Non-Invasive Ultrasound Therapy for Severe Aortic Stenosis: Early Effects on the Valve, Ventricle, and Cardiac Biomarkers (A Case Series)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Device and Procedure
2.3. Endpoints and Definitions
2.4. Statistical Analysis
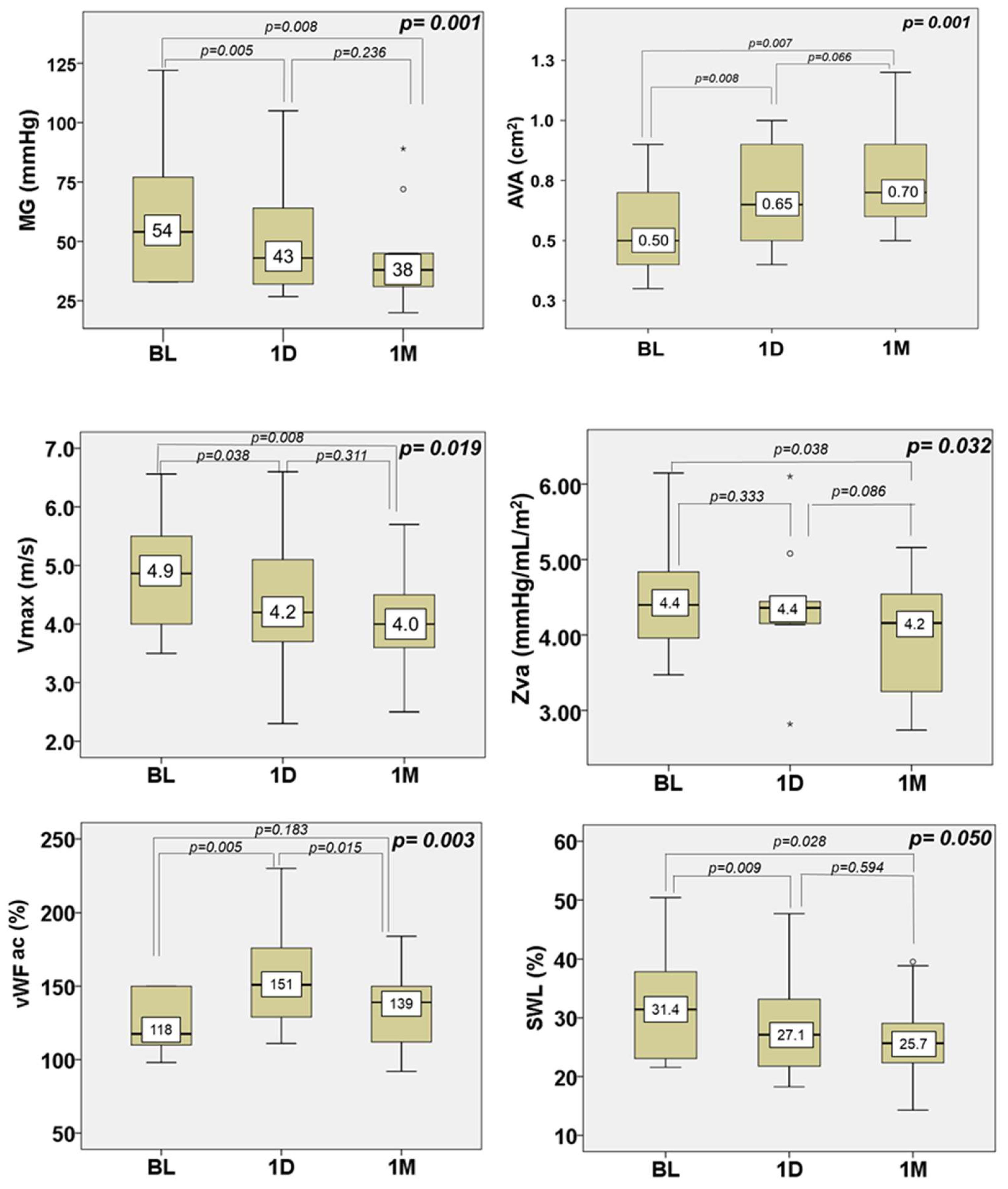
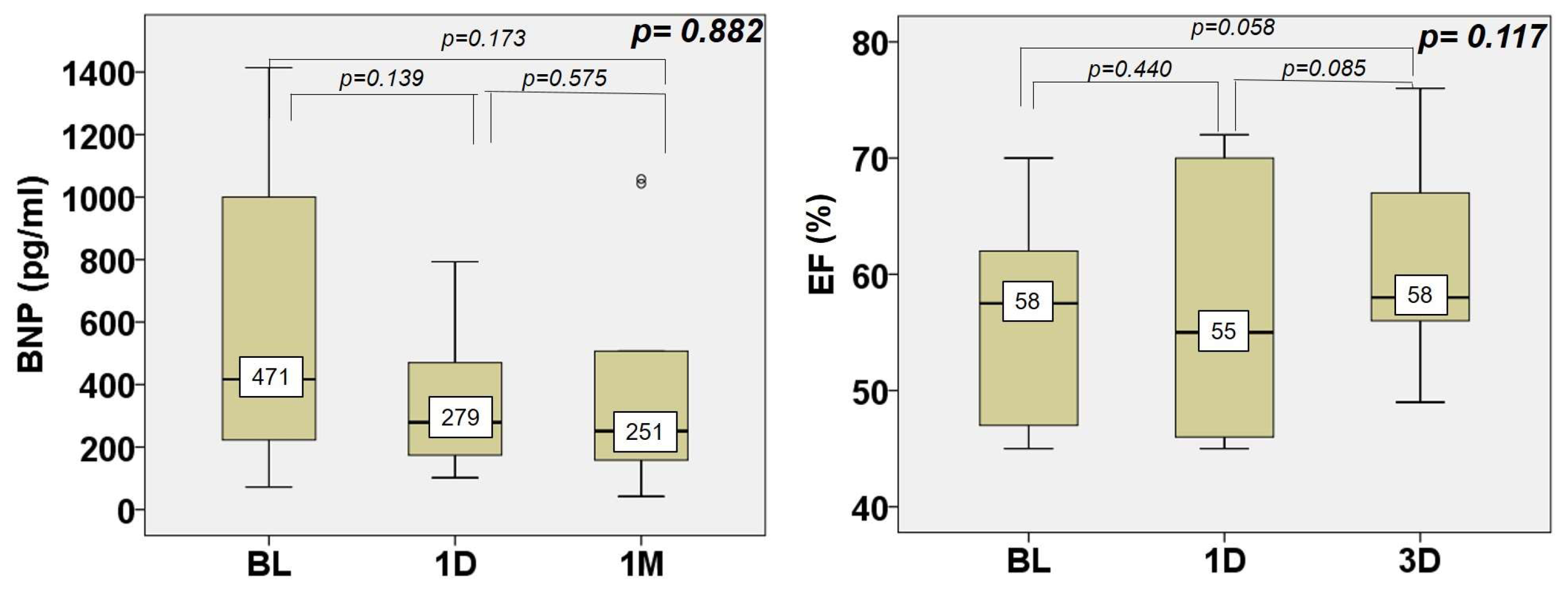
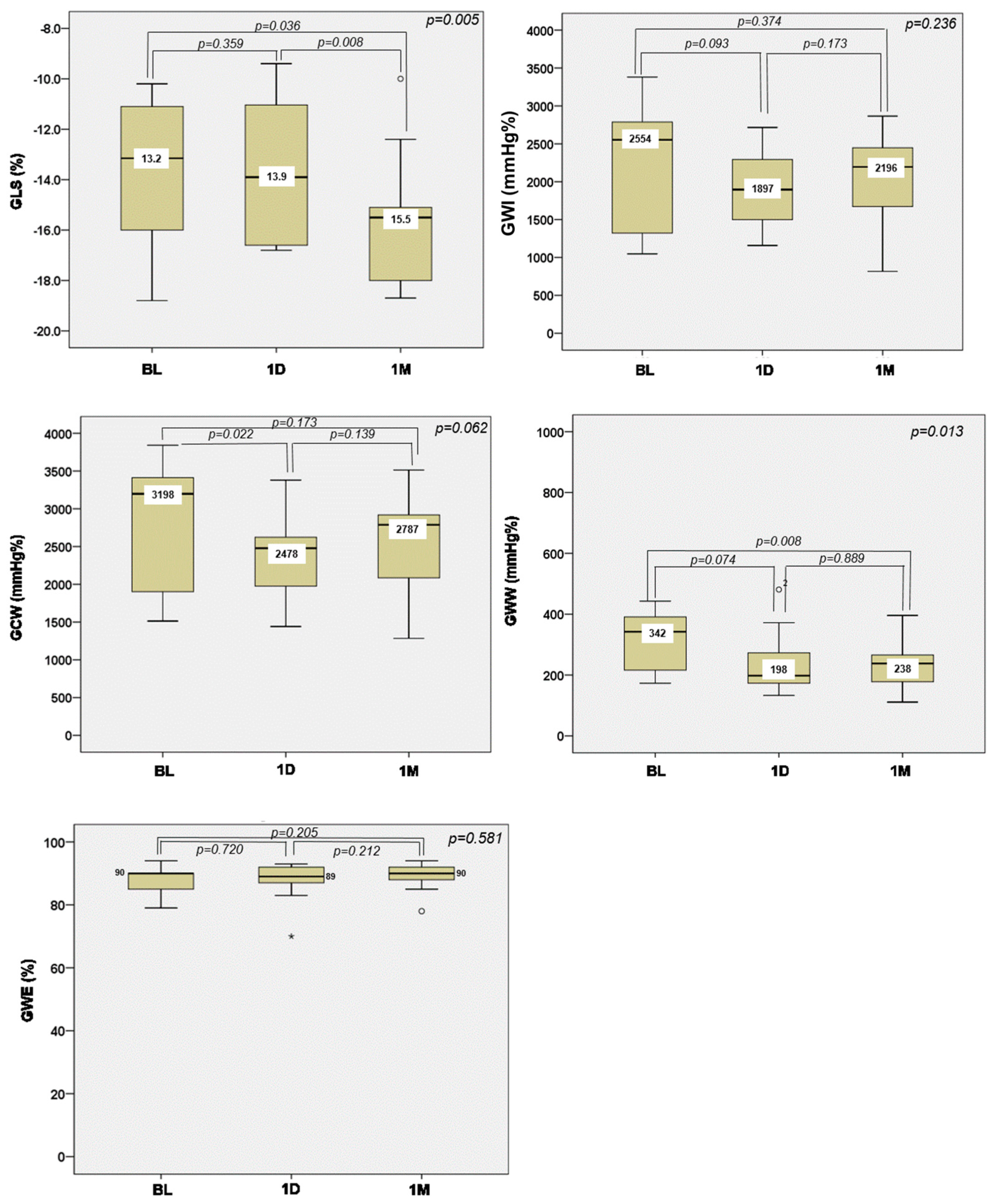
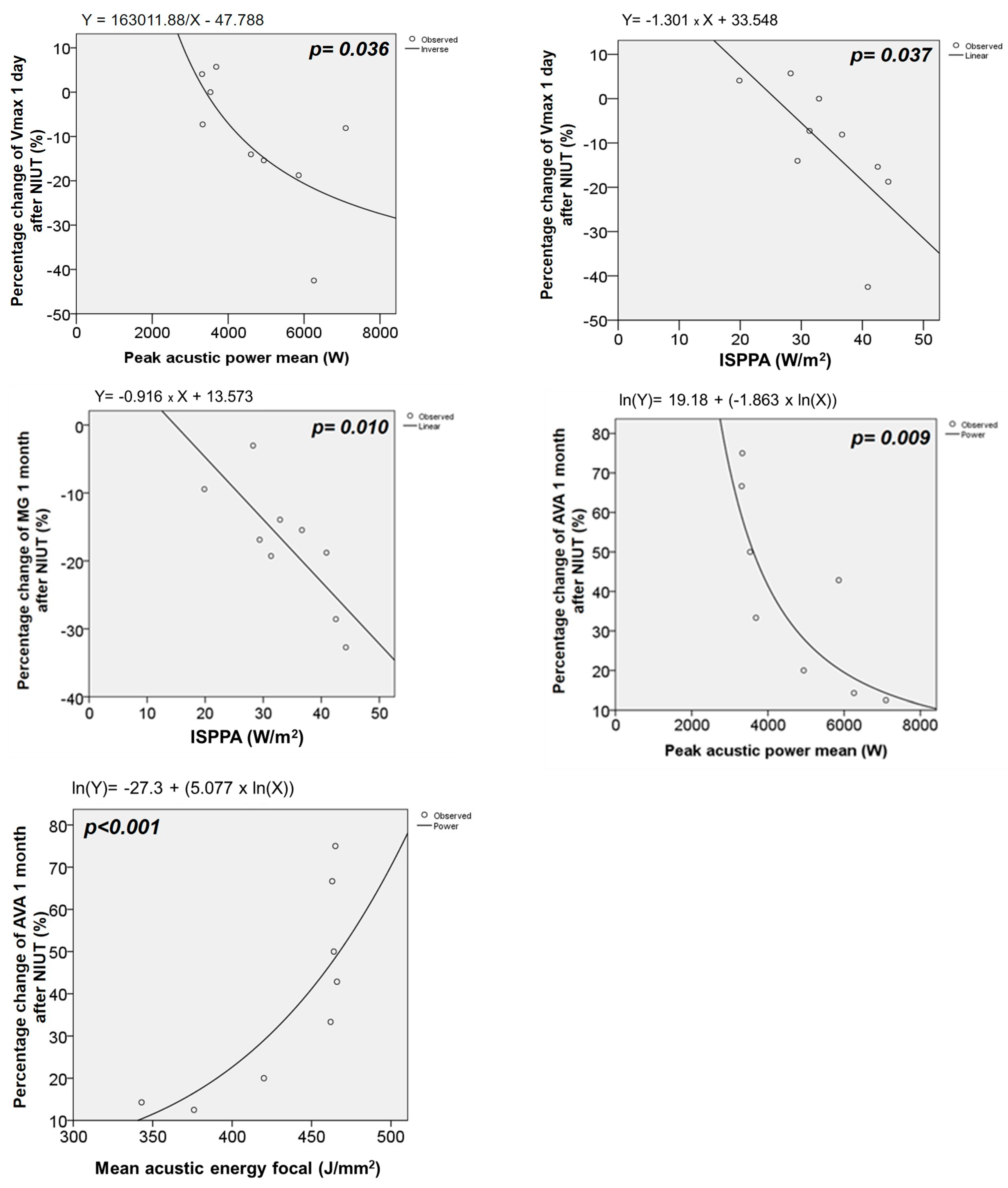
3. Results
4. Discussion
4.1. Effect of NIUT on Aortic Valve Hemodynamics and Valve Shear Stress
4.2. Impact of NIUT on the Left Ventricle
4.3. Safety
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. EuroIntervention 2022, 17, e1126–e1196. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar]
- Durko, A.P.; Osnabrugge, R.L.; Van Mieghem, N.M.; Milojevic, M.; Mylotte, D.; Nkomo, V.T.; Kappetein, A.P. Annual number of candidates for transcatheter aortic valve implantation per country: Current estimates and future projections. Eur. Heart J. 2018, 39, 2635–2642. [Google Scholar] [CrossRef] [PubMed]
- Villemain, O.; Robin, J.; Bel, A.; Kwiecinski, W.; Bruneval, P.; Arnal, B.; Rémond, M.; Tanter, M.; Messas, E.; Pernot, M. Pulsed cavitational ultrasound softening: A new non-invasive therapeutic approach of calcified bioprosthetic valve stenosis. JACC Basic. Transl. Sci. 2017, 2, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Curini, L.; Pesce, M. Shockwaves delivery for aortic valve therapy-Realistic perspective for clinical translation? Front. Cardiovasc. Med. 2023, 10, 1160833. [Google Scholar] [CrossRef] [PubMed]
- Bernava, G.; Fermi, E.; Gelpi, G.; Rizzi, S.; Benettin, D.; Barbuto, M.; Romagnoni, C.; Ventrella, D.; Palmierie, M.C.; Agrifoglio, M.; et al. Lithotripsy of Calcified Aortic Valve Leaflets by a Novel Ultrasound Transcatheter-Based Device. Front. Cardiovasc. Med. 2022, 9, 850393. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bertog, S.; Mbai, M. Transcatheter aortic valve lithotripsy in severely calcified bicuspid aortic stenosis prior to transcatheter aortic valve implantation. Eur. Heart J. 2021, 42, 358. [Google Scholar] [CrossRef]
- Sanz-Ruiz, R.; González-Mansilla, A.; Rivera-Juárez, A.; Bermejo, J.; Fernández-Avilés, F. 1-Step Percutaneous Treatment of Heavily Calcified Left-Heart Valve Stenoses. JACC Cardiovasc. Interv. 2021, 14, e335–e337. [Google Scholar] [CrossRef]
- Messas, E.; Rémond, M.C.; Goudot, G.; Zarka, S.; Penot, R.; Mateo, P.; Kwiecinski, W.; Suarez Escudero, D.; Bel, A.; Ialy-Radio, M.; et al. Feasibility and safety of non-invasive ultrasound therapy (NIUT) on a porcine aortic valve. Phys. Med. Biol. 2020, 65, 215004. [Google Scholar] [CrossRef]
- Trifunovic-Zamaklar, D.; Velinović, M.; Kovačević-Kostić, N.; Messas, E. Systematic brain magnetic resonance imaging and safety evaluation of non-invasive ultrasound therapy for patients with severe symptomatic aortic valve stenosis. Eur. Heart J. Cardiovasc. Imaging 2023, 24, e108–e109. [Google Scholar] [CrossRef]
- Messas, E.; Ijsselmuiden, A.; Trifunović-Zamaklar, D.; Cholley, B.; Puymirat, E.; Halim, J.; Karan, R.; van Gameren, M.; Terzić, D.; Milićević, V.; et al. Treatment of severe symptomatic aortic valve stenosis using non-invasive ultrasound therapy: A cohort study. Lancet 2023, 402, 2317–2325. [Google Scholar] [CrossRef]
- Pibarot, P.; Dumesnil, J.G. New concepts in valvular hemodynamics: Implications for diagnosis and treatment of aortic stenosis. Can. J. Cardiol. 2007, 23 (Suppl. B), 40b–47b. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Van Belle, E.; Vincent, F.; Rauch, A.; Casari, C.; Jeanpierre, E.; Loobuyck, V.; Rosa, M.; Delhaye, C.; Spillemaeker, H.; Paris, C.; et al. von Willebrand factor and management of heart valve disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 1078–1088. [Google Scholar] [CrossRef]
- Van Belle, E.; Rauch, A.; Vincent, F.; Robin, E.; Kibler, M.; Labreuche, J.; Jeanpierre, E.; Levade, M.; Hurt, C.; Rousse, N.; et al. Von Willebrand factor multimers during transcatheter aortic-valve replacement. N. Engl. J. Med. 2016, 375, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Van Belle, E.; Rauch, A.; Vincentelli, A.; Jeanpierre, E.; Legendre, P.; Juthier, F.; Hurt, C.; Banfi, C.; Rousse, N.; Godier, A.; et al. Von Willebrand factor as a biological sensor of blood flow to monitor percutaneous aortic valve interventions. Circ. Res. 2015, 116, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Smadja, D.M.; Goudot, G.; Gendron, N.; Zarka, S.; Puymirat, E.; Philippe, A.; Spaulding, C.; Peronino, C.; Tanter, M.; Pernot, M.; et al. Von Willebrand factor multimers during non-invasive ultrasound therapy for aortic valve stenosis. Angiogenesis 2021, 24, 715–717. [Google Scholar] [CrossRef]
- James, P.D.; Connell, N.T.; Ameer, B.; Di Paola, J.; Eikenboom, J.; Giraud, N.; Haberichter, S.; Jacobs-Pratt, V.; Konkle, B.; McLintock, C.; et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021, 5, 280–300. [Google Scholar] [CrossRef]
- Jain, R.; Bajwa, T.; Roemer, S.; Huisheree, H.; Allaqaband, S.Q.; Kroboth, S.; Perez Moreno, A.C.; Tajik, A.J.; Khandheria, B.K. Myocardial work assessment in severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 715–721. [Google Scholar] [CrossRef]
- Chen, J.S.; Huang, J.H.; Chiu, K.M.; Chiang, C.Y. Extent of Left Ventricular Mass regression and impact of global left ventricular afterload on cardiac events and mortality after aortic valve replacement. J. Clin. Med. 2022, 11, 7842. [Google Scholar] [CrossRef] [PubMed]
- Rosenhek, R.; Zilberszac, R.; Schemper, M.; Czerny, M.; Mundigler, G.; Graf, S.; Bergler-Klein, J.; Grimm, M.; Gabriel, H.; Maurer, G. Natural history of very severe aortic stenosis. Circulation 2010, 121, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Freeman, W.K.; Schaff, H.V.; Orszulak, T.A.; Tajik, A.J. Ultrasonic aortic valve decalcification: Serial Doppler echocardiographic follow-up. J. Am. Coll. Cardiol. 1990, 16, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Vincentelli, A.; Susen, S.; Le Tourneau, T.; Six, I.; Fabre, O.; Juthier, F.; Bauters, A.; Decoene, C.; Goudemand, J.; Prat, A.; et al. Acquired von Willebrand syndrome in aortic stenosis. N. Engl. J. Med. 2003, 349, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Grodecki, K.; Zbroński, K.; Przybyszewska-Kazulak, E.; Olasińska-Wiśniewska, A.; Wilimski, R.; Rymuza, B.; Scisło, P.; Czub, P.; Koper, D.; Kochman, J.; et al. Pre-procedural abnormal function of von Willebrand Factor is predictive of bleeding after surgical but not transcatheter aortic valve replacement. J. Thromb. Thrombolysis 2019, 48, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Selvam, S.N.; Bowman, M.; Inglis, M.; Kloosterman, R.; Grabell, J.; Casey, L.; Johri, A.M.; James, P. Patients with aortic stenosis have von Willebrand factor abnormalities and increased proliferation of endothelial colony forming cells. J. Thromb. Haemost. 2020, 18, 593–603. [Google Scholar] [CrossRef]
- Panagopoulou, V.; Deftereos, S.; Kossyvakis, C.; Raisakis, K.; Giannopoulos, G.; Bouras, G.; Pyrgakis, V.; Cleman, M.W. NTproBNP: An important biomarker in cardiac diseases. Curr. Top. Med. Chem. 2013, 13, 82–94. [Google Scholar] [CrossRef]
- Iwanaga, Y.; Nishi, I.; Furuichi, S.; Noguchi, T.; Sase, K.; Kihara, Y.; Goto, Y.; Nonogi, H. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: Comparison between systolic and diastolic heart failure. J. Am. Coll. Cardiol. 2006, 47, 742–748. [Google Scholar] [CrossRef]
- Shiino, K.; Yamada, A.; Scalia, G.M.; Putrino, A.; Chamberlain, R.; Poon, K.; Walters, D.L.; Chan, J. Early changes of myocardial function after transcatheter aortic valve implantation using multilayer strain speckle tracking echocardiography. Am. J. Cardiol. 2019, 123, 956–960. [Google Scholar] [CrossRef]
- Thellier, N.; Altes, A.; Appert, L.; Binda, C.; Leman, B.; Marsou, W.; Debry, N.; Joly, C.; Ennezat, P.V.; Tribouilloy, C.; et al. Prognostic importance of left ventricular global longitudinal strain in patients with severe aortic stenosis and preserved ejection fraction. J. Am. Soc. Echocardiogr. 2020, 33, 1454–1464. [Google Scholar] [CrossRef]
- De Rosa, S.; Sabatino, J.; Strangio, A.; Leo, I.; Romano, L.R.; Spaccarotella, C.A.; Mongiardo, A.; Polimeni, A.; Sorrentino, S.; Indolfi, C. Non-invasive myocardial work in patients with severe aortic stenosis. J. Clin. Med. 2022, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Schindler, M.; Stöckli, F.; Brütsch, R.; Jakob, P.; Holy, E.; Michel, J.; Manka, R.; Vogt, P.; Templin, C.; Kasel, M.; et al. Postprocedural troponin elevation and mortality after transcatheter aortic valve implantation. J. Am. Heart Assoc. 2021, 10, e020739. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; Paradies, V.; Barbato, E.; Baumbach, A.; Bøtker, H.E.; Capodanno, D.; De Caterina, R.; Cavallini, C.; Davidson, S.M.; Feldman, D.N.; et al. Prognostically relevant periprocedural myocardial injury and infarction associated with percutaneous coronary interventions: A consensus document of the ESC working group on cellular biology of the heart and European association of percutaneous cardiovascular interventions (EAPCI). Eur. Heart J. 2021, 42, 2630–2642. [Google Scholar]


| N = 10 | |
|---|---|
| Age (years) | 80.0 (74.5–83.0) |
| Female | 6 (60%) |
| Male | 4 (40%) |
| Systemic hypertension | 10 (100%) |
| Coronary artery disease | 7 (70%) |
| Angina | 4 (40%) |
| Diabetes mellitus | 4 (40%) |
| Renal insufficiency | 4 (40%) |
| Peripheral artery disease | 4 (40%) |
| Prior cerebrovascular event | 1 (10%) |
| New York Heart Association | |
| 2 | 4 (40.0%) |
| 3 | 6 (60.0%) |
| 4 | 0 (0.0%) |
| STS score (%) | 6.7 (4.6–8.2) |
| N = 10 | |
|---|---|
| Cumulative focal energy (J/mm2) | 463 (387–465) |
| Gain IHM mean (%) | 52 (48–58) |
| Steering mean (mm) | 122 (113–130) |
| Peak acoustic power max (W) | 8506 (7317–8922) |
| Peak acoustic power mean (W) | 4143 (3379–5628) |
| ISPPA max (W/mm2) | 58 (58–58) |
| ISPPA mean (W/mm2) | 33 (30–40) |
| Mean acoustic energy focal (J/mm2) | 463 (387–465) |
| TZ surface (from computation, mm2) | 125 (110–166) |
| Peak Acoustic Power Mean (W) | ISPPA Mean (W/mm2) | Mean Acoustic Energy Focal (J/mm2) | ||||
|---|---|---|---|---|---|---|
| Correlation Coefficient | p | Correlation Coefficient | p | Correlation Coefficient | p | |
| ∆ 1D | ||||||
| Vmax (m/s) | −0.697 | 0.025 | −0.794 | 0.006 | 0.321 | 0.365 |
| MG (mmHg) | −0.504 | 0.138 | −0.745 | 0.013 | −0.030 | 0.934 |
| AVA (cm2) | −0.232 | 0.519 | 0.030 | 0.933 | 0.311 | 0.382 |
| SWL (%) | −0.309 | 0.385 | −0.673 | 0.033 | −0.188 | 0.603 |
| Zva (mmHg/mL/m2) | 0.030 | 0.934 | −0.188 | 0.603 | 0.176 | 0.627 |
| ∆ 1M | ||||||
| Vmax (m/s) | −0.367 | 0.332 | −0.433 | 0.244 | 0.350 | 0.356 |
| MG (mmHg) | −0.017 | 0.966 | −0.067 | 0.865 | −0.017 | 0.066 |
| AVA (cm2) | −0.733 | 0.025 | −0.517 | 0.154 | 0.700 | 0.0036 |
| SWL (%) | 0.100 | 0.798 | 0.200 | 0.606 | 0.283 | 0.460 |
| Zva (mmHg/mL/m2) | −0.5678 | 0.112 | −0.700 | 0.036 | −0.100 | 0.798 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifunović-Zamaklar, D.; Karan, R.; Kovačević-Kostić, N.; Terzić, D.; Milićević, V.; Petrović, O.; Canić, I.; Pernot, M.; Tanter, M.; Wang, L.Z.; et al. Non-Invasive Ultrasound Therapy for Severe Aortic Stenosis: Early Effects on the Valve, Ventricle, and Cardiac Biomarkers (A Case Series). J. Clin. Med. 2024, 13, 4607. https://doi.org/10.3390/jcm13164607
Trifunović-Zamaklar D, Karan R, Kovačević-Kostić N, Terzić D, Milićević V, Petrović O, Canić I, Pernot M, Tanter M, Wang LZ, et al. Non-Invasive Ultrasound Therapy for Severe Aortic Stenosis: Early Effects on the Valve, Ventricle, and Cardiac Biomarkers (A Case Series). Journal of Clinical Medicine. 2024; 13(16):4607. https://doi.org/10.3390/jcm13164607
Chicago/Turabian StyleTrifunović-Zamaklar, Danijela, Radmila Karan, Nataša Kovačević-Kostić, Duško Terzić, Vladimir Milićević, Olga Petrović, Ivana Canić, Mathieu Pernot, Mickael Tanter, Louise Z. Wang, and et al. 2024. "Non-Invasive Ultrasound Therapy for Severe Aortic Stenosis: Early Effects on the Valve, Ventricle, and Cardiac Biomarkers (A Case Series)" Journal of Clinical Medicine 13, no. 16: 4607. https://doi.org/10.3390/jcm13164607
APA StyleTrifunović-Zamaklar, D., Karan, R., Kovačević-Kostić, N., Terzić, D., Milićević, V., Petrović, O., Canić, I., Pernot, M., Tanter, M., Wang, L. Z., Goudot, G., Velinović, M., & Messas, E. (2024). Non-Invasive Ultrasound Therapy for Severe Aortic Stenosis: Early Effects on the Valve, Ventricle, and Cardiac Biomarkers (A Case Series). Journal of Clinical Medicine, 13(16), 4607. https://doi.org/10.3390/jcm13164607






