Symptom Persistence Relates to Volume and Asymmetry of the Limbic System after Mild Traumatic Brain Injury
Abstract
:1. Introduction
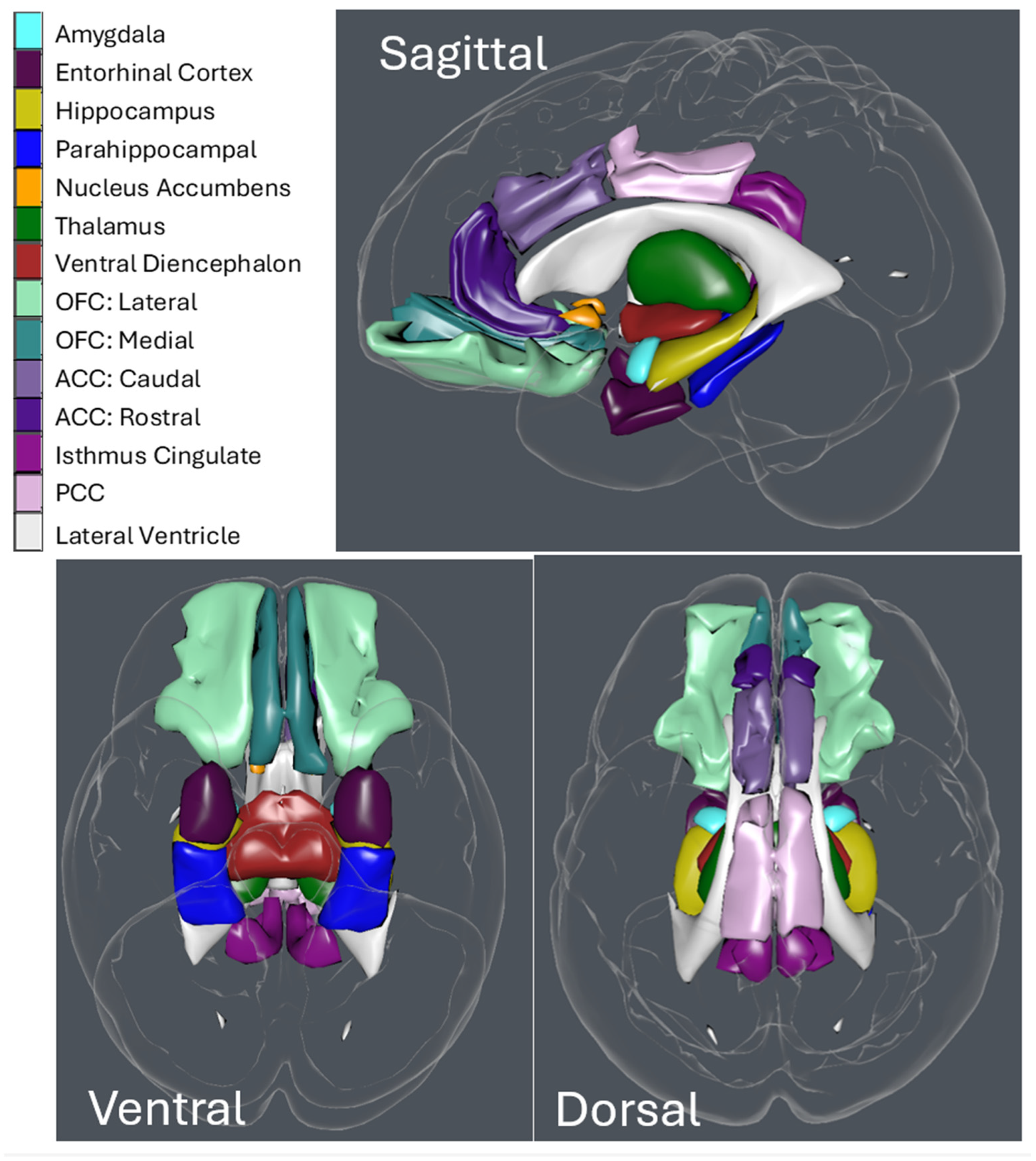
2. Materials and Methods
3. Results
3.1. Symptom Presentation
3.2. Symptom Persistence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The epidemiology and impact of traumatic brain injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, I.; Wood, R.L.; Phillips, C.J.; Macey, S. The costs of traumatic brain injury: A literature review. Clinicoecon. Outcomes Res. 2013, 5, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.R. Traumatic brain injury: Current treatment strategies and future endeavors. Cell Transpl. 2017, 26, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Bigler, E.D. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J. Int. Neuropsychol. Soc. 2008, 14, 1–22. [Google Scholar] [CrossRef]
- McInnes, K.; Friesen, C.L.; MacKenzie, D.E.; Westwood, D.A.; Boe, S.G. Mild Traumatic Brain Injury (MTBI) and chronic cognitive impairment: A scoping review. PLoS ONE 2017, 12, e0174847. [Google Scholar] [CrossRef]
- Seabury, S.A.; Gaudette, E.; Goldman, D.P.; Markowitz, A.J.; Brooks, J.; McCrea, M.A.; Okonkwo, D.O.; Manley, G.T.; Track-Tbi Investigators; Adeoye, O.; et al. Assessment of follow-up care after emergency department presentation for mild traumatic brain injury and concussion: Results from the Track-Tbi Study. JAMA Netw. Open 2018, 1, e180210. [Google Scholar] [CrossRef]
- WHO. Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research; World Health Organization: Geneva, Swtizerland, 1993. [Google Scholar]
- Nelson, L.D.; Temkin, N.R.; Dikmen, S.; Barber, J.; Giacino, J.T.; Yuh, E.; Levin, H.S.; McCrea, M.A.; Stein, M.B.; Mukherjee, P.; et al. Recovery after mild traumatic brain injury in patients presenting to US Level I trauma centers: A transforming research and clinical knowledge in traumatic brain injury (Track-Tbi) study. JAMA Neurol. 2019, 76, 1049–1059. [Google Scholar] [CrossRef]
- McMahon, P.; Hricik, A.; Yue, J.K.; Puccio, A.M.; Inoue, T.; Lingsma, H.F.; Beers, S.R.; Gordon, W.A.; Valadka, A.B.; Manley, G.T.; et al. Symptomatology and functional outcome in mild traumatic brain injury: Results from the prospective Track-Tbi study. J. Neurotrauma 2014, 31, 26–33. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Liu, C.J.; Huang, H.C.; Lin, J.H.; Chen, P.Y.; Su, Y.K.; Chen, C.T.; Chiu, H.Y. A meta-analysis of dynamic prevalence of cognitive deficits in the acute, subacute, and chronic phases after traumatic brain injury. J. Neurosci. Nurs. 2021, 53, 63–68. [Google Scholar] [CrossRef]
- Boake, C.; McCauley, S.R.; Levin, H.S.; Pedroza, C.; Contant, C.F.; Song, J.X.; Brown, S.A.; Goodman, H.; Brundage, S.I.; Diaz-Marchan, P.J. Diagnostic Criteria for Postconcussional Syndrome after Mild to Moderate Traumatic Brain Injury. J. Neuropsych. Clin. Neurosci. 2005, 17, 350–356. [Google Scholar] [CrossRef]
- Vogt, B.A. Chapter 3—Cingulate cortex in the three limbic subsystems. In Handbook of Clinical Neurology; Vogt, B.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 39–51. [Google Scholar]
- Vogt, B.A. Chapter 1—The cingulate cortex in neurologic diseases: History, structure, overview. In Handbook of Clinical Neurology; Vogt, B.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–21. [Google Scholar]
- Rolls, E.T. Limbic systems for emotion and for memory, but no single limbic system. Cortex 2015, 62, 119–157. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Cheng, W.; Feng, J. The orbitofrontal cortex: Reward, emotion and depression. Brain Commun. 2020, 2, fcaa196. [Google Scholar] [CrossRef]
- Banwinkler, M.; Theis, H.; Prange, S.; van Eimeren, T. Imaging the limbic system in Parkinson’s Disease- A review of limbic pathology and clinical symptoms. Brain Sci. 2022, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Bigler, E.D. Volumetric MRI findings in mild traumatic brain injury (mTBI) and neuropsychological outcome. Neuropsychol. Rev. 2023, 33, 5–41. [Google Scholar] [CrossRef] [PubMed]
- Ropper, A.H.; Gorson, K.C. Clinical practice: Concussion. N. Engl. J. Med. 2007, 356, 166–172. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Domel, A.G.; Dennis, E.L.; Georgiadis, M.; Liu, Y.; Raymond, S.J.; Grant, G.; Kleiven, S.; Camarillo, D.; et al. The presence of the temporal horn exacerbates the vulnerability of hippocampus during head impacts. Front. Bioeng. Biotechnol. 2022, 10, 754344. [Google Scholar] [CrossRef]
- Stokum, J.A.; Sours, C.; Zhuo, J.; Kane, R.; Shanmuganathan, K.; Gullapalli, R.P. A longitudinal evaluation of diffusion kurtosis imaging in patients with mild traumatic brain injury. Brain Inj. 2015, 29, 47–57. [Google Scholar] [CrossRef]
- Fortier-Lebel, O.; Jobin, B.; Lecuyer-Giguere, F.; Gaubert, M.; Giguere, J.F.; Gagnon, J.F.; Boller, B.; Frasnelli, J. Verbal Episodic Memory Alterations and Hippocampal Atrophy in Acute Mild Traumatic Brain Injury. J. Neurotrauma 2021, 38, 1506–1514. [Google Scholar] [CrossRef]
- Leh, S.E.; Schroeder, C.; Chen, J.K.; Mallar Chakravarty, M.; Park, M.T.; Cheung, B.; Huntgeburth, S.C.; Gosselin, N.; Hock, C.; Ptito, A.; et al. Microstructural Integrity of Hippocampal Subregions Is Impaired after Mild Traumatic Brain Injury. J. Neurotrauma 2017, 34, 1402–1411. [Google Scholar] [CrossRef]
- Derakhshan, I. Laterality of Motor Control Revisited: Directionality of Callosal Traffic and Its Rehabilitative Implications. Top Stroke Rehabil. 2005, 12, 76–82. [Google Scholar] [CrossRef]
- Ledig, C.; Kamnitsas, K.; Koikkalainen, J.; Posti, J.P.; Takala, R.S.K.; KReatila, A.; Frantzen, J.; Ala-Seppala, H.; Kyllonen, A.; Maanpaa, H.R.; et al. Regional brain morphometry in patients with traumatic brain injury based on acute- and chronic-phase magnetic resonance imaging. PLoS ONE 2017, 12, e0188152. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Vanier, C.; Pandey, T.; Parikh, S.; Rodriguez, A.; Knoblauch, T.; Peralta, J.; Hertzler, A.; Ma, L.; Nam, R.; Musallam, S.; et al. Interval-censored survival analysis of mild traumatic brain injury with outcome based neuroimaging clinical applications. J. Concussion 2020, 4, 2059700220947194. [Google Scholar] [CrossRef]
- Lei-Rivera, L.; Sutera, J.; Galatioto, J.A.; Hujsak, B.D.; Gurley, J.M. Special tools for the assessment of balance and dizziness in individuals with mild traumatic brain injury. NeuroRehabilitation 2013, 32, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Harasym, J.; Miguel-Cruz, A.; Chisholm, S.; Smith-MacDonald, L.; Bremault-Phillips, S. Neurocognitive assessment tools for military personnel with mild traumatic brain injury: Scoping literature review. JMIR Ment. Health 2021, 8, e26360. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.E.; Ochs, A.L.; DeSmit, M.E.; Seabaugh, J.M.; Havranek, M.D.; Initiative Alzheimer’s Disease Neuroimaging. Man versus Machine part 2: Comparison of radiologists’ interpretations and NeuroQuant measures of brain asymmetry and progressive atrophy in patients with traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2015, 27, 147–152. [Google Scholar] [CrossRef]
- Ross, D.E.; Ochs, A.L.; Seabaugh, J.M.; Shrader, C.R. Man versus machine: Comparison of radiologists’ interpretations and NeuroQuant(R) volumetric analyses of brain MRIs in patients with traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2013, 25, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.E.; Seabaugh, J.D.; Seabaugh, J.M.; Alvarez, C.; Ellis, L.P.; Powell, C.; Reese, C.; Cooper, L.; Shepherd, K.; Alzheimer’s Disease Neuroimaging Initiative. Journey to the other side of the brain: Asymmetry in patients with chronic mild or moderate traumatic brain injury. Concussion 2022, 8, CNC101. [Google Scholar] [CrossRef]
- Voevodskaya, O.; Simmons, A.; Nordenskjöld, R.; Kullberg, J.; Ahlström, H.; Lind, L.; Wahlund, L.O.; Larsson, E.M.; Westman, E. The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 264. [Google Scholar] [CrossRef]
- Seghier, M.L. Laterality index in functional MRI: Methodological issues. Magn. Reson. Imaging 2008, 26, 594–601. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, M.; Ambler, G.; Seaman, S.R.; Guttmann, O.; Elliott, P.; King, M.; Omar, R.Z. How to develop a more accurate risk prediction model when there are few events. BMJ 2015, 351, h3868. [Google Scholar] [CrossRef]
- Anderson-Bergman, C. Icenreg: Regression models for interval censored data in R. J. Stat. Softw. 2017, 81, 1–23. [Google Scholar] [CrossRef]
- Marek, S.; Tervo-Clemmens, B.; Calabro, F.J.; Montez, D.F.; Kay, B.P.; Hatoum, A.S.; Donohue, M.R.; Foran, W.; Miller, R.L.; Hendrickson, T.J.; et al. Reproducible brain-wide association studies require thousands of individuals. Nature 2022, 603, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, R.E.; Winzeck, S.; Luppi, A.I.; Kelleher-Unger, I.R.; Spindler, L.R.B.; Wilson, J.T.L.; Newcombe, V.F.J.; Coles, J.P.; Amrein, K.; Andelic, N.; et al. Acute thalamic connectivity precedes chronic post-concussive symptoms in mild traumatic brain injury. Brain 2023, 146, 3484–3499. [Google Scholar] [CrossRef]
- Lindemer, E.R.; Salat, D.H.; Leritz, E.C.; McGlinchey, R.E.; Milberg, W.P. Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF Veterans and the impact of comorbid TBI. NeuroImage Clin. 2013, 2, 601–611. [Google Scholar] [CrossRef]
- Santhanam, P.; Teslovich, T.; Wilson, S.H.; Yeh, P.H.; Oakes, T.R.; Weaver, L.K. Decreases in white matter integrity of ventro-limbic pathway linked to post-traumatic stress disorder in mild traumatic brain injury. J. Neurotrauma 2019, 36, 1093–1098. [Google Scholar] [CrossRef]
- Sydnor, V.J.; Bouix, S.; Pasternak, O.; Hartl, E.; Levin-Gleba, L.; Reid, B.; Tripodis, Y.; Guenette, J.P.; Kaufmann, D.; Makris, N.; et al. Mild traumatic brain injury impacts associations between limbic system microstructure and post-traumatic stress disorder symptomatology. NeuroImage Clin. 2020, 26, 102190. [Google Scholar] [CrossRef]
- Iraji, A.; Chen, H.; Wiseman, N.; Zhang, T.; Welch, R.; O’Neil, B.; Kulek, A.; Ayaz, S.I.; Wang, X.; Zuk, C.; et al. Connectome-scale assessment of structural and functional connectivity in mild traumatic brain injury at the acute stage. NeuroImage Clin. 2016, 12, 100–115. [Google Scholar] [CrossRef]
- Wolf, J.A.; Koch, P.F. Disruption of network synchrony and cognitive dysfunction after traumatic brain injury. Front. Syst. Neurosci. 2016, 10, 43. [Google Scholar] [CrossRef]
- Anand, K.S.; Dhikav, V. Hippocampus in health and disease: An overview. Ann. Indian Acad. Neurol. 2012, 15, 239–246. [Google Scholar] [CrossRef]
- Aungst, S.L.; Kabadi, S.V.; Thompson, S.M.; Stoica, B.A.; Faden, A.I. Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J. Cereb. Blood Flow Metab. 2014, 34, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Epstein, D.J.; Legarreta, M.; Bueler, E.; King, J.; McGlade, E.; Yurgelun-Todd, D. Orbitofrontal cortical thinning and aggression in mild traumatic brain injury patients. Brain Behav. 2016, 6, e00581. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, N.D.; Gardner, A.J.; Brubacher, J.R.; Panenka, W.J.; Li, J.J.; Iverson, G.L. Systematic review of multivariable prognostic models for mild traumatic brain injury. J. Neurotrauma 2015, 32, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Cnossen, M.C.; Winkler, E.A.; Yue, J.K.; Okonkwo, D.O.; Valadka, A.B.; Steyerberg, E.W.; Lingsma, H.F.; Manley, G.T.; Track-Tbi Investigators. Development of a prediction model for post-concussive symptoms following mild traumatic brain injury: A Track-Tbi pilot study. J. Neurotrauma 2017, 34, 2396–2409. [Google Scholar] [CrossRef] [PubMed]
- Polinder, S.; Cnossen, M.C.; Real, R.G.L.; Covic, A.; Gorbunova, A.; Voormolen, D.C.; Master, C.L.; Haagsma, J.A.; Diaz-Arrastia, R.; Von Steinbuechel, N. A multidimensional approach to post-concussion symptoms in mild traumatic brain injury. Front. Neurol. 2018, 9, 1113. [Google Scholar] [CrossRef] [PubMed]
- Verboon, L.N.; Patel, H.C.; Greenhalgh, A.D. The Immune System’s Role in the Consequences of Mild Traumatic Brain Injury (Concussion). Front. Immunol. 2021, 12, 620698. [Google Scholar] [CrossRef]
- Lu, J.; Moochhala, S.; Kaur, C.; Ling, E.A. Cellular inflammatory response associated with breakdown of the blood-brain barrier after closed head injury in rats. J. Neurotrauma 2001, 18, 399–408. [Google Scholar] [CrossRef]
- Bolte, A.C.; Dutta, A.B.; Hurt, M.E.; Smirnov, I.; Kovacs, M.A.; McKee, C.A.; Ennerfelt, H.E.; Shapiro, D.; Nguyen, B.H.; Frost, E.L.; et al. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat. Commun. 2020, 11, 4524. [Google Scholar] [CrossRef]
- Chong, C.D.; Nikolova, S.; Dumkrieger, G.; Wu, T.; Berisha, V.; Li, J.; Ross, K.; Schwedt, T.J. Thalamic subfield iron accumulation after acute mild traumatic brain injury as a marker of future post-traumatic headache intensity. Headache 2023, 63, 156–164. [Google Scholar] [CrossRef]
- Burrowes, S.A.B.; Rhodes, C.S.; Meeker, T.J.; Greenspan, J.D.; Gullapalli, R.P.; Seminowicz, D.A. Decreased grey matter volume in mTBI patients with post-traumatic headache compared to headache-free mTBI patients and healthy controls: A longitudinal MRI study. Brain Imaging Behav. 2020, 14, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.V.; Wood, D.M.; Bigler, E.D.; Blatter, D.D. Lesion volume, injury severity, and thalamic integrity following head injury. J. Neurotrauma 1996, 13, 35–40. [Google Scholar] [CrossRef]
- Zhuo, J.; Jiang, L.; Sours Rhodes, C.; Roys, S.; Shanmuganathan, K.; Chen, H.; Prince, J.L.; Badjatia, N.; Gullapalli, R.P. Early stage longitudinal subcortical volumetric changes following mild traumatic brain injury. Brain Inj. 2021, 35, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Catenaccio, E.; Lipton, M.L. Neuroimaging in blast-related mild traumatic brain injury. J. Head Trauma Rehabil. 2017, 32, 55–69. [Google Scholar] [CrossRef]
- Ge, Y.; Patel, M.B.; Chen, Q.; Grossman, E.J.; Zhang, K.; Miles, L.; Babb, J.S.; Reaume, J.; Grossman, R.I. Assessment of thalamic perfusion in patients with mild traumatic brain injury by true FISP arterial spin labelling MR imaging at 3T. Brain Inj. 2009, 23, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Obermann, M.; Nebel, K.; Schumann, C.; Holle, D.; Gizewski, E.R.; Maschke, M.; Goadsby, P.J.; Diener, H.C.; Katsarava, Z. Gray matter changes related to chronic posttraumatic headache. Neurology 2009, 73, 978–983. [Google Scholar] [CrossRef]
- Schwedt, T.J. Structural and functional brain alterations in post-traumatic headache attributed to mild traumatic brain injury: A narrative review. Front. Neurol. 2019, 10, 615. [Google Scholar] [CrossRef]
- Schwedt, T.J.; Chong, C.D.; Peplinski, J.; Ross, K.; Berisha, V. Persistent post-traumatic headache vs. migraine: An MRI study demonstrating differences in brain structure. J. Headache Pain 2017, 18, 87. [Google Scholar] [CrossRef]
- Surgent, O.J.; Dadalko, O.I.; Pickett, K.A.; Travers, B.G. Balance and the brain: A review of structural brain correlates of postural balance and balance training in humans. Gait Posture 2019, 71, 245–252. [Google Scholar] [CrossRef]
- Taubert, M.; Draganski, B.; Anwander, A.; Müller, K.; Horstmann, A.; Villringer, A.; Ragert, P. Dynamic properties of human brain structure: Learning-related changes in cortical areas and associated fiber connections. J. Neurosci. 2010, 30, 11670–11677. [Google Scholar] [CrossRef]
- Macfarlane, M.D.; Looi, J.C.; Walterfang, M.; Spulber, G.; Velakoulis, D.; Styner, M.; Crisby, M.; Orndahl, E.; Erkinjuntti, T.; Waldemar, G.; et al. Shape abnormalities of the caudate nucleus correlate with poorer gait and balance: Results from a subset of the LADIS study. Am. J. Geriatr. Psychiatry 2015, 23, 59–71.e1. [Google Scholar] [CrossRef]
- Boisgontier, M.P.; Cheval, B.; Chalavi, S.; van Ruitenbeek, P.; Leunissen, I.; Levin, O.; Nieuwboer, A.; Swinnen, S.P. Individual differences in brainstem and basal ganglia structure predict postural control and balance loss in young and older adults. Neurobiol. Aging 2017, 50, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Dean, P.J.; Sterr, A. Long-term effects of mild traumatic brain injury on cognitive performance. Front. Hum. Neurosci. 2013, 7, 30. [Google Scholar] [CrossRef]
- O’Jile, J.R.; Ryan, L.M.; Betz, B.; Parks-Levy, J.; Hilsabeck, R.C.; Rhudy, J.L.; Gouvier, W.D. Information processing following mild head injury. Arch. Clin. Neuropsychol. 2006, 21, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Sterr, A.; Herron, K.A.; Hayward, C.; Montaldi, D. Are mild head injuries as mild as we think? Neurobehavioral concomitants of chronic post-concussion syndrome. BMC Neurol. 2006, 6, 7. [Google Scholar] [CrossRef]
- Konrad, C.; Geburek, A.J.; Rist, F.; Blumenroth, H.; Fischer, B.; Husstedt, I.; Arolt, V.; Schiffbauer, H.; Lohmann, H. Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol. Med. 2011, 41, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.C.; Wilde, E.A.; Bigler, E.D.; Yallampalli, R.; McCauley, S.R.; Troyanskaya, M.; Chu, Z.; Li, X.; Hanten, G.; Hunter, J.V.; et al. Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. J. Neurotrauma 2010, 27, 303–307. [Google Scholar] [CrossRef]
- Wilde, E.A.; Hyseni, I.; Lindsey, H.M.; Faber, J.; McHenry, J.M.; Bigler, E.D.; Biekman, B.D.; Hollowell, L.L.; McCauley, S.R.; Hunter, J.V.; et al. A Preliminary DTI Tractography Study of Developmental Neuroplasticity 5–15 Years After Early Childhood Traumatic Brain Injury. Front. Neurol. 2021, 12, 734055. [Google Scholar] [CrossRef] [PubMed]
- Wilde, E.A.; Ramos, M.A.; Yallampalli, R.; Bigler, E.D.; McCauley, S.R.; Chu, Z.; Wu, T.C.; Hanten, G.; Scheibel, R.S.; Li, X.; et al. Diffusion tensor imaging of the cingulum bundle in children after traumatic brain injury. Dev. Neuropsychol. 2010, 35, 333–351. [Google Scholar] [CrossRef]
- Yount, R.; Raschke, K.A.; Biru, M.; Tate, D.F.; Miller, M.J.; Abildskov, T.; Gandhi, P.; Ryser, D.; Hopkins, R.O.; Bigler, E.D. Traumatic brain injury and atrophy of the cingulate gyrus. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 416–423. [Google Scholar] [CrossRef]
- Zhou, Y.; Kierans, A.; Kenul, D.; Ge, Y.; Rath, J.; Reaume, J.; Grossman, R.I.; Lui, Y.W. Mild traumatic brain injury: Longitudinal regional brain volume changes. Radiology 2013, 267, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Wang, L.; Zhao, Y.; Tong, W.; Wang, J.; Li, G.; Cheng, W.; Gao, L.; Dong, Y. Cortical and subcortical alterations and clinical correlates after traumatic brain injury. J. Clin. Med. 2022, 11, 4421. [Google Scholar] [CrossRef] [PubMed]
- Spitz, G.; Bigler, E.D.; Abildskov, T.; Maller, J.J.; O’Sullivan, R.; Ponsford, J.L. Regional cortical volume and cognitive functioning following traumatic brain injury. Brain Cogn. 2013, 83, 34–44. [Google Scholar] [CrossRef]
- Ariza, M.; Serra-Grabulosa, J.M.; Junque, C.; Ramirez, B.; Mataro, M.; Poca, A.; Bargallo, N.; Sahuquillo, J. Hippocampal head atrophy after traumatic brain injury. Neuropsychologia 2006, 44, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M.; Voss, M.W.; Pence, A.; McAuley, E.; Kramer, A.F.; Cohen, N.J. History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in life. Front. Aging Neurosci. 2013, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Misquitta, K.; Dadar, M.; Tarazi, A.; Hussain, M.W.; Alatwi, M.K.; Ebraheem, A.; Multani, N.; Khodadadi, M.; Goswami, R.; Wennberg, R.; et al. The relationship between brain atrophy and cognitive-behavioural symptoms in retired Canadian football players with multiple concussions. NeuroImage Clin. 2018, 19, 551–558. [Google Scholar] [CrossRef]
- Prak, R.F.; Marsman, J.B.C.; Renken, R.; van der Naalt, J.; Zijdewind, I. Fatigue following mild traumatic brain injury relates to visual processing and effort perception in the context of motor performance. NeuroImage Clin. 2021, 32, 102783. [Google Scholar] [CrossRef]
- Norrie, J.; Heitger, M.; Leathem, J.; Anderson, T.; Jones, R.; Flett, R. Mild traumatic brain injury and fatigue: A prospective longitudinal study. Brain Inj. 2010, 24, 1528–1538. [Google Scholar] [CrossRef]
- Clark, A.L.; Sorg, S.F.; Holiday, K.; Bigler, E.D.; Bangen, K.J.; Evangelista, N.D.; Bondi, M.W.; Schiehser, D.M.; Delano-Wood, L. Fatigue is associated with global and regional thalamic morphometry in veterans with a history of mild traumatic brain injury. J. Head Trauma Rehabil. 2018, 33, 382–392. [Google Scholar] [CrossRef]
- Berginstrom, N.; Nordstrom, P.; Ekman, U.; Eriksson, J.; Andersson, M.; Nyberg, L.; Nordstrom, A. Using functional magnetic resonance imaging to detect chronic fatigue in patients with previous traumatic brain injury: Changes linked to altered striato-thalamic-cortical functioning. J. Head Trauma Rehabil. 2018, 33, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Schubert, F.; Gallinat, J. Structural correlates of trait anxiety: Reduced thickness in medial orbitofrontal cortex accompanied by volume increase in nucleus accumbens. J. Affect. Disord. 2011, 134, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Hellewell, S.C.; Beaton, C.S.; Welton, T.; Grieve, S.M. Characterizing the Risk of Depression Following Mild Traumatic Brain Injury: A Meta-Analysis of the Literature Comparing Chronic mTBI to Non-mTBI Populations. Front. Neurol. 2020, 11, 350. [Google Scholar] [CrossRef]
- Hudak, A.; Warner, M.; Marquez de la Plata, C.; Moore, C.; Harper, C.; Diaz-Arrastia, R. Brain morphometry changes and depressive symptoms after traumatic brain injury. Psychiatry Res. 2011, 191, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Maller, J.J.; Thomson, R.H.; Pannek, K.; Bailey, N.; Lewis, P.M.; Fitzgerald, P.B. Volumetrics relate to the development of depression after traumatic brain injury. Behav. Brain Res. 2014, 271, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, G.C.; Twose, C.; Weller, A.; Dougherty, J.W.; Goes, F.S.; Sair, H.I.; Smith, G.S.; Roy, D. Neuroimaging correlates of depression after traumatic brain injury: A systematic review. J. Neurotrauma 2022, 39, 755–772. [Google Scholar] [CrossRef]
- Daniela, A.E.O.; Khawlah, A.l.; Nicolas, C. Volumetric brain differences in clinical depression in association with anxiety: A systematic review with meta-analysis. J. Psych. Neurosci. 2020, 45, 406. [Google Scholar] [CrossRef]
- Bryant, B.R.; Richey, L.N.; Jahed, S.; Heinzerling, A.; Stevens, D.A.; Pace, B.D.; Tsai, J.; Bray, M.J.C.; Esagoff, A.I.; Adkins, J.; et al. Behavioral and emotional dyscontrol following traumatic brain injury: A systematic review of neuroimaging and electrophysiological correlates. J Acad. Consult. Liaison Psychiatry 2022, 63, 579–598. [Google Scholar] [CrossRef]
- Koski, L.; Kolivakis, T.; Yu, C.; Chen, J.-K.; Delaney, S.; Ptito, A. Noninvasive Brain Stimulation for Persistent Postconcussion Symptoms in Mild Traumatic Brain Injury. J. Neurotrauma 2014, 32, 38–44. [Google Scholar] [CrossRef]
- Zaninotto, A.L.; El-Hagrassy, M.M.; Green, J.R.; Babo, M.; Paglioni, V.M.; Benute, G.G.; Paiva, W.S. Transcranial direct current stimulation (tDCS) effects on traumatic brain injury (TBI) recovery: A systematic review. Dement. Neuropsychol. 2019, 13, 172–179. [Google Scholar] [CrossRef]
- Page, S.J.; Cunningham, D.A.; Plow, E.; Blazak, B. It takes two: Noninvasive brain stimulation combined with neurorehabilitation. Arch. Phys. Med. Rehabil. 2015, 96, S89–S93. [Google Scholar] [CrossRef]



| All | no LOC | LOC | p | |
|---|---|---|---|---|
| Sample size | 524 | 375 (72%) | 149 (28%) | |
| Age (mean, SD, % under 40) | 42 (14) 45.6% | 43 (15) 44.3% | 40 (13) 49.0% | 0.026 |
| Sex (F) | 302 (58%) | 234 (65%) | 68 (48%) | <0.001 |
| Days from injury to first exam (median, IQR) | 69 (34, 132) | 70 (35, 129) | 66 (32, 139) | 0.778 |
| Days from injury to scan (median, IQR) | 156 (56, 174) | 102 (57, 173) | 94(53, 190) | 0.515 |
| Diagnosed with PTSD | 108 (21%) | 68 (18%) | 40 (27%) | 0.031 |
| Cause of injury | 0.019 | |||
| Assault | 15 (3%) | 10 (3%) | 5 (3%) | |
| Falling | 40 (8%) | 20 (5%) | 20 (13%) | |
| Motor vehicle accident | 430 (82%) | 315 (84%) | 115 (77%) | |
| All other causes, or unknown | 39 (7%) | 30 (8%) | 9 (6%) |
| Number (%) Diagnosed | Number (%) with Symptoms Improved or Resolved | |||||
|---|---|---|---|---|---|---|
| All | No LOC | LOC | All | No LOC | LOC | |
| Headache | 503 96% | 359 96% | 144 97% | 199 40% | 152 42% | 47 33% |
| Balance | 375 72% | 257 69% | 118 79% | 141 38% | 109 42% | 32 27% |
| Cognitive | 405 77% | 278 74% | 127 85% | 141 35% | 107 38% | 34 27% |
| Fatigue | 118 23% | 75 20% | 43 29% | 18 15% | 15 20% | 3 7% |
| Anxiety | 180 34% | 120 32% | 60 40% | 32 18% | 23 19% | 9 15% |
| Depression | 153 29% | 91 24% | 62 42% | 25 16% | 16 18% | 9 15% |
| Emotional Lability | 90 17% | 52 14% | 38 26% | 10 11% | 5 10% | 5 13% |
| Presence | |||||||
|---|---|---|---|---|---|---|---|
| Headache | Balance | Cognitive | Fatigue | Anxiety | Depression | Emot. Lab. | |
| Age | X | X | |||||
| LOC | X | X | X | X | X | X | |
| Thalamus | Vol | ||||||
| Persistence | |||||||
| Age | X | ||||||
| LOC | X | X | X | ||||
| Amygdala | |||||||
| Parahipp. Gyrus | Vol | ||||||
| Hippocampus | siLI | ||||||
| Entorh Cort. | LI | ||||||
| Rostral ACC | |||||||
| Caudal ACC | siLI | ||||||
| PCC | |||||||
| Isthmus Cing. | siLI | ||||||
| Lateral OFC | Vol | Vol | |||||
| Medial OFC | Vol | ||||||
| Ventral Dienc. | |||||||
| Nucleus Accum. | |||||||
| Thalamus | Vol | Vol | Vol | Vol |
| Headache | Balance Problems | Cognitive Deficits | Fatigue | Anxiety | Depression | Emotional Lability | ||
|---|---|---|---|---|---|---|---|---|
| Cov | Age (<40) | 0.79 0.32, 1.95 | 1.55 1.05, 2.28 | 1.51 1.00, 2.29 | 1.10 0.72, 1.66 | 1.10 0.76, 1.58 | 1.10 0.75, 1.62 | 1.28 0.80, 2.05 |
| Sex (F) | 0.41 0.16, 1.02 | 0.68 0.46, 1.02 | 0.91 0.60, 1.40 | 1.22 0.80, 1.85 | 0.81 0.56, 1.18 | 0.92 0.62, 1.37 | 1.22 0.77, 1.95 | |
| LOC (none) | 1.48 0.52, 4.19 | 1.91 1.20, 3.04 | 2.11 1.26, 3.53 | 1.58 1.01, 2.45 | 1.50 1.00, 2.23 | 2.28 1.51, 3.44 | 2.09 1.29, 3.36 | |
| DOI to DOS (z-score) | 0.69 0.45, 1.05 | 0.88 0.72, 1.06 | 1.11 0.89, 1.37 | 1.00 0.81, 1.23 | 1.06 0.88, 1.27 | 1.12 0.92, 1.35 | 0.99 0.79, 1.25 | |
| Vol | ||||||||
| Amygdala | 0.92 0.59, 73.71 ASLD | 0.84 0.54, 1.21 ASLD | 0.77 0.31, 1.11 ASLD D* | 0.78 0.60, 1.35 ASLD | 0.88 0.73, 5.16 ASLD | 0.86 0.69, 3.26 ASLD | 0.89 0.22, 1.84 ASL | |
| Parahipp. Gyrus | 0.90 0.61, 5.73 ASLD | 0.96 0.81, 2.60 ASLD | 0.85 0.03, 1.03 ASLD | 0.91 0.74, 1.50 ASL | 0.98 0.86, 6.83 ASLD | 1.01 0.00, 1.08 ASLD | 0.78 0.53, 1.03 ASLD | |
| Hippocampus | 0.80 0.52, 1.98 ASLD | 0.85 0.70, 1.15 ASLD | 0.93 0.75, 1.82 ASLD | 0.80 0.67, 4.77 ASLD | 0.99 0.88, 429.77 ASLD | 0.95 0.64, 2.24 ASLD | 0.80 0.57, 1.10 ASLD | |
| Entorhinal cortex | 1.04 0.00, 1.36 ASLD | 0.89 0.73, 1.26 ASLD | 0.78 0.64, 1.96 ASLD | 1.00 0.00, 1.45 ASL | 0.91 0.74, 1.35 ASLD | 0.93 0.26, 1.53 ASLD | 0.96 0.80, 6.29 ASLD | |
| Rostral ACC | 1.30 0.58, 2.03 ASLD | 0.92 0.76, 1.44 ASLD | 0.83 0.64, 1.19 ASLD L* | 0.89 0.70, 1.32 ASL | 1.11 0.78, 1.33 ASLD | 1.07 0.61, 1.29 ASLD | 1.16 0.80, 1.46 ASLD | |
| Caudal ACC | 0.69 0.00, 0.92 ASLD | 0.94 0.78, 1.90 ASLD | 0.85 0.67, 1.25 ASLD L* | 0.82 0.66, 1.09 ASL | 0.99 0.96, >100 ASLD | 0.94 0.78, 1.81 ASLD | 0.93 0.75, 2.21 ASLD | |
| PCC | 1.04 0.00, 1.35 ASLD | 1.27 0.97, 1.66 ASLD | 1.26 0.92, 1.71 ASLD | 1.02 0.11, 2.28 ASL | 1.34 1.00, 1.64 ASLD | 1.19 0.39, 1.43 ASLD | 1.05 0.30, 1.43 ASLD | |
| Isthmus Cingulate | 0.87 0.55, 3.26 ASLD | 1.01 0.00, 1.05 ASLD | 0.86 0.63, 1.18 ASLD | 1.03 0.27, 1.24 ASL | 1.13 0.82, 1.38 ASLD | 0.92 0.72, 1.46 ASLD | 0.75 0.59, 0.98 ASLD | |
| Lateral OFC | 1.06 0.02, 3.15 ASLD | 0.75 0.58, 0.95 ASLD | 0.79 0.60, 1.04 ASLD | 0.91 0.74, 1.48 SL | 0.99 0.84, >100 ASLD | 1.03 0.20, 1.22 ASLD | 0.91 0.61, 1.78 ASLD | |
| Medial OFC | 1.27 0.54, 23.1 ASLD | 0.94 0.78, 2.00 ASLD | 0.85 0.68, 1.20 ASLD | 0.91 0.02, 1.30 ASL | 1.05 0.40, 1.24 ASLD | 1.11 0.73, 1.37 ASLD | 0.96 0.77, 4.83 ASLD | |
| Ventral Diencephalon | 1.18 0.32, 3.83 ASLD | 0.87 0.71, 1.24 ASLD | 1.03 0.18, 1.25 ASLD | 0.85 0.69, 1.14 SL | 0.81 0.67, 1.84 ASLD | 0.82 0.69, 4.27 ASLD | 0.85 0.61, 1.28 ASL | |
| Nucleus Accumbens | 1.03 0.00, 3.91 ASLD | 0.83 0.60, 1.16 ASLD | 0.63 0.49, 1.39 ASLD | 0.69 0.53, 1.17 ASLD | 0.74 0.59, 1.00 ASLD | 0.71 0.55, 0.96 ASLD | 0.80 0.63, 1.08 SL | |
| Thalamus | 0.51 0.32, 0.85 ASLD | 1.04 0.35, 1.24 ASLD | 0.94 0.68, 2.01 ASLD | 1.16 0.80, 1.44 ASL | 1.22 0.96, 1.48 ASLD | 1.22 0.94, 1.49 ASLD | 0.92 0.71, 1.87 ASLD |
| Headache | Balance | Cognitive | Fatigue | Anxiety | Depression | Emotional Lability | |
|---|---|---|---|---|---|---|---|
| Covariates | |||||||
| Age (<40) | 1.19 (0.89, 1.59) | 1.30 (0.92, 1.83) | 1.57 (1.11, 2.22) | 1.43 (0.48, 4.26) | 1.46 (0.67, 3.19) | 1.69 (0.67, 4.31) | 0.54 (0, 282.13) |
| Sex (F) | 1.22 (0.92, 1.62) | 1.01 (0.72, 1.42) | 0.97 (0.68, 1.39) | 1.28 (0.46, 3.61) | 1.30 (0.61, 2.76) | 1.33 (0.55, 3.21) | 2.50 (0.03,217.64) |
| LOC (none) | 1.46 (1.05, 2.03) | 1.70 (1.13, 2.54) | 1.49 (1.02, 2.17) | 3.03 (0.08, 119.58) | 1.38 (0.56, 3.39) | 1.45 (0.61, 3.48) | 0.68 (0.04, 11.94) |
| Volume z-scores | |||||||
| Amygdala | 1.06 (0.92, 1.23) 0.391 | 1.17 (0.98, 1.39) 0.076 | 0.98 (0.79, 1.22) 0.845 | 0.91 (0.48, 1.73) 0.777 | 1.08 (0.73, 1.58) 0.704 | 1.29 (0.84, 1.99) 0.247 | 0.75 (0.28, 2.05) 0.578 |
| Parahippocampal Gyrus | 1.12 (0.97, 1.30) 0.113 | 1.17 (0.98, 1.39) 0.077 | 0.98 (0.81, 1.17) 0.797 | 1.49 (0.75, 2.97) 0.259 | 1.67 (1.14, 2.44) 0.008 | 1.64 (1.06, 2.56) 0.028 | 1.99 (1.09, 3.62) 0.025 |
| Hippocampus | 1.14 (0.98, 1.32) 0.084 | 1.17 (0.98, 1.39) 0.082 | 1.09 (0.91, 1.30) 0.338 | 1.06 (0.67, 1.68) 0.811 | 1.66 (1.09, 2.53) 0.018 | 1.77 (1.08, 2.91) 0.023 | 1.27 (0.62, 2.58) 0.516 |
| Entorhinal Cortex | 1.14 (1.00, 1.30) 0.042 | 1.09 (0.92, 1.30) 0.328 | 1.14 (0.95, 1.36) 0.150 | 1.22 (0.81, 1.84) 0.345 | 1.06 (0.74, 1.54) 0.742 | 1.01 (0.65, 1.58) 0.954 | 1.02 (0.46, 2.27) 0.970 |
| Rostral ACC | 0.95 (0.82, 1.09) 0.437 | 0.90 (0.76, 1.06) 0.211 | 0.93 (0.78, 1.09) 0.362 | 0.97 (0.63, 1.47) 0.869 | 1.22 (0.84, 1.78) 0.297 | 1.58 (0.90, 2.77) 0.114 | 1.15 (0.51, 2.58) 0.738 |
| Caudal ACC | 0.96 (0.84, 1.10) 0.571 | 0.96 (0.81, 1.13) 0.586 | 1.03 (0.86, 1.24) 0.754 | 0.67 (0.44, 1.02) 0.062 | 1.15 (0.79, 1.68) 0.460 | 0.93 (0.62, 1.39) 0.728 | 1.27 (0.01, 223.67) 0.928 |
| PCC | 0.97 (0.85, 1.12) 0.711 | 0.89 (0.76, 1.05) 0.172 | 0.92 (0.76, 1.11) 0.386 | 0.59 (0.32, 1.08) 0.089 | 0.90 (0.61, 1.35) 0.620 | 0.89 (0.57, 1.39) 0.600 | 0.89 (0.39, 2.01) 0.773 |
| Isthmus Cingulate | 1.20 (1.04, 1.39) 0.014 | 1.07 (0.90, 1.27) 0.461 | 1.00 (0.83, 1.21) 0.995 | 0.92 (0.51, 1.67) 0.787 | 1.06 (0.70, 1.61) 0.791 | 0.91 (0.52, 1.61) 0.756 | 1.78 (0.77, 4.13) 0.178 |
| Lateral OFC | 1.19 (1.02, 1.39) 0.031 | 1.33 (1.11, 1.58) 0.002 | 1.04 (0.86, 1.25) 0.719 | 1.73 (1.07, 2.77) 0.024 | 1.97 (1.27, 3.06) 0.002 | 1.34 (0.81, 2.23) 0.260 | 1.54 (0.82, 2.90) 0.178 |
| Medial OFC | 1.16 (1.01, 1.34) 0.037 | 1.23 (1.04, 1.44) 0.013 | 1.05 (0.87, 1.27) 0.631 | 1.53 (0.94, 2.49) 0.090 | 2.23 (1.44, 3.45) <0.001 | 1.64 (1.01, 2.65) 0.045 | 1.54 (0.92, 2.58) 0.099 |
| Ventral Diencephalon | 1.02 (0.89, 1.17) 0.771 | 1.06 (0.90, 1.26) 0.499 | 1.00 (0.83, 1.20) 0.983 | 0.92 (0.54, 1.57) 0.765 | 1.23 (0.85, 1.78) 0.265 | 1.25 (0.77, 2.02) 0.358 | 1.24 (0.52, 2.94) 0.623 |
| Nucleus Accumbens | 1.15 (1.00, 1.32) 0.051 | 1.20 (1.01, 1.42) 0.039 | 0.92 (0.74, 1.13) 0.420 | 1.73 (0.96, 3.10) 0.066 | 1.50 (1.05, 2.14) 0.026 | 1.33 (0.93, 1.90) 0.123 | 1.05 (0.51, 2.18) 0.899 |
| Thalamus | 1.25 (1.08, 1.44) 0.002 | 1.28 (1.07, 1.53) 0.006 | 1.17 (0.99, 1.38) 0.062 | 1.50 (0.83, 2.73) 0.181 | 1.94 (1.32, 2.85) 0.001 | 1.79 (1.21, 2.64) 0.003 | 1.75 (0.72, 4.24) 0.217 |
| LI | |||||||
| Amygdala | 1.04 (0.79, 1.36) 0.788 | 1.05 (0.77, 1.43) 0.766 | 0.89 (0.66, 1.19) 0.426 | 0.96 (0.44, 2.12) 0.926 | 1.06 (0.55, 2.01) 0.871 | 0.90 (0.42, 1.92) 0.788 | 0.83 (0.18, 3.88) 0.811 |
| Parahippocampal Gyrus | 1.30 (1.00, 1.70) 0.049 | 1.19 (0.89, 1.60) 0.239 | 1.02 (0.75, 1.40) 0.894 | 2.51 (0.99, 6.40) 0.054 | 1.30 (0.70, 2.41) 0.411 | 1.20 (0.55, 2.58) 0.650 | 0.99 (0.19, 5.14) 0.990 |
| Hippocampus | 1.03 (0.69, 1.56) 0.876 | 1.26 (0.78, 2.02) 0.353 | 1.02 (0.65, 1.61) 0.917 | 1.55 (0.49, 4.94) 0.457 | 1.09 (0.33, 3.58) 0.888 | 1.92 (0.60, 6.14) 0.271 | 1.74 (0.20, 14.84) 0.613 |
| Entorhinal Cortex | 0.99 (0.86, 1.15) 0.912 | 0.91 (0.76, 1.09) 0.315 | 0.91 (0.78, 1.06) 0.234 | 0.78 (0.47, 1.30) 0.346 | 0.52 (0.33, 0.83) 0.006 | 0.50 (0.29, 0.87) 0.014 | 0.41 (0.08, 1.97) 0.264 |
| Rostral ACC | 1.03 (0.83, 1.28) 0.797 | 1.10 (0.82, 1.46) 0.530 | 0.90 (0.67, 1.22) 0.506 | 1.17 (0.49, 2.80) 0.717 | 1.47 (0.91, 2.38) 0.115 | 1.39 (0.70, 2.76) 0.347 | 1.10 (0.42, 2.83) 0.851 |
| Caudal ACC | 1.06 (0.93, 1.20) 0.409 | 1.07 (0.92, 1.23) 0.395 | 1.04 (0.88, 1.22) 0.651 | 1.42 (0.91, 2.21) 0.125 | 1.21 (0.90, 1.63) 0.215 | 1.29 (0.85, 1.96) 0.238 | 1.01 (0.50, 2.03) 0.982 |
| PCC | 1.01 (0.81, 1.25) 0.941 | 0.97 (0.74, 1.26) 0.796 | 0.97 (0.73, 1.28) 0.803 | 0.38 (0.16, 0.88) 0.025 | 0.56 (0.30, 1.03) 0.064 | 0.65 (0.31, 1.35) 0.244 | 0.69 (0.13, 3.70) 0.664 |
| Isthmus Cingulate | 1.07 (0.81, 1.41) 0.657 | 0.99 (0.70, 1.40) 0.940 | 1.00 (0.71, 1.41) 0.983 | 1.23 (0.32, 4.76) 0.761 | 0.98 (0.45, 2.14) 0.963 | 0.82 (0.28, 2.41) 0.719 | 2.46 (0.53, 11.54) 0.252 |
| Lateral OFC | 0.90 (0.66, 1.24) 0.532 | 0.73 (0.50, 1.06) 0.099 | 1.00 (0.70, 1.42) 0.985 | 0.85 (0.29, 2.52) 0.773 | 1.17 (0.54, 2.51) 0.691 | 1.04 (0.39, 2.78) 0.936 | 0.84 (0.10, 7.19) 0.870 |
| Medial OFC | 1.01 (0.68, 1.49) 0.965 | 0.94 (0.57, 1.56) 0.820 | 0.88 (0.53, 1.45) 0.603 | 2.58 (0.57, 11.74) 0.220 | 2.59 (0.83, 8.10) 0.101 | 1.51 (0.37, 6.09) 0.562 | 1.29 (0.05, 31.48) 0.878 |
| Ventral Diencephalon | 0.93 (0.68, 1.26) 0.625 | 1.00 (0.69, 1.46) 0.998 | 0.94 (0.64, 1.37) 0.734 | 1.22 (0.42, 3.53) 0.711 | 1.07 (0.48, 2.39) 0.877 | 1.31 (0.44, 3.86) 0.624 | 0.42 (0.06, 2.92) 0.379 |
| Nucleus Accumbens | 0.94 (0.75, 1.18) 0.614 | 0.85 (0.65, 1.10) 0.218 | 1.02 (0.77, 1.36) 0.873 | 0.67 (0.20, 2.29) 0.524 | 0.81 (0.45, 1.45) 0.479 | 0.72 (0.37, 1.43) 0.348 | 1.15 (0.25, 5.27) 0.860 |
| Thalamus | 0.93 (0.61, 1.41) 0.740 | 1.20 (0.74, 1.96) 0.465 | 1.13 (0.70, 1.81) 0.617 | 0.43 (0.07, 2.79) 0.374 | 0.40 (0.13, 1.22) 0.108 | 0.85 (0.22, 3.25) 0.809 | 1.41 (0.08, 24.38) 0.813 |
| siLI | |||||||
| Amygdala | 0.75 (0.49, 1.17) 0.206 | 0.68 (0.40, 1.17) 0.167 | 0.78 (0.47, 1.31) 0.350 | 0.26 (0.04, 1.73) 0.163 | 0.41 (0.11, 1.55) 0.188 | 0.34 (0.07, 1.68) 0.184 | 1.03 (0.07, 14.19) 0.982 |
| Parahippocampal Gyrus | 0.99 (0.66, 1.50) 0.973 | 0.84 (0.51, 1.38) 0.485 | 1.03 (0.65, 1.64) 0.901 | 0.55 (0.14, 2.07) 0.375 | 0.62 (0.20, 1.93) 0.405 | 1.22 (0.36, 4.14) 0.751 | 1.68 (0.24, 11.76) 0.600 |
| Hippocampus | 0.51 (0.25, 1.06) 0.072 | 0.27 (0.11, 0.68) 0.006 | 0.37 (0.15, 0.92) 0.032 | 0.01 (0.00, 0.40) 0.015 | 0.57 (0.04, 7.33) 0.665 | 0.15 (0.01, 2.66) 0.194 | 0.25 (0.00, 21.32) 0.543 |
| Entorhinal Cortex | 0.97 (0.77, 1.22) 0.777 | 0.86 (0.66, 1.13) 0.274 | 0.88 (0.68, 1.15) 0.359 | 0.54 (0.22, 1.35) 0.190 | 0.84 (0.43, 1.63) 0.599 | 0.99 (0.48, 2.05) 0.981 | 1.12 (0.30, 4.19) 0.871 |
| Rostral ACC | 0.84 (0.57, 1.24) 0.381 | 0.66 (0.38, 1.16) 0.149 | 0.87 (0.53, 1.43) 0.578 | 0.85 (0.18, 4.03) 0.836 | 0.38 (0.13, 1.13) 0.082 | 0.58 (0.17, 2.03) 0.398 | 0.10 (0.00, 6.01) 0.270 |
| Caudal ACC | 0.78 (0.63, 0.98) 0.032 | 0.74 (0.56, 0.96) 0.025 | 0.67 (0.50, 0.90) 0.008 | 0.69 (0.28, 1.75) 0.438 | 0.62 (0.34, 1.13) 0.119 | 0.88 (0.43, 1.80) 0.733 | 1.16 (0.03, 40.25) 0.936 |
| PCC | 0.67 (0.43, 1.06) 0.087 | 0.64 (0.38, 1.09) 0.103 | 0.48 (0.27, 0.85) 0.012 | 1.68 (0.38, 7.38) 0.490 | 0.64 (0.18, 2.30) 0.499 | 0.58 (0.08, 4.26) 0.594 | 1.33 (0.07, 24.08) 0.847 |
| Isthmus Cingulate | 1.04 (0.65, 1.67) 0.866 | 1.04 (0.60, 1.82) 0.883 | 1.38 (0.82, 2.34) 0.224 | 2.14 (0.44, 10.38) 0.345 | 1.60 (0.53, 4.89) 0.407 | 5.39 (1.63, 17.78) 0.006 | 1.03 (0.08, 12.98) 0.983 |
| Lateral OFC | 1.09 (0.64, 1.86) 0.752 | 0.62 (0.30, 1.27) 0.190 | 0.73 (0.37, 1.46) 0.378 | 0.66 (0.12, 3.64) 0.633 | 0.28 (0.05, 1.49) 0.135 | 0.40 (0.05, 3.03) 0.376 | 0.31 (0.01, 16.38) 0.560 |
| Medial OFC | 1.01 (0.51, 2.00) 0.971 | 0.86 (0.35, 2.13) 0.749 | 1.24 (0.56, 2.74) 0.596 | 0.92 (0.09, 9.61) 0.947 | 1.30 (0.19, 9.04) 0.789 | 1.23 (0.12, 12.46) 0.862 | 3.20 (0.04, 292.33) 0.613 |
| Ventral Diencephalon | 1.14 (0.74, 1.76) 0.550 | 0.84 (0.47, 1.51) 0.563 | 1.35 (0.85, 2.14) 0.209 | 0.49 (0.09, 2.57) 0.396 | 0.40 (0.08, 2.06) 0.271 | 1.18 (0.28, 4.92) 0.817 | 1.46 (0.24, 8.97) 0.682 |
| Nucleus Accumbens | 1.13 (0.78, 1.62) 0.526 | 1.51 (1.02, 2.23) 0.040 | 1.16 (0.75, 1.78) 0.510 | 1.84 (0.47, 7.25) 0.383 | 0.98 (0.31, 3.16) 0.979 | 1.55 (0.67, 3.60) 0.309 | 0.69 (0.03, 17.92) 0.822 |
| Thalamus | 0.86 (0.41, 1.80) 0.686 | 0.87 (0.39, 1.92) 0.729 | 1.02 (0.48, 2.16) 0.957 | 1.18 (0.05, 30.33) 0.922 | 1.78 (0.40, 7.89) 0.450 | 1.69 (0.25, 11.57) 0.595 | 6.00 (0.24, 149.37) 0.275 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanier, C.; Santhanam, P.; Rochester, N.; Carter, L.; Lim, M.; Kilani, A.; Venkatesh, S.; Azad, S.; Knoblauch, T.; Surti, T.; et al. Symptom Persistence Relates to Volume and Asymmetry of the Limbic System after Mild Traumatic Brain Injury. J. Clin. Med. 2024, 13, 5154. https://doi.org/10.3390/jcm13175154
Vanier C, Santhanam P, Rochester N, Carter L, Lim M, Kilani A, Venkatesh S, Azad S, Knoblauch T, Surti T, et al. Symptom Persistence Relates to Volume and Asymmetry of the Limbic System after Mild Traumatic Brain Injury. Journal of Clinical Medicine. 2024; 13(17):5154. https://doi.org/10.3390/jcm13175154
Chicago/Turabian StyleVanier, Cheryl, Priya Santhanam, Nicholas Rochester, Lauren Carter, Mike Lim, Amir Kilani, Shivani Venkatesh, Sherwin Azad, Thomas Knoblauch, Tapasya Surti, and et al. 2024. "Symptom Persistence Relates to Volume and Asymmetry of the Limbic System after Mild Traumatic Brain Injury" Journal of Clinical Medicine 13, no. 17: 5154. https://doi.org/10.3390/jcm13175154





