Evolution of Cervical Endoscopic Spine Surgery: Current Progress and Future Directions—A Narrative Review
Abstract
:1. Introduction
1.1. Cervical Spondylosis
1.2. Traditional Open Surgery for Cervical Radiculopathy and Myelopathy
1.3. Minimally Invasive Cervical Spine Surgery
1.4. Cervical Endoscopic Spine Surgery
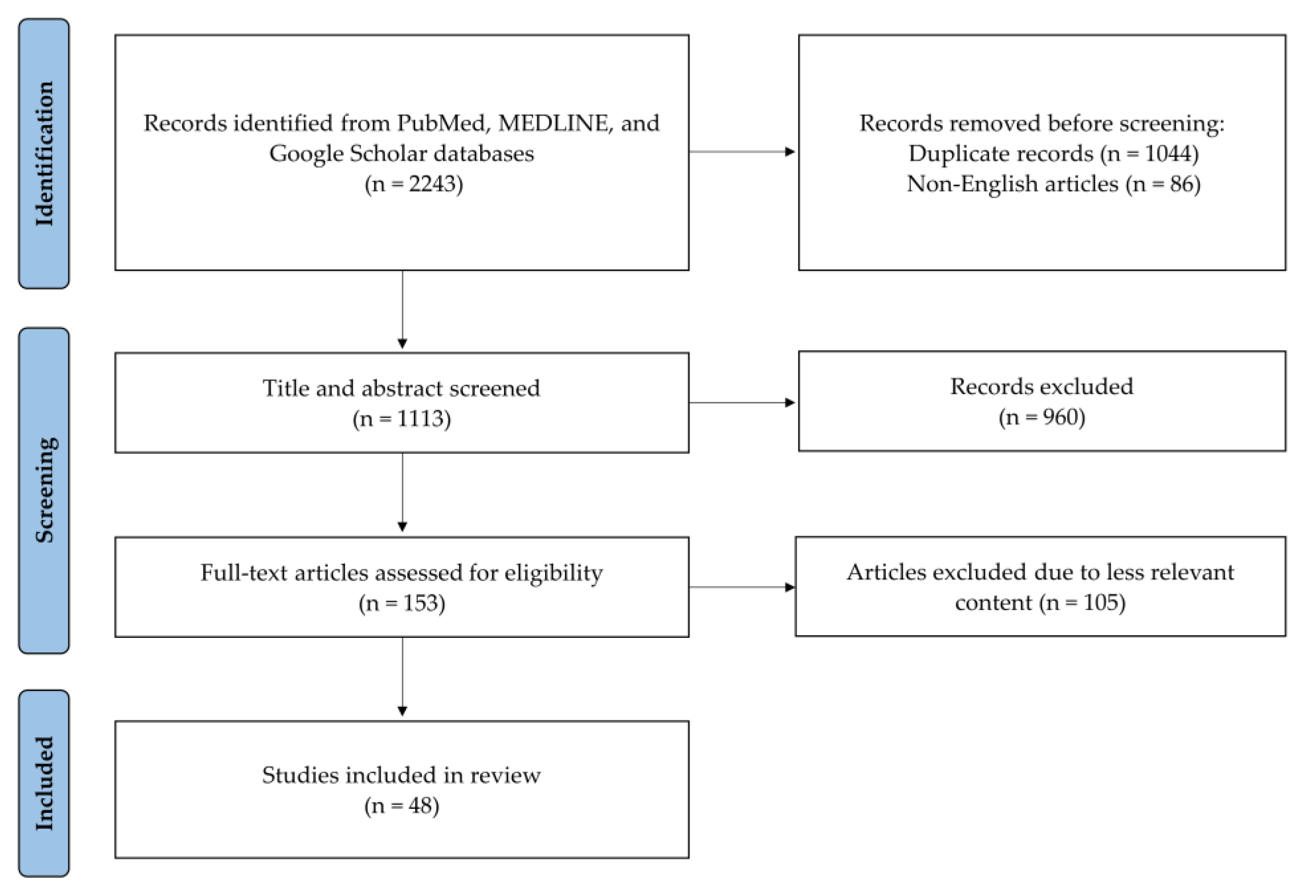
2. Methods
3. Classification of Endoscopic Spine Surgery
3.1. Nomenclature System
3.2. Advantages of Full-Endoscopic Cervical Spine Surgery
4. Common Endoscopic Techniques in the Cervical Spine
4.1. Posterior Endoscopic Cervical Foraminotomy/Discectomy (PECF/PECD)
4.2. Anterior Endoscopic Cervical Discectomy (AECD)
4.3. Cervical Endoscopic Unilateral Laminotomy for Bilateral Decompression (CE-ULBD)
5. Limitations of Cervical Endoscopic Spine Surgery
5.1. Steep Learning Curve
5.2. Radiation Exposure
5.3. Limitations with Multiple-Level Lesions
6. Facilitating Endoscopic Surgery
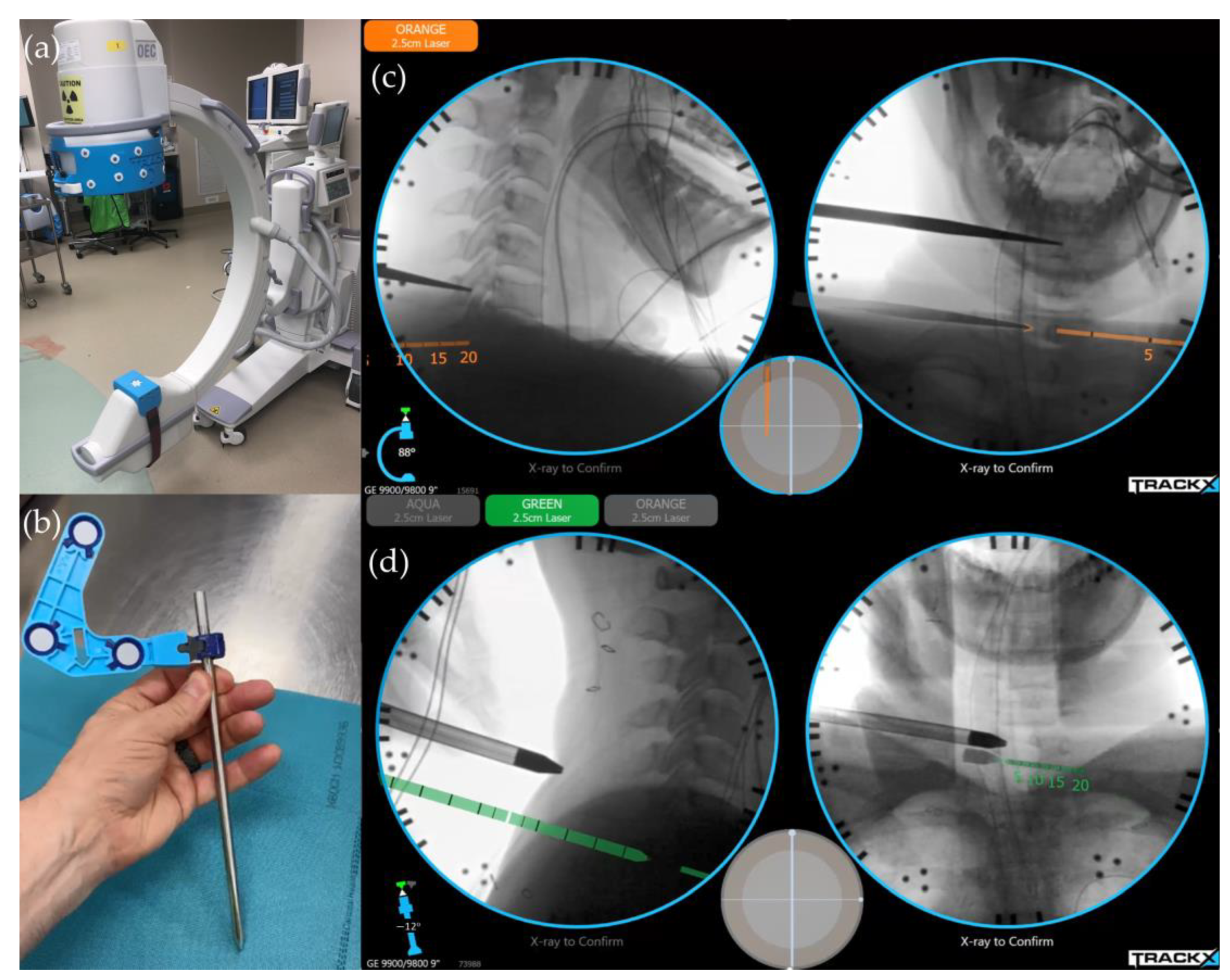
6.1. Image-Guided Navigation System/Instrument Tracking System
6.2. Regional Anesthesia for Perioperative Pain Control
7. Future Directions in Cervical Endoscopic Spine Surgery
7.1. Minimally Invasive Posterior Cervical Decompression and Fusion
7.2. Endoscopic Odontoidectomy and Atlantoaxial Fusion
7.3. Patient-Specific Surgical Planning
7.4. Application of Augmented Reality (AR) Technology
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Theodore, N. Degenerative cervical spondylosis. N. Engl. J. Med. 2020, 383, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.M.; Yoon, T.; Riew, K.D. Cervical radiculopathy. J. Am. Acad. Orthop. Surg. 2007, 15, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Azad, T.D.; Tharin, S. Cervical spondylotic myelopathy. Clin. Spine Surg. 2016, 29, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Caridi, J.M.; Pumberger, M.; Hughes, A.P. Cervical radiculopathy: A review. HSS J. 2011, 7, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Gutman, G.; Rosenzweig, D.H.; Golan, J.D. Surgical treatment of cervical radiculopathy: Meta-analysis of randomized controlled trials. Spine 2018, 43, E365–E372. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.F.; Badhiwala, J.H.; Moghaddamjou, A.; Martin, A.R.; Fehlings, M.G. Degenerative cervical myelopathy; A review of the latest advances and future directions in management. Neurospine 2019, 16, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Litrico, S.; Lonjon, N.; Riouallon, G.; Cogniet, A.; Launay, O.; Beaurain, J.; Blamoutier, A.; Pascal-Mousselard, H.; French Society of Spine Surgery (SFCR). Adjacent segment disease after anterior cervical interbody fusion: A multicenter retrospective study of 288 patients with long-term follow-up. Orthop. Traumatol. Surg. Res. 2014, 100, S305–S309. [Google Scholar] [CrossRef] [PubMed]
- Skovrlj, B.; Qureshi, S.A. Minimally invasive cervical spine surgery. J. Neurosurg. Sci. 2017, 61, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Platt, A.; Gerard, C.S.; O’Toole, J.E. Comparison of outcomes following minimally invasive and open posterior cervical foraminotomy: Description of minimally invasive technique and review of literature. J. Spine Surg. 2020, 6, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Adamson, T.E. Microendoscopic posterior cervical laminoforaminotomy for unilateral radiculopathy: Results of a new technique in 100 cases. J. Neurosurg. 2001, 95, 51–57. [Google Scholar] [CrossRef] [PubMed]
- McAnany, S.J.; Kim, J.S.; Overley, S.C.; Baird, E.O.; Anderson, P.A.; Qureshi, S.A. A meta-analysis of cervical foraminotomy: Open versus minimally-invasive techniques. Spine J. 2015, 15, 849–856. [Google Scholar] [CrossRef]
- Kim, K.T.; Kim, Y.B. Comparison between open procedure and tubular retractor assisted procedure for cervical radiculopathy: Results of a randomized controlled study. J. Korean Med. Sci. 2009, 24, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.; Suezawa, Y.; Leu, H. Does percutaneous nucleotomy with discoscopy replace conventional discectomy? Eight years of experience and results in treatment of herniated lumbar disc. Clin. Orthop. Relat. Res. 1989, 35–42. [Google Scholar] [CrossRef]
- Kambin, P. Arthroscopic microdiskectomy. Mt. Sinai J. Med. 1991, 58, 159–164. [Google Scholar]
- Mayer, H.M.; Brock, M. Percutaneous endoscopic discectomy: Surgical technique and preliminary results compared to microsurgical discectomy. J. Neurosurg. 1993, 78, 216–225. [Google Scholar] [CrossRef]
- Mayer, H.M. A History of Endoscopic Lumbar Spine Surgery: What Have We Learnt? Biomed. Res. Int. 2019, 4583943. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.T.; Yeung, C.A. Advances in endoscopic disc and spine surgery: Foraminal approach. Surg. Technol. Int. 2003, 11, 255–263. [Google Scholar]
- Ruetten, S.; Komp, M.; Godolias, G. A New full-endoscopic technique for the interlaminar operation of lumbar disc herniations using 6-mm endoscopes: Prospective 2-year results of 331 patients. Minim. Invasive Neurosurg. 2006, 49, 80–87. [Google Scholar] [CrossRef]
- Choi, G.; Lee, S.H.; Raiturker, P.P.; Lee, S.; Chae, Y.S. Percutaneous endoscopic interlaminar discectomy for intracanalicular disc herniations at L5-S1 using a rigid working channel endoscope. Neurosurgery 2006, 58, ONS59–ONS68. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Liu, B.; Hao, J.; Zhou, P.; Yao, Y.; Zhang, F.; Chen, X. Percutaneous endoscopic lumbar discectomy versus posterior open lumbar microdiscectomy for the treatment of symptomatic lumbar disc herniation: A systemic review and meta-analysis. World Neurosurg. 2018, 120, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Jarebi, M.; Awaf, A.; Lefranc, M.; Peltier, J. A matched comparison of outcomes between percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for the treatment of lumbar disc herniation: A 2-year retrospective cohort study. Spine J. 2021, 21, 114–121. [Google Scholar] [CrossRef] [PubMed]
- McGrath, L.B.; White-Dzuro, G.A.; Hofstetter, C.P. Comparison of clinical outcomes following minimally invasive or lumbar endoscopic unilateral laminotomy for bilateral decompression. J. Neurosurg. Spine 2019, 30, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Chen, W.J.; Chen, H.S.; Kao, Y.H.; Yu, S.W.; Tu, Y.K. Extended indications of percutaneous endoscopic lavage and drainage for the treatment of lumbar infectious spondylitis. Eur. Spine J. 2014, 23, 846–853. [Google Scholar] [CrossRef]
- Joo, Y.C.; Ok, W.K.; Baik, S.H.; Kim, H.J.; Kwon, O.S.; Kim, K.H. Removal of a vertebral metastatic tumor compressing the spinal nerve roots via a single-port, transforaminal, endoscopic approach under monitored anesthesia care. Pain. Physician 2012, 15, 297–302. [Google Scholar] [PubMed]
- Wan, Q.; Zhang, D.; Li, S.; Liu, W.; Wu, X.; Ji, Z.; Ru, B.; Cai, W. Posterior percutaneous full-endoscopic cervical discectomy under local anesthesia for cervical radiculopathy due to soft-disc herniation: A preliminary clinical study. J. Neurosurg. Spine 2018, 29, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, K.; Chu, L.; Chen, L.; Deng, Z. Posterior percutaneous endoscopic cervical discectomy through lamina-hole approach for cervical intervertebral disc herniation. Int. J. Neurosci. 2019, 129, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Lee, S.H.; Shin, S.W. Percutaneous endoscopic cervical discectomy: Clinical outcome and radiographic changes. Photomed. Laser Surg. 2005, 23, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Ruetten, S.; Komp, M.; Merk, H.; Godolias, G. A new full-endoscopic technique for cervical posterior foraminotomy in the treatment of lateral disc herniations using 6.9-mm endoscopes: Prospective 2-year results of 87 patients. Minim. Invasive Neurosurg. 2007, 50, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.L.; Chu, L.; Chen, L.; Yang, J.S. Anterior transcorporeal approach of percutaneous endoscopic cervical discectomy for disc herniation at the C4–C5 levels: A technical note. Spine J. 2016, 16, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, S.H. Clinical and radiographic changes after percutaneous endoscopic cervical discectomy: A long-term follow-up. Photomed. Laser Surg. 2014, 32, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.A.; Abecassis, I.J.; Hofstetter, C.P. Full endoscopic unilateral laminotomy for bilateral decompression of the cervical spine: Surgical technique and early experience. J. Spine Surg. 2020, 6, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, J.; Zhao, Z.; Li, J.; Zhao, H.; Gao, Y.; Chen, C. Microscopic anterior cervical discectomy and fusion versus posterior percutaneous endoscopic cervical keyhole foraminotomy for single-level unilateral cervical radiculopathy: A systematic review and meta-analysis. Clin. Spine Surg. 2023, 36, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, C.P.; Ahn, Y.; Choi, G.; Gibson, J.N.A.; Ruetten, S.; Zhou, Y.; Li, Z.Z.; Siepe, C.J.; Wagner, R.; Lee, J.H.; et al. AOSpine consensus paper on nomenclature for working-channel endoscopic spinal procedures. Glob. Spine J. 2020, 10, 111S–121S. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.W.; Lee, D.G.; Park, C.K. Rationale and advantages of endoscopic spine surgery. Int. J. Spine Surg. 2021, 15, S11–S20. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y. The Current state of cervical endoscopic spine surgery: An updated literature review and technical considerations. Expert. Rev. Med. Devices 2020, 17, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Shim, C.S.; Ahn, Y.; Choi, Y.G.; Kim, H.J.; Lee, S.H. Comparison of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for recurrent disc herniation. J. Korean Neurosurg. Soc. 2009, 46, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Ruetten, S.; Komp, M.; Merk, H.; Godolias, G. Full-endoscopic anterior decompression versus conventional anterior decompression and fusion in cervical disc herniations. Int. Orthop. 2009, 33, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.I.; Lee, S.M. Complications and Management of endoscopic spinal surgery. Neurospine 2023, 20, 56–77. [Google Scholar] [CrossRef] [PubMed]
- Bohlman, H.H.; Emery, S.E.; Goodfellow, D.B.; Jones, P.K. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J. Bone Jt. Surg. Am. 1993, 75, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Sahai, N.; Changoor, S.; Dunn, C.J.; Sinha, K.; Hwang, K.S.; Faloon, M.; Emami, A. Minimally invasive posterior cervical foraminotomy as an alternative to anterior cervical discectomy and fusion for unilateral cervical radiculopathy: A systematic review and meta-analysis. Spine 2019, 44, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Huang, L.; Feng, F.; Yang, B.; He, L.; Du, G.; Xie, P.; Chen, Z. Anterior cervical discectomy and fusion versus posterior cervical foraminotomy for the treatment of single-level unilateral cervical radiculopathy: A meta-analysis. J. Orthop. Surg. Res. 2020, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Wu, P.H.; Lee, Y.J.; Kim, D.H.; Kim, J.Y.; Lee, J.H.; Jeon, J.B.; Jang, I.T. Safe route for cervical approach: Partial pediculotomy, partial vertebrotomy approach for posterior endoscopic cervical foraminotomy and discectomy. World Neurosurg. 2020, 140, e273–e282. [Google Scholar] [CrossRef] [PubMed]
- Ruetten, S.; Komp, M.; Merk, H.; Godolias, G. Full-endoscopic cervical posterior foraminotomy for the operation of lateral disc herniations using 5.9-mm endoscopes: A prospective, randomized, controlled study. Spine 2008, 33, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Ji-Jun, H.; Hui-Hui, S.; Zeng-Wu, S.; Liang, Z.; Qing, L.; Heng-Zhu, Z. Posterior full-endoscopic cervical discectomy in cervical radiculopathy: A prospective cohort study. Clin. Neurol. Neurosurg. 2020, 195, 105948. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, J.; Sherman, J.H.; Szabo, T.; Shaffrey, C.I.; Jane, J.A. The posterior cervical foraminotomy in the treatment of cervical disc/osteophyte disease: A single-surgeon experience with a minimum of 5 years’ clinical and radiographic follow-up. J. Neurosurg. Spine 2009, 10, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Won, S.J.; Kim, C.H.; Chung, C.K.; Choi, Y.; Park, S.B.; Moon, J.H.; Heo, W.; Kim, S.M. Clinical outcomes of single-level posterior percutaneous endoscopic cervical foraminotomy for patients with less cervical lordosis. J. Minim. Invasive Spine Surg. Tech. 2016, 1, 11–17. [Google Scholar] [CrossRef]
- Chen, P.H.; Yang, T.H.; Chen, S.Y. Application of posterior endoscopic cervical foraminotomy for recurrent radiculopathy after anterior cervical discectomy and fusion surgery. J. Minim. Invasive Spine Surg. Tech. 2023, 8, S56–S61. [Google Scholar] [CrossRef]
- Siebert, W. Percutaneous laser discectomy of cervical discs: Preliminary clinical results. J. Clin. Laser Med. Surg. 1995, 13, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.C.; Hansraj, K.K.; Akiyama, C.; Greenspan, M. Percutaneous (endoscopic) decompression discectomy for non-extruded cervical herniated nucleus pulposus. Surg. Technol. Int. 1997, 6, 405–411. [Google Scholar] [PubMed]
- Yu, K.X.; Chu, L.; Yang, J.S.; Deng, R.; Chen, L.; Shi, L.; Hao, D.J.; Deng, Z.L. Anterior transcorporeal approach to percutaneous endoscopic cervical diskectomy for single-level cervical intervertebral disk herniation: Case series with 2-Year follow-up. World Neurosurg. 2019, 122, e1345–e1353. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Xin, Z.; Du, Q.; Cao, G.; Liao, W. Anterior percutaneous full-endoscopic transcorporeal decompression of the spinal cord for single-segment cervical spondylotic myelopathy: The technical interpretation and 2 years of clinical follow-up. J. Orthop. Surg. Res. 2019, 14, 461. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yang, J.; Chen, C.M.; Liu, K.; Wang, X.F.; Wei, J.M.; Shi, L.; Liu, W.; Jiang, H.; Zhou, H.; et al. Outcomes of discectomy by using full-endoscopic visualization technique via the transcorporeal and transdiscal approaches in the treatment of cervical intervertebral disc herniation: A comparative study. Biomed. Res. Int. 2020, 2020, 5613459. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Keum, H.J.; Shin, S.H. Percutaneous endoscopic cervical discectomy versus anterior cervical discectomy and fusion: A comparative cohort study with a five-year follow-up. J. Clin. Med. 2020, 9, 371. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.I.; Kim, P.; Seo, J.H.; Kim, S.W.; Lee, S.M. Complications of Cervical Endoscopic Spinal Surgery: A Systematic Review and Narrative Analysis. World Neurosurg. 2023, 178, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Chin, B.Z.; Yong, J.H.; Wang, E.; Sim, S.I.; Lin, S.; Wu, P.H.; Hey, H.W.D. Full-endoscopic versus microscopic spinal decompression for lumbar spinal stenosis: A systematic review & meta-analysis. Spine J. 2024, in press. [Google Scholar] [CrossRef]
- Perez-Roman, R.J.; Gaztanaga, W.; Lu, V.M.; Wang, M.Y. Endoscopic decompression for the treatment of lumbar spinal stenosis: An updated systematic review and meta-analysis. J. Neurosurg. Spine 2021, 36, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshian, J.; Mehta, V.A.; Liu, J.C. Current diagnosis and management of cervical spondylotic myelopathy. Glob. Spine J. 2017, 7, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, X.; Zhang, L.M.; Yan, Y.Q.; Liu, Y.K.; Lewandrowski, K.U. Comparative study of curative effect of spinal endoscopic surgery and anterior cervical decompression for cervical spondylotic myelopathy. J. Spine Surg. 2020, 6, S186–S196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.B.; Ma, Y.J.; Ma, H.J.; Zhang, X.Y.; Zhou, H.G. Clinical efficacy of posterior percutaneous endoscopic unilateral laminotomy with bilateral decompression for symptomatic cervical spondylotic myelopathy. Orthop. Surg. 2022, 14, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Kim, C.H.; Lee, J.H.; Lee, S.H.; Kim, J.S. Radiation exposure to the surgeon during percutaneous endoscopic lumbar discectomy: A prospective study. Spine 2013, 38, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, D.; Than, K.D.; Wang, A.C.; La Marca, F.; Wang, P.I.; Schermerhorn, T.C.; Park, P. Radiation safety and spine surgery: Systematic review of exposure limits and methods to minimize radiation exposure. World Neurosurg. 2014, 82, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Iwai, H.; Oka, H.; Otomo, K.; Inanami, H. A protective method to reduce radiation exposure to the surgeon during endoscopic lumbar spine surgery. J. Spine Surg. 2019, 5, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Higashino, K.; Hayashi, H.; Hayashi, F.; Fukui, Y.; Sairyo, K. Pulsation and Collimation During Fluoroscopy to Decrease Radiation: A Cadaver Study. JBJS Open Access 2017, 2, e0039. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Koga, H. Early experience of single level full endoscopic posterior cervical foraminotomy and comparison with microscope-assisted open surgery. J. Spine Surg. 2020, 6, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Ao, S.; Wu, J.; Tang, Y.; Zhang, C.; Li, J.; Zheng, W.; Zhou, Y. Percutaneous endoscopic lumbar discectomy assisted by O-arm-based navigation improves the learning curve. Biomed. Res. Int. 2019, 6509409. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T.; Song, M.S.; Kim, J.S. How I do it? Interlaminar contralateral endoscopic lumbar foraminotomy assisted with the O-arm navigation. Acta Neurochir. 2020, 162, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, C.; Zhou, Y.; Huang, B. Minimally invasive computer navigation-assisted endoscopic transforaminal interbody fusion with bilateral decompression via a unilateral approach: Initial clinical experience at one-year follow-up. World Neurosurg. 2017, 106, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, J.; Xu, C.; Zheng, W.; Pan, Y.; Li, C.; Zhou, Y. Minimally invasive full-endoscopic posterior cervical foraminotomy assisted by O-arm-based Navigation. Pain. Physician 2018, 21, E215–E223. [Google Scholar] [PubMed]
- Pennington, Z.; Cottrill, E.; Westbroek, E.M.; Goodwin, M.L.; Lubelski, D.; Ahmed, A.K.; Sciubba, D.M. Evaluation of surgeon and patient radiation exposure by imaging technology in patients undergoing thoracolumbar fusion: Systematic review of the literature. Spine J. 2019, 19, 1397–1411. [Google Scholar] [CrossRef] [PubMed]
- Rawicki, N.; Dowdell, J.E.; Sandhu, H.S. Current state of navigation in spine surgery. Ann. Transl. Med. 2021, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, F.; Wang, T.Y.; Gabr, M.; Mehta, V.A.; Bwensa, A.M.; Foster, N.; Than, K.D.; Goodwin, R.C.; Abd-El-Barr, M.M. A Prospective comparison of the effects of instrument tracking on time and radiation during minimally invasive lumbar interbody fusion. World Neurosurg. 2021, 152, e101–e111. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Tabarestani, T.Q.; Mehta, V.A.; Sankey, E.W.; Karikari, I.O.; Goodwin, C.R.; Than, K.D.; Abd-El-Barr, M.M. A comparison of percutaneous pedicle screw accuracy between robotic navigation and novel fluoroscopy-based instrument tracking for patients undergoing instrumented thoracolumbar surgery. World Neurosurg. 2023, 172, e389–e395. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.K.; Chadwick, A.L.; Nwaneshiudu, C.; Aggarwal, A.; Salmasi, V.; Lii, T.R.; Hah, J.M. Management of postoperative pain in patients following spine surgery: A narrative review. Int. J. Gen. Med. 2022, 15, 4535–4549. [Google Scholar] [CrossRef]
- Goel, V.K.; Chandramohan, M.; Murugan, C.; Shetty, A.P.; Subramanian, B.; Kanna, R.M.; Rajasekaran, S. Clinical efficacy of ultrasound guided bilateral erector spinae block for single-level lumbar fusion surgery: A prospective, randomized, case-control study. Spine J. 2021, 21, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, H.; Ince, I.; Hamadnalla, H.; Drake, R.L.; Tsui, B.C.H. Cervical erector spinae plane block: A cadaver study. Reg. Anesth. Pain. Med. 2020, 45, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, J.; Yang, J. Letter to the editor regarding, “Peri-operative analgesic efficacy and safety of erector spinae plane block in posterior cervical spine surgery-a double blinded, randomized controlled study” by Kanna et al. Spine J. 2022, 22, 1922. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Kalgudi, P.; Sriganesh, K. Ultrasound-guided erector spinae plane block for perioperative analgesia in cervical and thoracic spine surgeries—A case series. Neurol. Ind. 2021, 69, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Kanna, R.M.; Ramachandran, K.; Subramanian, J.B.; Shetty, A.P.; Rajasekaran, S. Perioperative analgesic efficacy and safety of erector spinae plane block in posterior cervical spine surgery-a double blinded, randomized controlled study. Spine J. 2023, 23, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Kanna, R.M.; Ramachandran, K.; Subramanian, J.B.; Shetty, A.P.; Rajasekaran, S. Reply to the letter to editor regarding “Peri-operative analgesic efficacy and safety of erector spinae plane block in posterior cervical spine surgery—A double blinded, randomized controlled study”. Spine J. 2022, 22, 1923–1924. [Google Scholar] [CrossRef] [PubMed]
- Badiee, R.K.; Mayer, R.; Pennicooke, B.; Chou, D.; Mummaneni, P.V.; Tan, L.A. Complications following posterior cervical decompression and fusion: A review of incidence, risk factors, and prevention strategies. J. Spine Surg. 2020, 6, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Coric, D.; Rossi, V. Percutaneous posterior cervical pedicle instrumentation (C1 to C7) with navigation guidance: Early series of 27 cases. Glob. Spine J. 2022, 12, 27S–33S. [Google Scholar] [CrossRef] [PubMed]
- Farah, K.; Meyer, M.; Prost, S.; Albader, F.; Dufour, H.; Blondel, B.; Fuentes, S. Robotic assistance for minimally invasive cervical pedicle instrumentation: Report on feasibility and safety. World Neurosurg. 2021, 150, e777–e782. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Li, Y. Percutaneous Endoscopic Posterior Lateral Approach for the Treatment of Central Cervical Disc Herniation. World Neurosurg. 2024, 181, e376–e383. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, A.; Jho, H.D.; Tschabitscher, M. Endoscopic endonasal approach to the ventral cranio-cervical junction: Anatomical study. Acta Neurochir. 2002, 144, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Re, M.; Iacoangeli, M.; Di Somma, L.; Alvaro, L.; Nasi, D.; Magliulo, G.; Gioacchini, F.M.; Fradeani, D.; Scerrati, M. Endoscopic endonasal approach to the craniocervical junction: The importance of anterior C1 arch preservation or its reconstruction. Acta Otorhinolaryngol. Ital. 2016, 36, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Taghva, A.; Attenello, F.J.; Zada, G.; Khalessi, A.A.; Hsieh, P.C. Minimally invasive posterior atlantoaxial fusion: A cadaveric and clinical feasibility study. World Neurosurg. 2013, 80, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Gelinne, A.; Piazza, M.; Bhowmick, D.A. Minimally invasive modification of the Goel-Harms atlantoaxial fusion technique: A case series and illustrative guide. Neurosurg. Focus 2023, 54, E14. [Google Scholar] [CrossRef]
- Lvov, I.; Grin, A.; Godkov, I.; Kordonskiy, A.; Krylov, V. Posterior percutaneous transarticular stand-alone screw instrumentation of C1-C2 with endoscopic assistance: A report of two cases. Neurocirugia 2021, 32, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Penner, F.; De Marco, R.; Di Perna, G.; Portonero, I.; Baldassarre, B.; Garbossa, D.; Zenga, F. Endoscopic endonasal odontoidectomy: A long-term follow-up results for a cohort of 21 patients. Eur. Spine J. 2022, 31, 2693–2703. [Google Scholar] [CrossRef]
- Mendes, G.A.; Dickman, C.A.; Rodriguez-Martinez, N.G.; Kalb, S.; Crawford, N.R.; Sonntag, V.K.; Preul, M.C.; Little, A.S. Endoscopic endonasal atlantoaxial transarticular screw fixation technique: An anatomical feasibility and biomechanical study. J. Neurosurg. Spine 2015, 22, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Tabarestani, T.Q.; Sykes, D.A.W.; Kouam, R.W.; Salven, D.S.; Wang, T.Y.; Mehta, V.A.; Shaffrey, C.I.; Wiggins, W.F.; Chi, J.H.; Abd-El-Barr, M.M. Novel Approach to Percutaneous Lumbar Surgeries via Kambin’s Triangle-Radiographic and Surgical Planning Analysis with Nerve Segmentation Technology. World Neurosurg. 2023, 177, e385–e396. [Google Scholar] [CrossRef] [PubMed]
- Tabarestani, T.Q.; Sykes, D.A.W.; Maquoit, G.; Wang, T.Y.; Ayoub, C.M.; Shaffrey, C.I.; Wiggins, W.F.; Abd-El-Barr, M.M. Novel Merging of CT and MRI to Allow for Safe Navigation into Kambin’s Triangle for Percutaneous Lumbar Interbody Fusion-Initial Case Series Investigating Safety and Efficacy. Oper. Neurosurg. 2023, 24, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Chen, R.E.; Han, P.K.; Si, P.; Freeman, W.D.; Pirris, S.M. Technical feasibility and safety of an intraoperative head-up display device during spine instrumentation. Int. J. Med. Robot. 2017, 13, e1770. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J., 3rd; Shepard, N.; Nolte, M.; Siemionow, K.; Phillips, F. Augmented reality in spine surgery: Current state of the art. Int. J. Spine Surg. 2022, 16, S22–S27. [Google Scholar] [CrossRef] [PubMed]
- Dennler, C.; Jaberg, L.; Spirig, J.; Agten, C.; Götschi, T.; Fürnstahl, P.; Farshad, M. Augmented reality-based navigation increases precision of pedicle screw insertion. J. Orthop. Surg. Res. 2020, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.J.; Colman, M.W.; Lynch, J.; Phillips, F.M. Augmented reality in minimally invasive spine surgery: Early efficiency and complications of percutaneous pedicle screw instrumentation. Spine J. 2023, 23, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Schwendner, M.; Ille, S.; Wostrack, M.; Meyer, B. Evaluating a cutting-edge augmented reality-supported navigation system for spinal instrumentation. Eur. Spine J. 2024, 33, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Mollica, C.; Guatta, R.; Scarone, P. Augmented reality surgical navigation in hybrid operating room for minimally invasive lumbar fusion: 2-dimensional operative video. Oper. Neurosurg. 2023, 25, e107. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Egawa, S.; Sakai, K.; Onuma, H.; Hashimoto, J.; Utagawa, K.; Morishita, S.; Yamada, K.; Matsukura, Y.; Arai, Y.; et al. Novel technique of anterior foraminotomy based on augmented reality with computed tomography vavigation system: A case report. Spine Surg. Relat. Res. 2023, 8, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Onuma, H.; Sakai, K.; Arai, Y.; Torigoe, I.; Tomori, M.; Sakaki, K.; Hirai, T.; Egawa, S.; Kobayashi, Y.; Okawa, A.; et al. Augmented Reality Support for Anterior Decompression and Fusion Using Floating Method for Cervical Ossification of the Posterior Longitudinal Ligament. J. Clin. Med. 2023, 12, 2898. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-C.; Fitts, J.; Huie, D.; Bhowmick, D.A.; Abd-El-Barr, M.M. Evolution of Cervical Endoscopic Spine Surgery: Current Progress and Future Directions—A Narrative Review. J. Clin. Med. 2024, 13, 2122. https://doi.org/10.3390/jcm13072122
Huang C-C, Fitts J, Huie D, Bhowmick DA, Abd-El-Barr MM. Evolution of Cervical Endoscopic Spine Surgery: Current Progress and Future Directions—A Narrative Review. Journal of Clinical Medicine. 2024; 13(7):2122. https://doi.org/10.3390/jcm13072122
Chicago/Turabian StyleHuang, Chuan-Ching, Jamal Fitts, David Huie, Deb A. Bhowmick, and Muhammad M. Abd-El-Barr. 2024. "Evolution of Cervical Endoscopic Spine Surgery: Current Progress and Future Directions—A Narrative Review" Journal of Clinical Medicine 13, no. 7: 2122. https://doi.org/10.3390/jcm13072122
APA StyleHuang, C.-C., Fitts, J., Huie, D., Bhowmick, D. A., & Abd-El-Barr, M. M. (2024). Evolution of Cervical Endoscopic Spine Surgery: Current Progress and Future Directions—A Narrative Review. Journal of Clinical Medicine, 13(7), 2122. https://doi.org/10.3390/jcm13072122




