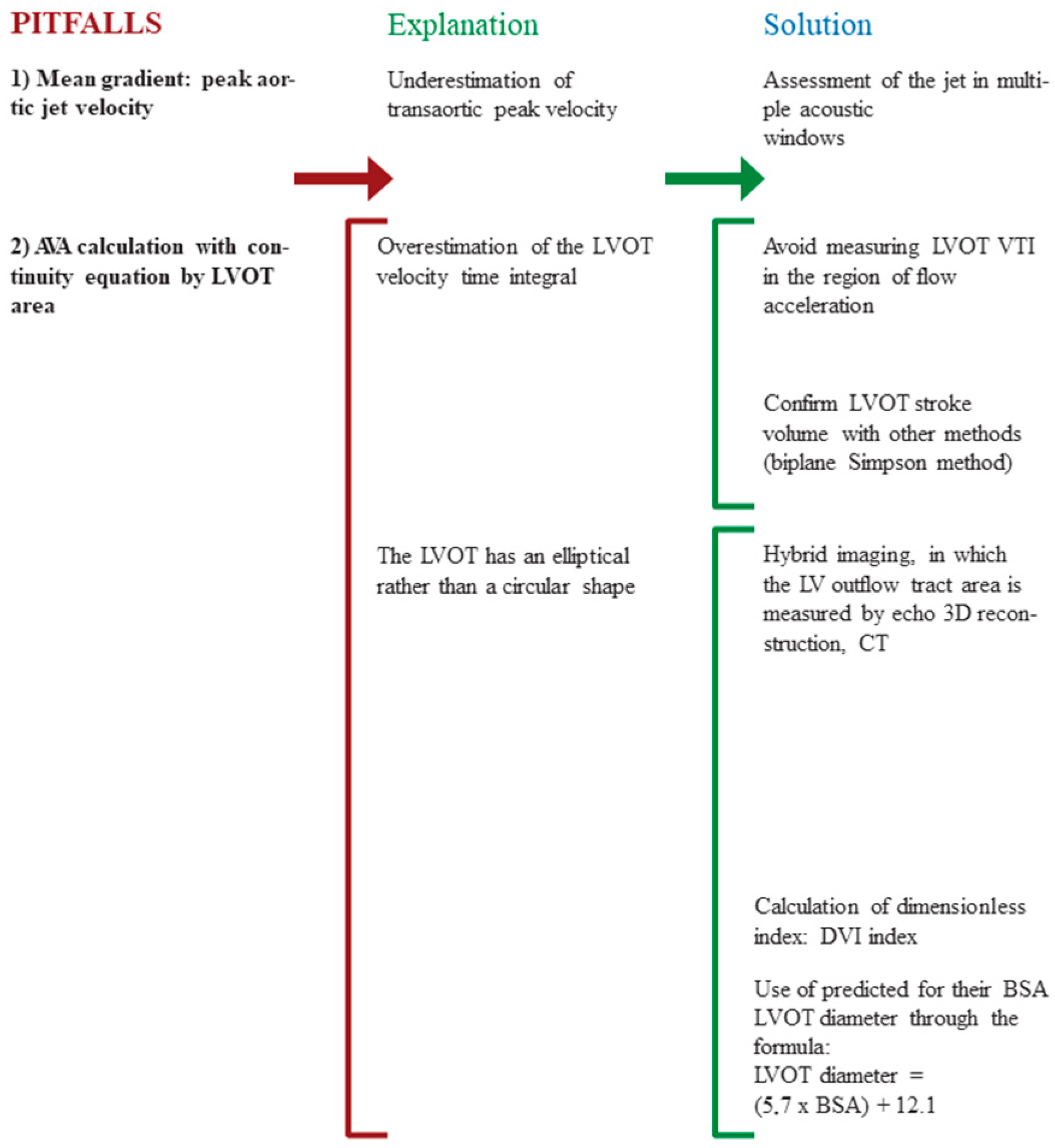
3.1. Pitfalls
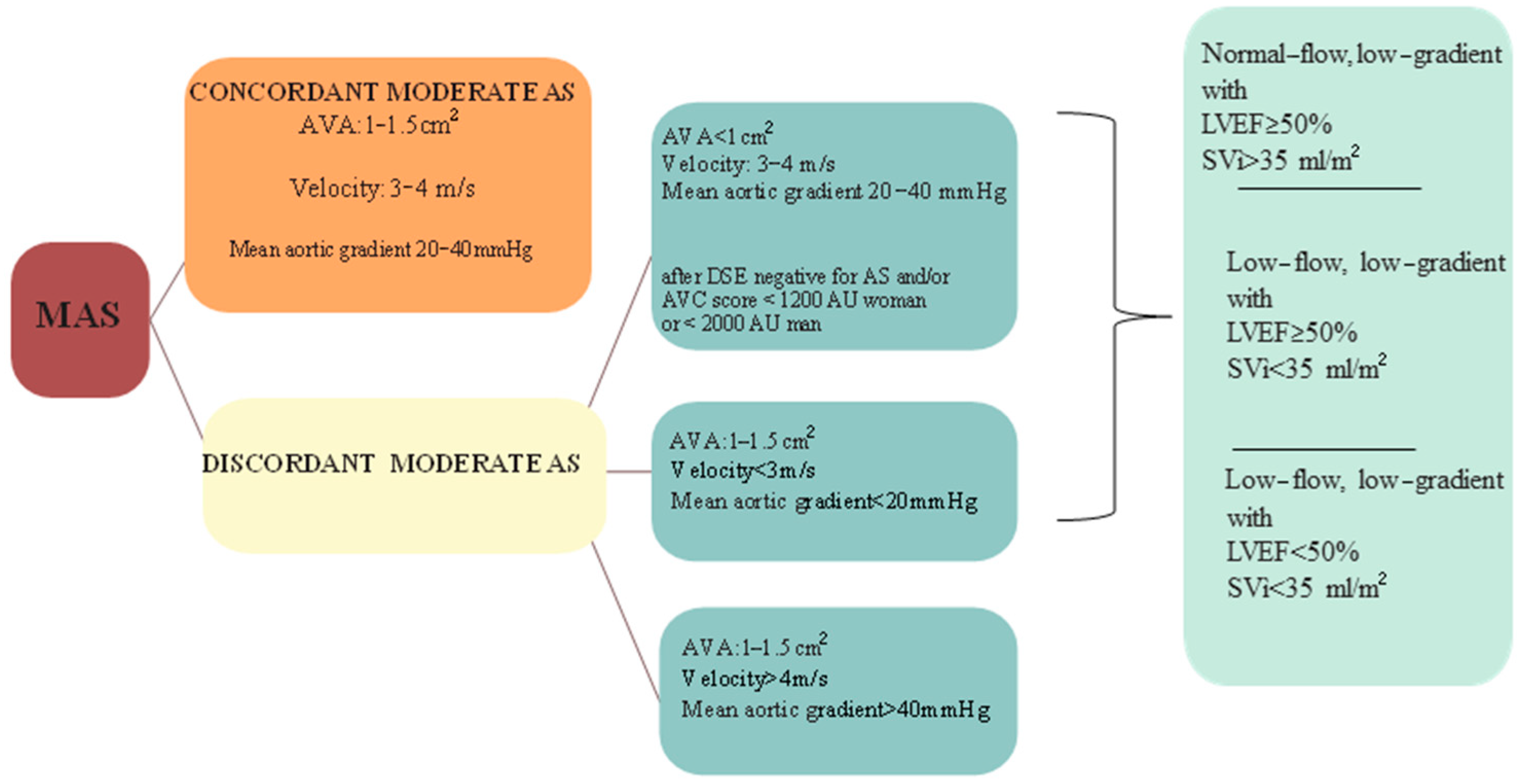
3.2. Discordant Moderate AS
- Severe AS by AVA and moderate AS by pressure gradient (AVA < 1 cm2, mean pressure gradient < 40 mmHg) in the presence of low-flow state (SV index < 35 mL/m2): low-flow low gradient AS; subclassified in “classical” when LVEF is <50% or “paradoxical” when LVEF is >50%.
- Moderate AS by AVA and severe by high pressure gradient (AVA 1–1.5 cm2 and mean pressure gradient > 40 mmHg).
- Moderate AS by AVA and mild AS by pressure gradient (AVA 1–1.5 cm2, mean pressure gradient < 20 mmHg).
4. Progression and Prognosis
Moderate Aortic Stenosis and Left Ventricular Disfunction
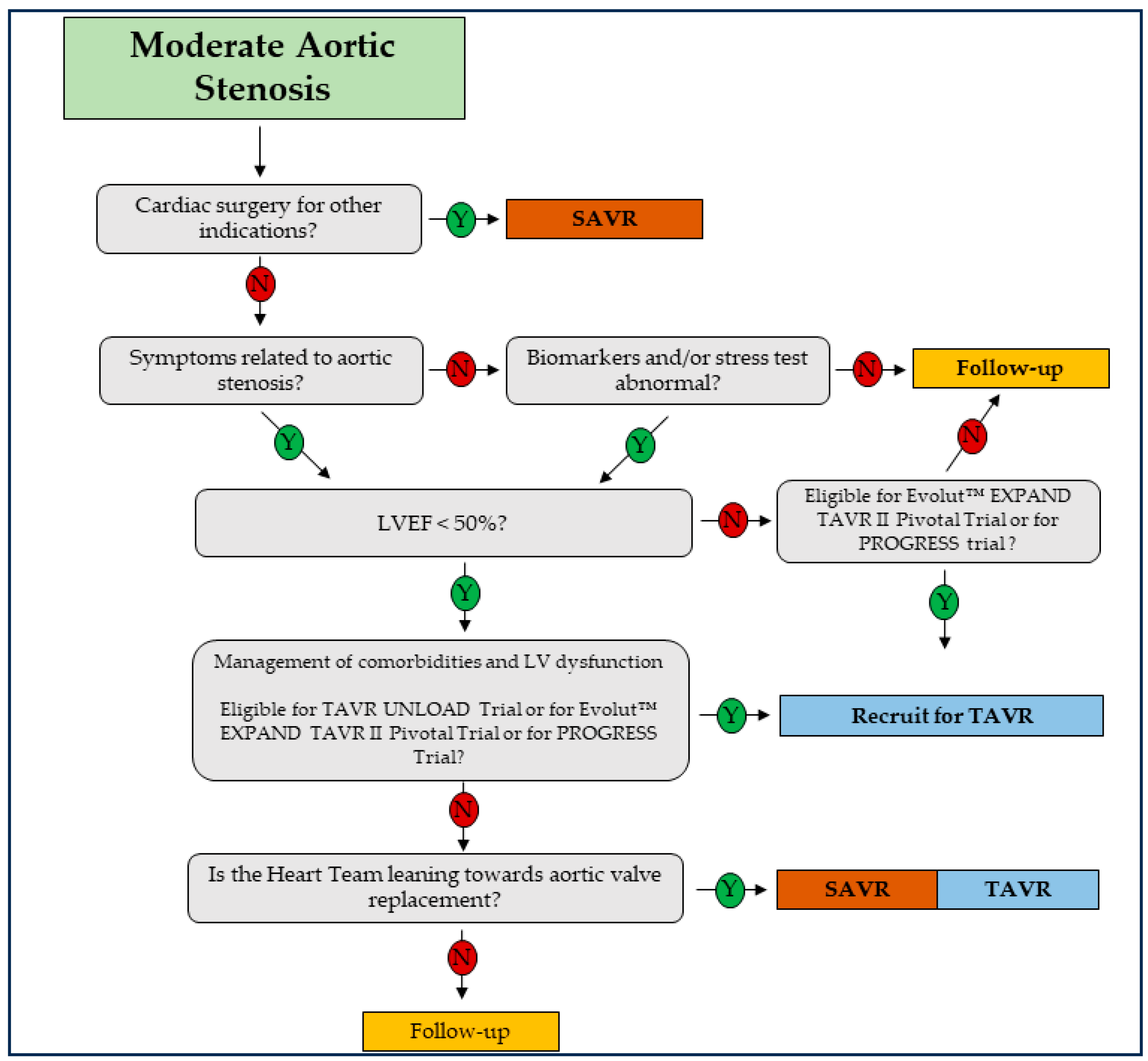
5. Therapy
Non-Medical Therapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F.; Otto, C.M. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 18, 254–275. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Pankayatselvan, V.; Raber, I.; Playford, D.; Stewart, S.; Strange, G.; Strom, J.B. Moderate aortic stenosis: Culprit or bystander? Open Heart 2022, 9, e001743. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, Y.; Coisne, A.; Altes, A.; Levy, F.; Di Lena, C.; Aghezzaf, S.; Maréchaux, S.; Rusinaru, D.; Tribouilloy, C. Is “moderate” aortic stenosis still the right name? A review of the literature. Arch. Cardiovasc. Dis. 2023, 116, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Dweck, M.R.; Boon, N.A.; Newby, D.E. Calcific aortic stenosis: A disease of the valve and the myocardium. J. Am. Coll. Cardiol. 2012, 60, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Ambrosy, A.P.; Go, A.S.; Leong, T.K.; Garcia, E.A.; Chang, A.J.; Slade, J.J.; McNulty, E.J.; Mishell, J.M.; Rassi, A.N.; Ku, I.A.; et al. Temporal trends in the prevalence and severity of aortic stenosis within a contemporary and diverse community-based cohort. Int. J. Cardiol. 2023, 384, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic stenosis in the elderly: Disease prevalence and number of candidates for transcatheter aortic valve replacement: A meta-analysis and modeling study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.D.; Loewenstein, I.; Ben Assa, E.; Topilsky, Y. Natural History of Moderate Aortic Stenosis with Preserved and Low Ejection Fraction. J. Am. Soc. Echocardiogr. 2021, 34, 735–743. [Google Scholar] [CrossRef]
- Strange, G.; Stewart, S.; Celermajer, D.; Prior, D.; Scalia, G.M.; Marwick, T.; Ilton, M.; Joseph, M.; Codde, J.; Playford, D. Poor Long-Term Survival in Patients with Moderate Aortic Stenosis. J. Am. Coll. Cardiol. 2019, 74, 1851–1863. [Google Scholar] [CrossRef]
- Yan, A.T.; Koh, M.; Chan, K.K.; Guo, H.; Alter, D.A.; Austin, P.C.; Tu, J.V.; Wijeysundera, H.C.; Ko, D.T. Association between Cardiovascular Risk Factors and Aortic Stenosis: The CANHEART Aortic Stenosis Study. J. Am. Coll. Cardiol. 2017, 69, 1523–1532. [Google Scholar] [CrossRef]
- Kaltoft, M.; Langsted, A.; Nordestgaard, B.G. Obesity as a Causal Risk Factor for Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2020, 75, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Kontogeorgos, S.; Thunstrom, E.; Lappas, G.; Rosengren, A.; Fu, M. Cumulative incidence and predictors of acquired aortic stenosis in a large population of men followed for up to 43 years. BMC Cardiovasc. Disord. 2022, 22, 43. [Google Scholar] [CrossRef] [PubMed]
- Mangner, N.; Woitek, F.J.; Haussig, S.; Holzhey, D.; Stachel, G.; Schlotter, F.; Hollriegel, R.; Mohr, F.W.; Schuler, G.; Linke, A. Impact of active cancer disease on the outcome of patients undergoing transcatheter aortic valve replacement. J. Interv. Cardiol. 2018, 31, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Frattini, S.; Troise, G.; Fucci, C.; Pressman, G.S.; Faggiano, P. Aortic valve stenosis and cancer: A common and complex association. Expert. Rev. Cardiovasc. Ther. 2021, 19, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, P.; Frattini, S.; Zilioli, V.; Rossi, A.; Nistri, S.; Dini, F.L.; Lorusso, R.; Tomasi, C.; Cas, L.D. Prevalence of comorbidities and associated cardiac diseases in patients with valve aortic stenosis. Potential implications for the decision-making process. Int. J. Cardiol. 2012, 159, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Capoulade, R.; Chan, K.L.; Yeang, C.; Mathieu, P.; Bosse, Y.; Dumesnil, J.G.; Tam, J.W.; Teo, K.K.; Mahmut, A.; Yang, X.; et al. Oxidized Phospholipids, Lipoprotein(a), and Progression of Calcific Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2015, 66, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, Y.; van der Toorn, J.E.; Singh, S.S.; Zheng, K.H.; Kavousi, M.; Sijbrands, E.J.G.; Stroes, E.S.G.; Vernooij, M.W.; de Rijke, Y.B.; Boekholdt, S.M.; et al. Lipoprotein(a) is associated with the onset but not the progression of aortic valve calcification. Eur. Heart J. 2022, 43, 3960–3967. [Google Scholar] [CrossRef]
- Stulnig, T.M.; Morozzi, C.; Reindl-Schwaighofer, R.; Stefanutti, C. Looking at Lp(a) and Related Cardiovascular Risk: From Scientific Evidence and Clinical Practice. Curr. Atheroscler. Rep. 2019, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, P.; Perrone-Filardi, P. Dangerous relationships: Aortic stenosis and transthyretin cardiac amyloidosis. Eur. Heart J. 2017, 38, 2888–2889. [Google Scholar] [CrossRef]
- Pesko, G.; Jenei, Z.; Varga, G.; Apor, A.; Vago, H.; Czibor, S.; Prohaszka, Z.; Masszi, T.; Pozsonyi, Z. Coexistence of aortic valve stenosis and cardiac amyloidosis: Echocardiographic and clinical significance. Cardiovasc. Ultrasound 2019, 17, 32. [Google Scholar] [CrossRef]
- Stassen, J.; Ewe, S.H.; Pio, S.M.; Pibarot, P.; Redfors, B.; Leipsic, J.; Genereux, P.; Van Mieghem, N.M.; Kuneman, J.H.; Makkar, R.; et al. Managing Patients with Moderate Aortic Stenosis. JACC Cardiovasc. Imaging 2023, 16, 837–855. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Munoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Kebed, K.; Sun, D.; Addetia, K.; Mor-Avi, V.; Markuzon, N.; Lang, R.M. Measurement errors in serial echocardiographi.c assessments of aortic valve stenosis severity. Int. J. Cardiovasc. Imaging 2020, 36, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Blais, C.; Burwash, I.G.; Mundigler, G.; Dumesnil, J.G.; Loho, N.; Rader, F.; Baumgartner, H.; Beanlands, R.S.; Chayer, B.; Kadem, L.; et al. Projected Valve Area at Normal Flow Rate Improves the Assessment of Stenosis Severity in Patients With Low-Flow, Low-Gradient Aortic Stenosis: The multicenter TOPAS (Truly or Pseudo-Severe Aortic Stenosis) study. Circulation 2006, 113, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Unger, P.; Powers, A.; Le Nezet, E.; Lacasse-Rioux, E.; Galloo, X.; Clavel, M.A. Prevalence and Outcomes of Patients with Discordant High-Gradient Aortic Stenosis. J. Am. Coll. Cardiol. 2024, 83, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- O’sullivan, C.J.; O’sullivan, D. Discordant High-Gradient Aortic Stenosis: Trust the Gradient. J. Am. Coll. Cardiol. 2024, 83, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- Vulesevic, B.; Burwash, I.G.; Beauchesne, L.M.; Cimadevilla, C.; Siouti, L.; Tubiana, S.; Duval, X.; Nguyen, V.; Arangalage, D.; Chan, K.L.; et al. Outcomes of Patients with Discordant High-Gradient Aortic Valve Stenosis. JACC Cardiovasc. Imaging 2020, 13, 1636–1638. [Google Scholar] [CrossRef] [PubMed]
- Stassen, J.; Ewe, S.H.; Singh, G.K.; Butcher, S.C.; Hirasawa, K.; Amanullah, M.R.; Pio, S.M.; Sin, K.Y.K.; Ding, Z.P.; Sia, C.H.; et al. Prevalence and Prognostic Implications of Discordant Grading and Flow-Gradient Patterns in Moderate Aortic Stenosis. J. Am. Coll. Cardiol. 2022, 80, 666–676. [Google Scholar] [CrossRef]
- Seo, J.H.; Kim, K.H.; Chun, K.J.; Lee, B.K.; Cho, B.R.; Ryu, D.R. How can progression be predicted in patients with mild to moderate aortic valve stenosis? Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1146–1153. [Google Scholar] [CrossRef]
- Lancellotti, P.; Magne, J.; Dulgheru, R.; Clavel, M.A.; Donal, E.; Vannan, M.A.; Chambers, J.; Rosenhek, R.; Habib, G.; Lloyd, G.; et al. Outcomes of Patients with Asymptomatic Aortic Stenosis Followed Up in Heart Valve Clinics. JAMA Cardiol. 2018, 3, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, P.; Ghizzoni, G.; Sorgato, A.; Sabatini, T.; Simoncelli, U.; Gardini, A.; Rusconi, C. Rate of progression of valvular AS in adults. Am J Cardiol. 1992, 70, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, P.; Antonini-Canterin, F.; Erlicher, A.; Romeo, C.; Cervesato, E.; Pavan, D.; Piazza, R.; Huang, G.; Nicolosi, G.L. Progression of aortic valve sclerosis to aortic stenosis. Am. J. Cardiol. 2003, 91, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.W.; Gershlick, A.H.; Balcon, R. Progression of valvar AS: A long-term retrospective study. Eur. Heart J. 1991, 12, 10–14. [Google Scholar] [CrossRef]
- Kebed, K.; Sun, D.; Addetia, K.; Mor-Avi, V.; Markuzon, N.; Lang, R.M. Progression of aortic stenosis and echocardiographic criteria for its severity. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Kearney, L.G.; Ord, M.; Buxton, B.F.; Matalanis, G.; Patel, S.K.; Burrell, L.M.; Srivastava, P.M. Progression of aortic stenosis in elderly patients over long-term follow up. Int. J. Cardiol. 2013, 167, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Voisine, M.; Hervault, M.; Shen, M.; Boilard, A.J.; Filion, B.; Rosa, M.; Bosse, Y.; Mathieu, P.; Cote, N.; Clavel, M.A. Age, Sex, and Valve Phenotype Differences in Fibro-Calcific Remodeling of Calcified Aortic Valve. J. Am. Heart Assoc. 2020, 9, e015610. [Google Scholar] [CrossRef] [PubMed]
- Willner, N.; Prosperi-Porta, G.; Lau, L.; Nam Fu, A.Y.; Boczar, K.; Poulin, A.; Di Santo, P.; Unni, R.R.; Visintini, S.; Ronksley, P.E.; et al. Aortic Stenosis Progression: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2023, 16, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Kim, W.D.; Kim, S.; Ko, K.Y.; Seong, Y.; Kim, D.Y.; Seo, J.; Shim, C.Y.; Ha, J.W.; Mori, M.; et al. Reclassification of moderate aortic stenosis based on data-driven phenotyping of hemodynamic progression. Sci. Rep. 2023, 13, 6694. [Google Scholar] [CrossRef]
- Mason, D.T.; Braunwald, E.; Ross, J., Jr.; Morrow, A.G. Diagnostic value of the first and second derivatives of the arteriale pressure pulse in aortic valve disease and hypertrophic subas. Circulation 1964, 30, 90–100. [Google Scholar] [CrossRef]
- Alzubi, J.; Pressman, G.S. Aortic stenosis: New insights into predicting disease progression. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1154–1155. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Pearlman, A.S.; Gardner, C.L. Hemodynamic progression of aortic stenosis in adults assessed by Doppler echocardiography. J. Am. Coll. Cardiol. 1989, 13, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Murtazalieva, P.; Ryzhkova, D.; Malev, E.; Zhiduleva, E.; Moiseeva, O. Prediction of AS Progression by 18F-FDG and 18F-NaF PET/CT in Different Aortic Valve Phenotypes. Front. Pharmacol. 2022, 13, 909975. [Google Scholar] [CrossRef] [PubMed]
- Peeters, F.E.C.M.; Van Mourik, M.J.W.; Meex, S.J.R.; Bucerius, J.; Schalla, S.M.; Gerretsen, S.C.; Mihl, C.; Dweck, M.R.; Schurgers, L.J.; Wildberger, J.E.; et al. Bicuspid Aortic Valve Stenosis and the Effect of Vitamin K2 on Calcification Using 18F-Sodium Fluoride Positron Emission Tomography/Magnetic Resonance: The BASIK2 Rationale and Trial Design. Nutrients 2018, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Hadziselimovic, E.; Greve, A.M.; Sajadieh, A.; Olsen, M.H.; Kesäniemi, Y.A.; Nienaber, C.A.; Ray, S.G.; Rossebø, A.B.; Willenheimer, R.; Wachtell, K.; et al. N-Terminal Pro-Brain Natriuretic Peptide Measurements with Clinical Events in Patients with Asymptomatic Nonsevere AS: A Post Hoc Substudy of the SEAS Trial. JAMA Cardiol. 2022, 7, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.; Quezada-Feijoó, M.; Ayala, R.; Manzano, A.; Gómez-Pavón, F.J.; Jaramillo, J.; Herrera, C.; López Vazquez de la Torre, M.; Toro, R. Value of NT-proBNP and Galectin-3 as Biomarkers in the Follow-Up of Asymptomatic Elderly Patients with Severe AS. J. Clin. Med. 2023, 12, 2987. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, Y.; Renard, C.; Manrique, A.; Levy, F.; Marechaux, S.; Gerber, B.L.; Tribouilloy, C. Usefulness of Cardiac Magnetic Resonance Imaging in Aortic Stenosis. Circ. Cardiovasc. Imaging 2020, 13, e010356. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.W.L.; Everett, R.J.; Kwiecinski, J.; Vesey, A.T.; Yeung, E.; Esson, G.; Jenkins, W.; Koo, M.; Mirsadraee, S.; White, A.C.; et al. Myocardial Fibrosis and Cardiac Decompensation in Aortic Stenosis. JACC Cardiovasc. Imaging 2017, 10, 1320–1333. [Google Scholar] [CrossRef]
- Stassen, J.; Ewe, S.H.; Hirasawa, K.; Butcher, S.C.; Singh, G.K.; Amanullah, M.R.; Sin, K.Y.K.; Ding, Z.P.; Pio, S.M.; Chew, N.W.S.; et al. Left ventricular remodelling patterns in patients with moderate aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1326–1335. [Google Scholar] [CrossRef]
- Zhu, D.; Ito, S.; Miranda, W.R.; Nkomo, V.T.; Pislaru, S.V.; Villarraga, H.R.; Pellikka, P.A.; Crusan, D.J.; Oh, J.K. Left Ventricular Global Longitudinal Strain Is Associated with Long-Term Outcomes in Moderate Aortic Stenosis. Circ. Cardiovasc. Imaging 2020, 13, e009958. [Google Scholar] [CrossRef]
- Tastet, L.; Tribouilloy, C.; Marechaux, S.; Vollema, E.M.; Delgado, V.; Salaun, E.; Shen, M.; Capoulade, R.; Clavel, M.A.; Arsenault, M.; et al. Staging Cardiac Damage in Patients with Asymptomatic Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2019, 74, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Rosenhek, R.; Klaar, U.; Schemper, M.; Scholten, C.; Heger, M.; Gabriel, H.; Binder, T.; Maurer, G.; Baumgartner, H. Mild and moderate aortic stenosis: Natural history and risk stratification by echocardiography. Eur. Heart J. 2004, 25, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Fougeres, E.; Tribouilloy, C.; Monchi, M.; Petit-Eisenmann, H.; Baleynaud, S.; Pasquet, A.; Chauvel, C.; Metz, D.; Adams, C.; Rusinaru, D.; et al. Outcomes of pseudo-severe aortic stenosis under conservative treatment. Eur. Heart J. 2012, 33, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- Stassen, J.; Ewe, S.H.; Butcher, S.C.; Amanullah, M.R.; Mertens, B.J.; Hirasawa, K.; Singh, G.K.; Sin, K.Y.; Ding, Z.P.; Pio, S.M.; et al. Prognostic implications of left ventricular diastolic dysfunction in moderate aortic stenosis. Heart 2022, 108, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.A.; Burwash, I.G.; Pibarot, P. Cardiac Imaging for Assessing Low-Gradient Severe Aortic Stenosis. JACC Cardiovasc. Imaging 2017, 10, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Samad, Z.; Vora, A.N.; Dunning, A.; Schulte, P.J.; Shaw, L.K.; Al-Enezi, F.; Ersboll, M.; McGarrah, R.W., 3rd; Vavalle, J.P.; Shah, S.H.; et al. Aortic valve surgery and survival in patients with moderate or severe aortic stenosis and left ventricular dysfunction. Eur. Heart J. 2016, 37, 2276–2286. [Google Scholar] [CrossRef]
- Spitzer, E.; Ren, B.; Kroon, H.; van Gils, L.; Manintveld, O.; Daemen, J.; Zijlstra, F.; de Jaegere, P.P.; Geleijnse, M.L.; Van Mieghem, N.M. Moderate Aortic Stenosis and Reduced Left Ventricular Ejection Fraction: Current Evidence and Challenges Ahead. Front. Cardiovasc. Med. 2018, 5, 111. [Google Scholar] [CrossRef] [PubMed]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Huded, C.P.; Desai, M.Y. Moderate aortic valve stenosis in patients with left ventricular systolic dysfunction-insights on prognosis and the potential role of early aortic valve replacement. J. Thorac. Dis. 2017, 9, 3590–3593. [Google Scholar] [CrossRef]
- van Gils, L.; Clavel, M.A.; Vollema, E.M.; Hahn, R.T.; Spitzer, E.; Delgado, V.; Nazif, T.; De Jaegere, P.P.; Geleijnse, M.L.; Ben-Yehuda, O.; et al. Prognostic Implications of Moderate Aortic Stenosis in Patients with Left Ventricular Systolic Dysfunction. J. Am. Coll. Cardiol. 2017, 69, 2383–2392. [Google Scholar] [CrossRef]
- Généreux, P.; Sharma, R.P.; Cubeddu, R.J.; Aaron, L.; Abdelfattah, O.M.; Koulogiannis, K.P.; Marcoff, L.; Naguib, M.; Kapadia, S.R.; Makkar, R.R.; et al. The Mortality Burden of Untreated AS. J. Am. Coll. Cardiol. 2023, 82, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.R.; Khan, O.A.; Rassa, A.C.; Elman, M.R.; Chadderdon, S.M.; Song, H.K.; Golwala, H.; Cigarroa, J.E.; Zahr, F.E. Clinical and Echocardiographic Predictors of Outcomes in Patients with Moderate (Mean Transvalvular Gradient 20 to 40 mm Hg) Aortic Stenosis. Am. J. Cardiol. 2019, 124, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Elmariah, S.; Patel, N.K. Aortic Stenosis and LV Dysfunction: Not Everything in Moderation. J. Am. Coll. Cardiol. 2021, 77, 2804–2806. [Google Scholar] [CrossRef] [PubMed]
- Sabini, A.; Bolognese, L. Moderate aortic stenosis and left ventricular dysfunction: An ominous association. Eur. Heart J. Suppl. 2019, 21, B15–B16. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Magne, J. Valvuloarterial impedance in aortic stenosis: Look at the load, but do not forget the flow. Eur. J. Echocardiogr. 2011, 12, 354–357. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Monin, J.L.; Quere, J.P.; Monchi, M.; Petit, H.; Baleynaud, S.; Chauvel, C.; Pop, C.; Ohlmann, P.; Lelguen, C.; Dehant, P.; et al. Low-gradient aortic stenosis: Operative risk stratification and predictors for long-term outcome: A multicenter study using dobutamine stress hemodynamics. Circulation 2003, 108, 319–324. [Google Scholar] [CrossRef]
- Pibarot, P.; Messika-Zeitoun, D.; Ben-Yehuda, O.; Hahn, R.T.; Burwash, I.G.; Van Mieghem, N.M.; Spitzer, E.; Leon, M.B.; Bax, J.; Otto, C.M. Moderate Aortic Stenosis and Heart Failure with Reduced Ejection Fraction: Can Imaging Guide Us to Therapy? JACC Cardiovasc. Imaging 2019, 12, 172–184. [Google Scholar] [CrossRef]
- Smith, N.; McAnulty, J.H.; Rahimtoola, S.H. Severe aortic stenosis with impaired left ventricular function and clinical heart failure: Results of valve replacement. Circulation 1978, 58, 255–264. [Google Scholar] [CrossRef]
- Stewart, W.J. Aortic Stenosis Is Still Very Tricky, Especially When it Is Moderate. J. Am. Coll. Cardiol. 2017, 69, 2393–2396. [Google Scholar] [CrossRef]
- Cowell, S.J.; Newby, D.E.; Prescott, R.J.; Bloomfield, P.; Reid, J.; Northridge, D.B.; Boon, N.A. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N. Engl. J. Med. 2005, 352, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Rossebo, A.B.; Pedersen, T.R.; Boman, K.; Brudi, P.; Chambers, J.B.; Egstrup, K.; Gerdts, E.; Gohlke-Barwolf, C.; Holme, I.; Kesaniemi, Y.A.; et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N. Engl. J. Med. 2008, 359, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.; Teo, K.; Dumesnil, J.G.; Ni, A.; Tam, J.; ASTRONOMER Investigators. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: Results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010, 121, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Roos, C.; Hagler, M.; Verzosa, G.; Zhang, H.; Schaff, H.; Sarano, M.; Miller, J. Activation of oxidized soluble guanylate cyclase slows progression of aortic valve calcification. Arterioscler. Thromb. Vasc. Biol. 2019, 39, A123. [Google Scholar]
- Lee, S.; Lee, S.A.; Choi, B.; Kim, Y.J.; Oh, S.J.; Choi, H.M.; Kim, E.K.; Kim, D.H.; Cho, G.Y.; Song, J.M.; et al. Dipeptidyl peptidase-4 inhibition to prevent progression of calcific aortic stenosis. Heart 2020, 106, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Pawade, T.A.; Doris, M.K.; Bing, R.; White, A.C.; Forsyth, L.; Evans, E.; Graham, C.; Williams, M.C.; van Beek, E.J.R.; Fletcher, A.; et al. Effect of Denosumab or Alendronic Acid on the Progression of Aortic Stenosis: A Double-Blind Randomized Controlled Trial. Circulation 2021, 143, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Merki, E.; Graham, M.J.; Mullick, A.E.; Miller, E.R.; Crooke, R.M.; Pitas, R.E.; Witztum, J.L.; Tsimikas, S. Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein(a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein(a) transgenic mice. Circulation 2008, 118, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Muth, A.N.; Ransom, J.F.; Schluterman, M.K.; Barnes, R.; King, I.N.; Grossfeld, P.D.; Srivastava, D. Mutations in NOTCH1 cause aortic valve disease. Nature 2005, 437, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Toshima, T.; Watanabe, T.; Narumi, T.; Otaki, Y.; Shishido, T.; Aono, T.; Goto, J.; Watanabe, K.; Sugai, T.; Takahashi, T.; et al. Therapeutic inhibition of microRNA-34a ameliorates aortic valve calcification via modulation of Notch1-Runx2 signalling. Cardiovasc. Res. 2020, 116, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.C.; Bowen, C.J.; Vaidya, K.A.; Zhou, J.; Chapurin, N.; Recknagel, A.; Zhou, B.; Chen, J.; Kotlikoff, M.; Butcher, J.T. Cadherin-11 Overexpression Induces Extracellular Matrix Remodeling and Calcification in Mature Aortic Valves. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1627–1637. [Google Scholar] [CrossRef]
- Clark, C.R.; Bowler, M.A.; Snider, J.C.; Merryman, W.D. Targeting Cadherin-11 Prevents Notch1-Mediated Calcific Aortic Valve Disease. Circulation 2017, 135, 2448–2450. [Google Scholar] [CrossRef] [PubMed]
- Jean, G.; Van Mieghem, N.M.; Gegenava, T.; van Gils, L.; Bernard, J.; Geleijnse, M.L.; Vollema, E.M.; El Azzouzi, I.; Spitzer, E.; Delgado, V.; et al. Moderate Aortic Stenosis in Patients with Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2021, 77, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, E.; Van Mieghem, N.M.; Pibarot, P.; Hahn, R.T.; Kodali, S.; Maurer, M.S.; Nazif, T.M.; Rodes-Cabau, J.; Paradis, J.M.; Kappetein, A.P.; et al. Rationale and design of the Transcatheter Aortic Valve Replacement to UNload the Left ventricle in patients with ADvanced heart failure (TAVR UNLOAD) trial. Am. Heart J. 2016, 182, 80–88. [Google Scholar] [CrossRef] [PubMed]
| Mild | Moderate | Severe | |
|---|---|---|---|
| EOA | >1.5 cm2 | 1.0–1.5 cm2 | <1.0 cm2 |
| EOAi | >0.85 cm2/m2 | 0.6–0.85 cm2/m2 | <0.6 cm2/m2 |
| Gradient | <20 mmHg | 20–40 mmHg | >40 mmHg |
| Velocity | <3.0 m/s | 3.0–4.0 m/s | >4.0 m/s |
| DVI | >0.5 | 0.25–0.5 | <0.25 |
| Study Name | Study Design | Study Population | Number of Patient | Primary End-Point | Result | Conclusion | Cit |
|---|---|---|---|---|---|---|---|
| Poor Long-Term Survival in PatientsWith Moderate Aortic Stenosis | Retrospective, multicentric | All patients with and without AS | 241,303 | All-cause and cardiovascular-related mortality | Increased risk of 5-year mortality: severe AS 3.0-fold and moderate 2.6-fold. | Moderate AS had a high risk of dying in the longer term that was similar to the risk in patients presenting with severe AS at baseline. | [9] |
| Mild and moderate aortic stenosis Natural history and risk stratification by echocardiography | Retrospective, single centre | Moderate and severe AS, normal EF | 176 | All-cause mortality | Survival of patients with mild and moderate AS was significantly worse than that predicted for age- and gender-matched control subjects, with an overall mortality that was 80% higher than that of the general population. | The presence of moderate to severe aortic valve calcification appears to be the most powerful predictor of outcome in mild and moderate AS and should, therefore, be determined in all patients | [52] |
| Natural History of Moderate Aortic Stenosis with Preserved and Low Ejection Fraction | Retrospective, single centre | Moderate AS with a propensity-matched cohort (1:3 ratio) without AS | 952 | All-cause mortality | Patients with moderate AS have increased mortality rates compared with control subjects in an unadjusted and adjusted analysis. | Patients with moderate AS have increased mortality rates in comparison with matched control subjects. This increased mortality is observed in patients with low or preserved EFs and even in patients with low transaortic gradients. | [8] |
| Outcomes of pseudo-severe aortic stenosis under conservative treatment | Retrospective, multicentric | Pseudo-severe low-flow/low-gradient AS defined with stress Echo Dobutamine | 305 | All-cause mortality | Pseudo-severe AS has a better survival rate than true-severe AS and comparable with that of propensity-matched patients with LV systolic dysfunction and no evidence of valve disease. | [53] | |
| Prevalence and Prognostic implications of Discordant Grading and Flow-Gradient Patterns in Moderate Aortic Stenosis | Retrospective, multicentric | Moderate AS with concordant or discordant mean gradient | 1974 | All-cause mortality | Patients with discordant moderate AS showed significantly higher mortality when compared with patients with concordant moderate AS; on multivariable analysis, discordant moderate AS was independently associated with all-cause mortality. | Discordant grading is frequently observed in patients with moderate AS and is associated with increased risk of mortality compared with concordant moderate AS; among patients with discordant grading, the paradoxical and classical low-flow, low-gradient patterns but not the normal-flow, low-gradient pattern, are independently associated with worse outcomes. | [29] |
| Prognostic Implications of Moderate Aortic Stenosis in Patients With Left Ventricular Systolic Dysfunction | Retrospective, multicentric | Moderate and severe AS, reduced EF | 305 | All-cause death, AVR, and HF hospitalization at 4 year follow-up | All-cause death was 36%, HF hospitalization was 27%, and AVR occurred in 24% of patients. | Patients with concomitant moderate AS and LV systolic dysfunction are at high risk for clinical events. | [54] |
| Study Name | Device | Population | Study Design | Type of Study | Primary End-Point | Study Start | Study Completion (Estimated) | Cit |
|---|---|---|---|---|---|---|---|---|
| TAVR UNLOAD | Edwards SAPIEN 3 THV | Moderate AS | Randomized 1:1 Treatment vs. OHFT | International, multicenter, randomized, open-label | Non-hierarchical composite of all-cause death, disabling stroke, hospitalizations related to HF. | 09-2016 | 02-2025 | [11] |
| PROGRESS | Edwards SAPIEN 3/SAPIEN 3 Ultra/SAPIEN 3 Ultra RESILIA THV | Moderate AS | Randomized 1:1 Treatment vs. OHFT | International, multicenter, prospective, randomized, open-label | Non-hierarchical composite of death, and hf hospitalization or event. | 12-10-2021 | 06-2037 | ClinicalTrials.gov ID NCT04889872 |
| EXPAND TAVR II | Medtronic Evolut PRO+ TAVR System, or Evolut FX TAVR System | Moderate AS | Randomized 1:1 Treatment vs. OHFT | International, multicenter, prospective, randomized, open-label | Composite rate of all-cause mortality, hf hospitalization or event, or medical instability leading to aortic valve replacement or re-intervention. | 27-04-2022 | 12-2034 | ClinicalTrials.gov ID NCT05149755 |
| Clinical Medicine Updates for Aortic Valve Stenosis |
|---|
| Despite many observational studies have reported substantial morbidity and mortality associated with moderate AS, guidelines recommended a “watchful waiting” approach |
| Recent data indicates that around 40% of patients with moderate AS develop severe stenosis within approximately 2.5–3 years |
| Advancements in multimodal valve imaging technology have provided fresh insights into predicting disease progression and prognosis |
| AI algorithm capable of predicting progression rate from moderate to severe AS was developed |
| Prognostic value of LV remodeling patterns in moderate AS is associated with an increased risk of all-cause mortality |
| NT-proBNP concentrations are associated with clinical risk in the subsequent two years in individuals with non-severe AS |
| The risk/benefit profile of AVR in moderate AS warrants re-evaluation and should be properly addressed through dedicated clinical trials, several of which are currently ongoing |
| A shift from “standardization” to “individualization” in defining the severity of AS and management strategy is necessary to improve clinical outcomes and quality of life in patients with AS |
| Early interventional approach for moderate AS should be considered in some scenarios |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santangelo, G.; Tumminello, G.; Barbieri, L.; Mallardi, G.P.F.; Faggiano, A.; Moscardelli, S.; Rossi, A.; Cozza, F.; Carugo, S.; Faggiano, P. Unraveling the Enigma of Moderate Aortic Stenosis: Challenges and Future Prospects. J. Clin. Med. 2024, 13, 3478. https://doi.org/10.3390/jcm13123478
Santangelo G, Tumminello G, Barbieri L, Mallardi GPF, Faggiano A, Moscardelli S, Rossi A, Cozza F, Carugo S, Faggiano P. Unraveling the Enigma of Moderate Aortic Stenosis: Challenges and Future Prospects. Journal of Clinical Medicine. 2024; 13(12):3478. https://doi.org/10.3390/jcm13123478
Chicago/Turabian StyleSantangelo, Gloria, Gabriele Tumminello, Lucia Barbieri, Giulio Pio Federico Mallardi, Andrea Faggiano, Silvia Moscardelli, Andrea Rossi, Fabiana Cozza, Stefano Carugo, and Pompilio Faggiano. 2024. "Unraveling the Enigma of Moderate Aortic Stenosis: Challenges and Future Prospects" Journal of Clinical Medicine 13, no. 12: 3478. https://doi.org/10.3390/jcm13123478
APA StyleSantangelo, G., Tumminello, G., Barbieri, L., Mallardi, G. P. F., Faggiano, A., Moscardelli, S., Rossi, A., Cozza, F., Carugo, S., & Faggiano, P. (2024). Unraveling the Enigma of Moderate Aortic Stenosis: Challenges and Future Prospects. Journal of Clinical Medicine, 13(12), 3478. https://doi.org/10.3390/jcm13123478







