Abstract
Background: Post-cholecystectomy bile duct injuries (BDIs) represent a challenging complication, with negative impacts on clinical outcomes. Several surgical and endoscopic/interventional radiologist (IR) approaches have been proposed to manage these damages, though with high failure rates. This individual patient data (IPD) systematic review analyzes the potential risk factors for failure after treatment interventions for BDIs, both surgical and endoscopic/IR. Methods: An extensive literature search was conducted on MEDLINE and Scopus for relevant articles published in English on the management of BDIs after cholecystectomy, between 1 January 2010 and 31 December 2023. Our series of BDIs was included. BDIs were always categorized according to the Strasberg’s classification. The composite primary endpoints evaluated were the failure of treatment interventions, defined as patient death or the requirement of any other procedure, whatever surgical and/or endoscopic/IR, after the primary treatment. Results: A total of 342 cases were retrieved from our literature analysis, including our series of 19 patients. Among these, three groups were identified: “upfront surgery”, “upfront endoscopy and/or IR” and “no upfront treatment”, consisting of 224, 109 and 9 patients, respectively. After eliminating the third group, treatment intervention failure was observed overall in 34.2% (114/333) of patients, of whom 80.7% (92/114) and 19.3% (22/114) in the “upfront surgery” and in the “upfront endoscopy/IR” groups, respectively. At multivariable analysis, injury type D and E, and repair in a non-specialized center represented independent predictors of treatment failure in both groups, whereas laparoscopic cholecystectomy (LC) converted to open and immediate attempt of surgical repair exclusively in the first group. Conclusions: Significant treatment failure rates are responsible for remarkable negative effects on immediate and longer-term clinical outcomes of post-cholecystectomy BDIs. Understanding the important risk factors for this outcome may better guide the most appropriate therapeutical approach and improve clinical decisions in case this serious complication occurs.
1. Introduction
Since the first description in 1985 [1], laparoscopic cholecystectomy (LC) has gradually emerged as the gold standard surgical technique in the treatment of gallstone disease and acute acalculous cholecystitis, representing one of the most common procedures performed worldwide in both elective and urgent settings [2,3].
Though with improving surgical experience and technological advances, bile duct injuries (BDIs) might still occur at a frequency ranging between 0.1 and 5.2%, with the highest percentages reported in the context of acute cholecystitis [2,3,4,5], compared to the 0.2–0.3% rate historically documented in open cholecystectomy (OC) [6].
BDIs are associated with significant morbidity, mortality, and considerable costs for the healthcare system [2,3,4,5,7], meaning at the same time a very common cause of litigation against general surgeons [8].
Several classifications of BDIs have been proposed by different authors [9,10,11,12], with Strasberg’s [10] currently representing the most used in clinical practice [13], allowing a simple and quick differentiation between minor and major BDIs [4].
Nevertheless, since vascular injuries might be found in 10–60% of iatrogenic BDIs [14,15], the more recent ATOM (anatomic, time of detection, mechanism) classification, proposed by the European Association for Endoscopic Surgery (EAES) in 2013 [16], integrates the main previously described codification systems into a composite, all-inclusive, nominal scheme combining bile tract anatomical damage, vascular injury, timing of detection, and mechanism of damage, with the purpose of standardizing BDI definitions, though limited by a poor clinical application due to its complexity [4].
Previous systematic reviews have focused their attention on the analysis of large-scale nationally validated databases, population-based cohort studies [5,17], and pooled data extracted through selectively screening major medical databases [18,19,20], with the associated weaknesses mainly consisting of limited accuracy, reporting bias and several confounders.
In order to overcome these limitations, we decided to conduct an individual patient data (IPD) systematic review as an alternative to a conventional systematic review, based on the search of all available case reports and series describing BDIs after cholecystectomy, aiming at providing a valid estimate of the main features inherent this subject, the treatment effects and their outcomes. In addition, the predictors of outcome when a surgical and/or an endoscopic/interventional radiology (IR) approach was adopted, were evaluated.
2. Materials and Methods
2.1. Data Sources and Extraction
An extensive literature search was conducted on MEDLINE and Scopus for published relevant articles. MeSH terms for the search string are provided in Appendix A. The search was limited to papers published in English between 1 January 2010 and 31 December 2023.
The case series of BDIs managed at our institution during the study period was also included.
The abstracts of the citations identified by the database search were independently screened by two authors (P.V. and A.B.C.). When it was certain from the abstract that the article was not of use, it was excluded. From all other studies, we obtained the full article to decide whether the study was potentially eligible. Discrepancies were resolved by discussion.
In addition, reference lists in all extracted studies were manually searched for eligible citations.
All published clinical studies that included the management of patients with BDIs after cholecystectomy were selected for further analysis. The exclusion criteria were as follows: (1) cholecystectomy not indicated for acute or chronic onset of cholelithiasis and for acute acalculous cholecystitis; (2) reviews, including those with pooled data, and editorials; (3) studies not precisely describing the type of BDI; (4) studies reporting a BDI not related to a procedure of cholecystectomy; (5) studies not describing the diagnostic or treatment modalities employed; (6) studies without sufficient data for analysis.
Duplicate studies were also identified and excluded.
Patients with multiple concomitant injuries to the biliary tree and/or vascular injuries (VIs) were included in the analysis, and exclusively the most serious damage to the biliary tree was recorded.
BDIs were always categorized according to the Strasberg classification [10]. In case a different or any classification was used in the paper included in the analysis, all the information provided were accurately reviewed in order to sort the BDI by the Strasberg codification [10].
The authors of the included studies were contacted only when the IPD needed for the analysis were incompletely described in the manuscript.
The available published studies regarding BDIs after cholecystectomy consist of case reports or single-center case series (Table A1, Appendix A). The choice of management of these injuries, whether surgical, endoscopic, IR, or conservative, was based on local expert opinion.
The PRISMA (preferred reporting items for systematic reviews and Meta-analyses) checklist was used to perform this systematic review [21] (Figure 1).
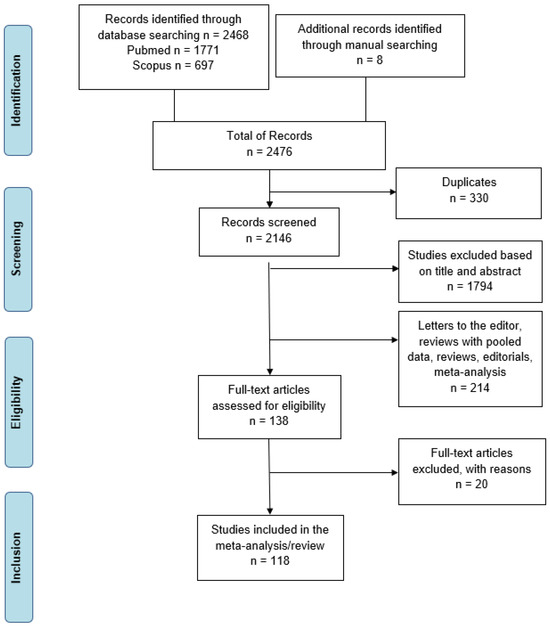
Figure 1.
Literature search PRISMA flow diagram.
The methodological quality and the risk of bias of the included studies were assessed by two independent reviewers (P.V. and A.B.C.) through the JBI critical appraisal tools for case series and case reports studies [22].
This review was registered as PROSPERO CRD42024551515.
2.2. Study Details and Endpoints
The following details were recorded for each study: year of publication, authors, title, journal, design (case report or case series), and number of cases. The study population demographics as well as clinical parameters, such as type of cholecystectomy (open or laparoscopic), conversion to open during LC and causes, the interval between BDI occurrence and recognition (timing of diagnosis), type of surgical treatment applied, timing of surgical repair, type of endoscopic, and IR treatment employed and referral to an hepato-pancreato-biliary (HPB) or tertiary care center, were also extracted. Mortality directly correlated to the event analyzed and long-term outcomes were also documented.
Some baseline variables, such as emergent and elective cholecystectomy, were not analyzed due to the high percentage of missing data.
All the baseline and outcome variables analyzed are listed in Table 1.

Table 1.
Variables and endpoints analyzed.
Three main groups were identified: group one, named “upfront surgery”, was composed of all patients undergoing a primary attempt of surgical repair of the BDI while in group two, named “upfront endoscopy and/or IR”, all cases were included that were initially treated by endoscopic or IR methods. The third group was defined “no upfront treatment”, since neither a surgical or an endoscopic/IR approach was undertaken, and a conservative management was pursued.
The techniques included in the first two groups were all those meant to provide an effective management of the BDI and therefore represented by suture or clips placement, T-tube insertion, end-to-end duct-to-duct anastomosis, hepatico-jejunostomy (HJ), hepatic resection (HR) in the group “upfront surgery” and by the placement of endoprosthesis or naso-biliary (NB) drainage through endoscopic retrograde colangiopancreatography (ERCP), by percutaneous transhepatic biliary drainage (PTBD) or other IR methodic, such as embolization coils, sclerotherapy and fibrin glue, in the other group.
Timing of BDI diagnosis was divided in two intervals: within one week and after one week the initial cholecystectomy.
Timing of surgical repair of BDI was defined as immediate if occurring within one day from the cholecystectomy, early if after one day and within one week, delayed if after one week and within 6 weeks and late if after 6 weeks [23,24].
The composite primary endpoints evaluated were the failure of treatment interventions, defined as patient death or the requirement of any other procedure, whatever surgical and/or endoscopic/IR, after the primary treatment.
2.3. Statistical Analysis
IPD extracted from eligible studies were entered in an Excel spreadsheet (Microsoft, Redmond, WA, USA).
Frequency distributions were determined for baseline categorical variables, and the arithmetic mean along with standard error (±SE) was calculated for baseline continuous variables [with median and corresponding interquartile (IQ) range being used for baseline continuous variables having skewed distributions].
Tests of association between baseline variables and treatment intervention failure development (No: no failure/Yes: failure) were performed using Pearson (uncorrected) chi-squared tests for dichotomous baseline variables and standard t-tests or Mann–Whitney tests as appropriate for continuous baseline variables (using natural logarithmic transformed values for skewed distributions).
Multivariable analysis was performed using stepwise logistic and linear regression on those variables with statistical relevance at the univariable analysis (p-value < 0.05).
The optimal cut-off for continuous variables was obtained from analyses of receiver operating characteristic (ROC) curves.
The statistical analysis was performed using IBM SPSS Statistics for Windows, version 24 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Study Population
The above-mentioned key words yielded a total of 323 cases from 118 manuscripts [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142], as displayed in Table A1 (Appendix A). Most of the case studies (n = 88, 74.6%) were isolated case reports, with the remaining 30 (25.4%) reporting between 2 and 43 cases of BDI.
Adding our case series composed of 19 cases (Table A2, Appendix A), the entire population analyzed in the review was made of 342 cases.
In more than half of the studies (67/118, 56.7%), the authors did not utilize any classification system to report the BDI, whereas in 28.8% (n = 34) of the studies identified, the Strasberg’s classification was used to catalogue the injury type [10]. The Bismuth–Corlette [9], Stewart–Way [11], ATOM [16], Hannover [12], Neuhaus [143], and Bergman [144] codification systems were applied in the remaining studies (Table A1, Appendix A).
Among the cases identified, the group “upfront surgery” consisted of 224 patients (65.5%), whereas the groups “upfront endoscopy and/or IR” and “no upfront treatment” of 109 (31.9%) and 9 (2.6%) cases, respectively.
Due to the scarce number of patients belonging to the “no upfront treatment” group and since this topic was not incorporated among our primary endpoints, it was excluded from further analysis.
At the comparative analysis, the overall rate of LC: converted to open and, specifically, the conversion rate after recognizing a BDI, the degree of bile duct damage, according to the Strasberg’s classification [10], and the percentage of associated VIs differed between the two groups analyzed, with significantly higher percentages of converted procedures, and of complex injuries, i.e., types D and E, with concomitant vascular damage in the “upfront surgery” group (p = 0.001 and <0.0001, respectively), as outlined in Table 2.

Table 2.
Comparisons of variables by group (upfront surgery vs. upfront endoscopy/IR) [1].
Likewise, among the other baseline variables investigated, a significantly higher rate of early diagnosis, i.e., within one week, and of surgical repair in a non-specialized center, were observed in the same group (p < 0.0001 and 0.025, respectively) (Table 2).
Accordingly, when comparing the primary endpoint, the occurrence of treatment intervention failure was significantly worse in the surgical group compared to the endoscopy/IR group (p < 0.0001) and consequently the mortality rate (p = 0.025), as shown in Table 2.
3.2. Upfront Surgery Group
3.2.1. Distributions of Baseline Characteristics
Overall, median patient age was 47 (IQ range: 36.2–62) years, with 13% of patients (24/184) aged more than 70 years old and 37.1% (75/202) of male sex, as shown in Table 2. Indeed, age and sex were not reported in 40 and 22 cases, respectively.
Cholecystectomy was performed laparoscopically in the majority of cases (n = 197, 87.9%), and converted to open in approximately one quarter of cases (n = 55, 27.9%), mainly because of intraoperative recognition of a BDI, as shown in Table 2.
Type E (n = 152, 67.9%) and D (n = 44, 19.6%) lesions, according to the Strasberg’s classification [10], represented the most frequent indications for surgery, followed by type C (n = 13, 5.8%), A (n = 11, 4.9%), and B (n = 4, 1.8%) injuries, as displayed in Table 2.
Of note, in the type E category of BDIs, the E2 and E4 subtypes were the most common damage patterns, reported in 58 (38.2%) and 40 (26.3%) cases, respectively, followed by the E3 (26/152, 17.1%) and the E1 (23/152, 15.1%) patterns. The most serious BDI, i.e., the E5 subtype, was recorded in only five cases (3.3%).
An associated VI was documented in almost one quarter of patients (51/224, 22.8%) (Table 2), with the RHA involved in 82.3% (n = 42) of cases and the proper (PHA) or common hepatic artery (CHA) in approximately 10% of cases (n = 5). A combination of hepatic artery (HA) and PV damage was reported in 11 patients (21.6%).
Of the 190 patients (84.8%) with mentioned timing of BDI diagnosis, in the majority (n = 151, 79.5%), the BDI was recognized within one week from the cholecystectomy (Table 2).
HJ was the most common surgical technique adopted for the repair of BDIs, utilized in half of cases (113/224, 50.4%), followed by leak repair through application of stitches or clips and end-to-end duct-to-duct anastomosis, chosen in 43 (19.2%) and 25 (11.2%) cases, respectively.
HR, generally consisting of right hepatectomy or right posterior sectorectomy, alone or in association with HJ, comprised the repair methods applied in 3.6% (n = 8) and 5.4% (n = 12) of cases, respectively, whereas in 4.5% of patients (n = 10), stitches or clips removal at the level of the common bile duct (CBD), omental patch or hepatico-duodenostomy, were the other methodic employed to fix the damage.
Lastly, a T-tube placement, alone or combined with other procedures, was carried out in 38 patients (17%).
Of the 204 (91%) patients with reported timing of surgical repair, an immediate repair was performed in 44.1% (n = 90) of cases, whereas an early, delayed, and late approach of reconstruction was chosen in 39 (19.1%), 43 (21.1%) and 32 (15.7%) cases, respectively.
In the majority of patients (79%, 177/224), the BDI was repaired in a specialized HPB center or in a tertiary care facility, whereas in the remaining cases (21%, 47/224), the surgical repair was attempted at the community hospital where the procedure of cholecystectomy was performed, as outlined in Table 2.
3.2.2. Distribution of the Primary Endpoint
In total, 92 patients (41%) developed a failure of the initial surgical treatment intervention (Table 2).
The most frequent reason for failure was the development of a secondary stricture at the level of the CBD or of the previous anastomosis (n = 53, 57.6%), followed by a persistent bile leak (n = 23, 25%) and the occurrence of a secondary biliary cirrhosis (SBC) over time (n = 7, 7.6%).
Perioperative mortality was reported in 10 patients (10.9%) (Table 2).
In the 82 alive patients who required a secondary intervention, HJ was carried out in 57.3% (n = 47) of cases, whereas an isolated HR and IR/endoscopic procedures were completed in approximately 33% (n = 27) and 42% (n = 34) of cases, respectively.
In 22 patients (26.8%), HJ was combined with HR, whereas 7 patients (8.5%) underwent a liver transplantation as a result of SBC.
3.2.3. Univariable Comparisons of Baseline Variables between Failure and Non-Failure of the Surgical Treatment Intervention
Tests of the association of baseline variables with development of surgical treatment intervention failure found that patients undergoing upfront OC, LC converted to open for any reason, experiencing a major BDI, i.e., type D and E, and hence sustaining an immediate repair, i.e., within one day from cholecystectomy, were more likely to develop this outcome (p = 0.024, 0.001, 0.014, and 0.001, respectively) (Table 3).

Table 3.
Comparisons of variables by group (surgical failure vs. surgical non-failure) [1].
Among the other variables analyzed, a repair of the injury in an HPB/tertiary care institution was associated with a significantly lower percentage of failure (31.6%, 56/177) compared to a repair attempted in a non-specialized center (76.6%, 36/47) [p < 0.0001], as displayed in Table 3.
3.2.4. Multivariable Analysis Results
In a stepwise logistic regression analysis of the baseline predictors of surgical failure, three variables were selected containing independent predictive value: injury type D and E (p = 0.0094), immediate attempt of surgical repair (p = 0.0113), and repair in a non-specialized center (p = 0.001) (Table 4).

Table 4.
Multivariable analysis results.
Once these three multivariable predictors of surgical treatment intervention failure were controlled, none of the other baseline variables offered additional predictive value.
Of note, three of the baseline variables were highly correlated and collinear: LC converted to open, and LC converted to open because of BDI recognition and immediate attempt of surgical repair (p = 0.001). Thus, it was statistically impossible to determine which of these three baseline variables were the most appropriate predictor of surgical failure development.
However, it appeared that clinically, the immediate attempt of surgical repair was the most appropriate variable to be selected into the surgical failure logistic model, as the timing of surgical repair clearly provides the most accurate information on the management of BDIs.
3.3. Upfront Endoscopy/IR Group
3.3.1. Distributions of Baseline Characteristics
Overall, median patient age was 52 (IQ range: 42–65) years, with 15.8% of patients (16/101) aged more than 70 years old and 42.2% (43/102) of male sex, as shown in Table 2. Indeed, age and sex were not reported in eight and seven cases, respectively.
Cholecystectomy was performed laparoscopically in the majority of cases (n = 96, 88%) and converted to open in only ten percent of cases (10/96), as shown in Table 2.
The most frequent indications for an endoscopic and/or IR treatment were represented by type E (n = 50, 45.9%) and A (n = 30, 27.5%) lesions, followed by type C (n = 18, 16.5%), D (n = 10, 9.2%) and B (n = 1, 0.9%) injuries, as outlined in Table 2.
Of note, in the type E category of BDIs, the E2 and E1 subtypes were the most common damage patterns, reported in approximately one third (n = 18, 36%) and one quarter (n = 12, 24%) of cases, respectively, followed by the E4 (10/50, 20%) and the E3 (7/50, 14%) patterns. The most serious BDI, i.e., the E5 subtype, was recorded in only three cases (6%).
Similarly, an associated VI was documented in only three patients (3/109, 2.8%), with the exclusive involvement of the RHA (Table 2).
Of the 101 patients (92.7%) with the mentioned timing of BDI diagnosis, in more than half (n = 57, 56.4%), the BDI was recognized at least one week after the initial operation of cholecystectomy (Table 2).
Among the endoscopic procedures chosen for the management of BDIs, ERCP with stenting and/or NB drainage was the most common technique, adopted in almost all cases (92/93, 98.9%).
IR methods were employed in almost half of the patients (53/109, 48.6%), mainly consisting of PTBD, utilized in approximately three quarter of cases (39/53, 73.6%), followed by leak repair through coil embolization, sclerotherapy with acetic acid or ethanol, and fibrin glue application, employed in 9 (17%), 2 (3.8%), and 1 (1.8%) case.
Combined ERCP and IR, but mainly PTBD, were delivered in approximately one third of cases (38/109, 34.9%), whereas a real rendez-vous procedure was described in almost one quarter of patients (24/109, 22%).
In the majority of patients (97/109, 89%), the BDI was managed in a specialized HPB center or in a tertiary care facility, whereas in the remaining cases (12/109, 11%), the endoscopic/IR procedure was carried out at the community hospital where cholecystectomy was performed (Table 2).
3.3.2. Distribution of the Primary Endpoint
In total, 22 patients (20.2%) developed a failure of the initial endoscopic/IR treatment intervention (Table 2).
The most frequent reason for failure was the persistence of the bile leak (n = 15, 68.2%), followed by a secondary stricture at the level of the CBD (n = 7, 31.8%).
Perioperative mortality was not reported in this group (Table 2).
In the 22 patients who required a secondary intervention, HJ was carried out in the majority of cases (n = 18, 81.8%), whereas an isolated HR and a combined procedure of HJ and HR were completed in approximately half (n = 10, 45.5%) and one third of cases (n = 7, 31.8%). In the only remaining case (4.5%), another ERCP with stent placement was performed in order to treat the CBD secondary stricture.
3.3.3. Univariable Comparisons of Baseline Variables between Failure and Non-Failure of the Endoscopic/IR Treatment Intervention
Tests of association of baseline variables with the development of endoscopic/IR treatment intervention failure found that patients presenting with a higher degree of BDIs, i.e., type D and E, and with an associated VI, were more likely to develop this outcome (p = 0.001 and 0.006, respectively) (Table 5).

Table 5.
Comparisons of variables by group (endoscopy/IR failure vs. endoscopy/IR non-failure) [1].
Among the other variables analyzed, the management of the injury in an HPB/tertiary care institution was associated with a significantly lower percentage of failure (13.4%, 13/97) compared to that when attempted in a non-specialized center (75%, 9/12) [p < 0.0001], as displayed in Table 5.
3.3.4. Multivariable Analysis Results
In a stepwise logistic regression analysis of baseline predictors of endoscopic/IR failure, two variables were selected containing independent predictive value: injury type D and E (p = 0.02), and repair in a non-specialized center (p = 0.0002) (Table 6).

Table 6.
Multivariable analysis results.
Once these two multivariable predictors of endoscopic/IR treatment intervention failure were controlled, none of the other baseline variables offered additional predictive value.
3.4. Sensitivity Analysis
The sensitivity analysis was performed by including only the most recent studies, published between 2013 and 2023.
In the “upfront surgery group”, this sensitivity analysis confirmed the predictive factors emerged at the overall analysis, whereas in the “upfront endoscopy/IR group”, only the favorable effect of repair in an HPB/tertiary care center was still preserved (Table A3, Appendix A).
4. Discussion
Our study represents the first IPD review and one of the largest case series analyzing the current management of BDIs after cholecystectomy, with a particular focus on investigating the baseline predictors of treatment failure, both surgical and endoscopic/IR.
Among all the baseline variables assessed, we were able to identify specific risk factors for developing this outcome in those patients presenting with a biliary tract damage after cholecystectomy (LC converted to open for any reason, severity of BDI, immediate surgical reconstruction, and repair in a non-specialized center).
Overall, in our study population, significantly higher incidences of treatment failure and mortality were reported in the “upfront surgery” group when compared to the “upfront endoscopy/IR” group. However, this finding should not lead one to conclude that an upfront surgical approach in the case of BDI after cholecystectomy might actually increase the failure risk. In fact, higher rates of conversion to open, mainly because of BDI recognition, major degrees of damage patterns with concomitant vascular impairment, the early diagnosis of the injury and hence the immediate attempt of repair in a non-specialized center, emerged as significant baseline variables in the surgical group, suggesting that more complex injuries requiring conversion to open were recognized earlier and thus potentially repaired in the non-specialized hospital where the cholecystectomy was performed, confirming the literature findings [4,145].
Thus, the prognostic value of a surgical approach in terms of it implying a greater risk of failure appears to be more of a reflection of the patient’s clinical status at the time of the damage rather than a direct consequence of the surgical treatment itself.
When analyzing both groups separately, “upfront surgery” and “upfront endoscopy/IR”, the severity of BDI emerged as a significant risk factor for treatment failure.
Indeed, from what emerged in our IPD review, though the majority of the series included did not use any classification system to categorize the reported BDIs and though there is still no consensus on the “gold standard” classification for these injuries, we decided to adopt Strasberg’s classification [10], principally due to its reliable application in clinical practice, allowing to easily convert the clinical information detailed in each case report or series to the accurate grade of damage.
Conversely, more recent and extremely detailed codifications systems, such as the ATOM [16], properly developed to standardize the definition of BDI and improve the reproducibility of data in epidemiological and comparative studies, might lack applicability in the current clinical scenario due to its complexity [4], as emerged in the analyzed reports.
In our study, indeed, grade D and E carried an increased risk of treatment failure by approximately four times in both the groups investigated. While in the surgical group, these types of injuries represented the most common damage patterns, accounting alone for almost 90% of cases, in the other group, approximately half of patients presented with these degrees of damage.
In the original classification by Strasberg et al. [10], a type D injury was defined as a lateral injury to the major bile duct whereas a type E injury consisted of a stricture affecting the CBD and/or the confluence of lobar ducts and was further divided into five subtypes of increasing severity, directly correlated to the involvement of the right and left hepatic ducts.
Unfortunately, a wide range of injuries included within the D category, ranging from a small clean lateral laceration to a laceration greater than 50% of circumference or a thermal injury whose full extent may be difficult to determine initially, explains the reasons underlying an objective difficulty in classifying the severity of this damage, leading to concomitant controversies in their management [13].
The other three categories of BDIs in the Strasberg’s classification are represented by type A, B and C, the first one described as a leak from the cystic duct and/or the duct of Luschka while the second and the third as an occlusion and a transection, without ligation, respectively, of an aberrant right hepatic duct [10].
In our study, type A injury represented the second most common damage pattern in the “endoscopy/IR” group, documented in one quarter of cases, as a result of its approachability with ERCP, followed by the type C, whereas the grade B damage pattern was uncommon in both groups.
Since the last two refer to aberrant right hepatic ducts, it is often difficult to reach a correct diagnosis, and consequently, their management might be complex, independently from adopting an endoscopic or surgical treatment [4].
As stated above, Strasberg’s classification [10] did not mention an associated VI, which was very frequent in the surgical group, mainly involving the arterial side, though combined lesions of the HA and PV were described in one quarter of cases, and conversely quite rare in the other group analyzed. However, the concomitant presence of vascular damage did not interfere with the response to treatment.
Regarding the appropriate timing of surgical repair, it has been a common matter of debate in the literature, with two metanalyses demonstrating a significantly lower risk of treatment failure, post-repair bile leak, need for surgical revision, and overall morbidity when a delayed strategy, i.e., repair after 6 weeks from the cholecystectomy, was adopted, compared to an early approach, i.e., within 6 weeks [145,146].
In addition, on-table repair, by direct suture or bilio-enteric anastomosis and mainly if carried out by non-HPB specialists, presented a trend for a higher risk of failure in comparison to postoperative repair [145].
On the other hand, recent evidence from a large multicenter study conducted by the European-African Hepato-Pancreato-Biliary Association (E-AHPBA) [24], together with a randomized controlled trial [147], indicates that the timing of biliary reconstruction with HJ after major post-cholecystectomy BDIs seems not to have any impact on the analyzed outcomes: a successful reconstruction rate, anastomotic patency, re-intervention rate, morbidity, and mortality.
In a cost-effective analysis on more than 500,000 cholecystectomies, Sweigert et al. [148] demonstrated that a delayed repair strategy, i.e., after 6 weeks, though not associated with increased mortality, was responsible for significantly higher inpatient costs (+USD 45,111; 95% CI: USD 36,813–USD 53,409) compared to an early approach, defined in this study as within 3 days from the cholecystectomy, whereas significantly higher costs and mortality were reported when an intermediate repair, from 4 days to 6 weeks, was chosen, thus suggesting that the latter option be avoided.
However, due to the lack of a standardized definition for the timing of BDI repair, a recent systematic review stated that no definitive conclusions can be drawn on the optimal interval period to fix these damages, at the same time advocating for a uniform reporting system [23].
In our IPD review, an immediate repair strategy, i.e., within one day, thus including the on-table repair, emerged as a significant risk factor for treatment failure at the multivariable analysis, whereas the late approach displayed the lowest percentage of failure, in agreement with the two previously cited metanalysis [145,146].
Indeed, several weeks are usually required for the resolution of the acute inflammatory phase, allowing one to lower the risks associated with extensive reconstructive surgery by reducing inflammation, improving the nutritional status, recovering an adequate immunologic competence, and guaranteeing a correct assessment of the extent of ischemic injury in the case of associated VIs [4,145].
Nevertheless, repair success is strictly dependent upon surgical experience and skills [145,149]. Indeed, as emerged in the metanalysis by Wang et al. [145], when exclusively analyzing those patients undergoing repair in a specialized HPB center, a lower rate of repair failure, although not significant, was shown for on-table versus postoperative repair (18.9% vs. 24.7%), apparently contradicting their previous findings.
Therefore, the authors concluded that, since an early repair was more likely attempted by a non-HPB specialist, compared with late repair, nonspecialist attempts could obfuscate the benefits of an on-table repair, whereas more specialist involvement may facilitate the success of late repair [145].
Similar conclusions were also reached in our review, stressing that the repair of the BDI in a non-specialized center represented the most significant risk factor for treatment failure, independently from adopting a surgical or an endoscopic/IR approach.
Therefore, according to the already cited metanalysis [145], to the WSES guidelines [4] and to a recent multicenter study investigating the textbook outcomes of BDIs after cholecystectomy [149], an early referral to an HPB or a tertiary care center should be considered mandatory, aiming at decreasing the likelihood of repair failure, perioperative morbidity, and biliary strictures.
Nevertheless, when the diagnosis is not immediate or logistic constraints limit referral to an HPB/tertiary care center, the “drain now, fix later” strategy with percutaneous drainage of the biloma, targeted antibiotic therapy, and nutritional support seems to be the best approach, rather than attempting a surgical repair [4].
In case of major BDIs, the HJ technique represents the most common and preferred type of surgical reconstruction, being the end-to-end anastomosis potentially associated with increased failure rates, particularly when a concomitant VI is demonstrated [4,149].
An anastomotic stricture (AS) represents the most common long-term complication of the above-mentioned reconstructive method, generally occurring after ten to thirty months and in a range between 10 and 20% [150], though minimally invasive (MI) approaches, both laparoscopic and robotic, appear to show promising preliminary results in terms of stricture rate, approximately of 4.5% [151].
Main risk factors for stricture are represented by a concomitant VI, a post-repair bile leak and a repair by a non-HPB surgeon, as stated in a recent systematic review and meta-analysis [152].
SBC represents another significant long-term complication of BDI repair, with a reported incidence between 2.4 and 10.9%, and potentially requiring a liver transplantation as final treatment [150].
In our review, almost one quarter of open HJ developed an AS, in line with the literature [150], whereas no stenosis was reported after MI surgery, though only four cases were included, three robotic and one laparoscopic.
Similarly, SBC was documented in 2.1% of cases, with all patients undergoing subsequently a liver transplantation.
On the other hand, ERCP represents the primary treatment of minor BDIs, with a reported success rate ranging between 87% and 100%, in case of postoperative bile leaks, depending on the grade and location of the fistula [153].
Combining, in course of ERCP, biliary sphinterectomy with the placement of a transpapillary stent, both plastic and metallic, aiming to reduce the transpapillary pressure gradient, thus facilitating the bile flow to the duodenum opposite to the site of leak, and represents the preferred approach over a temporary NB drainage, that, although similar in efficacy, might be difficult to bear for the patient [153,154,155].
Endoscopic management should be attempted exclusively in those cases with at least a partially documented continuity of the bile tract damage or when the two stumps, proximal and distal, are very close [4,154,155].
In the case of non-feasibility or failure of the ERCP, IR procedures, mainly PTBD, can be pursued, though complex, in relation to non-dilated bile ducts, and with success rates ranging between 70% and 80% [156], which are slightly inferior to endoscopic techniques.
Moreover, PTBD allows one to perform an extraluminal percutaneous endoscopic rendez-vous procedure with stent placement in order to restore the continuity of the bile duct [157,158].
Lastly, ERCP represents the first-line treatment of benign biliary strictures after cholecystectomy, with the multi-stenting approach considered to be the most effective method, although impaired by a recurrence rate up to 30% within 2 years from stent removal [159].
The major advancements currently reported in the endoscopic and IR management of BDIs and the associated improved outcomes [160,161] might explain the results emerged at the sensitivity analysis of the non-surgical group, showing a no longer existing correlation between the severity of the damage pattern and the treatment failure over the latest years.
Even if we have taken a thorough and systematic approach to individual predictive factors using all obtainable IPD collected in published studies until December 2023, making this review a unique contribution to the global evidence base for the risk assessment of treatment failure in the case of BDI after cholecystectomy, there are several limitations to this study.
First, since the pooled estimates are based on a retrospective case series and case reports only, the possibility of bias inherent to the original studies, cannot be excluded and might still have influenced the results of this review.
Second, IPD could not be obtained for all studies that the search strategy retrieved, causing to include some missing data in our analysis or to exclude some baseline variables, i.e., emergent and elective cholecystectomies, and despite efforts to contact authors, preventing us to obtain the data from some potentially eligible studies.
Third, predictions were not generated for concomitant multiple BDIs, which represents a limitation of most of the current classifications systems, including that adopted in our study. We indeed decided to assign to each individual case the most serious damage pattern reported, with the risk of missing less severe injuries, that might have influenced the outcome.
5. Conclusions
In our IPD review, the management of BDIs after cholecystectomy in a non-specialized center resulted the strongest multivariable predictor of treatment failure, followed by the severity of the damage pattern recorded.
Additionally, the failure of the surgical treatment was associated with the timing of repair adopted, with higher chances of failure when an early approach was endorsed, supporting the conclusion of two previous meta-analyses, and adding more exactness to this topic.
Further prospective studies adopting uniform codification systems and more rigorous definitions, particularly with regard to the time intervals applied, are required to validate and confirm these findings.
Author Contributions
Conceptualization: P.V. and F.M.; Methodology: P.V. and F.M.; Software: P.V. and D.G.; Validation: P.V., F.M., D.N., and A.B.C.; Formal analysis: D.N. and A.B.C.; Investigation: D.G.; Resources: M.V.; Data curation: P.V., F.M., and D.G.; Writing—original draft preparation: P.V. and F.M.; Writing—review and editing: P.V., F.M., D.N., A.B.C., and M.V.; Visualization: P.V. and D.G.; Supervision: P.V., F.M., and M.V.; Project administration: P.V. and F.M.; Funding acquisition: M.V., P.V., and F.M. have contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to being a systematic review.
Informed Consent Statement
Written informed consent has been obtained from our patients to publish this paper.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
- MEDLINE
(((((Laparoscop* OR Celioscop* OR Coelioscop* OR Abdominoscop* OR Peritoneoscop* OR lap) AND (cholecystectom* OR colecystecto* OR chole) OR “Cholecystectomy, Laparoscopic” [Mesh]) AND ((((injur* OR bile leak* OR biliary leak* OR transection* OR occlusion* OR stricture* OR stenosis* OR obstruct* OR laceration* OR damage*) OR (harm* OR convers* OR “Peritonitis” [MeSH] OR peritonitis OR “Cholestasis” [Mesh] OR Cholestas*) OR ((biliary OR bile) AND stas*)) AND (bile duct* OR biliary tract*)) OR “Biliary Tract/injuries” [Mesh])) AND (English [LA]))) NOT (animals [MeSH] NOT (humans [MeSH] AND animals [MeSH])).
- SCOPUS
- All fields
(Laparoscop* OR Celioscop* OR Coelioscop* OR Abdominoscop* OR Peritoneoscop* OR lap) AND (cholecystectom* OR colecystecto* OR chole) AND (injur* OR bile leak* OR biliary leak* OR transection* OR occlusion* OR stricture* OR stenosis* OR obstruct* OR laceration* OR damage*) OR (harm* OR convers* OR peritonitis OR Cholestas*) OR (biliary OR bile AND stas*) AND (bile duct* OR biliary tract*) AND (English) AND NOT (animals) AND NOT (humans AND animals).

Table A1.
Basic characteristics of the included studies.
Table A1.
Basic characteristics of the included studies.
| Author | Publication Year | Country | N Cases | M/F | Age (y) ° | Initial Classification Used | Primary Repair Site (HPB-H/C-H) |
|---|---|---|---|---|---|---|---|
| Parmeggiani et al. [137] | 2010 | Italy | 3 | 1/2 | 52 (45–56) | none | 1/2 |
| Bernhardt et al. [120] | 2010 | Austria | 1 | 0/1 | 49 | none | 1/0 |
| Chiruvella et al. [121] | 2010 | USA | 1 | 0/1 | 65 | Bismuth | 1/0 |
| Moo-Young et al. [102] | 2010 | USA | 1 | 0/1 | 64 | none | 1/0 |
| Humes et al. [107] | 2010 | England | 3 | 2/1 | 42 (35–63) | none | 3/0 |
| Marin et al. [110] | 2010 | USA | 1 | 1/0 | 68 | none | 0/1 |
| Selvaggi et al. [60] | 2010 | Italy | 1 | 0/1 | 40 | none | 0/1 |
| Ganguly et al. [40] | 2010 | USA | 1 | 1/0 | 51 | none | 1/0 |
| Schmidt et al. [32] | 2010 | Germany | 14 | n.r. | n.r. | Neuhaus | 14/0 |
| Hwang J.C. et al. [37] | 2011 | Republic of Korea | 1 | 0/1 | 31 | none | 1/0 |
| Ball et al. [125] | 2011 | USA | 1 | 0/1 | 27 | none | 1/0 |
| Brunet et al. [103] | 2011 | Canada | 1 | 0/1 | 48 | none | 1/0 |
| Palermo et al. [94] | 2011 | USA | 1 | 0/1 | 43 | none | 0/1 |
| Lau et al. [119] | 2011 | USA | 1 | 1/0 | 30 | none | 1/0 |
| Mazer et al. [91] | 2011 | USA | 7 | 1/6 | 41 (22–71) | none | 7/0 |
| Yan et al. [73] | 2011 | China | 1 | 1/0 | 41 | Strasberg | 1/0 |
| Maurea et al. [93] | 2011 | Italy | 1 | 0/1 | 41 | Bismuth | 1/0 |
| Hwang S. et al. [58] | 2011 | Republic of Korea | 1 | 0/1 | 48 | none | 1/0 |
| Romano O. et al. [51] | 2011 | Italy | 2 | 1/1 | 50 (45–55) | none | 0/2 |
| Choi et al. [39] | 2011 | Republic of Korea | 1 | 0/1 | 34 | none | 1/0 |
| Donatelli et al. [126] | 2012 | France | 1 | 1/0 | 51 | none | 1/0 |
| Strasberg et al. [118] | 2012 | USA | 8 | 0/8 | n.r. | Strasberg | 8/0 |
| Truant et al. [75] | 2012 | France | 3 | 2/1 | 35 (25–43) | Strasberg | 1/2 |
| Mohamadnejad et al. [109] | 2012 | Iran | 1 | 0/1 | 68 | none | 1/0 |
| Sasahira et al. [76] | 2012 | Japan | 1 | 0/1 | 58 | none | 0/1 |
| Olmez et al. [77] | 2012 | Turkey | 1 | 1/0 | 53 | Strasberg | 1/0 |
| Addeo et al. [101] | 2013 | France | 6 | 2/4 | 39 (28–46) | Strasberg | 0/6 |
| De Werra et al. [122] | 2013 | Italy | 9 | n.r. | n.r. | Strasberg | 9/0 |
| Denjalic et al. [96] | 2013 | Bosnia | 1 | 1/0 | 43 | none | 1/0 |
| Wojcicki et al. [79] | 2013 | Poland | 2 | 0/2 | 18 (15–21) | Strasberg | 2/0 |
| Mungai et al. [92] | 2013 | Italy | 1 | 0/1 | 42 | none | 1/0 |
| Macedo et al. [66] | 2013 | USA | 1 | 0/1 | 58 | none | 1/0 |
| Majumder et al. [59] | 2013 | USA | 1 | 0/1 | 58 | none | 1/0 |
| Parlak et al. [61] | 2013 | Turkey | 7 | 1/6 | 44 (30–82) | none | 7/0 |
| Paik et al. [28] | 2013 | Republic of Korea | 1 | 1/0 | 76 | none | 1/0 |
| Gluszek et al. [138] | 2014 | Poland | 14 | 3/11 | 45 (29–84) | ATOM | 13/1 |
| Buturovic et al. [106] | 2014 | Bosnia | 1 | 1/0 | 40 | none | 1/0 |
| Crema et al. [116] | 2014 | Brazil | 1 | 1/0 | 41 | Bismuth | 1/0 |
| Odemis et al. [105] | 2014 | Turkey | 1 | 0/1 | 45 | Stewart-Way | 1/0 |
| Donatelli et al. [98] | 2014 | France | 16 | 6/10 | 55.5 (31–89) | Strasberg | 16/0 |
| Shokouh-Amiri et al. [111] | 2014 | USA | 1 | 0/1 | 71 | none | 1/0 |
| Jadrijevic et al. [80] | 2014 | Croatia | 1 | 0/1 | 36 | Bismuth | 0/1 |
| Judd et al. [104] | 2014 | USA | 1 | 1/0 | 68 | none | 1/0 |
| Laux et al. [83] | 2014 | USA | 1 | 0/1 | 48 | none | 1/0 |
| Jalali et al. [97] | 2014 | USA | 1 | 0/1 | 45 | Strasberg | 0/1 |
| Yaqub et al. [52] | 2014 | Norway | 1 | 1/0 | 32 | Strasberg | 1/0 |
| Sofi et al. [33] | 2014 | USA | 3 | 2/1 | 46 (27–61) | none | 3/0 |
| Anantha et al. [34] | 2014 | USA | 2 | 1/1 | 39.5 (29–52) | none | 2/0 |
| Sugawara et al. [29] | 2014 | Japan | 14 | 11/3 | 51 (27–81) | Strasberg | 14/0 |
| Artifon et al. [141] | 2015 | Brazil | 1 | 0/1 | 63 | none | 1/0 |
| Velidedeoglu et al. [81] | 2015 | Turkey | 12 | 2/10 | n.r. | Strasberg | 12/0 |
| Odemis et al. [142] | 2015 | Turkey | 1 | 1/0 | 53 | none | 1/0 |
| Prasad et al. [67] | 2015 | India | 1 | 0/1 | 36 | Strasberg | 1/0 |
| Rai et al. [57] | 2015 | USA | 1 | 0/1 | 54 | none | 1/0 |
| Pekolj et al. [30] | 2015 | Argentina | 15 | 7/8 | 44 (23–65) | Strasberg | 5/10 |
| Curcio et al. [124] | 2016 | Italy | 1 | 0/1 | 36 | none | 1/0 |
| Merrick et al. [85] | 2016 | England | 1 | 0/1 | 48 | Bismuth | 0/1 |
| Takahashi et al. [49] | 2016 | Japan | 2 | 0/2 | 78 (73–83) | none | 2/0 |
| Hoepfner et al. [38] | 2016 | USA | 1 | 1/0 | 79 | none | 1/0 |
| Sasaki et al. [131] | 2017 | Japan | 1 | 1/0 | 78 | none | 1/0 |
| Dokmak et al. [88] | 2017 | France | 3 | 1/2 | 38 (26–45) | none | 3/0 |
| Dokmak et al. [87] | 2017 | France | 1 | 0/1 | 59 | none | 1/0 |
| Ko et al. [108] | 2017 | Republic of Korea | 1 | 1/0 | 51 | none | 1/0 |
| Zoričić et al. [86] | 2017 | Croatia | 1 | 1/0 | 78 | Strasberg | 1/0 |
| Park et al. [70] | 2017 | Republic of Korea | 1 | 1/0 | 67 | none | 1/0 |
| Xu et al. [140] | 2017 | USA | 1 | 0/1 | 32 | none | 1/0 |
| Odemis et al. [45] | 2017 | Turkey | 1 | 1/0 | 58 | none | 1/0 |
| Meek et al. [53] | 2018 | USA | 1 | 0/1 | 44 | none | 1/0 |
| Kohn et al. [132] | 2018 | USA | 7 | n.r. | n.r. | Strasberg | 7/0 |
| Dousse et al. [112] | 2018 | France | 1 | 0/1 | 55 | Bismuth | 0/1 |
| Elmunzer et al. [127] | 2018 | USA | 1 | 0/1 | 43 | none | 1/0 |
| Rifatbegovic et al. [41] | 2018 | Bosnia | 1 | 0/1 | 21 | Strasberg | 1/0 |
| Runge et al. [65] | 2018 | USA | 1 | 0/1 | 24 | none | 1/0 |
| Lim et al. [54] | 2018 | Australia | 1 | 0/1 | 44 | none | 0/1 |
| Kotecha et al. [113] | 2019 | Australia | 1 | 0/1 | 56 | none | 1/0 |
| Nezami et al. [136] | 2019 | USA | 7 | 5/2 | 60 (44–76) | none | 7/0 |
| Machado et al. [134] | 2019 | Brazil | 1 | 0/1 | 24 | none | 1/0 |
| Bonati et al. [133] | 2019 | Italy | 1 | 0/1 | 45 | none | 1/0 |
| Acquafresca et al. [129] | 2019 | Argentina | 1 | 1/0 | 64 | none | 1/0 |
| Ayloo et al. [128] | 2019 | USA | 1 | 0/1 | 36 | Strasberg | 1/0 |
| Battal et al. [100] | 2019 | Turkey | 13 | 4/9 | 46 (19–65) | Strasberg | 13/0 |
| Desai et al. [123] | 2019 | India | 1 | 0/1 | 35 | none | 1/0 |
| Kumar et al. [82] | 2019 | India | 1 | 0/1 | 42 | Strasberg | 1/0 |
| Kwak et al. [71] | 2019 | Republic of Korea | 8 | 2/6 | 49 (29–77) | Bismuth | 8/0 |
| Özdemir et al. [63] | 2019 | France | 1 | 0/1 | 42 | Strasberg | 0/1 |
| Kohga et al. [56] | 2019 | Japan | 2 | 1/1 | 82.5 (81–84) | none | 0/2 |
| Gallagher et al. [36] | 2019 | USA | 1 | 0/1 | 67 | Strasberg | 1/0 |
| Martines et al. [27] | 2019 | Italy | 43 | 19/24 | 50 (15–81) | Strasberg | 34/9 |
| Lubikowski et al. [130] | 2019 | Poland | 1 | 0/1 | 26 | Bismuth | 1/0 |
| Drs et al. [117] | 2020 | Czech Republic | 1 | 1/0 | 45 | Stewart-Way | 1/0 |
| Tsai et al. [95] | 2020 | Taiwan | 2 | 1/1 | 55.5 (53–58) | Strasberg | 1/1 |
| Hite et al. [115] | 2020 | USA | 1 | 0/1 | 31 | none | 0/1 |
| Fan et al. [62] | 2020 | China | 1 | 1/0 | 65 | Stewart-Way | 0/1 |
| Vasilidias et al. [64] | 2020 | Greece | 1 | 0/1 | 46 | Strasberg | 0/1 |
| Sondhi et al. [47] | 2020 | USA | 1 | 1/0 | 87 | Bergman | 1/0 |
| Ferrada et al. [31] | 2020 | Chile | 1 | 1/0 | 65 | Strasberg | 1/0 |
| Bowen et al. [99] | 2021 | Ireland | 1 | 0/1 | 30 | none | 1/0 |
| Romano L. et al. [69] | 2021 | Italy | 1 | 0/1 | 18 | none | 1/0 |
| Wei et al. [90] | 2021 | USA | 1 | 1/0 | 85 | Strasberg | 0/1 |
| Lin et al. [135] | 2021 | Taiwan | 1 | 1/0 | 50 | none | 1/0 |
| Mistry et al. [26] | 2021 | India | 2 | 0/2 | 53 (30–76) | Strasberg | 2/0 |
| Anwar et al. [84] | 2022 | Indonesia | 1 | 0/1 | 32 | none | 1/0 |
| Mayer et al. [114] | 2022 | France | 1 | 0/1 | 70 | none | 1/0 |
| Torretta et al. [68] | 2022 | Italy | 1 | 1/0 | 65 | Strasberg | 1/0 |
| Garcia et al. [74] | 2022 | Brazil | 1 | 0/1 | 39 | none | 1/0 |
| Sharma et al. [72] | 2022 | USA | 1 | 0/1 | 62 | none | 1/0 |
| Kakati et al. [42] | 2022 | Lebanon | 4 | 2/2 | 38 (30–50) | none | 4/0 |
| Emara et al. [46] | 2022 | Egypt | 1 | 1/0 | 58 | none | 1/0 |
| D’Ovidio et al. [35] | 2022 | Italy | 1 | 1/0 | 60 | Hannover | 0/1 |
| Robles-Medrandra et al. [25] | 2022 | Ecuador | 1 | 0/1 | 24 | Strasberg | 1/0 |
| Sucandy et al. [89] | 2023 | USA | 1 | 0/1 | 43 | Strasberg | 1/0 |
| Swallow et al. [139] | 2023 | Tanzania | 2 | 0/2 | 43 (40–46) | Bismuth | 2/0 |
| Hristov et al. [55] | 2023 | Bulgaria | 1 | 0/1 | 33 | Strasberg | 1/0 |
| Rott et al. [48] | 2023 | Germany | 1 | 1/0 | 68 | none | 1/0 |
| Abughararah et al. [43] | 2023 | Saudi Arabia | 1 | 1/0 | 85 | Strasberg | 1/0 |
| Rathore et al. [78] | 2023 | India | 1 | 0/1 | 43 | Bismuth | 0/1 |
| Selleslag et al. [50] | 2023 | Belgium | 1 | 0/1 | 74 | none | 1/0 |
| Marti Romero et al. [44] | 2023 | Spain | 1 | 0/1 | 79 | Strasberg | 0/1 |
° If more than one case, age was expressed in median with range. HPB-H, hepato-pancreato-biliary hospital; C-H, community hospital; n.r., not reported.

Table A2.
Our series of patients with BDI.
Table A2.
Our series of patients with BDI.
| Pt n. | Age (y)/ Sex | Indication for Surgery | Primary Surgery | BDI Type ° | VI | Timing Diagnosis | Initial Treatment/ Site | Timing Surgical Repair | Further Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 78/M | acute calculous cholecystitis | LC, converted | E2 | - | 4 d | HJ/ HPB-H | 9 d | - | death |
| 2 | 42/F | lithiasis, elective | OC | E2 | - | io | HJ/ HPB-H | 14 d | PTBD, re-do HJ for AS | no further complications |
| 3 | 50/F | lithiasis, elective | LC | D | - | 5 d | ERCP + stenting/ HPB-H | - | ERCP + ballooning for stricture | no further complications |
| 4 | 68/F | lithiasis, elective | LC | E2 | - | 58 d | ERCP + stenting, PTBD/HPB-H | - | - | no further complications |
| 5 | 40/F | lithiasis, elective | OC | D | - | 4 d | E-E A/ C-H | 5 d | PTBD, HJ for AS | no further complications |
| 6 | 22/M | acute calculous cholecystitis | LC | E2 | - | 26 d | HJ/ HPB-H | 29 d | re-do HJ + LH for AS | no further complications |
| 7 | 67/M | lithiasis, elective | LC, converted | E2 | - | 45 d | HJ/ HPB-H | 49 d | - | no further complications |
| 8 | 79/M | acute calculous cholecystitis | LC, converted | D | - | io | HJ/ HPB-H | 1 d | - | no further complications |
| 9 | 84/M | acute calculous cholecystitis | LC | E4 | - | 3 d | ERCP + stenting/ C-H | 9 d | HJ | no further complications |
| 10 | 49/M | acute acalculous cholecystitis | LC | E4 | - | 11 d | RH + HJ/ HPB-H | 81 d | - | no further complications |
| 11 | 62/M | lithiasis, elective | LC | E2 | - | 148 d | HJ/ HPB-H | 153 d | - | no further complications |
| 12 | 67/M | lithiasis, elective | LC | E3 | - | 10 d | ERCP + stenting/ C-H | 24 d | HJ | no further complications |
| 13 | 34/F | lithiasis, elective | LC | C | - | 5 d | clips placement/C-H | 7 d | ERCP + stenting/ E-lap | no further complications |
| 14 | 32/F | lithiasis, elective | LC | E4 | - | io | T-tube insertion/C-H | i.o. | HJ | no further complications |
| 15 | 33/F | lithiasis, elective | LC | E2 | - | 3 d | HJ/ HPB-H | 4 d | - | no further complications |
| 16 | 87/M | lithiasis, elective | LC | E4 | RHA | io | HJ + VR/ HPB-H | 1 d | - | no further complications |
| 17 | 36/M | acute calculous cholecystitis | LC | E2 | - | 4 d | HJ/ C-H | 12 d | PTBD/ ERCP + stenting for stricture | recurrent cholangitis |
| 18 | 49/M | acute calculous cholecystitis | LC converted | D | - | io | primary suture repair/C-H | i.o. | PTBD/ robotic HJ | no further complications |
| 19 | 73/F | lithiasis, elective | LC | C | - | 16 d | ERCP + stenting/ C-H | 36 d | laparoscopic leak repair | no further complications |
° According to the Strasberg’s classification. BDI, bile duct injury; VI, vascular injury; LC, laparoscopic cholecystectomy; HJ, hepatico-jejunostomy; HPB-H, hepato-pancreato-biliary hospital; OC, open cholecystectomy; io, intraoperative; PTBD, percutaneous transhepatic biliary drainage; AS, anastomotic stricture; ERCP, endoscopic retrograde colangio-pancreatography; E-E A, end-to-end anastomosis; C-H, community hospital; LH, left hepatectomy; RH, right hepatectomy; E-lap, exploratory laparoscopy; RHA, right hepatic artery; VR, vascular reconstruction.

Table A3.
Sensitivity analysis: the pooled OR was reassessed after excluding the studies published before 2013.
Table A3.
Sensitivity analysis: the pooled OR was reassessed after excluding the studies published before 2013.
| 1. Upfront surgery group | ||
| Preoperative risk factors contributing to Surgical Failure Variable | p-value | Odds ratio (95% CI) |
| Injury type D and E ° | 0.028 | 3.845 (1.153 to 12.82) |
| Immediate attempt of surgical repair | 0.0015 | 3.373 (1.594 to 7.14) |
| Repair in a non-specialized center | 0.0001 | 8.317 (2.878 to 24.035) |
| 2. Upfront endoscopy/IR upfront group | ||
| Preoperative risk factors contributing to rndoscopy/IR gailure Variable | p-value | Odds ratio (95% CI) |
| Repair in a non-specialized center | <0.0001 | 34.2 (6.45 to 181.32) |
° According to the Strasberg’s classification. IR, interventional radiology.
References
- Reynolds, W. The First Laparoscopic Cholecystectomy. JSLS 2001, 5, 89–94. [Google Scholar] [PubMed]
- Alexander, H.C.; Bartlett, A.S.; Wells, C.I.; Hannam, J.A.; Moore, M.R.; Poole, G.H.; Merry, A.F. Reporting of complications after laparoscopic cholecystectomy: A systematic review. HPB 2018, 20, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Brunt, L.M.; Deziel, D.J.; Telem, D.A.; Strasberg, S.M.; Aggarwal, R.; Asbun, H.; Bonjer, J.; McDonald, M.; Alseidi, A.; Ujiki, M.; et al. Safe Cholecystectomy Multi-society Practice Guideline and State of the Art Consensus Conference on Prevention of Bile Duct Injury during Cholecystectomy. Ann. Surg. 2020, 272, 3–23. [Google Scholar] [CrossRef] [PubMed]
- de Angelis, N.; Catena, F.; Memeo, R.; Coccolini, F.; Martínez-Pérez, A.; Romeo, A.M.; De Simone, B.; Di Saverio, S.; Brustia, R.; Rhaiem, R.; et al. 2020 WSES guidelines for the detection and management of bile duct injury during cholecystectomy. World J. Emerg. Surg. 2021, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Mangieri, C.W.; Hendren, B.P.; Strode, M.A.; Bandera, B.C.; Faler, B.J. Bile duct injuries (BDI) in the advanced laparoscopic cholecystectomy era. Surg. Endosc. 2019, 33, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Roslyn, J.J.; Binns, G.S.; Hughes, E.F.X.; Saunders-Kirkwood, K.; Zinner, M.J.; Cates, J.A. Open Cholecystectomy A Contemporary Analysis of 42,474 Patients. Ann. Surg. 1993, 218, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Booij, K.A.C.; De Reuver, P.R.; Van Dieren, S.; van Delden, O.M.; Rauws, A.M.; Busch, O.R.; van Gulik, T.M.; Gouma, D.J. Long-term Impact of Bile Duct Injury on Morbidity, Mortality, Quality of Life, and Work Related Limitations. Ann. Surg. 2018, 268, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Gartland, R.M.; Bloom, J.P.; Fong, Z.V.; DeRoo, C.; Dwyer, K.; Quinn, G.; Lillemoe, K.; Mort, E. What Have We Learned from Malpractice Claims Involving the Surgical Management of Benign Biliary Disease?: A 128 Million Dollar Question. Ann. Surg. 2019, 269, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Bismuth, H.; Majno, P.E. Biliary strictures: Classification based on the principles of surgical treatment. World J. Surg. 2001, 25, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Strasberg, S.; Hertl, M.; Soper, N. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J. Am. Coll. Surg. 1995, 180, 101–125. [Google Scholar] [PubMed]
- Stewart, L. Iatrogenic biliary injuries: Identification, classification, and management. Surg. Clin. N. Am. 2014, 94, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Bektas, H.; Schrem, H.; Winny, M.; Klempnauer, J. Surgical treatment and outcome of iatrogenic bile duct lesions after cholecystectomy and the impact of different clinical classification systems. Br. J. Surg. 2007, 94, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Baron, T.H.; Carr-Locke, D.L.; Chapman, W.C.; Costamagna, G.; de Santibanes, E.; Dominguez Rosado, I.; Garden, O.J.; Gouma, D.; Lillemoe, K.H.; et al. Proposed standards for reporting outcomes of treating biliary injuries. HPB 2018, 20, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Pesce, A.; Fabbri, N.; Feo, C.V. Vascular injury during laparoscopic cholecystectomy: An often-overlooked complication. World J. Gastrointest. Surg. 2023, 15, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, V.; Kuemmerli, C.; Cutillas, J.; Maupoey, J.; López-Andujar, R.; Ramos, E.; Mils, K.; Valdivieso, A.; Perfecto Valero, A.; Martinez, P.A.; et al. Vascular injury during cholecystectomy: A multicenter critical analysis behind the drama. Surgery 2022, 172, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Fingerhut, A.; Dziri, C.; Garden, O.J.; Gouma, D.; Millat, B.; Neugebauer, E.; Paganini, A.; Targarona, E. ATOM, the all-inclusive, nominal EAES classification of bile duct injuries during cholecystectomy. Surg. Endosc. 2013, 27, 4608–4619. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Wei, D.; Bhutiani, N.; Rao, M.K.; Johnston, S.S.; Patkar, A.; Vitale, G.C.; Martin, R.C.G. Adverse outcomes and short-term cost implications of bile duct injury during cholecystectomy. Surg. Endosc. 2020, 34, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hu, S.; Gu, X.; Zhang, X. Analysis of risk factors for bile duct injury in laparoscopic cholecystectomy in China: A systematic review and meta-analysis. Medicine 2022, 101, E30365. [Google Scholar] [CrossRef] [PubMed]
- Pucher, P.H.; Brunt, L.M.; Davies, N.; Linsk, A.; Munshi, A.; Rodriguez, H.A.; Fingerhut, A.; Fanelli, R.D.; Asbun, H.; Aggarwal, R. Outcome trends and safety measures after 30 years of laparoscopic cholecystectomy: A systematic review and pooled data analysis. Surg. Endosc. 2018, 32, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Temperley, H.C.; O’Sullivan, N.J.; Grainger, R.; Bolger, J.C. Is the use of a routine intraoperative cholangiogram necessary in laparoscopic cholecystectomy? Surgeon 2023, 21, e242–e248. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Kambakamba, P.; Cremen, S.; Möckli, B.; Linecker, M. Timing of surgical repair of bile duct injuries after laparoscopic cholecystectomy: A systematic review. World J. Hepatol. 2022, 14, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Rystedt, J.M.L.; Kleeff, J.; Salvia, R.; Besselink, M.G.; Prasad, R.; Lesurtel, M.; Sturesson, C.; Abu Hilal, M.; Aljaiuossi, A.; Antonucci, A.; et al. Post cholecystectomy bile duct injury: Early, intermediate or late repair with hepaticojejunostomy—An E-AHPBA multi-center study. HPB 2019, 21, 1641–1647. [Google Scholar] [CrossRef]
- Robles-Medranda, C.; Oleas, R.; Arevalo-Mora, M.; Alcivar-Vasquez, J.; Del Valle, R. EUS-guided hepaticoduodenostomy for the management of postsurgical bile duct injury: An alternative to surgery (with video). Endosc. Ultrasound. 2022, 11, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Vala, H. Combined Biliary and Right Hepatic Artery Injury during Laparoscopic Cholecystectomy. Indian J. Surg. 2021, 83, 846–849. [Google Scholar] [CrossRef]
- Martines, G.; Musa, N.; Lagouvardou, E.; Aquilino, F.; Caputi Iambrenghi, O. Iatrogenic bile duct injuries in the era of laparoscopic cholecystectomy: A single center experience. Chirurgia 2019, 32, 69–76. [Google Scholar] [CrossRef]
- Paik, K.Y. Biliary injury after cholecystectomy in a patient with severe right liver atrophy. J. Korean Surg. Soc. 2013, 84, 185–188. [Google Scholar] [CrossRef]
- Sugawara, G.; Ebata, T.; Yokoyama, Y.; Igami, T.; Mizuno, T.; Nagino, M. Management strategy for biliary stricture following laparoscopic cholecystectomy. J. Hepatobiliary Pancreat. Sci. 2014, 21, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Pekolj, J.; Yanzón, A.; Dietrich, A.; Del Valle, G.; Ardiles, V.; De Santibañes, E. Major liver resection as definitive treatment in post-cholecystectomy common bile duct injuries. World J. Surg. 2015, 39, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Ferrada, P.I.S.M.; Morales, H.F.L.; Abarca, J.A.S.; Muñoz, P.I.F. Major biliovascular injury associated with cholecystectomy with the need for percutaneous arterial revascularization and staged right hepatectomy: Case report. Arq. Bras. Cir. Dig. 2020, 33, e1493. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.C.; Fikatas, P.; Denecke, T.; Schumacher, G.; Aurich, F.; Neumann, U.; Seehofer, D. Hepatic resection for patients with cholecystectomy related complex bile duct injury. Eur. Surg. 2010, 42, 77–82. [Google Scholar] [CrossRef]
- Sofi, A.A.; Tang, J.; Alastal, Y.; Nawras, A.T. A simultaneous endoscopic and laparoscopic approach for management of early iatrogenic bile duct obstruction. Gastrointest. Endosc. 2014, 80, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Anantha Sathyanarayana, S.; Lee, C.; Lobko, I.; Febles, A.; Madariaga, J.R. Modified rendezvous biliary procedure involving the hepatobiliary surgeon, endoscopist, and interventional radiologist: A novel solution for complex bile duct injuries. J. Am. Coll. Surg. 2014, 219, e51–e54. [Google Scholar] [CrossRef] [PubMed]
- D’Ovidio, V.; Pompa, V.; Maltzeff, N.; Sodani, G.; Cancellieri, R.; Bazuro, M.E. Impossible but true: Complete transection of common bile duct treated with ERCP/percutaneous biliodigestive rendezvous. Endoscopy 2022, 54, E427–E428. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, S.P.; Imagawa, D.K. Spontaneous choledochoduodenal fistula in a patient with a bile duct injury following laparoscopic cholecystectomy. J. Surg. Case Rep. 2019, 2019, rjz141. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.C.; Kim, J.H.; Yoo, B.M.; Lim, S.; Kim, J.H.; Kim, W.H.; Kim, M.W. Temporary placement of a newly designed, fully covered, self-expandable metal stent for refractory bile leaks. Gut. Liver. 2011, 5, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Hoepfner, L.; Sweeney, M.K.; White, J.A. Duplicated extrahepatic bile duct identified following cholecystectomy injury. J. Surg. Case Rep. 2016, 2016, rjw064. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Eun, C.K.; Choi, H.W. Acetic acid sclerotherapy for treatment of a bile leak from an isolated bile duct after laparoscopic cholecystectomy. Cardiovasc. Interv. Radiol. 2011, 34 (Suppl. 2), 303–306. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, E.K.; Najarian, K.E.; Vecchio, J.A.; Moses, P.L. Endoscopic occlusion of cystic duct using N-butyl cyanoacrylate for postoperative bile leakage. Dig. Endosc. 2010, 22, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Rifatbegovic, Z.; Kovacevic, M.; Nikic, B. Treatment of late identified iatrogenic injuries of the right and left hepatic duct after laparoscopic cholecystectomy without transhepatic stent and Witzel drainage: Case report. Int. J. Surg. Case Rep. 2018, 48, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Kakati, R.T.; Othman, M.; Kharroubi, H.; Ataya, K.; Nassar, H.; Hafez, B.; Faraj, W.; Khalife, M.J. The implications and management of complex biliary injuries at a tertiary hepatobiliary specialty center. SAGE Open Med. Case Rep. 2022, 10, 2050313X221119587. [Google Scholar] [CrossRef] [PubMed]
- Abughararah, M.Z.; Justaniah, A.; Ahmad, N.; Ashour, M.; Alqarni, H. Percutaneous Management of Hepatic Duct Injury Using Extra-Anatomic Biliary Catheters. Cureus 2023, 15, e35012. [Google Scholar] [CrossRef] [PubMed]
- Marti Romero, L.; Boix Clemente, C.; Alemany Perez, G.; Martinez Escapa, V. Nonoperative repair of complete transection of the common bile duct using single-operator cholangioscopy. Endoscopy 2023, 55, E53–E54. [Google Scholar] [CrossRef] [PubMed]
- Odemis, B.; Oztas, E.; Akpinar, M.Y.; Ozdemir, E.; Torun, S.; Coskun, O. An alternative treatment for biliary injuries characterized by complete transection of the common bile duct: Intraperitoneal rendezvous. Therap. Adv. Gastroenterol. 2017, 10, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Emara, M.H.; Elbatae, H.E.; Ali, R.F.; Ahmed, M.H.; Radwan, M.S.; Elhawary, A. Laparoscopy-Assisted Endoscopic Retrograde Cholangiopancreatography: New Insight in Management of Iatrogenic Bile Duct Injury. Middle East J. Dig. Dis. 2022, 14, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, A.R.; Pomerantz, B.J.; Kazanjian, S.; Nathan, H.; Law, R. Recanalization of the bile duct by using percutaneous and endoscopic methods after iatrogenic injury. VideoGIE 2020, 5, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Rott, G.; Boecker, F. Embolization of an incomplete isolated right segmental hepatic duct injury (incomplete IRSHDI)-A case report. Radiol. Case Rep. 2023, 18, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Yokoyama, N.; Matsuzawa, N.; Sato, D.; Otani, T. Effectiveness of a barbed suture in the repair of bile duct injury during laparoscopic cholecystectomy: Report of two cases. Int. J. Surg. Case Rep. 2016, 26, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Selleslag, S.; Vandeputte, M.; Walgraeve, M.S. Unveiling the Hidden Culprit: A Case of Bile Leakage Post-Cholecystectomy Caused by a Luschka Duct. J. Belg. Soc. Radiol. 2023, 107, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Romano, O.; Romano, C.; Cerbone, D.; Sperlongano, P.; Caserta, L.; Frega, N.; Cimmino, G.; D’Agostino, A.; Addeo, R. Two Case Reports of Biliary Tract Injuries during Laparoscopic Cholecystectomy. ISRN Gastroenterol. 2011, 2011, 868471. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, S.; Mala, T.; Mathisen, Ø.; Edwin, B.; Fosby, B.; Berntzen, D.T.K.; Abildgaard, A.; Labori, K.J. Management of Injury to the Common Bile Duct in a Patient with Roux-en-Y Gastric Bypass. Case Rep. Surg. 2014, 2014, 938532. [Google Scholar] [CrossRef] [PubMed]
- Meek, J.; Fletcher, S.; Crumley, K.; Culp, W.C.; Meek, M. Percutaneous rendezvous technique for the management of a bile duct injury. Radiol. Case Rep. 2018, 13, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.Z.; Wong, E.; Hassen, S.; Al-Habbal, Y. Retroperitoneal bile leak after laparoscopic cholecystectomy. BMJ Case Rep. 2018, 2018, bcr-2017. [Google Scholar] [CrossRef] [PubMed]
- Hristov, B.; Doykov, D.; Stanchev, D.; Kraev, K.; Uchikov, P.; Kostov, G.; Valova, S.; Tilkiyan, E.; Doykova, K.; Doykov, M. Hepatico-Duodenal Fistula Following Iatrogenic Strasberg Type E4 Bile Duct Injury: A Case Report. Medicina 2023, 59, 1621. [Google Scholar] [CrossRef] [PubMed]
- Kohga, A.; Suzuki, K.; Okumura, T.; Yajima, K.; Yamashita, K.; Isogaki, J.; Kawabe, A. Two Cases of Subvesical Bile Duct Injury Detected and Repaired Simultaneously during Laparoscopic Cholecystectomy. Case Rep. Med. 2019, 2019, 3873876. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Beckley, A.; Fabre, A.; Bellows, C.F. Successful Treatment of Persistent Postcholecystectomy Bile Leak Using Percutaneous Cystic Duct Coiling. Case Rep. Surg. 2015, 2015, 273198. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Yoon, S.Y.; Jung, S.W.; Namgoong, J.; Park, G.; Gwon, D.; Lee, S. Therapeutic induction of hepatic atrophy for isolated injury of the right anterior sectoral duct following laparoscopic cholecystectomy. Korean J. Hepatobiliary Pancreat Surg. 2011, 15, 189–193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Majumder, S.; Habibi, H.; Garcia, C.M. Subvesical bile duct injury: An often missed cause of postcholecystectomy bile leak. Surg. Laparosc. Endosc. Percutan Tech. 2013, 23, e168–e169. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, G.; Cappello, G.; Astolfi, A.; Di Sebastiano, P.; Del Ciotto, N.; Di Bartolomeo, N.; Innocenti, P. Endoscopic therapy for type B surgical biliary injury in a patient with short cystic duct. G Chir. 2010, 31, 229–232. [Google Scholar] [PubMed]
- Parlak, E.; Odemis, B.; Disibeyaz, S.; Oztas, E.; Kalkan, I.H.; Oguz Onder, F.; Kucukay, F.; Sasmaz, N.; Sahin, B. Cholecystectomy-related aberrant bile duct injuries and their endoscopic treatment. Surg. Laparosc. Endosc. Percutan Tech. 2013, 23, e119–e123. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Pan, J.Y.; Zhang, Y.W. Recovery from a biliary stricture of a common bile duct ligature injury: A case report. World J. Clin. Cases. 2020, 8, 3567–3572. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, T.; Schmitt, A.; Cesaretti, M.; Le Bian, A.Z.; Jiao, L.R. Intraoperatively malpositioned stent as a complication of common bile duct injury during laparoscopic cholecystectomy. Ann. Hepatobiliary Pancreat. Surg. 2019, 23, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadis, K.; Moschou, E.; Papaioannou, S.; Tzitzis, P.; Totsi, A.; Dimou, S.; Lazaridou, E.; Kapetanos, D.; Papavasiliou, C. Isolated aberrant right cysticohepatic duct injury during laparoscopic cholecystectomy: Evaluation and treatment challenges of a severe postoperative complication associated with an extremely rare anatomical variant. Ann. Hepatobiliary Pancreat. Surg. 2020, 24, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Runge, T.M.; French, J.B.; Grimm, I.S.; Baron, T.H. Endoscopic repair of complete bile duct transection by use of transpapillary cholangioperitoneoscopy. VideoGIE 2018, 3, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.I.B.; Casillas, V.J.; Davis, J.S.; Levi, J.U.; Sleeman, D. Biliary-colonic fistula caused by cholecystectomy bile duct injury. Hepatobiliary Pancreatic Dis. Int. 2013, 12, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; De, S.; Mishra, P.; Tiwari, A. Robotic assisted Roux-en-Y hepaticojejunostomy in a post-cholecystectomy type E2 bile duct injury. World J. Gastroenterol. 2015, 21, 1703–1706. [Google Scholar] [CrossRef] [PubMed]
- Torretta, A.; Kaludova, D.; Roy, M.; Bhattacharya, S.; Valente, R. Simultaneous early surgical repair of post-cholecystectomy major bile duct injury and complex abdominal evisceration: A case report. Int. J. Surg. Case Rep. 2022, 94, 107110. [Google Scholar] [CrossRef] [PubMed]
- Romano, L.; Lazzarin, G.; Varrassi, M.; Di Sibio, A.; Vicentini, V.; Schietroma, M.; Carlei, F.; Giuliani, A. Haemobilia secondary to a cystic artery pseudoaneurysm as complication of VLC. Acta Biomed. 2021, 92, 92–96. [Google Scholar] [CrossRef]
- Park, J.I.; Choi, Y.K.; Jung, B.H. Acetic acid sclerotherapy for treatment of biliary leak from an isolated right posterior sectoral duct after cholecystectomy. Ann. Surg. Treat Res. 2017, 92, 221–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwak, B.J.; Choi, H.J.; You, Y.K.; Kim, D.G.; Hong, T.H. Laparoscopic end-to-end biliary reconstruction with T-tube for transected bile duct injury during laparoscopic cholecystectomy. Ann. Surg. Treat Res. 2019, 96, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Ruch, B.; Alwatari, Y.; Lele, S.; Bouhaidar, D.S. Delayed, recurrent bile leak from isolated right posterior sectoral duct injury after laparoscopic cholecystectomy: An unusual presentation. Clin. Case Rep. 2022, 10, e6032-36. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.Q.; Peng, C.H.; Shen, B.; Zhou, G.; Yang, W.; Chen, Y.; Li, H. Liver transplantation as a treatment for complicated bile duct injury. Hepatogastroenterology 2011, 58, 8–13. [Google Scholar] [PubMed]
- Garcia, S.; Concepción, A.M.; Wakoff, C. Bile Leak Due to Luschka Duct Injury After Laparoscopic Cholecystectomy: A Case Report. Cureus 2022, 14, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Truant, S.; Boleslawski, E.; Zerbib, P.; Sergent, G.; Buob, D.; Leteurtre, E.; Pruvot, F. Liver resection in management of post-cholecystectomy biliary injury: A case series. Hepatogastroenterology 2012, 59, 2403–2406. [Google Scholar] [PubMed]
- Sasahira, N.; Isayama, H.; Kogure, H.; Tsujino, T.; Koike, K. Endoscopic management with inside stent for proximal benign biliary stricture after laparoscopic cholecystectomy. Dig. Endosc. 2012, 24, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Olmez, A.; Hatipoglu, S.; Itik, V.; Kotan, C. T-tube bridging for the management of biliary tree injuries. Am. J. Case Rep. 2012, 13, 247–249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rathore, K.S.; Varshney, P.; Soni, S.C.; Varshney, V.K.; Selvakumar, B.; Agarwal, L.; Birda, C.L. Open injury, robotic repair–moving ahead! Total robotic Roux-en-Y hepaticojejunostomy for post-open cholecystectomy Bismuth type 2 biliary stricture using indocyanine green dye. J. Minim. Invasive Surg. 2023, 26, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Wojcicki, M.; Patkowski, W.; Chmurowicz, T.; Bialek, A.; Wiechowska-Kozlowska, A.; Stankiewicz, R.; Milkiewicz, P.; Krawczyk, M. Isolated right posterior bile duct injury following cholecystectomy: Report of two cases. World J. Gastroenterol. 2013, 19, 6118–6121. [Google Scholar] [CrossRef] [PubMed]
- Jadrijevic, S.; Sef, D.; Kocman, B.; Mrzljak, A.; Matasic, H.; Dinko, S. Right hepatectomy due to portal vein thrombosis in vasculobiliary injury following laparoscopic cholecystectomy: A case report. J. Med. Case Rep. 2014, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Velidedeoglu, M.; Arikan, A.; Uludag, S.; Olgun, D.; Kilic, F.; Kapan, M. Clinical Application of Six Current Classification Systems for Iatrogenic Bile Duct Injuries after Cholecystectomy. Hepatogastroenterology 2015, 62, 577–584. [Google Scholar] [PubMed]
- Kumar, S.; Kumar, P.; Chandra, A. Bile duct injury: To err is human; To refer is divine. BMJ Case Rep. 2019, 12, e228361. [Google Scholar] [CrossRef] [PubMed]
- Laux, A.; Testa, G.; Goldstein, R.; Cavaness, K.M. The Management of a Complex Bile Duct Injury after Laparoscopic Cholecystectomy. Am. Surg. 2014, 80, 175–178. [Google Scholar] [CrossRef]
- Anwar, A.D.; Nugrahani, A.D.; Mulyawan, A.; Santoso, D.P.J.; Rachmawati, A.; Aziz, M.A.; Usman, N.; Effendi, J.S. Common bile duct injury following open conversion of laparoscopic cholecystectomy in 14–15 Weeks pregnancy: A rare case report. Ann. Med. Surg. 2022, 84, 104930. [Google Scholar] [CrossRef] [PubMed]
- Merrick, B.; Yue, D.; Sodergren, M.H.; Jiao, L.R. Portobiliary fistula following laparoscopic cholecystectomy. Ann. R. Coll. Surg. Engl. 2016, 98, e123–e125. [Google Scholar] [CrossRef] [PubMed]
- Zoričić, I.; Soldo, I.; Simović, I.; Sever, M.; Bakula, B.; Grbavac, M.; Marušić, M.; Soldo, A. Common bile duct stricture after laparoscopic cholecystectomy: Case report. Acta Clin. Croat. 2017, 56, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Dokmak, S.; Aussilhou, B.; Ragot, E.; Tantardini, C.; Cauchy, F.; Ponsot, P.; Belghiti, J.; Sauvanet, A.; Soubrane, O. Reconstruction of Bile Duct Injury and Defect with the Round Ligament. J. Gastrointest. Surg. 2017, 21, 1540–1543. [Google Scholar] [CrossRef] [PubMed]
- Dokmak, S.; Amharar, N.; Aussilhou, B.; Ponsot, P.; Belghiti, J.; Soubrane, O. Laparoscopic Repair of Post-cholecystectomy Bile Duct Injury: An Advance in Surgical Management. J. Gastrointest. Surg. 2017, 21, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Sucandy, I.; Castro, M.; Krill, E.; Ross, S.; Rosemurgy, A. Robotic RY Hepaticojejunostomy for Strasberg E4 Iatrogenic Bile Duct Injury: A Modern Minimally Invasive Technique. Am. Surg. 2023, 89, 1239–1240. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Fanous, M. Omentoplasty Combined with Endoscopic Stenting in the Treatment of Lateral Duct Injury at Hepatic Duct Bifurcation. Am. Surg. 2021, 87, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Mazer, L.M.; Tapper, E.B.; Sarmiento, J.M. Non-Operative Management of Right Posterior Sectoral Duct Injury Following Laparoscopic Cholecystectomy. J. Gastrointest. Surg. 2011, 15, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Mungai, F.; Berti, V.; Colagrande, S. Bile leak after elective laparoscopic cholecystectomy: Role of MR imaging. J. Radiol. Case Rep. 2013, 7, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Maurea, S.; Imbriaco, M.; Mollica, C.; Fusari, M.; Salvatore, M. Magnetic resonance cholangiography to evaluate biliary tree integrity after cholecystectomy: A case report. J. Dig. Dis. 2011, 12, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Palermo, M.; Trelles, N.; Gagner, M. Laparoscopic revisional hepaticojejunostomy for biliary stricture after open repair following common bile duct injury: A case report. Surg. Innov. 2011, 18, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Chen, M.Y.; Yeh, T.S. Hemi-hepatectomy for E4 major bile duct injury following laparoscopic cholecystectomy. Asian J. Surg. 2020, 43, 1212–1213. [Google Scholar] [CrossRef] [PubMed]
- Denjalić, A.; Škiljo, H.; Bečulić, H.; Jusić, A.; Avdagić, N.; Oruč, M. Bile duct injury during laparoscopic cholecystectomy: Risk of procedure or professional negligence? Case report. Med. Glas. 2013, 10, 413–415. [Google Scholar]
- Jalali, F.; Lee, J.G. Endoscopic ultrasound-guided antegrade cholangiography for diagnosis of aberrant right intrahepatic duct injury after laparoscopic cholecystectomy. Endoscopy 2014, 46 (Suppl. 1), E465. [Google Scholar] [CrossRef] [PubMed]
- Donatelli, G.; Vergeau, B.M.; Derhy, S.; Dumont, J.L.; Tuszynski, T.; Dhumane, P.; Meduri, B. Combined endoscopic and radiologic approach for complex bile duct injuries (with video). Gastrointest. Endosc. 2014, 79, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Bowen, G.; Hannan, E.; Harding, T.; Maguire, D.; Stafford, A.T. Aberrant right posterior sectoral duct injury necessitating liver resection. Ann. R. Coll. Surg. Engl. 2021, 103, E241–E243. [Google Scholar] [CrossRef] [PubMed]
- Battal, M.; Yazici, P.; Bostanci, O.; Karatepe, O. Early Surgical Repair of Bile Duct Injuries following Laparoscopic Cholecystectomy: The Sooner the Better. Surg. J. 2019, 5, e154–e158. [Google Scholar] [CrossRef] [PubMed]
- Addeo, P.; Saouli, A.C.; Ellero, B.; Woehl-Jaegle, M.L.; Oussoultzoglou, E.; Rosso, E.; Cesaretti, M.; Bachellier, P. Liver transplantation for iatrogenic bile duct injuries sustained during cholecystectomy. Hepatol. Int. 2013, 7, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Moo-Young, T.A.; Picus, D.D.; Teefey, S.; Strasberg, S.M. Common bile duct injury following laparoscopic cholecystectomy in the setting of sinistroposition of the galladder and biliary confluence: A case report. J. Gastrointest. Surg. 2010, 14, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Brunet, M.; Mosimann, F. Hepatobiliary and pancreatic: Bile duct stricture after cholecystectomy. J. Gastroenterol. Hepatol. 2011, 26, 1466. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Judd, S.; Antaki, F. Novel application of electrohydraulic lithotripsy probe in managing a refractory cystic duct bile leak. Gastrointest. Endosc. 2014, 80, 743–744. [Google Scholar] [CrossRef] [PubMed]
- Ödemiş, B.; Köksal, A.Ş.; Torun, S.; Yurdakul, M.; Kayaçetin, E. Complete biliary obstruction without jaundice due to an anatomic variation. Turk J. Gastroenterol. 2014, 25 (Suppl. 1), 187–190. [Google Scholar] [CrossRef] [PubMed]
- Buturovic, S. Iatrogenic injury to the common bile duct. Med. Arch. 2014, 68, 291–293. [Google Scholar] [CrossRef]
- Humes, D.J.; Ahmed, I. The Pedicle Effect and Direct Coupling Delayed Thermal Injuries to the Bile Duct After Laparoscopic Cholecystectomy. Arch. Surg. 2010, 145, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.Y.; Lee, J.R.; Wang, J.H. Endoscopic Nasobiliary Drainage for Bile Leak Caused by Injury to the Ducts of Luschka. Korean J. Gastroenterol. 2017, 69, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnejad, M.; Kazemi, A.; Khatibian, M.; Eloubeidi, M.A. Endoscopic ultrasonography to diagnose common bile duct transection after laparoscopic cholecystectomy: With video. Dig. Endosc. 2012, 24, 475. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.; Bova, V.; Agnello, F.; Youngblood, R.; Midiri, M.; Brancatelli, G. Gadoxetate DisodiumY Enhanced Magnetic Resonance Cholangiography for the Noninvasive Detection of an Active Bile Duct Leak After Laparoscopic Cholecystectomy. J. Comput. Assist. Tomogr. 2010, 34, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Shokouh-Amiri, H.; Mohammad, F.; Fallahzadeh, K.; Abdehou, S.T.; Zibari, G.B. Aberrant Left Main Bile Duct Draining Directly Into the Cystic Duct or Gallbladder: An Unreported Anatomical Variation and Cause of Bile Duct Injury During Laparoscopic Cholecystectomy. J. La State Med. Soc. 2014, 166, 203–206. [Google Scholar] [PubMed]
- Dousse, D.; Otal, P.; Suc, B. Biliary repair after main bile duct injury in a patient with “fusion of hepatic planes” anatomical variation (with video). J. Visc. Surg. 2018, 155, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, K.; Kaushal, D.; Low, W.; Townend, P.; Das, A.; Apostolou, C.; Merrett, N. Modified Longmire procedure: A novel approach to bile duct injury repair. ANZ J. Surg. 2019, 89, E554–E555. [Google Scholar] [CrossRef] [PubMed]
- Mayer, P.; Héroin, L.; Habersetzer, F.; Vix, M.; Pessaux, P.; Felli, E.; Mathis, G. Combined endoscopic and surgical management of a right intrahepatic bile duct injury during laparoscopic cholecystectomy. Endoscopy 2022, 54, E682–E683. [Google Scholar] [CrossRef] [PubMed]
- Hite, M.; Chung, C.; Lancaster, W. Surgical Management of Iatrogenic Bile Duct Injury in Patients with Atypical Ductal Anatomy. Am. Surg. 2020, 86, e64–e66. [Google Scholar] [CrossRef] [PubMed]
- Crema, E.; Trentini, E.A.; Teles, C.J.O.; Monti, P.R.; Lacerda, C.F.; Terra, J.A.; Silva, A.A. Laparoscopic reconstruction of the extrahepatic bile duct using a jejunal tube: An innovative, more physiological and anatomical technique for biliodigestive derivation. J. Surg. Case Rep. 2014, 2014, rjt106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drs, A.; Kocík, M.; Chlupac, J.; Froněk, J. Extensive bile duct injury resulting from a laparoscopic cholecystectomy resolved by a Roux-en-Y tri-hepaticojejunostomy: A case report. Rozhl. Chir. 2020, 99, 403–407. [Google Scholar] [CrossRef]
- Strasberg, S.M.; Gouma, D.J. “Extreme” vasculobiliary injuries: Association with fundus-down cholecystectomy in severely inflamed gallbladders. HPB 2012, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.N.; Sindram, D.; Agee, N.; Martinie, J.B.; Iannitti, D.A. Bile duct injury after single incision laparoscopic cholecystectomy. JSLS 2011, 14, 587–591. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bernhardt, G.A.; Kornprat, P.; Schweiger, W.; Cerwenka, H.; Mischinger, H.J. Late onset bile duct stricture caused by iatrogenic injury during laparoscopic cholecystectomy, mimicking cholangiocellular carcinoma. Endoscopy 2010, 42 (Suppl. 2), E148–E149. [Google Scholar] [CrossRef] [PubMed]
- Chiruvella, A.; Sarmiento, J.M.; Sweeney, J.F.; Lin, E.; Davis, S.S. Iatrogenic combined bile duct and right hepatic artery injury during single incision laparoscopic cholecystectomy. JSLS 2010, 14, 268–271. [Google Scholar] [CrossRef] [PubMed]
- De Werra, C.; Del Giudice, R.; Di Micco, R.; Aloia, S.; Bracciano, L.; Cervotti, M.; Galloro, G.; Bucci, L. Biliary duct injuries in the laparoscopic era: Our experience. G Chir. 2013, 34, 59–63. [Google Scholar] [PubMed]
- Desai, G.S.; Pande, P.; Narkhede, R.; Kulkarni, D.R. Revision Roux-en-y hepaticojejunostomy for a post-cholecystectomy complex vasculobiliary injury with complete proper hepatic artery occlusion: A case report literature review. Int. J. Surg. Case Rep. 2019, 58, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Curcio, G.; Miraglia, R.; Ligresti, D.; Granata, A.; Traina, M. Intraperitoneal rendezvous: A mini-invasive biliary reconstruction. Gastrointest. Endosc. 2016, 84, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Ball, C.G.; House, M.G.; Lillemoe, K.D. Image of the month--quiz case. Isolated common hepatic duct injury with anomalous right hepatic duct anatomy. Arch. Surg. 2011, 146, 989–990. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Donatelli, G.; Mutter, D.; Dhumane, P.; Callari, C.; Marescaux, J. Transhepatic metallic stenting for hepaticojejunostomy stricture following laparoscopic cholecystectomy biliary injury: A case of successful 20 years follow-up. J. Min. Access Surg. 2012, 8, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Elmunzer, J.B.; Feussner, D.J.; Payne, M.K.; Nadig, S.N.; Yamada, R. Combined Endoscopic-Percutaneous Biliary Restoration Following Severe Bile Duct Injury During Cholecystectomy. Am. J. Gastroenterol. 2018, 113, 328. [Google Scholar] [CrossRef] [PubMed]
- Ayloo, S.; Schwartzman, J. Robot-Assisted Repair of E1 Biliary Ductal Injury with Roux-en-Y Hepaticojejunostomy. J. Laparo Adv. Surg. Tech. 2019, 29, 817–819. [Google Scholar] [CrossRef]
- Acquafresca, P.A. Minimal invasive treatment of biliary leak after laparoscopic cholecystectomy. Int. J. Gastrointest. Int. 2019, 8, 45–48. [Google Scholar] [CrossRef]
- Lubikowski, J.; Piotuch, B.; Stadnik, A.; Przedniczek, M.; Remiszewski, P.; Milkiewicz, P.; Silva, M.A.; Wojcicki, M. Difficult iatrogenic bile duct injuries following different types of upper abdominal surgery: Report of three cases and review of literature. BMC Surg. 2019, 19, 162. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Hori, T.; Furuyama, H.; Machimoto, T.; Hata, T.; Kadokawa, Y.; Ito, T.; Kato, S.; Yasukawa, D.; Aisu; et al. Postoperative biliary leak treated with chemical bile duct ablation using absolute ethanol: A report of two cases. Am. J. Case Rep. 2017, 18, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Kohn, J.F.; Trenk, A.; Kuchta, K.; Lapin, B.; Denham, W.; Linn, J.G.; Haggerty, S.; Joehl, R.; Ujiki, M.B. Characterization of common bile duct injury after laparoscopic cholecystectomy in a high-volume hospital system. Surg. Endosc. 2018, 32, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Bonati, E.; Gnocchi, A.; Cremaschi, E.; Viani, L.; Dell’abate, P. Treatment of postoperative accessory bile duct injury by fibrin glue and balloon tamponade: A case report. Acta Biomed. 2019, 90, 559–563. [Google Scholar] [CrossRef]
- Machado, M.A.; Surjan, R.C.; Makdissi, F.F. Laparoscopic right hepatectomy for complex biliary injury after laparoscopic cholecystectomy (with video). J. Visc. Surg. 2019, 156, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lin, C.W.; Yin, W.Y. Subvesical Duct Detected by Magnetic Resonance Cholangiopancreatography (MRCP) in a Patient with Bile Leak after Laparoscopic Cholecystectomy. CRSLS 2021, 8, e2020.00074. [Google Scholar] [CrossRef] [PubMed]
- Nezami, N.; Jarmakani, H.; Arici, M.; Latich, I.; Mojibian, H.; Ayyagari, R.R.; Pollak, J.S.; Perez Lozada, J.C.L. Selective Trans-Catheter Coil Embolization of Cystic Duct Stump in Post-Cholecystectomy Bile Leak. Dig. Dis. Sci. 2019, 64, 3314–3320. [Google Scholar] [CrossRef] [PubMed]
- Parmeggiani, D.; Cimmino, G.; Cerbone, D.; Avenia, N.; Ruggero, R.; Gubitosi, A.; Docimo, G.; Mordente, S.; Misso, C.; Parmeggiani, U. Biliary tract injuries during laparoscopic cholecystectomy: Three case reports and literature review. G Chir. 2010, 31, 9–16. [Google Scholar]
- Głuszek, S.; Kot, M.; Bałchanowski, N.; Matykiewicz, J.; Kuchinka, J.; Kozieł, D.; Wawrzycka, I. Iatrogenic bile duct injuries-Clinical problems. Pol. Przegl. Chir. 2014, 86, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Swallow, A.Y.; Mwanga, A.H.; Kitembo, K.; Mchele, G.; Akoko, L.; Awadh, A. Hepaticojejunostomy in proximal bile duct injury by left hepatic duct approach for patients attended at Muhimbili National Hospital. Egypt. Liver J. 2023, 13, 18–22. [Google Scholar] [CrossRef]
- Xu, M.; Carames, C.; Novikov, A.; Saumoy, M.; Afaneh, C.; Kahaleh, M.; Sharahia, R.Z. One-step endoscopic ultrasound-directed gastro-gastrostomy ERCP for treatment of bile leak. Endoscopy 2017, 49, 715–716. [Google Scholar] [CrossRef] [PubMed]
- Artifon, E.L.A.; Silva, G.L.R.; Uemura, R.S.; Teran, A.; Buch, M. Percutaneous passage of an extraction balloon to assist recanalization of the common bile duct after surgical transection. Gastrointest. Endosc. 2015, 81, 1017–1018. [Google Scholar] [CrossRef] [PubMed]
- Odemis, B.; Oztas, E.; Torun, S.; Suna, N. Endoscopic treatment of a cystic duct stump leak complicated by aberrant bile duct communication. Endoscopy 2015, 47, E141–E142. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, P.; Schmidt, S.C.; Hintze, R.E.; Adler, A.; Veltzke, W.; Raakow, R.; Langrehr, J.M.; Bechstein, W.O. Classification and treatment of bile duct injuries after laparoscopic cholecystectomy. Der. Chirurg. 2000, 71, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.J.G.H.M.; Van Den Brink, G.R.; Rauws, E.A.J.; de Wit, L.; Obertop, H.; Huibregtse, K.; Tytgat, G.N.; Gouma, D.J. Treatment of bile duct lesions after laparoscopic cholecystectomy. Gut 1996, 38, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, W.L.; Fu, X.H.; Zhu, B.; Zhao, T.; Zhang, Y.J. Early Versus Delayed Surgical Repair and Referral for Patients with Bile Duct Injury: A Systematic Review and Meta-analysis. Ann. Surg. 2020, 271, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, A.M.; Nunez Vas, B.C.; Booij, K.A.C.; van Dieren, S.; Besselink, M.G.; Busch, O.R.; van Gulik, T.M. Optimal timing for surgical reconstruction of bile duct injury: Meta-analysis. BJS Open 2020, 4, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.A.; Kamal, A.; Redwan, A.A.; Alansary, M.N.; Ahmed, E.A. Post-cholecystectomy major bile duct injury: Ideal time to repair based on a multicentre randomized controlled trial with promising results. Int. J. Surg. 2023, 109, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- Sweigert, P.J.; Eguia, E.; Nelson, M.H.; Bunn, C.; Kulshrestha, S.; Luchette, F.A.; Baker, M.S. Biliary Enteric Reconstruction After Biliary Injury: Delayed Repair Is More Costly Than Early Repair. J. Surg. Res. 2021, 257, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, V.; Kuemmerli, C.; Maupoey, J.; Lopez-Andujar, R.; Llado’, L.; Mils, K.; Muller, P.; Valdivieso, A.; Garces-Albir, M.; Sabater, L.; et al. Textbook outcome in patients with biliary duct injury during cholecystectomy. J. Gastrointest. Surg. 2024, 28, 725–730. [Google Scholar] [CrossRef]
- Marichez, A.; Adam, J.P.; Laurent, C.; Chiche, L. Hepaticojejunostomy for bile duct injury: State of the art. Langenbeck Arch. Surg. 2023, 408, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Cubisino, A.; Dreifuss, N.H.; Cassese, G.; Bianco, F.M.; Panaro, F. Minimally invasive biliary anastomosis after iatrogenic bile duct injury: A systematic review. Updates Surg. 2023, 75, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Halle-Smith, J.M.; Hall, L.A.; Mirza, D.F.; Roberts, K.J. Risk factors for anastomotic stricture after hepaticojejunostomy for bile duct injury–A systematic review and meta-analysis. Surgery 2021, 170, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Dumonceau, J.M.; Tringali, A.; Papanikolaou, I.S.; Blero, D.; Mangiavillano, B.; Schmidt, A.; Vanbiervliet, G.; Costamagna, G.; Deviere, J.; Garcia-Cano, J.; et al. Endoscopic biliary stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline—Updated October 2017. Endoscopy 2018, 50, 910–930. [Google Scholar] [CrossRef] [PubMed]
- Canena, J.; Liberato, M.; Meireles, L.; Marques, I.; Romao, C.; Pereira Coutinho, A.; Costa Nevez, B.; Mota Veiga, P. A non-randomized study in consecutive patients with postcholecystectomy refractory biliary leaks who were managed endoscopically with the use of multiple plastic stents or fully covered self-expandable metal stents (with videos). Gastrointest. Endosc. 2015, 82, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.G.; Papachristou, G.I.; Taylor, L.J.; McVay, T.; Birch, M.; Francis, G.; Zabolotsky, A.; Laique, S.N.; Hayat, U.; Zhan, T.; et al. Clinical outcomes in patients with bile leaks treated via ERCP with regard to the timing of ERCP: A large multicenter study. Gastrointest. Endosc. 2017, 85, 766–772. [Google Scholar] [CrossRef] [PubMed]
- De Jong, E.A.; Moelker, A.; Leertouwer, T.; Spronk, S.; Van Dijk, M.; Van Eijck, C.H.J. Percutaneous transhepatic biliary drainage in patients with postsurgical bile leakage and nondilated intrahepatic bile ducts. Dig. Surg. 2014, 30, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, J.; Kloppers, C.; Burmeister, S.; Bernon, M.; Jonas, E. Mind the gap! Extraluminal percutaneous-endoscopic rendezvous with a self-expanding metal stent for restoring continuity in major bile duct injury: A case series. Int. J. Surg. Case Rep. 2019, 60, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, A.; Booij, K.; de Reuver, P.; van Delden, O.M.; van Lienden, K.P.; Besselink, M.G.; Busch, O.R.; Gouma, D.J.; Rauws, E.A.; van Gulik, T.M. Percutaneous-endoscopic rendezvous procedure for the management of bile duct injuries after cholecystectomy: Short- and long-term outcomes. Endoscopy 2018, 50, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, G.; Tringali, A.; Perri, V.; Familiari, P.; Boskoski, I.; Barbaro, F.; Landi, R. Endotherapy of postcholecystectomy biliary strictures with multiple plastic stents: Long-term results in a large cohort of patients. Gastrointest. Endosc. 2020, 91, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Emara, M.H.; Ahmed, M.H.; Radwan, M.I.; Emara, E.H.; Basheer, M.; Ali’, A.; Elfert, A.A. Post-cholecystectomy iatrogenic bile duct injuries: Emerging role for endoscopic management. World J. Gastrointest. Surg. 2023, 15, 2709–2718. [Google Scholar] [CrossRef] [PubMed]
- Schizas, D.; Papaconstantinou, D.; Moris, D.; Koliakos, N.; Tsilimigras, D.I.; Bakopoulos, A.; Karaolanis, G.; Spartalis, E.; Dimitroulis, D.; Felekouras, E. Management of Segmental Bile Duct Injuries After Cholecystectomy: A Systematic Review. J. Gastrointest. Surg. 2019, 23, 408–416. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).