Abstract
Background: Managing shock, a life-threatening emergency, is challenging. The influence of the initial misclassification of undifferentiated hypotension (UH) in the emergency department (ED) on patients’ outcomes remains uninvestigated. The aim of this study was to investigate whether the initial misclassification of UH in the ED affects patients’ clinical outcomes. Materials and Methods: This prospective observational study enrolled 270 non-traumatic adult patients with UH who had visited the ED of National Taiwan University Hospital between July 2020 and January 2022. The patients were divided into same-diagnosis and different-diagnosis groups, depending on the consistency between the initial and final classifications of shock. The outcome was survival to discharge. The clinical variables, management, and outcomes were compared between the groups. Results: A total of 39 of 270 patients (14.4%) were in the different-diagnosis group. Most misclassified patients were initially diagnosed as having hypovolemic shock (HS, n = 29) but finally diagnosed as having distributive shock (DS, n = 28) or cardiogenic shock (n = 1). When compared with the same-diagnosis group, the different-diagnosis group had higher hospitalization (94.9% vs. 81.4%, p = 0.023) but lower ED discharge (5.1% vs. 16.5%, p = 0.046) rates. Logistic regression analysis showed that the HS initially diagnosed was associated with an increased risk of misclassification (odds ratio [OR] = 14.731, 95% confidence interval [CI] = 3.572–60.749, p < 0.001). However, the survival to discharge did not differ between the two groups. DS, when finally diagnosed instead of the initial misclassification, was associated with in-hospital mortality (OR = 0.317, 95%CI = 0.124–0.810, p = 0.016). Conclusions: The misclassification of UH in the ED is not rare, particularly in patients with DS, who are likely to be initially misdiagnosed with HS. Although misclassification may increase hospitalization and decrease ED discharge, it does not affect survival to discharge.
1. Introduction
Shock, a life-threatening medical emergency caused by the inequality of oxygen supply and demand, is reversible initially but rapidly becomes irreversible, causing multiorgan failure and death [1,2,3,4,5]. Etiologically, shock is classified into cardiogenic shock (CS), hypovolemic shock (HS), obstructive shock (OS), and distributive shock (DS) [1,2,3,4,5]. The assessment and management of patients with shock remain challenging for emergency and critical care specialists, who need to appropriately treat diverse and variable clinical manifestations of shock through immediate evaluation, accurate diagnosis, precise etiological classification, and continuous maintenance of patients’ medical conditions, thereby preventing the development of reversible shock to irreversible organ failure or even death.
In a university hospital’s emergency department (ED) in Denmark, the average annual incidence of shock in adults was 63.2 cases per 100,000 population, increasing by 2.6% annually from 2000 to 2011, with a 7-day mortality rate of 23.1% and a 90-day mortality rate of approximately 40.7% [6]. The most common etiologies of shock in the ED in Denmark was DS (50.6%), followed by HS (30.8%), CS (14.0%), other conditions (3.7%), and OS (0.9%) [7]. A European multicenter study involving adults with shock who required vasopressors demonstrated that the most frequent type of shock was septic shock (62.2%), followed by CS (16.7%) and HS (15.7%); the least common type was OS (2%) [1]. In the ED, patients with shock or hypotension may fail to offer a detailed history initially and require resuscitation, making it challenging for the first-line physicians to adequately evaluate and classify the etiology of shock and ensure the proper management. The use of point-of-care ultrasound (PoCUS) on patients with shock has improved the clinical diagnostic accuracy for shock to 80–89% [8,9,10,11]. However, some studies have demonstrated that PoCUS does not improve survival and may worsen prognosis in hypotensive or critically ill patients in the ED [12,13].
Further examination and continuous patient management facilitate the accurate classification of shock type by the time of discharge. Studies have demonstrated the serious consequences of initial misdiagnosis; nonetheless, diagnostic errors are not uncommon in the ED [14,15,16,17]. However, the prevalence of the misclassification of shock and its effects on patients’ clinical outcomes remain unclear. Therefore, the current study aimed to investigate whether the initial misclassification of undifferentiated hypotension in the ED affects patients’ clinical outcomes.
2. Materials and Methods
2.1. Study Design and Patient Enrollment
The primary objective of this study was to explore whether the initial misclassification of undifferentiated hypotension in the ED has a significant impact on the clinical outcomes of patients. This prospective observational study was conducted in the ED of National Taiwan University Hospital (NTUH), a 2500-bed medical center that provides both primary and tertiary care in Taipei [18]. NTUH provides 24 h emergency care, and its ED has an average number of 300 visits per day. Critical patients are initially treated and constantly monitored in the critical area of the ED by experienced senior residents and attending physicians. The primary care physicians in the critical area of the ED may reserve the decisions to follow the standard operating protocols of the ED and might adjust the examinations and management according to the specific needs of each patient. This study was approved by the Institutional Review Board (IRB) of NTUH (IRB number: 202005121RINB; ClinicalTrials.gov Identifier: NCT04478045), and the written informed consents from all subjects and/or their legally authorized representative were obtained.
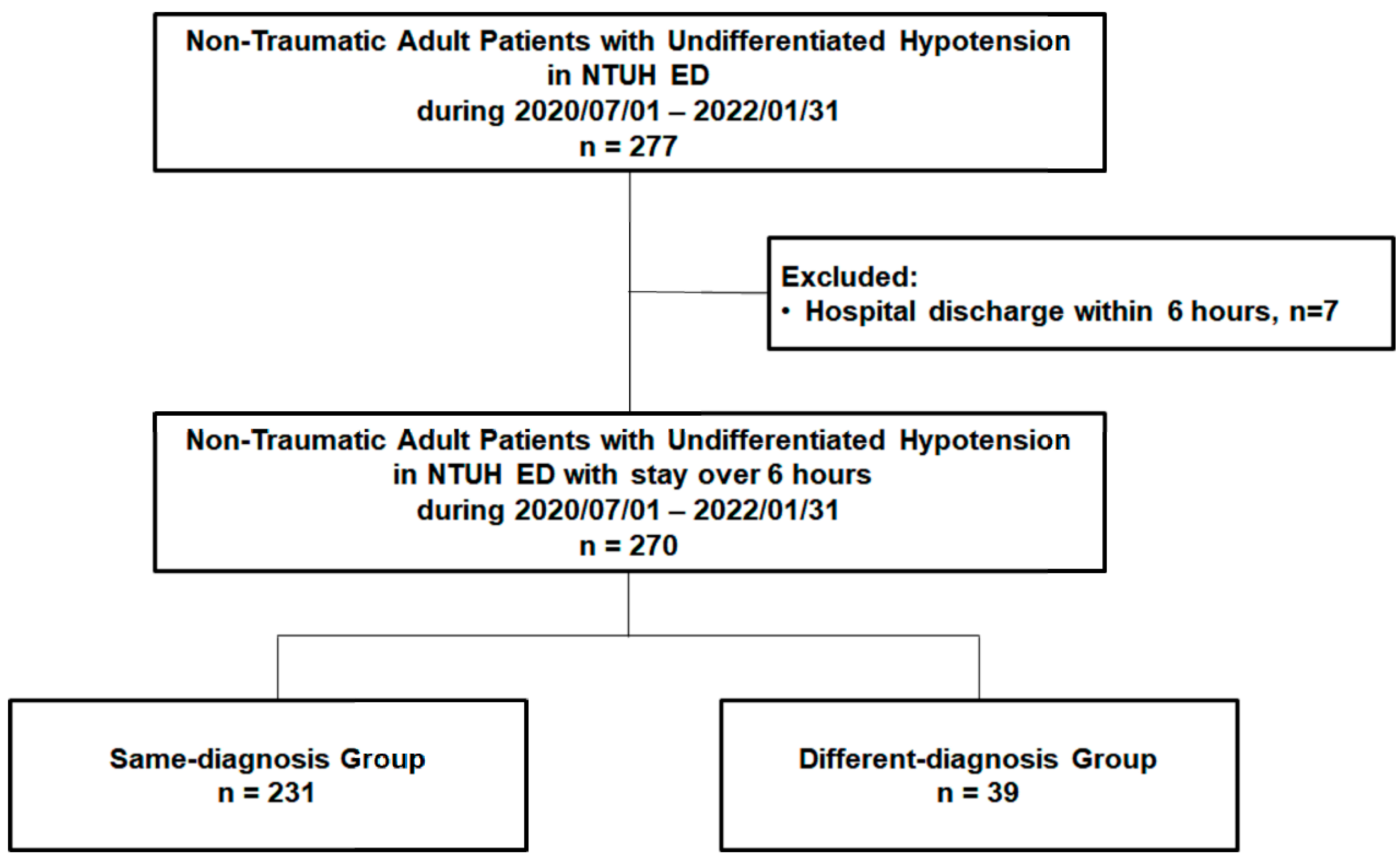
Patients with undifferentiated hypotension were initially managed and observed by experienced senior residents and attending physicians in the critical area of the ED, who were blinded to the current study. They were then recruited, informed, and enrolled in the study by the staff of this study group and provided their consent. Usually, 4 to 5 patients with undifferentiated hypotension were admitted to the critical area of the ED each day, and 2 to 3 of these patients were recruited. Approximately 15–20% of the recruited patients were informed of the study and enrolled during the study period with their informed consent. The undifferentiated hypotension in the current study was defined as systolic blood pressure of <90 mmHg with a superimposed lactic acid level of >2.2 mmol/L or a clinical presentation of inadequate/insufficient tissue perfusion, as evaluated by ED physicians. Patients who experienced trauma, were pregnant, were aged <18 years, or had a do-not-resuscitate status were excluded from this study. Between July 2020 and January 2022, 277 non-traumatic adult patients visited the ED and were diagnosed as having undifferentiated hypotension. After excluding patients discharged within 6 h (n = 7), we included 270 non-traumatic adult patients with undifferentiated hypotension (Figure 1).
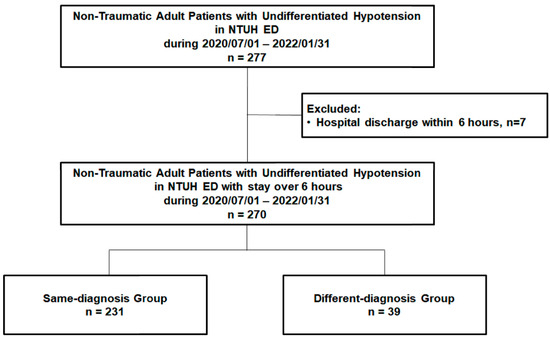
Figure 1.
The flow chart of patient enrollment. ED: emergency department; NTUH: National Taiwan University Hospital.
2.2. Data Collection
The following data were prospectively collected from the patients’ medical records: baseline characteristics, pre-existing comorbidities, clinical manifestations, physical examinations and laboratory results, initial diagnosis by ED physicians, clinical events and management during ED stay and hospitalization, and final diagnosis. Malignancy was defined as active or stable cancer. The Charlson comorbidity index (CCI) [19] and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores [20] were used to evaluate comorbidity and clinical severity. The patients’ vital signs, clinical features, examinations, and laboratory results when presenting hypotension were evaluated by primary care physicians who were blinded to the current study. For assessing the cause and classification of hypotension, PoCUS was performed by trained and qualified doctors at the critical area, who were also blinded to this study. Fluid challenge was defined as the initial administration of >250 mL of intravenous crystalloid solutions in this study. A response to fluid challenge was defined as an increase >10 mmHg in systolic blood pressure after fluid challenge. The vasopressors and inotropes included dopamine, norepinephrine, epinephrine, vasopressin, or dobutamine. Patients with high respiratory support were defined when patients required bilevel positive airway pressure, high-flow oxygen therapy, or endotracheal intubation [21]. The definition of transfusion was the supplementation of blood products, including packed red blood cells, platelets, fresh frozen plasma, or cryoprecipitates. Emergent renal replacement therapy (RRT), such as sustained low-efficiency dialysis, continuous veno-venous hemofiltration, or hemodialysis, was indicated for refractory fluid overload with respiratory distress, severe electrolyte imbalance or metabolic acidosis, or overt uremic symptoms [21].
2.3. Outcome Measurements and Patient Grouping
The primary outcome was survival to hospital discharge. The main etiology of undifferentiated hypotension was evaluated and classified by ED physicians and by the primary care physicians in the intensive care unit (ICU) or ward separately, both who were blinded to the present study. The enrolled patients were divided into two groups—same-diagnosis and different-diagnosis groups—based on whether their shock type was misclassified (according to the consistency in shock classification between ED and ICU/ward physicians).
2.4. Statistical Analysis
We assumed that 80% of shock patients at the ED with the same diagnosis had an in-hospital mortality rate of 15%, whereas the 20% ones with a different diagnosis had a mortality rate of 35%. When the alpha level was 0.05 and the power was 0.85, the estimated sample size was about 270. Continuous variables with an approximately normal distribution between the two groups were presented as mean ± standard deviation values and compared using an independent t test. Continuous variables without a normal distribution between the two groups were presented as median (interquartile range [IQR]) and compared using the Mann–Whitney U test. Categorical variables were presented as number and percentage values and compared using the chi-squared test. A multiple logistic regression analysis, adjusted for the statistically significant variables of between-group differences in univariate analysis, was performed to identify the correlations between the independent variables and outcomes. Survival curves were plotted through the Kaplan–Meier analysis and compared between groups by using the log-rank test [22]. Statistical significance was set at p < 0.05. All statistical analyses were performed using Statistical Package for Social Sciences Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY, USA).
3. Results
Of the enrolled 270 patients (mean age: 68.77 ± 15.34 years; men: 166 [61.5%]), 231 (85.6%) were included in the same-diagnosis group and 39 (14.4%) in the different-diagnosis group (Figure 1).
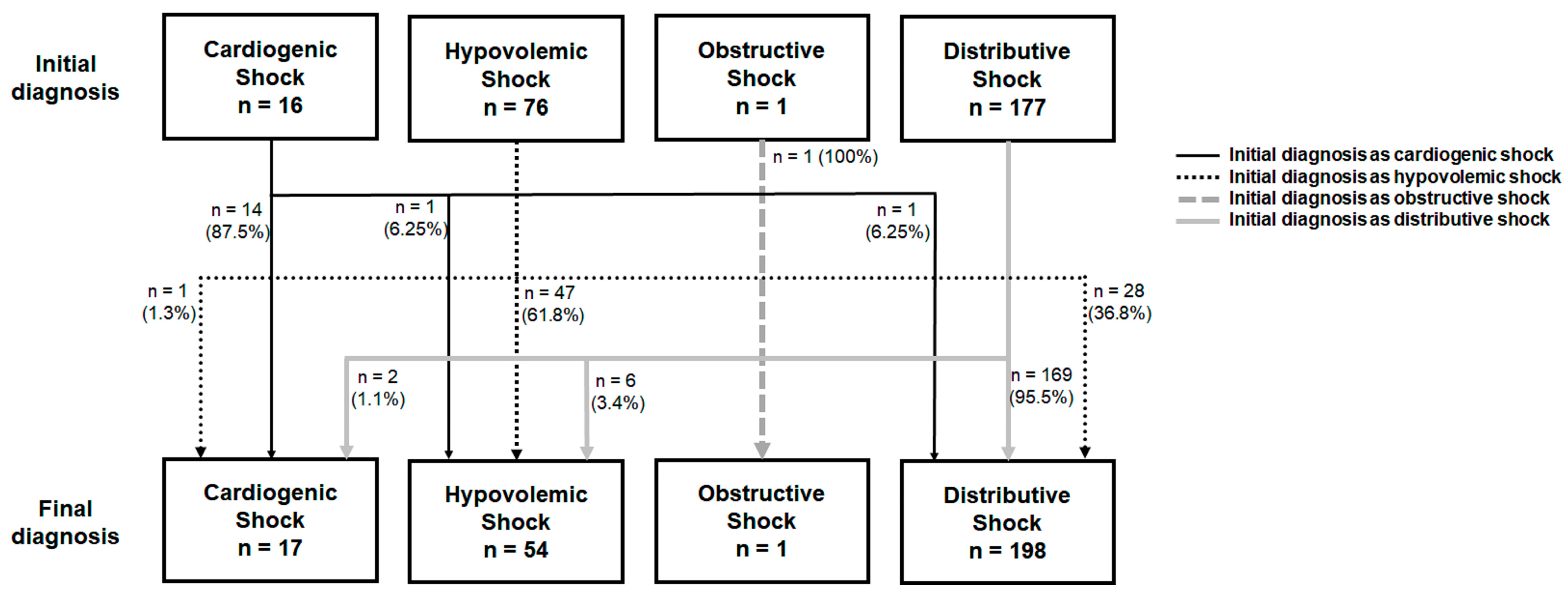
The baseline characteristics, clinical manifestations, and the PoCUS findings between the groups are listed in Table 1, and the management, outcomes, and classifications of shock between the groups were listed in Table 2. The different-diagnosis group had higher proportions of patients with a medical history of hypertension (53.8% vs. 37.2%, p = 0.050) and lower pulse rates when initially presenting with undifferentiated hypotension (99.86 ± 23.67/min vs. 108.95 ± 25.56/min, p = 0.044) than the same-diagnosis group. The CCI, the highest APACHE II score during hospitalization, and PoCUS findings did not differ between the two groups (Table 1). The management methods, including fluid challenge, the use of vasopressors and inotropes, and respiratory support were similar in these two groups (Table 2). The different-diagnosis group had higher proportions of patients initially diagnosed as having HS (74.4% vs. 20.3%, p < 0.001) and lower proportions of patients initially diagnosed as having DS (20.5% vs. 73.2%, p < 0.001) than the same-diagnosis group (Table 2). Most patients with misclassification were initially diagnosed as having hypovolemic shock (n = 29, 74.4%), but finally diagnosed as having distributive shock (n = 28) or cardiogenic shock (n = 1) (Figure 2). The multiple logistic regression analysis revealed that an initial diagnosis of HS (odds ratio [OR] = 14.731; 95% confidence interval [CI] = 3.572–60.749; p < 0.001) was significantly associated with an altered classification of shock (Table 3).

Table 1.
Baseline characteristics, initial clinical features, and PoCUS findings between the groups.

Table 2.
Management, outcomes, and diagnosis between the groups.
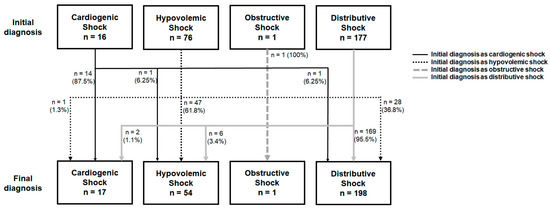
Figure 2.
The numbers of patients with different types of shock between initial diagnosis and final diagnosis.

Table 3.
The association between the clinical factors and the misclassification of shock.
For the patients initially diagnosed with HS (Supplementary Table S1), those misclassified as having HS (n = 29) showed a higher prevalence of diabetes mellitus, bedridden status, altered conscious state, cachexia, dry skin turgor, inotrope use, and ICU admission than those with a consistent HS diagnosis (n = 47); additionally, these misclassified patients also had higher body temperatures and APACHE II scores during hospitalization than those consistently diagnosed with HS. Notably, many patients who misclassified HS exhibited inferior vena cava (IVC) collapse level of >50% when presenting hypotension, but only approximately one-third of them ultimately responded to fluid challenge. In our study, the sensitivity, specificity, and accuracy were, respectively, 82.4%, 99.2%, and 98.2% for CS; 87.0%, 86.6%, and 86.7% for HS; 100.0%, 100.0%, and 100.0% for OS; and 85.4%, 88.9%, and 86.3% for DS.
When compared with the same-diagnosis group, the different-diagnosis group had a higher percentage of hospitalization (94.9% vs. 81.4%, p = 0.023) but a lower percentage of ED discharge (5.1% vs. 16.5%, p = 0.046). These two groups did not differ in ICU admission, survival to discharge, and the hospital length of stay (Table 2). The survival curves did not differ between the two groups (Supplementary Figure S1). The 7-day and 30-day survival rates in the same-diagnosis group were 93.5% and 84.8%, respectively, whereas the 7-day and 30-day survival rates in the different-diagnosis group were 92.3% and 82.1%, respectively. A total of 207 (76.7%) patients survived to hospital discharge, and 63 (23.3%) patients failed. Supplementary Tables S2 and S3 indicate the clinical variables grouped by survival to discharge or not. No significant difference in the misclassification of hypotension was noted between the survivor and non-survivor groups. The multiple logistic regression analysis revealed that DS as the final diagnosis (OR = 0.317; 95% CI = 0.124–0.810; p = 0.016) was significantly associated with the lower possibility of survival to discharge (Table 4).

Table 4.
The association between the clinical factors and survival to discharge.
4. Discussion
This prospective observational study enrolled 270 non-traumatic adult patients who presented with undifferentiated hypotension in our ED and identified that the initial misclassification of shock was not rare. Most patients with misclassification were initially diagnosed as having hypovolemic shock, but the initial misclassification did not change the patients’ survival to discharge.
Diagnostic errors are not uncommon in the ED and may cause further complications or mortalities [16,17]. Given the heterogeneous symptoms and pathophysiology of shock, the etiologies of shock in some patients could be misclassified in the ED. In our study, 85.6% of all shock cases in the ED were accurately classified, similar to the accuracy of 89% observed in another study which included non-traumatic ED patients with undifferentiated hypotension [15]. In our study, HS and DS (particularly septic shock) were the most common types of shock, consistent with the finding of other studies [1,7], and DS was often initially misclassified as HS. In addition to history-taking and physical examination of patients with undifferentiated hypotension in the ED, PoCUS is currently extensively applied in clinical settings for rapid evaluations and quick diagnoses in the ED; this helps ED physicians to further stabilize and appropriately treat patients initially [8,9,10,11]. However, Atkinson PR et al. reported that PoCUS did not significantly improve the survival of patients with undifferentiated hypotension in the ED [12], and Mosier JM et al. indicated that the PoCUS results obtained in the ED might be associated with increased mortality in critically ill patients [13]. Our results showed that an initial misclassification of shock with PoCUS did not significantly affect survival to discharge in patients with undifferentiated hypotension in the ED.
A systematic review and meta-analysis conducted by Yoshida T et al. revealed different sensitivities and specificities of PoCUS among patients with shock (78% and 96% for CS, 90% and 92% for HS, 82% and 98% for OS, and 79% and 96% for DS) [23]. In our study, both sensitivity and specificity for OS were 100.0%, and Yoshida T et al. also indicated that PoCUS usually exhibited a high diagnostic accuracy for OS [23]. However, the specificity was only 89% for DS in our study, and DS was a major type prone to misclassification in the ED. When cardiac output or contractility reduced, the structural obstruction of the heart or large vessels, or distended IVC were observed in patients with shock, and CS or OS could be correctly and rapidly diagnosed [23,24,25]. For the patients with shock who were initially misclassified as having HS or DS, PoCUS might indicate a flat IVC with or without a hypercontractile heart [24,25]. In our study, the 28 patients who were initially misclassified as having HS and were later diagnosed as having DS exhibited no typical objective symptoms or signs of septic shock, such as fever, tachycardia, or leukocytosis [4,5,7]; furthermore, among these misclassified patients, increased proportions of patients were with altered mental status and cachexia, required inotrope use, and underwent ICU admission, indicating a severe shock status necessitating urgent treatment. Many of them reasonably indicated an IVC collapse level of >50% via PoCUS. This likely explained why they were initially misclassified as having HS. Only 10 patients from this patient population exhibited hemodynamic responses after fluid challenges. One of the possible hints for the discrimination of HS and DS is that fluid responsiveness is better for patients with HS; however, this requires time to observe and monitor, which may be limited in the ED.
Although taking a detailed history and monitoring patients’ responsiveness to fluid challenge and medications might ensure an accurate diagnosis, ED physicians might find it challenging to accomplish this in the crowded and busy ED. The number of ED visits has been gradually increasing worldwide [26,27]. Kim DU et al. demonstrated that ED overcrowding increased the rate of return visits within 72 h of ED discharge, likely because of medical mistakes, previous misdiagnosis, postponed diagnosis, and delay in alerting about disease progression [28]. ED overcrowding affects ED physicians’ accuracy in classifying the etiology of shock and the timely recognition of patient response to management strategies. In our study, approximately 92.6% of all patients underwent fluid challenge and 94.4% were prescribed antibiotics. For the patients who were initially misclassified as having HS and confirmed as having DS afterwards, the high prevalence of fluid challenge and empirical antibiotics in the ED might cover the misclassification. For the patients who were initially misclassified as having DS and diagnosed as having HS later, the initial fluid challenge under close hemodynamic monitor might not have led to severe outcomes. These findings suggest that ED physicians treating undifferentiated hypotensive patients may face the possibility of the misclassification of hypotension due to scant medical history, limited resources, and time constraints. Prompt and comprehensive critical management according to patients’ personalized dynamic clinical status may remedy the misclassification of hypotension in the ED.
This study has several limitations. First, the observational design precluded the verification of the clinical variables not listed in this study with outcomes. Second, the results of PoCUS, which are highly dependent on the accuracy and ability of the performers, might have caused information bias. Third, because our study outcome was based on survival to discharge rather than survival to a fixed time point after presenting shock, a categorical bias might have occurred because hospitalization duration might be influenced not only by shock but also by other factors. Finally, because of the diversities in patient characteristics, clinical manifestation, and management across health insurance systems, hospitals, and countries, further larger-scale prospective studies are needed to assess the generalizability of our findings.
5. Conclusions
The misclassification of undifferentiated hypotension in the ED is not rare, particularly in patients with DS, who are likely to receive an initial misdiagnosis of HS. Diagnostic errors might be due to atypical objective symptoms or signs, similar flat IVC results from PoCUS, or limited time to observe fluid responsiveness in the ED. Although misclassification may increase hospitalization and decrease ED discharge, it does not affect survival to hospital discharge.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13175293/s1, Table S1: Baseline characteristics, initial clinical features, PoCUS findings, management, outcomes, and final diagnosis between the groups among the patients with initial diagnosis as HS; Table S2: Baseline characteristics, initial clinical features, and PoCUS findings between the survivors and non-survivors; Table S3: Management, outcomes, and diagnosis between the survivors and non-survivors; Figure S1: The survival curves of the same-diagnosis and different-diagnosis groups.
Author Contributions
Conceptualization, J.-J.L., W.-T.C. (Wei-Ting Chen), H.-N.O., C.-S.H., W.-T.C. (Wei-Tien Chang), C.-H.H., and M.-S.T.; Data curation, J.-J.L. and M.-S.T.; Formal analysis, J.-J.L. and M.-S.T.; Investigation, J.-J.L., W.-T.C. (Wei-Ting Chen), H.-N.O., and M.-S.T.; Methodology, C.-S.H., W.-T.C. (Wei-Tien Chang), C.-H.H., and M.-S.T.; Project administration, M.-S.T.; Resources, C.-S.H., W.-T.C. (Wei-Tien Chang), C.-H.H., and M.-S.T.; Software, J.-J.L., W.-T.C. (Wei-Ting Chen), H.-N.O., and M.-S.T.; Supervision, C.-H.H.; Validation, J.-J.L. and M.-S.T.; Visualization, M.-S.T.; Writing—original draft, J.-J.L. and M.-S.T.; Writing—review and editing, C.-S.H., W.-T.C. (Wei-Tien Chang), C.-H.H., and M.-S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was performed in line with the principles of the Declaration of Helsinki. The data collection was approved by the Institutional Review Board of National Taiwan University Hospital. (Date: 17 June 2020; IRB number: 202005121RINB).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| APACHE II | Acute Physiology and Chronic Health Evaluation II |
| CCI | Charlson comorbidity index |
| CI | Confidence interval |
| CS | Cardiogenic shock |
| DS | Distributive shock |
| ED | Emergency department |
| HS | Hypovolemic shock |
| ICU | Intensive care unit |
| IQR | Interquartile range |
| IRB | Institutional Review Board |
| IVC | Inferior vena cava |
| NTUH | National Taiwan University Hospital |
| OR | Odds ratio |
| OS | Obstructive shock |
| PoCUS | Point-of-care ultrasound |
| RRT | Renal replacement therapy |
References
- De Backer, D.; Biston, P.; Devriendt, J.; Madl, C.; Chochrad, D.; Aldecoa, C.; Brasseur, A.; Defrance, P.; Gottignies, P.; Vincent, J.-L. Comparison of dopamine and norepinephrine in the treatment of shock. N. Engl. J. Med. 2010, 362, 779–789. [Google Scholar] [CrossRef]
- De Backer, D. Detailing the cardiovascular profile in shock patients. Crit. Care 2017, 21 (Suppl. 3), 311. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; De Backer, D. Circulatory shock. N. Engl. J. Med. 2013, 369, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.B.; Wilcox, S.R. Diagnosis and management of shock in the emergency department. Emerg. Med. Pract. 2014, 16, 1–22; quiz 22–23. [Google Scholar]
- Standl, T.; Annecke, T.; Cascorbi, I.; Heller, A.R.; Sabashnikov, A.; Teske, W. The Nomenclature, Definition and Distinction of Types of Shock. Dtsch. Arztebl. Int. 2018, 115, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Holler, J.G.; Henriksen, D.P.; Mikkelsen, S.; Rasmussen, L.M.; Pedersen, C.; Lassen, A.T. Shock in the emergency department; a 12 year population based cohort study. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 87. [Google Scholar] [CrossRef]
- Holler, J.G.; Jensen, H.K.; Henriksen, D.P.; Rasmussen, L.M.; Mikkelsen, S.; Pedersen, C.; Lassen, A.T. Etiology of Shock in the Emergency Department: A 12-Year Population-Based Cohort Study. Shock 2019, 51, 60–67. [Google Scholar] [CrossRef]
- Shokoohi, H.; Boniface, K.S.; Pourmand, A.; Liu, Y.T.; Davison, D.L.; Hawkins, K.D.; Buhumaid, R.E.; Salimian, M.; Yadav, K. Bedside Ultrasound Reduces Diagnostic Uncertainty and Guides Resuscitation in Patients with Undifferentiated Hypotension. Crit. Care Med. 2015, 43, 2562–2569. [Google Scholar] [CrossRef]
- Sasmaz, M.I.; Gungor, F.; Guven, R.; Akyol, K.C.; Kozaci, N.; Kesapli, M. Effect of Focused Bedside Ultrasonography in Hypotensive Patients on the Clinical Decision of Emergency Physicians. Emerg. Med. Int. 2017, 2017, 6248687. [Google Scholar] [CrossRef]
- Stickles, S.P.; Carpenter, C.R.; Gekle, R.; Kraus, C.K.; Scoville, C.; Theodoro, D.; Tran, V.H.; Ubiñas, G.; Raio, C. The diagnostic accuracy of a point-of-care ultrasound protocol for shock etiology: A systematic review and meta-analysis. Can. J. Emerg. Med. 2019, 21, 406–417. [Google Scholar] [CrossRef]
- Berg, I.; Walpot, K.; Lamprecht, H.; Valois, M.; Lanctôt, J.-F.; Srour, N.; Brand, C.V.D. A Systemic Review on the Diagnostic Accuracy of Point-of-Care Ultrasound in Patients with Undifferentiated Shock in the Emergency Department. Cureus 2022, 14, e23188. [Google Scholar] [CrossRef]
- Atkinson, P.R.; Milne, J.; Diegelmann, L.; Lamprecht, H.; Stander, M.; Lussier, D.; Pham, C.; Henneberry, R.; Fraser, J.M.; Howlett, M.K.; et al. Does Point-of-Care Ultrasonography Improve Clinical Outcomes in Emergency Department Patients with Undifferentiated Hypotension? An International Randomized Controlled Trial from the SHoC-ED Investigators. Ann. Emerg. Med. 2018, 72, 478–489. [Google Scholar] [CrossRef]
- Mosier, J.M.M.F.; Stolz, U.; Milligan, R.; Roy-Chaudhury, A.B.; Lutrick, K.; Hypes, C.D.; Billheimer, D.; Cairns, C.B. Impact of Point-of-Care Ultrasound in the Emergency Department on Care Processes and Outcomes in Critically Ill Nontraumatic Patients. Crit. Care Explor. 2019, 1, e0019. [Google Scholar] [CrossRef] [PubMed]
- Moonen, P.-J.; Mercelina, L.; Boer, W.; Fret, T. Diagnostic error in the Emergency Department: Follow up of patients with minor trauma in the outpatient clinic. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 13. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Srinivasarangan, M.; Javali, R.H.; Loganathan, A.; Siddappa, G.B.; Satyanarayana, N.; Bheemanna, A.S.; Jagadeesh, S.; Betkerur, S. Reliability of Emergency Department Diagnosis in Identifying the Etiology of Nontraumatic Undifferentiated Hypotension. Indian J. Crit. Care Med. 2020, 24, 313–320. [Google Scholar] [CrossRef]
- Pelaccia, T.; Messman, A.M.; Kline, J.A. Misdiagnosis and failure to diagnose in emergency care: Causes and empathy as a solution. Patient Educ. Couns. 2020, 103, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Horberg, M.A.; Nassery, N.; Rubenstein, K.B.; Certa, J.M.; Shamim, E.A.; Rothman, R.; Wang, Z.; Hassoon, A.; Townsend, J.L.; Galiatsatos, P.; et al. Rate of sepsis hospitalizations after misdiagnosis in adult emergency department patients: A look-forward analysis with administrative claims data using Symptom-Disease Pair Analysis of Diagnostic Error (SPADE) methodology in an integrated health system. Diagnosis 2021, 8, 479–488. [Google Scholar] [CrossRef]
- Lin, J.J.; Huang, C.H.; Chen, W.J.; Chuang, P.Y.; Chang, W.T.; Chen, W.T.; Tsai, M.S. Targeted temperature management and emergent coronary angiography are associated with improved outcomes in patients with prehospital return of spontaneous circulation. J. Formos. Med. Assoc. 2020, 119, 1259–1266. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Tien, Y.-T.; Chen, W.-J.; Huang, C.-H.; Wang, C.-H.; Chen, W.-T.; Hung, C.-S.; Lin, J.-J.; Huang, C.-C.; Chang, W.-T.; Tsai, M.-S. The CSP (Cardiogenic Shock Prognosis) Score: A Tool for Risk Stratification of Cardiogenic Shock. Front. Cardiovasc. Med. 2022, 9, 842056. [Google Scholar] [CrossRef]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 12: Survival analysis. Crit. Care 2004, 8, 389–394. [Google Scholar] [CrossRef]
- Yoshida, T.; Noma, H.; Nomura, T.; Suzuki, A.; Mihara, T. Diagnostic accuracy of point-of-care ultrasound for shock: A systematic review and meta-analysis. Crit. Care 2023, 27, 200. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.; Mailhot, T.; Riley, D.; Mandavia, D. The RUSH Exam: Rapid Ultrasound in Shock in the Evaluation of the Critically lll. Emerg. Med. Clin. N. Am. 2010, 28, 29–56. [Google Scholar] [CrossRef]
- Mok, K.L. Make it SIMPLE: Enhanced shock management by focused cardiac ultrasound. J. Intensiv. Care 2016, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Hoot, N.R.; Aronsky, D. Systematic review of emergency department crowding: Causes, effects, and solutions. Ann. Emerg. Med. 2008, 52, 126–136.e1. [Google Scholar] [CrossRef]
- Ruud, S.E.; Hjortdahl, P.; Natvig, B. Is it a matter of urgency? A survey of assessments by walk-in patients and doctors of the urgency level of their encounters at a general emergency outpatient clinic in Oslo, Norway. BMC Emerg. Med. 2016, 16, 22. [Google Scholar] [CrossRef]
- Kim, D.-u.; Park, Y.S.; Park, J.M.; Brown, N.J.; Chu, K.; Lee, J.H.; Kim, J.H.; Kim, M.J. Influence of Overcrowding in the Emergency Department on Return Visit within 72 H. J. Clin. Med. 2020, 9, 1406. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).