Association of Obesity with Telomere Length in Human Sperm
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants’ Characteristics and Semen Sample Collection
2.2. Sperm DNA Extraction and Quantification
2.3. Real-Time Quantitive PCR (qPCR)
2.4. Quantification Method: Comparative ΔΔCq Method Based on the AHTLQ Assay Kit
- The difference in the number of quantification cycles of telomeres (TEL) between the target and reference genomic DNA samples is known as Cq (TEL).
- 2.
- For single-copy references, Cq (SCR) is the number of quantification cycles that differ between the reference and target genomic DNA samples.
- 3.
- The target sample’s relative telomere length to the reference sample (fold) = 2−ΔΔCq.
- (a)
- By subtracting each sample’s mean nuclear Ct value from its mean mitochondrial Ct value, as shown by the formula ΔCt = mtDNA – nDNA;
- (b)
- In this case, the control group comprised men with normal body mass index (18.5 to 24.9 kg/m2) and normal semen characteristics, that is, the average ΔCt value;
- (c)
- A sample’s ΔΔCt is determined by deducting the control group’s ΔCt from the sample’s mean ΔCt. In other words, ΔΔCt = a sample’s ΔCt minus the control group’s ΔCt [28];
- (d)
- The fold difference is calculated using the formula 2−ΔΔCt.
2.5. Statistical Analysis
2.6. Inclusion and Exclusion Criteria
3. Results
3.1. Correlation between Mitochondrial DNA Content and the Relative Telomere Length
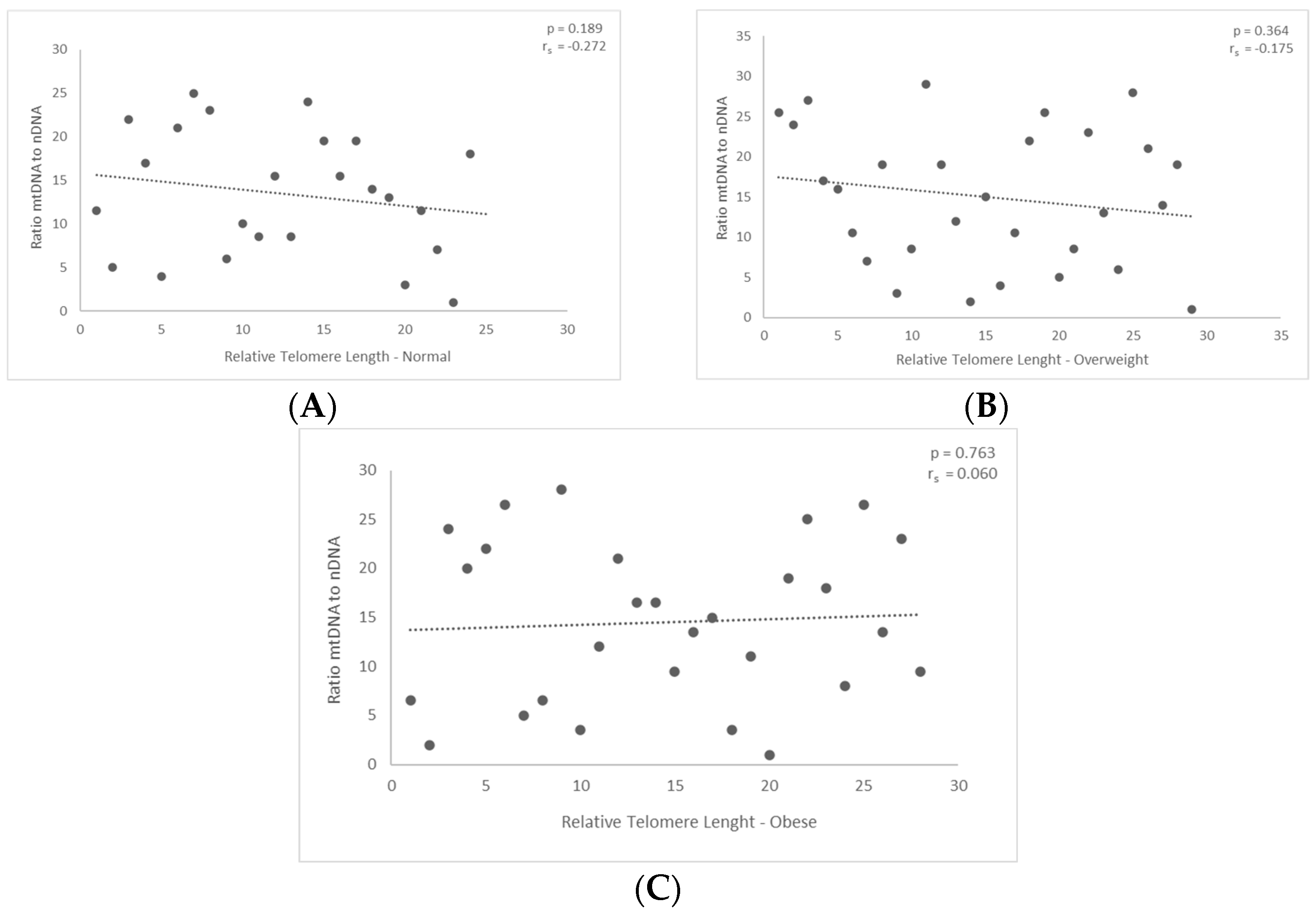
3.2. Correlation between mtDNA-to-nDNA Ratio and the Relative Telomere Length
4. Discussion
5. Conclusions
6. Limitations of This Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q.; et al. Brown and beige adipose tissue: A novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol.-Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Roberto, C.A.; Swinburn, B.; Hawkes, C.; Huang, T.T.-K.; Costa, S.A.; Ashe, M.; Zwicker, L.; Cawley, J.H.; Brownell, K.D. Patchy progress on obesity prevention: Emerging examples, entrenched barriers, and new thinking. Lancet 2015, 385, 2400–2409. [Google Scholar] [CrossRef]
- Santos, A.L.; Sinha, S. Obesity and aging: Molecular mechanisms and therapeutic approaches. Ageing Res. Rev. 2021, 67, 101268. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.G.A.; Faragher, R.G.A. Obesity and type-2 diabetes as inducers of premature cellular senescence and ageing. Biogerontology 2018, 19, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Welendorf, C.; Nicoletti, C.F.; Pinhel, M.A.D.S.; Noronha, N.Y.; De Paula, B.M.F.; Nonino, C.B. Obesity, weight loss, and influence on telomere length: New insights for personalized nutrition. Nutrition 2019, 66, 115–121. [Google Scholar] [CrossRef] [PubMed]
- García-Calzón, S.; Moleres, A.; Marcos, A.; Campoy, C.; Moreno, L.A.; Azcona-Sanjulián, M.C.; Martínez-González, M.A.; Martínez, J.A.; Zalba, G.; Marti, A.; et al. Telomere length as a biomarker for adiposity changes after a multidisciplinary intervention in overweight/obese adolescents: The EVASYON study. PLoS ONE 2014, 9, e89828. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Martin, H.; Firpo, M.A.; Demerath, E.W. Inverse association between adiposity and telomere length: The Fels Longitudinal Study. Am. J. Hum. Biol. Off. J. Hum. Biol. Counc. 2011, 23, 100–106. [Google Scholar] [CrossRef]
- Rode, L.; Nordestgaard, B.G.; Weischer, M.; Bojesen, S.E. Increased Body Mass Index, Elevated C-Reactive Protein, and Short Telomere Length. J. Clin. Endocrinol. Metab. 2014, 99, E1671–E1675. [Google Scholar] [CrossRef]
- Narasimhan, A.; Flores, R.R.; Camell, C.D.; Bernlohr, D.A.; Robbins, P.D.; Niedernhofer, L.J. Cellular Senescence in Obesity and Associated Complications: A New Therapeutic Target. Curr. Diab. Rep. 2022, 22, 537–548. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef] [PubMed]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Bhari, V.K.; Kumar, D.; Kumar, S.; Mishra, R. Shelterin complex gene: Prognosis and therapeutic vulnerability in cancer. Biochem. Biophys. Rep. 2021, 26, 100937. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Xu, D. Telomerase Reverse Transcriptase (TERT) in Action: Cross-Talking with Epigenetics. Int. J. Mol. Sci. 2019, 20, 3338. [Google Scholar] [CrossRef]
- Yuan, X.; Larsson, C.; Xu, D. Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: Old actors and new players. Oncogene 2019, 38, 6172–6183. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.D.; Comeau, L.; Rosenfield, S.; Stansel, R.M.; Bianchi, A.; Moss, H.; De Lange, T. Mammalian Telomeres End in a Large Duplex Loop. Cell 1999, 97, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 2021, 11, 630186. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Huang, J.; Wang, G. Mitochondria, Telomeres and Telomerase Subunits. Front. Cell Dev. Biol. 2019, 7, 274. [Google Scholar] [CrossRef]
- Green, P.D.; Sharma, N.K.; Santos, J.H. Telomerase Impinges on the Cellular Response to Oxidative Stress Through Mitochondrial ROS-Mediated Regulation of Autophagy. Int. J. Mol. Sci. 2019, 20, 1509. [Google Scholar] [CrossRef]
- Huang, J.; Liu, P.; Wang, G. Regulation of mitochondrion-associated cytosolic ribosomes by mammalian mitochondrial ribonuclease T2 (RNASET2). J. Biol. Chem. 2018, 293, 19633–19644. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, P.; Zheng, Q.; Gao, G.; Yuan, J.; Wang, P.; Huang, J.; Xie, L.; Lu, X.; Tong, T.; et al. Mitochondrial Trafficking and Processing of Telomerase RNA TERC. Cell Rep. 2018, 24, 2589–2595. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Y.; Ge, Y.; Liu, J.; Zhao, Y. TERC promotes cellular inflammatory response independent of telomerase. Nucleic Acids Res. 2019, 47, 8084–8095. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.R.W.L.; Blackburn, E.H. Telomeres and telomerase. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2004, 359, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Moustakli, E.; Zikopoulos, A.; Georgiou, I.; Dimitriadis, F.; Symeonidis, E.N.; Markou, E.; Michaelidis, T.M.; Tien, D.M.B.; Giannakis, I.; et al. Impact of Advanced Paternal Age on Fertility and Risks of Genetic Disorders in Offspring. Genes 2023, 14, 486. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Cherkas, L.F.; Kato, B.S.; Demissie, S.; Hjelmborg, J.B.; Brimacombe, M.; Cupples, A.; Hunkin, J.L.; Gardner, J.P.; Lu, X.; et al. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008, 4, e37. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.M.; Britt-Compton, B.; Rowson, J.; Amso, N.N.; Gregory, L.; Kipling, D. Telomere instability in the male germline. Hum. Mol. Genet. 2006, 15, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.J.; Watson, E.M.; Skinner, B.M.; Griffin, D.K. Telomere Distribution in Human Sperm Heads and Its Relation to Sperm Nuclear Morphology: A New Marker for Male Factor Infertility? Int. J. Mol. Sci. 2021, 22, 7599. [Google Scholar] [CrossRef] [PubMed]
- Quiros, P.M.; Goyal, A.; Jha, P.; Auwerx, J. Analysis of mtDNA/nDNA Ratio in Mice. Curr. Protoc. Mouse Biol. 2017, 7, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods San Diego Calif 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zia, S. Obesity: Impact and Outcome on Infertility—A Literature Review. Open J. Obstet. Gynecol. 2023, 13, 214–240. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, F.; Hu, L.; Bai, R.; Zhang, N.; Yao, G.; Sun, Y. Effect of paternal overweight or obesity on IVF treatment outcomes and the possible mechanisms involved. Sci. Rep. 2016, 6, 29787. [Google Scholar] [CrossRef] [PubMed]
- Raee, P.; Shams Mofarahe, Z.; Nazarian, H.; Abdollahifar, M.-A.; Ghaffari Novin, M.; Aghamiri, S.; Ghaffari Novin, M. Male obesity is associated with sperm telomere shortening and aberrant mRNA expression of autophagy-related genes. Basic Clin. Androl. 2023, 33, 13. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Chen, Y.; Fu, X.; Lin, C.-P.; Zheng, X.F.S.; Liu, L.F. TOR Regulates Cell Death Induced by Telomere Dysfunction in Budding Yeast. PLoS ONE 2008, 3, e3520. [Google Scholar] [CrossRef][Green Version]
- Ahmed, S.; Passos, J.F.; Birket, M.J.; Beckmann, T.; Brings, S.; Peters, H.; Birch-Machin, M.A.; von Zglinicki, T.; Saretzki, G. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J. Cell Sci. 2008, 121, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Singhapol, C.; Pal, D.; Czapiewski, R.; Porika, M.; Nelson, G.; Saretzki, G.C. Mitochondrial Telomerase Protects Cancer Cells from Nuclear DNA Damage and Apoptosis. PLoS ONE 2013, 8, e52989. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Prescott, J.; Kraft, P.; Han, J.; Giovannucci, E.; Hankinson, S.E.; De Vivo, I. Physical Activity, Sedentary Behavior, and Leukocyte Telomere Length in Women. Am. J. Epidemiol. 2012, 175, 414–422. [Google Scholar] [CrossRef]
- Cassidy, A.; De Vivo, I.; Liu, Y.; Han, J.; Prescott, J.; Hunter, D.J.; Rimm, E.B. Associations between diet, lifestyle factors, and telomere length in women. Am. J. Clin. Nutr. 2010, 91, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Puterman, E.; Lin, J.; Blackburn, E.; O’Donovan, A.; Adler, N.; Epel, E. The Power of Exercise: Buffering the Effect of Chronic Stress on Telomere Length. PLoS ONE 2010, 5, e10837. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh-Far, R. Association of Marine Omega-3 Fatty Acid Levels with Telomeric Aging in Patients with Coronary Heart Disease. JAMA 2010, 303, 250. [Google Scholar] [CrossRef]
- Ornish, D.; Lin, J.; Daubenmier, J.; Weidner, G.; Epel, E.; Kemp, C.; Magbanua, M.J.M.; Marlin, R.; Yglecias, L.; Carroll, P.R.; et al. Increased telomerase activity and comprehensive lifestyle changes: A pilot study. Lancet Oncol. 2008, 9, 1048–1057. [Google Scholar] [CrossRef]
- Wyrobek, A.J.; Eskenazi, B.; Young, S.; Arnheim, N.; Tiemann-Boege, I.; Jabs, E.W.; Glaser, R.L.; Pearson, F.S.; Evenson, D. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc. Natl. Acad. Sci. USA 2006, 103, 9601–9606. [Google Scholar] [CrossRef] [PubMed]
- Vagnini, L.; Baruffi, R.L.R.; Mauri, A.L.; Petersen, C.G.; Massaro, F.C.; Pontes, A.; Oliveira, J.B.A.; Franco, J.G. The effects of male age on sperm DNA damage in an infertile population. Reprod. Biomed. Online 2007, 15, 514–519. [Google Scholar] [CrossRef]
- Gunes, S.; Hekim, G.N.T.; Arslan, M.A.; Asci, R. Effects of aging on the male reproductive system. J. Assist. Reprod. Genet. 2016, 33, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, H.K.; Ko, J.-H.; Bang, H.; Lee, D.-C. The relationship between leukocyte mitochondrial DNA copy number and telomere length in community-dwelling elderly women. PLoS ONE 2013, 8, e67227. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Enquobahrie, D.A.; Gelaye, B.; Hevner, K.; Williams, M.A. The association between leukocyte telomere length and mitochondrial DNA copy number in pregnant women: A pilot study. Clin. Lab. 2015, 61, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Alegría-Torres, J.A.; Velázquez-Villafaña, M.; López-Gutiérrez, J.M.; Chagoyán-Martínez, M.M.; Rocha-Amador, D.O.; Costilla-Salazar, R.; García-Torres, L. Association of Leukocyte Telomere Length and Mitochondrial DNA Copy Number in Children from Salamanca, Mexico. Genet. Test. Mol. Biomark. 2016, 20, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Melicher, D.; Illés, A.; Littvay, L.; Tárnoki, Á.D.; Tárnoki, D.L.; Bikov, A.; Kunos, L.; Csabán, D.; Buzás, E.I.; Molnár, M.J.; et al. Positive association and future perspectives of mitochondrial DNA copy number and telomere length—A pilot twin study. Arch. Med. Sci. AMS 2021, 17, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar]
- Moustakli, E.; Zikopoulos, A.; Sakaloglou, P.; Bouba, I.; Sofikitis, N.; Georgiou, I. Functional association between telomeres, oxidation and mitochondria. Front. Reprod. Health 2023, 5, 1107215. [Google Scholar] [CrossRef]
- Fice, H.; Robaire, B. Telomere Dynamics throughout Spermatogenesis. Genes 2019, 10, 525. [Google Scholar] [CrossRef]
- Yim, H.W.; Slebos, R.J.C.; Randell, S.H.; Umbach, D.M.; Parsons, A.M.; Rivera, M.P.; Detterbeck, F.C.; Taylor, J.A. Smoking is associated with increased telomerase activity in short-term cultures of human bronchial epithelial cells. Cancer Lett. 2007, 246, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, D.; Collotta, A.; Carfi, M.; Bowe, G.; Vahter, M.; Hartung, T.; Gribaldo, L. Arsenic induces telomerase expression and maintains telomere length in human cord blood cells. Toxicology 2009, 260, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Xia, Y.; Ning, Z.; Wade, T.J.; Mumford, J.L. Elevated human telomerase reverse transcriptase gene expression in blood cells associated with chronic arsenic exposure in Inner Mongolia, China. Environ. Health Perspect. 2009, 117, 354–360. [Google Scholar] [CrossRef] [PubMed]
- López-Diazguerrero, N.E.; Pérez-Figueroa, G.E.; Martínez-Garduño, C.M.; Alarcón-Aguilar, A.; Luna-López, A.; Gutiérrez-Ruiz, M.C.; Königsberg, M. Telomerase activity in response to mild oxidative stress. Cell Biol. Int. 2012, 36, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Rhee, D.B.; Lu, J.; Bohr, C.T.; Zhou, F.; Vallabhaneni, H.; de Souza-Pinto, N.C.; Liu, Y. Characterization of oxidative guanine damage and repair in mammalian telomeres. PLoS Genet. 2010, 6, e1000951. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, R.; Malhotra, N.; Singh, N.; Dada, R. Mild oxidative stress is beneficial for sperm telomere length maintenance. World J. Methodol. 2016, 6, 163. [Google Scholar] [CrossRef]


| Primer Pair | Sequence (Forward/Reverse) | Amplicon Base Pair (bp) | Tm |
|---|---|---|---|
| Nuclear region (RPE gene) | Forward 5′-ATAGGAAGCCAGAGAAGAGAGACT-3′ Reverse 5′-TCTATCTCTGCGGACTTTGAGCAT-3′ | 200 | 60 °C |
| Mitochondrial region | Forward 5′-TAGAGGAGCCTGTTCTGTAATCG-3′ Reverse 5′-TAAGGGCTATCGTAGTTTTCTGG-3′ | 205 | 59 °C |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| IVF/ICSI participants | Males without informed consent |
| Lifestyle (smoking, alcohol, drugs, etc.) | Urological conditions (e.g., varicocele) |
| Abnormal Karyotype | |
| AZF deletions |
| Semen Characteristics | Normal | Overweight | Obese | p-Values |
|---|---|---|---|---|
| No. of participants | 25 | 29 | 28 | |
| Progressive motility (%), CI 95% | 47.71 ± 6.14 | 37.53 ± 5.61 | 4.2 ± 3.56 | 0.0062 |
| Sperm morphology (%), CI 95% | 13.48 ± 4.11 | 16 ± 9.04 | 10.16 ± 3.02 | 0.4073 |
| Sperm count, million/mL, CI 95% | 30.54 ± 14.84 | 16.98 ± 8.74 | 34.56 ± 12.67 | 0.0692 |
| Male age, CI 95% | 38.3 ± 1.75 | 43.25 ± 2.75 | 41 ± 2.55 | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moustakli, E.; Zikopoulos, A.; Skentou, C.; Dafopoulos, S.; Stavros, S.; Dafopoulos, K.; Drakakis, P.; Georgiou, I.; Zachariou, A. Association of Obesity with Telomere Length in Human Sperm. J. Clin. Med. 2024, 13, 2150. https://doi.org/10.3390/jcm13072150
Moustakli E, Zikopoulos A, Skentou C, Dafopoulos S, Stavros S, Dafopoulos K, Drakakis P, Georgiou I, Zachariou A. Association of Obesity with Telomere Length in Human Sperm. Journal of Clinical Medicine. 2024; 13(7):2150. https://doi.org/10.3390/jcm13072150
Chicago/Turabian StyleMoustakli, Efthalia, Athanasios Zikopoulos, Charikleia Skentou, Stefanos Dafopoulos, Sofoklis Stavros, Konstantinos Dafopoulos, Peter Drakakis, Ioannis Georgiou, and Athanasios Zachariou. 2024. "Association of Obesity with Telomere Length in Human Sperm" Journal of Clinical Medicine 13, no. 7: 2150. https://doi.org/10.3390/jcm13072150
APA StyleMoustakli, E., Zikopoulos, A., Skentou, C., Dafopoulos, S., Stavros, S., Dafopoulos, K., Drakakis, P., Georgiou, I., & Zachariou, A. (2024). Association of Obesity with Telomere Length in Human Sperm. Journal of Clinical Medicine, 13(7), 2150. https://doi.org/10.3390/jcm13072150








