Therapeutic Maintenance of Janus Kinase Inhibitors in Real Life for Rheumatoid Arthritis: Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data Recorded
- -
- Demographic features (age, sex, body mass index, history of pulmonary, digestive and urogenital infections, neoplastic history, smoking, and Charlson comorbidity index (Supplementary Tables S1 and S2) [13]).
- -
- RA characteristics (duration of disease, positivity of rheumatoid factor (RF) and anticitrullinated protein antibodies (ACPAs), presence of extra-articular manifestation (such as rheumatoid nodule, ocular involvement, cardiac involvement, pulmonary involvement, biological involvement, renal involvement, and vascular involvement), presence of erosion, and prior treatments and concomitant treatments).
- -
- Cardiovascular risk (CV) (age, systolic blood pressure (mmHg, total cholesterol (mmol/L), HDL (mmol/L)) with a SCORE2 risk calculation for patients aged 40–69 (with no history of cardiovascular disease or diabetes), which is predictive of the 10-year risk of lethal or non-lethal cardiovascular events. The SCORE2-OP risk was also calculated at the start of treatment for patients aged over 70 to predict the risk of cardiovascular events at 5 or 10 years [14,15]. The score was adapted to EULAR recommendations [16]. The CV risk was divided into four categories according to the SCORE2 and SCORE2-OP risk calculations and the patient’s personal history: low CV risk, moderate CV risk, high CV risk, and very high CV risk.
- -
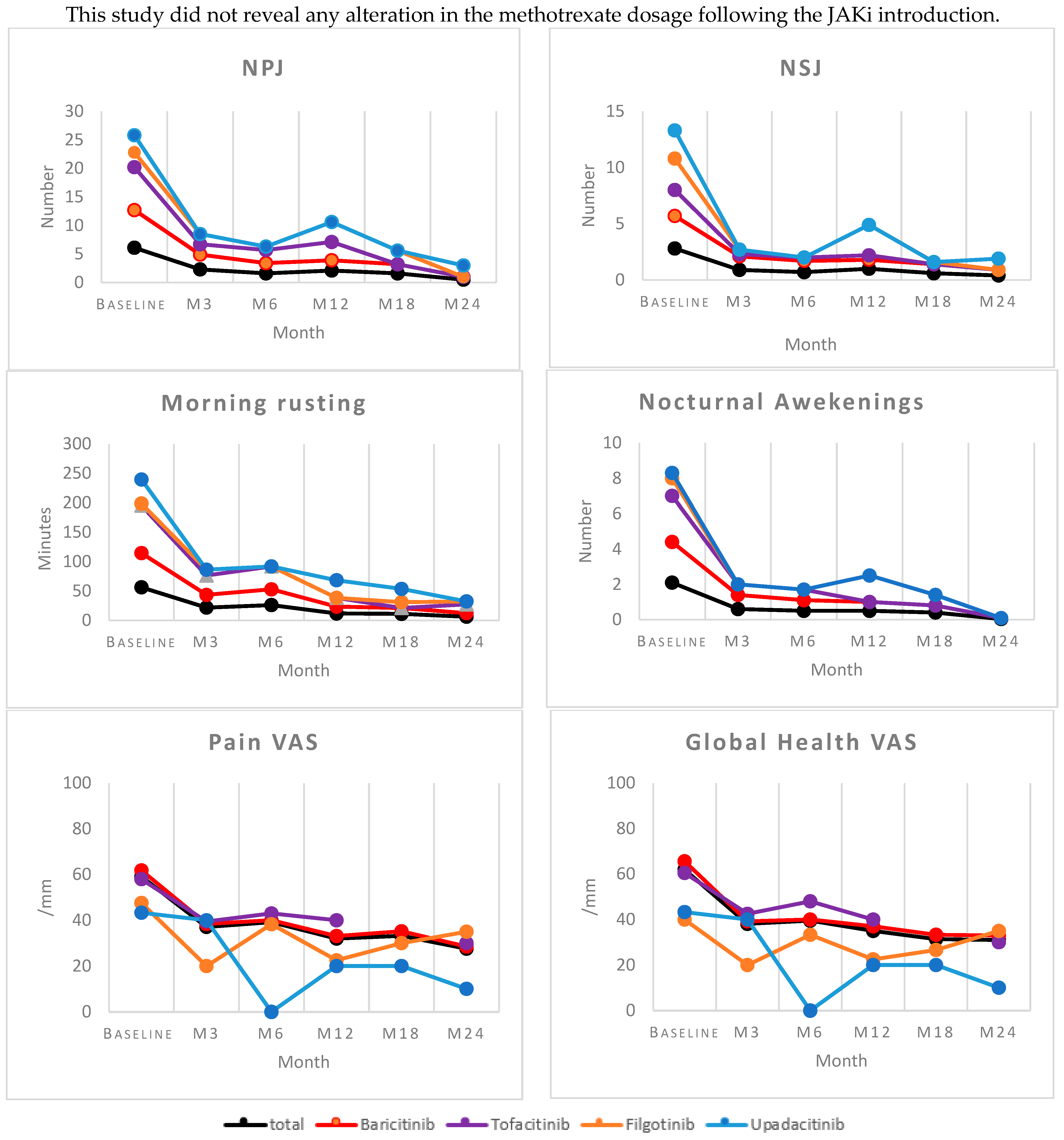
- Clinical signs and symptoms were assessed at baseline and 3, 6, 12, 18, and 24 months and included the following: number of nocturnal awakenings, duration of morning rusting, number of swollen joints (NSJ), number of tender joints (NTJ), pain visual analog scale (VAS), patient global health VAS (hVAS), disease activity score in 28 joints using the erythrocyte sedimentation rate (DAS-28 ESR), disease activity score in 28 joints using C-reactive protein (DAS-28 CRP), and secondary effects (infections, neoplasia, or cardiovascular events).
- -
- Laboratory findings were recorded at initiation and 3, 6, 12, 18, and 24 months, including the following: complete blood count (CBC), sedimentation rate (ESR), C-reactive protein (CRP), liver function, creatinine, and MDRD clearance.
2.3. Statistical Analysis
3. Results
3.1. Patient Selection and Characteristics
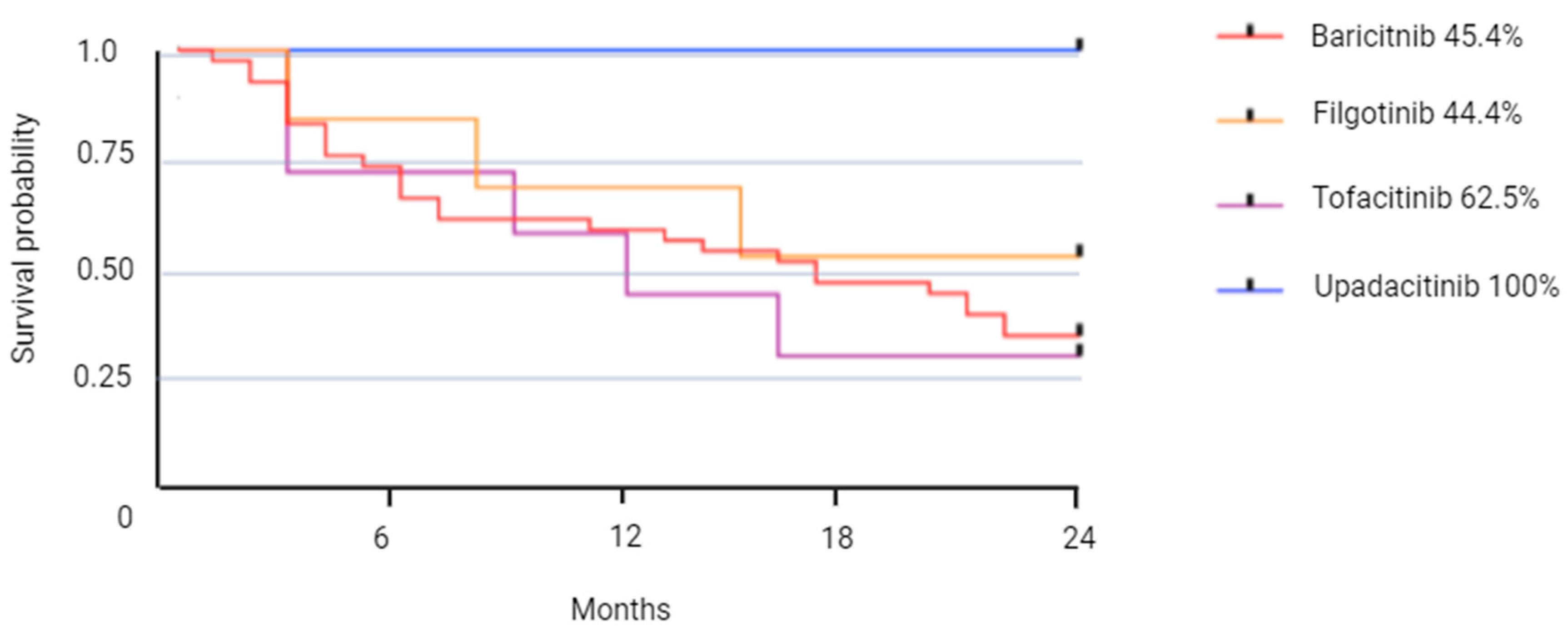
3.2. JAKI Persistence
3.3. Predictive Factors for Therapeutic Maintenance
3.4. Tolerance
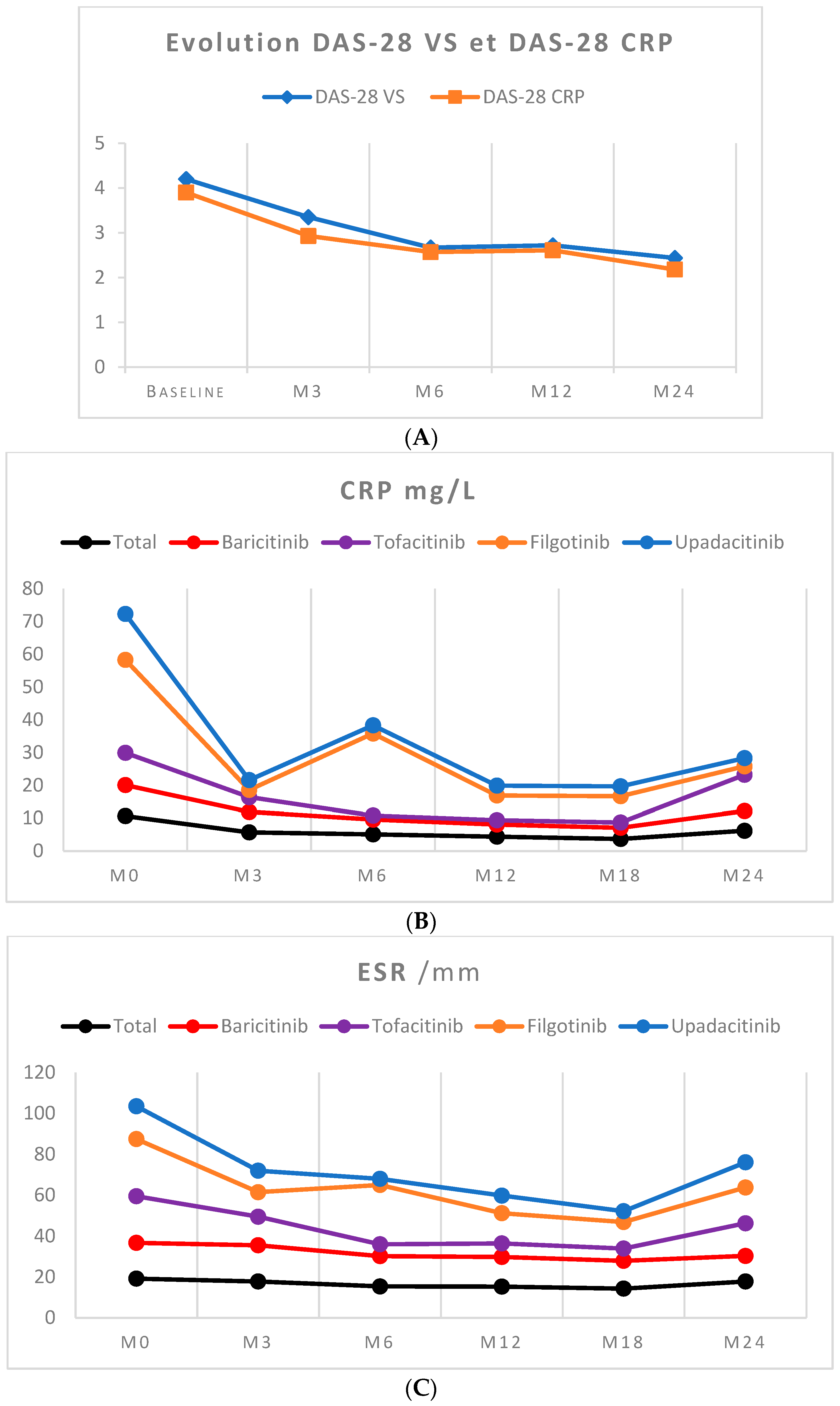
3.5. Biological Surveillance
3.6. Effectiveness Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Firestein, G.S. Immunologic Mechanisms in the Pathogenesis of Rheumatoid Arthritis. JCR J. Clin. Rheumatol. 2005, 11, S39–S44. [Google Scholar] [CrossRef]
- Angelini, J.; Talotta, R.; Roncato, R.; Fornasier, G.; Barbiero, G.; Dal Cin, L.; Brancati, S.; Scaglione, F. JAK-Inhibitors for the Treatment of Rheumatoid Arthritis: A Focus on the Present and an Outlook on the Future. Biomolecules 2020, 10, 1002. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malemud, C.J. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2018, 10, 117–127. [Google Scholar] [CrossRef]
- Dowty, M.E.; Lin, T.H.; Jesson, M.I.; Hegen, M.; Martin, D.A.; Katkade, V.; Menon, S.; Telliez, J.-B. Janus Kinase Inhibitors for the Treatment of Rheumatoid Arthritis Demonstrate Similar Profiles of in Vitro Cytokine Receptor Inhibition. Pharmacol. Res. Perspect. 2019, 7, e00537. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef]
- Walker, J.G.; Ahern, M.J.; Coleman, M.; Weedon, H.; Papangelis, V.; Beroukas, D.; Roberts-Thomson, P.J.; Smith, M.D. Changes in synovial tissue Jak-STAT expression in rheumatoid arthritis in response to successful DMARD treatment. Ann. Rheum. Dis. 2006, 65, 1558–1564. [Google Scholar] [CrossRef]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef]
- Meeting Highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 7–10 Febuary 2022. Available online: https://www.ema.europa.eu/en/news/ema-recommends-measures-minimise-risk-serious-side-effects-janus-kinase-inhibitors-chronic-inflammatory-disorders (accessed on 15 January 2023).
- Finckh, A.; Neto, D.; Iannone, F.; Loza, E.; Lie, E.; van Riel, P.; Hetland, M.L.; Pavelka, K.; Gottenberg, J.E.; Canhão, H.; et al. The impact of patient heterogeneity and socioeconomic factors on abatacept retention in rheumatoid arthritis across nine European countries. RMD Open 2015, 1, e000040. [Google Scholar] [CrossRef]
- Deprez, V.; Le Monnier, L.; Sobhy Danial, J.M.; Grados, F.; Henry Desailly, I.; Salomon, S.; Rabin, T.; Ristic, S.; Fumery, M.; Fardellone, P.; et al. Maintenance thérapeutique en vie réelle des inhibiteurs de Janus Kinases dans le traitement de la polyarthrite rhumatoïde. Rev. Rhum. 2020, 87, A124. [Google Scholar] [CrossRef]
- Hua, C.; Combe, B. Les nouveaux critères de classification ACR/EULAR 2010 pour un diagnostic plus précoce de la polyarthrite rhumatoïde. Rev. Rhum. Monogr. 2017, 84, 337–342. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- SCORE2 Working Group; ESC Cardiovascular Risk Collaboration. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef]
- SCORE2-OP Working Group; ESC Cardiovascular Risk Collaboration. SCORE2-OP risk prediction algorithms: Estimating incident cardiovascular event risk in older persons in four geographical risk regions. Eur. Heart J. 2021, 42, 2455–2467. [Google Scholar] [CrossRef]
- Peters, M.J.L.; Symmons, D.P.M.; McCarey, D.; Dijkmans, B.A.C.; Nicola, P.; Kvien, T.K.; McInnes, I.B.; Haentzschel, H.; Gonzalez-Gay, M.A.; Provan, S.; et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann. Rheum. Dis. 2010, 69, 325–331. [Google Scholar] [CrossRef]
- Combe, B.; Kivitz, A.; Tanaka, Y.; Van Der Heijde, D.; Simon, J.A.; Baraf, H.S.B.; Kumar, U.; Matzkies, F.; Bartok, B.; Ye, L.; et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: A phase III randomised clinical trial. Ann. Rheum. Dis. 2021, 80, 848–858. [Google Scholar] [CrossRef]
- Taylor, P.C.; Keystone, E.C.; Van Der Heijde, D.; Weinblatt, M.E.; Del Carmen Morales, L.; Gonzaga, J.R.; Yakushin, S.; Ishii, T.; Emoto, K.; Beattie, S.; et al. Baricitinib versus Placebo or Adalimumab in Rheumatoid Arthritis. N. Engl. J. Med. 2017, 376, 652–662. [Google Scholar] [CrossRef]
- Fleischmann, R.; Mysler, E.; Hall, S.; Kivitz, A.J.; Moots, R.J.; Luo, Z.; Demasi, R.; Soma, K.; Zhang, R.; Takiya, L.; et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): A phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 2017, 390, 457–468. [Google Scholar] [CrossRef]
- Rubbert-Roth, A.; Enejosa, J.; Pangan, A.L.; Haraoui, B.; Rischmueller, M.; Khan, N.; Zhang, Y.; Martin, N.; Xavier, R.M. Trial of Upadacitinib or Abatacept in Rheumatoid Arthritis. N. Engl. J. Med. 2020, 383, 1511–1521. [Google Scholar] [CrossRef]
- XELJANZ (tofacitinib): Restriction D’utilisation en cas de Risque élevé D’embolie Pulmonaire. Available online: https://www.vidal.fr/actualites/23360/xeljanz_tofacitinib_restriction_d_utilisation_en_cas_de_risque_eleve_d_embolie_pulmonaire/ (accessed on 5 August 2020).
- Diep, L.; Barbier, V.; Doussière, M.; Touboul, E.; Jesson, C.; Deprez, V.; Sobhy-Danial, J.-M.; Fardellone, P.; Goëb, V. Comparison of Rheumatoid Arthritis Patients’ 2-Year Infliximab, Abatacept, and Tocilizumab Persistence Rates. J. Clin. Med. 2022, 11, 5978. [Google Scholar] [CrossRef]
- Gottenberg, J.E.; Morel, J.; Constantin, A.; Bardin, T.; Cantagrel, A.G.; Combe, B.; Dougados, M.; Flipo, R.M.; Saraux, A.; Schaeverbeke, T.; et al. Long-Term Registry Data in 4498 Patients with Rheumatoid Arthritis Indicate a Similar Safety but a Different Drug Retention between Abatacept, Rituximab and Tocilizumab. In Proceedings of the ACR Meeting, Washington, DC, USA, 11–16 November 2016. Abstracts number 1998, 28 September 2016. [Google Scholar]
- Martinez-Molina, C.; Gich, I.; Diaz-Torné, C.; Park, H.S.; Feliu, A.; Vidal, S.; Corominas, H. Patient-related factors influencing the effectiveness and safety of Janus Kinase inhibitors in rheumatoid arthritis: A real-world study. Sci. Rep. 2024, 14, 172. [Google Scholar] [CrossRef] [PubMed]
- Mahlich, J.; Sruamsiri, R. Persistence with biologic agents for the treatment of rheumatoid arthritis in Japan. Patient Prefer Adherence 2016, 10, 1509–1519. [Google Scholar] [CrossRef]
- Mease, P.; Charles-Schoeman, C.; Cohen, S.; Fallon, L.; Woolcott, J.; Yun, H.; Kremer, J.; Greenberg, J.; Malley, W.; Onofrei, A.; et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann. Rheum. Dis. 2020, 79, 1400–1413. [Google Scholar] [CrossRef]
- Ketfi, C.; Boutigny, A.; Mohamedi, N.; Bouajil, S.; Magnan, B.; Amah, G.; Dillinger, J.G. Risk of venous thromboembolism in rheumatoid arthritis. Jt. Bone Spine 2021, 88, 105122. [Google Scholar] [CrossRef]
- CRIT-Net. Fiche Pratique Utlisation des Anti Jak. Available online: https://cri-net.com/ckfinder/userfiles/files/fiches-pratiques/JAKI-octobre2019/JAK_06_%20Cardio_MAJ_220719.pdf (accessed on 1 December 2023).
- Combe, B.; Aletaha, D.; Westhovens, R.; Gottenberg, J.E.; Buch, M.H.; Caporali, R.; Gómez-Puerta, J.A.; Van Hoek, P.; Rajendran, V.; Stiers, P.J.; et al. Efficacité et tolérance du filgotinib (FIL) chez les patients âgés de ≥ 75 ans: Une analyse post-hoc en sous-groupes de l’étude d’extension à long terme (ELT) FINCH 4. Rev. Rhum. 2022, 89, A271–A272. [Google Scholar] [CrossRef]
- Gottenberg, J.E.; Winthrop, K.; Tanaka, Y.; Takeuchi, T.; Burmester, G.R.; Kivitz, A.; Genovese, M.C.; Pechonkina, A.; Matzkies, F.; Bartok, B.; et al. Mise à jour de l’analyse intégrée de la tolérance du filgotinib (FIL) chez les patients atteints de polyarthrite rhumatoïde (PR) active modérée à sévère recevant un traitement (TT) sur une période médiane de 2,2 ans. Rev. Rhum. 2022, 89, A270–A271. [Google Scholar] [CrossRef]
| Total n = 76 | Baricitinib n = 55 | Tofacitinib n = 9 | Filgotinib n = 8 | Upadacitinib n = 4 | p-Value | |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Female sex, n (%) | 63 (82.9) | 45 (81.8) | 7 (77.7) | 7 (87.5) | 4 (100) | 0.82 |
| Age (years), mean ± SD | 56.8 (13.2) | 55.0 (13.7) | 64.6 (14.8) | 58.6 (6.9) | 60.0 (7.0) | 0.21 |
| BMI (kg/m2) | 26.4 | 27.1 | 21.3 | 27.6 | * | 0.19 |
| Systolic blood pressure (mmHg), mean ± SD | 132 (14.5) | 130 (13.2) | 128 (10.0) | 145 (19.0) | * | 0.08 |
| Personal medical history | ||||||
| Pulmonary infection, n (%) | 12 (15.8) | 9 (16.3) | 2 (22) | 1 (12.5) | 0 (0) | 0.85 |
| Digestive infection, n (%) | 8 (10.5) | 6 (10.9) | 1 (11.1) | 1 (12.5) | 0 (0) | 1.00 |
| Urogenital infection, n (%) | 7 (9.2) | 4 (7.2) | 1 (11.1) | 1 (12.5) | 1 (25) | 0.76 |
| Neoplasia, n (%) | 8 (10.5) | 5 (9.0) | 3 (33.3) | 0 (0) | 0 (0) | 0.12 |
| History of MACEs and venous thromboembolism, n (%) | 34 (44) | 22 (40) | 5 (55) | 4 (50) | 3 (75) | 0.43 |
| Anticoagulant treatment, n (%) | 1 (1.3) | 0 (0) | 0 (0) | 1 (12.5) | 0 (0) | 0.15 |
| Anti-lipid treatment, n (%) | 16 (21.1) | 12 (21.8) | 1 (11.1) | 2 (25) | 1 (25) | 0.90 |
| Smoking, n (%) | 17 (22.4) | 13 (23.6) | 2 (22) | 2 (25) | 0 (0) | 0.81 |
| Charlson comorbidity index, mean | 1.79 | 1.61 | 3.1 | 1.62 | 1.5 | 0.15 |
| Cardiovascular risk ** | n = 48 | n = 35 | n = 5 | n = 7 | n = 1 | 0.44 |
| - Low risk | 4 (8.3) | 4 (11.4) | 0 (0) | 0 (0) | 0 (0) | |
| - Moderate risk | 17 (35.4) | 13 (37.2) | 2 (40) | 2 (28.6) | 0 (0) | |
| - High risk | 19 (39.6) | 12 (34.3) | 3 (60) | 4 (57.1) | 0 (0) | |
| - Very high risk | 8 (16.7) | 6 (17.1) | 0 (0) | 1 (14.2) | 1 (100) | |
| Characteristics of rheumatoid arthritis | ||||||
| RF positive, n (%) | 58 (76.3) | 43 (78.1) | 7 (77.7) | 5 (62.5) | 3 (75) | 0.90 |
| ACPA positive, n (%) | 59 (77.6) | 42 (76.3) | 7 (77.7) | 6 (75) | 4 (100) | 0.66 |
| Disease duration, (years), mean ± SD | 14.2 (10.6) | 12.2 | 14.8 | 24.8 | 19 | 0.001 |
| Erosion presence, n (%) | 46 (60.5) | 34 (61.8) | 6 (66.6) | 5 (62.5) | 1 (25) | 0.50 |
| Extra-articular manifestations, n (%) | 9 (11.8) | 5 (9.0) | 4 (44) | 0 (0) | 0 (0) | 0.02 |
| DAS 28-ESR, mean | 4.2 (1.2) | 4.8 (1.1) | 4.1 (1.8) | 3.5 (1.3) | 3.7 (0.7) | 0.57 |
| DAS 28-CRP, mean | 3.9 (1.1) | 4.0 (1.1) | 3.8 (1.3) | 2.8 (0.9) | * | 0.14 |
| VAS pain, mean ± SD | 59 (23.8) | 61.6 (22.9) | 58 (17.3) | 47.6 (31.2) | 44.3 (37.8) | 0.59 |
| VAS disease, mean ± SD | 61 (22.9) | 65.6 (20.0) | 60.5 (23.1) | 40 (29.1) | 43.3 (37.8) | 0.12 |
| NPJ mean ± SD | 6.1 (5.4) | 6.5 (5.4) | 7.7 (7.3) | 2.6 (2.0) | 3 (1.6) | 0.24 |
| NSJ mean ± SD | 2.8 (3.2) | 2.9 (3.4) | 2.3 (2.7) | 2.8 (2.5) | 3.5 (2.6) | 0.96 |
| Number of nocturnal awakenings, mean ± SD | 2.1 (2.1) | 2.3 (2.2) | 2.6 (1.9) | 1 (1.7) | 0.3 (0.5) | 0.29 |
| Morning stiffness (minutes) mean ± SD | 56.4 (61) | 58.8 (63) | 81 (66.7) | 3.3 (5.7) | 40.6 (33.4) | 0.10 |
| Total n = 76 | Baricitinib n = 55 | Tofacitinib n = 9 | Filgotinib n = 8 | Upadacitinib n = 4 | ||
|---|---|---|---|---|---|---|
| Therapeutic maintenance, n (%) | 6 months | 55 (72.3) | 38 (69.0) | 6 (66.6) | 7 (87.5) | 4 (100.0) |
| 12 months | 50 (65.7) | 35 (63.6) | 5 (55.5) | 6 (66.6) | 4 (100.0) | |
| 18 months | 43 (56.5) | 30 (54.5) | 4 (44.4) | 5 (62.5) | 4 (100.0) | |
| 24 months | 38 (50.0) | 25(45.4) | 4 (44.4) | 5 (62.5) | 4 (100.0) | |
| Average retention time (months) [CI 95%] | 8.6 [6.4–10.9] | 8.6 [5.9–11.3] | 8.6 [3.6–13.5] | 8.6 [1.8–15.4] | ||
| Number of stops, n (%) | 38 (50.0) | 30 (54.4) | 5 (55.5) | 3 (37.5) | 0 (0) | |
| Parameter at M0 | HR [CI 95%] | p-Value |
|---|---|---|
| Age at initiation | 0.959 [0.835–1.101] | 0.55 |
| Disease duration at initiation | 0.975 [0.870–1.093] | 0.63 |
| RF positive | 1.493 [0.222–10.041] | 0.68 |
| ACPA positive | 1.736 [0.370–8.150] | 0.48 |
| DAS 28-ESR at initiation | 1.036 [0.438–2.448] | 0.93 |
| DAS 28-CRP at initiation | 0.990 [0.381–2.574] | 0.98 |
| Low-risk CV | 0.461 [0.011–19.394] | 0.68 |
| Moderate-risk CV | 0.667 [0.089–4.996] | 0.69 |
| High-risk CV | 0.368 [0.052–2.620] | 0.31 |
| Prior biotherapy | 0.62 | |
| 1–2 | 0.517 [0.056–4.766] | 0.56 |
| >=3 | 0.550 [0.153–1.969] | 0.33 |
| Charlson comorbidity index | 0.17 | |
| 1 unit | 0.018 [0.000–1.831] | 0.08 |
| 2 units | 0.026 [0.001–0.723] | 0.03 |
| 3 units | 0.028 [0.002–0.470] | 0.01 |
| 4 units | 0.096 [0.005–1.744] | 0.11 |
| 5 units | 0.539 [0.046–6.355] | 0.62 |
| 6 units | 0.120 [0.005–2.807] | 0.18 |
| Biological Evolution, Mean ± SD | Baseline | At 3 Months | At 6 Months |
|---|---|---|---|
| Hemoglobin (g/dL) | 13.6 (1.34) | 13.2 (1.2) * | 13.2 (1.1) * |
| Platelets (103/µL) | 291.6 (103.6) | 325.6 (94.7) * | 315.8 (107.1) * |
| Leukocytes (103/µL) | 8.0 (3.0) | 7.4 (2.1) | 7.4 (1.9) |
| Polynuclear neutrophils (103/µL) | 4.9 (2.5) | 4.3 (1.5) | 4.4 (1.5) |
| Eosinophilic cells (/mm3) | 207 (130) | 161 (93) * | 148 (84) * |
| Lymphocytes (/mm3) | 2453 (1030) | 2414 (1080) | 2263 (1012) |
| Aspartate transaminase (UI/L) | 21.9 (8.5) | 26 (9) * | 26 (9) * |
| Alanine transaminase (UI/L) | 24.1 (15.1) | 24 (14) | 28 (15) |
| Creatinine (µmol/L) | 68.8 (15.5) | 69 (14) | 69 (10) |
| Creatinine clearance (mL/min) | 86.7 (23.2) | 86 (15) | 86 (14) |
| Total cholesterol ** (g/L) | 2.08 (0.4) | 2.0 (0.4) | 2.1 (0.4) |
| Triglycerides ** (g/L) | 1.2 (0.6) | 1.2 (0.6) | 1.2 (0.4) |
| LDL cholesterol ** (g/L) | 1.1 (0.3) | 1.1 (0.3) | 1.2 (0.3) |
| HDL cholesterol ** (g/L) | 0.6 (0.1) | 0.6 (0.1) | 0.6 (0.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farnos, C.; Barbier, V.; Doussiere, M.; Deprez, V.; Hamidou, Y.; Bruy, P.A.; Sobhy Danial, J.M.; Goeb, V. Therapeutic Maintenance of Janus Kinase Inhibitors in Real Life for Rheumatoid Arthritis: Retrospective Study. J. Clin. Med. 2024, 13, 4608. https://doi.org/10.3390/jcm13164608
Farnos C, Barbier V, Doussiere M, Deprez V, Hamidou Y, Bruy PA, Sobhy Danial JM, Goeb V. Therapeutic Maintenance of Janus Kinase Inhibitors in Real Life for Rheumatoid Arthritis: Retrospective Study. Journal of Clinical Medicine. 2024; 13(16):4608. https://doi.org/10.3390/jcm13164608
Chicago/Turabian StyleFarnos, Camille, Vincent Barbier, Marie Doussiere, Valentine Deprez, Yannis Hamidou, Pierre Antoine Bruy, Jean Marc Sobhy Danial, and Vincent Goeb. 2024. "Therapeutic Maintenance of Janus Kinase Inhibitors in Real Life for Rheumatoid Arthritis: Retrospective Study" Journal of Clinical Medicine 13, no. 16: 4608. https://doi.org/10.3390/jcm13164608
APA StyleFarnos, C., Barbier, V., Doussiere, M., Deprez, V., Hamidou, Y., Bruy, P. A., Sobhy Danial, J. M., & Goeb, V. (2024). Therapeutic Maintenance of Janus Kinase Inhibitors in Real Life for Rheumatoid Arthritis: Retrospective Study. Journal of Clinical Medicine, 13(16), 4608. https://doi.org/10.3390/jcm13164608







