Multipoint Left Ventricular Pacing as Alternative Approach in Cases of Biventricular Pacing Failure
Abstract
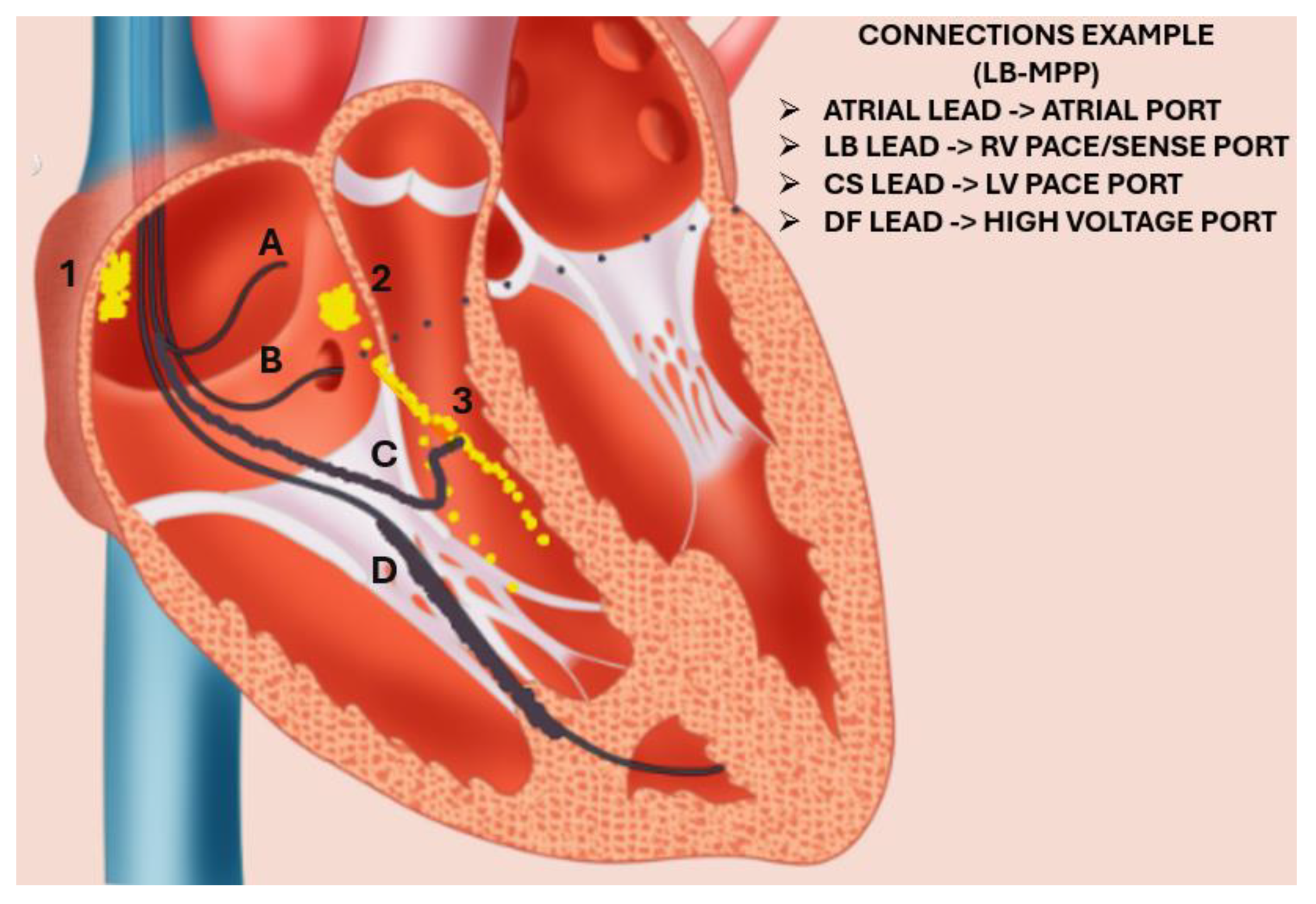
:1. Introduction
1.1. The Concept of Resequencing
1.2. The Notion of Multipoint Pacing
1.3. Clinical Evidence
1.4. Current Perspectives for Non-Responders
2. Conclusions
Funding
Conflicts of Interest
References
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; Marco, T.D.; Carson, P.; Di Carlo, L.; De Mets, D.; White, B.G.; et al. Cardiac-Resynchronization Therapy with or without an Implantable Defibrillator in Advanced Chronic Heart Failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Daubert, J.-C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L. The Effect of Cardiac Resynchronization on Morbidity and Mortality in Heart Failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A.M.; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-Resynchronization Therapy for the Prevention of Heart-Failure Events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.S.L.; Wells, G.A.; Talajic, M.; Arnold, M.O.; Sheldon, R.; Connolly, S.; Hohnloser, S.H.; Nichol, G.; Birnie, D.H.; Sapp, J.L.; et al. Cardiac-Resynchronization Therapy for Mild-to-Moderate Heart Failure. N. Engl. J. Med. 2010, 363, 2385–2395. [Google Scholar] [CrossRef]
- Hadwiger, M.; Dagres, N.; Haug, J.; Wolf, M.; Marschall, U.; Tijssen, J.; Katalinic, A.; Frielitz, F.S.; Hindricks, G. Survival of patients undergoing cardiac resynchronization therapy with or without defibrillator: The RESET-CRT project. Eur. Heart J. 2022, 43, 2591–2599. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA). Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar]
- Cho, H.; Barth, A.S.; Tomaselli, G.F. Basic science of cardiac resynchronization therapy: Molecular and electrophysiological mechanisms. Circ. Arrhythmia Electrophysiol. 2012, 5, 594–603. [Google Scholar] [CrossRef]
- Spragg, D.D.; Leclercq, C.; Loghmani, M.; Faris, O.P.; Tunin, R.S.; DiSilvestre, D.; McVeigh, E.R.; Tomaselli, G.F.; Kass, D.A. Regional alterations in protein expression in the dyssynchronous failing heart. Circulation 2003, 108, 929–932. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Wang, S.Q.; Chakir, K.; Yang, D.; Zhang, T.; Brown, J.H.; Devic, E.; Kobilka, B.K.; Cheng, H.; Xiao, R.P. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J. Clin. Investig. 2003, 111, 617–625. [Google Scholar] [CrossRef]
- Backs, J.; Song, K.; Bezprozvannaya, S.; Chang, S.; Olson, E.N. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J. Clin. Investig. 2006, 116, 1853–1864. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, T.; Bossuyt, J.; Li, X.; McKinsey, T.A.; Dedman, J.R.; Olson, E.N.; Chen, J.; Brown, J.H.; Bers, D.M. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J. Clin. Investig. 2006, 116, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Grueter, C.E.; Colbran, R.J.; Anderson, M.E. CaMKII, an emerging molecular driver for calcium homeostasis, arrhythmias, and cardiac dysfunction. J. Mol. Med. 2007, 85, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Khoo, M.S.; Wu, Y.; Yang, Y.; Grueter, C.E.; Ni, G.; Price, E.E., Jr.; Thiel, W.; Guatimosim, S.; Song, L.S.; et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat. Med. 2005, 11, 409–417. [Google Scholar] [CrossRef]
- Feldman, A.M.; Combes, A.; Wagner, D.; Kadakomi, T.; Kubota, T.; Li, Y.Y.; McTiernan, C. The role of tumor necrosis factor in the pathophysiology of heart failure. J. Am. Coll. Cardiol. 2000, 35, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Akar, F.G.; Rosenbaum, D.S. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ. Res. 2003, 93, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Beuckelmann, D.J.; Näbauer, M.; Erdmann, E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ. Res. 1993, 73, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Aiba, T.; Hesketh, G.G.; Barth, A.S.; Liu, T.; Daya, S.; Chakir, K.; Dimaano, V.L.; Abraham, T.P.; O’Rourke, B.; Akar, F.G.; et al. Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation 2009, 119, 1220–1230. [Google Scholar] [CrossRef]
- Nishijima, Y.; Sridhar, A.; Viatchenko-Karpinski, S.; Shaw, C.; Bonagura, J.D.; Abraham, W.T.; Joshi, M.S.; Bauer, J.A.; Hamlin, R.L.; Györke, S.; et al. Chronic cardiac resynchronization therapy and reverse ventricular remodeling in a model of nonischemic cardiomyopathy. Life Sci. 2007, 81, 1152–1159. [Google Scholar] [CrossRef]
- Antoniou, C.-K.; Manolakou, P.; Magkas, N.; Konstantinou, K.; Chrysohoou, C.; Dilaveris, P.; Gatzoulis, K.A.; Tousoulis, D. Cardiac Resynchronisation Therapy and Cellular Bioenergetics: Effects Beyond Chamber Mechanics. Eur. Cardiol. Rev. 2019, 14, 33–44. [Google Scholar] [CrossRef]
- Agnetti, G.; Kaludercic, N.; Kane, L.A.; Elliott, S.T.; Guo, Y.; Chakir, K.; Samantapudi, D.; Paolocci, N.; Tomaselli, G.F.; Kass, D.A.; et al. Modulation of mitochondrial proteome and improved mitochondrial function by biventricular pacing of dyssynchronous failing hearts. Circ. Cardiovasc. Genet. 2010, 3, 78–87. [Google Scholar] [CrossRef]
- Barth, A.S.; Aiba, T.; Halperin, V.; DiSilvestre, D.; Chakir, K.; Colantuoni, C.; Tunin, R.S.; Dimaano, V.L.; Yu, W.; Abraham, T.P.; et al. Cardiac resynchronization therapy corrects dyssynchrony-induced regional gene expression changes on a genomic level. Circ. Cardiovasc. Genet. 2009, 2, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Ståhlberg, M.; Rullman, E.; Pernow, J.; Nakagawa, R.; Nordin, H.; Braunschweig, F.; Ljung, K. Dyssynchrony and resynchronization in heart failure—Effects on regional and global gene expression in a murine pacemaker model. Eur. Heart J. Open 2023, 3, oead058. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, A.; Stellbrink, C.; Sack, S.; Block, M.; Vogt, J.; Bakker, P.; Mortensen, P.; Klein, H. The Pacing Therapies for Congestive Heart Failure (PATH-CHF) study: Rationale, design, and endpoints of a prospective randomized multicenter study. Am. J. Cardiol. 1999, 83, 130d–135d. [Google Scholar] [CrossRef] [PubMed]
- Linde, C.; Leclercq, C.; Rex, S.; Garrigue, S.; Lavergne, T.; Cazeau, S.; McKenna, W.; Fitzgerald, M.; Deharo, J.-C.; Alonso, C.; et al. Long-term benefits of biventricular pacing in congestive heart failure: Results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J. Am. Coll. Cardiol. 2002, 40, 111–118. [Google Scholar] [CrossRef]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Cardiac Resynchronization in Chronic Heart Failure. N. Engl. J. Med. 2002, 346, 1845–1853. [Google Scholar] [CrossRef]
- Young, J.B.; Abraham, W.T.; Smith, A.L.; Leon, A.R.; Lieberman, R.; Wilkoff, B.; Canby, R.C.; Schroeder, J.S.; Liem, L.B.; Hall, S.; et al. Combined Cardiac Resynchronization and Implantable Cardioversion Defibrillation in Advanced Chronic Heart FailureThe MIRACLE ICD Trial. JAMA 2003, 289, 2685–2694. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.L.; Hummel, J.D.; Niazi, I.K.; Giudici, M.C.; Worley, S.J.; Saxon, L.A.; Boehmer, J.P.; Higginbotham, M.B.; De Marco, T.; Foster, E.; et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J. Am. Coll. Cardiol. 2003, 42, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Kindermann, M.; Hennen, B.; Jung, J.; Geisel, J.; Böhm, M.; Fröhlig, G. Biventricular Versus Conventional Right Ventricular Stimulation for Patients With Standard Pacing Indication and Left Ventricular Dysfunction: The Homburg Biventricular Pacing Evaluation (HOBIPACE). J. Am. Coll. Cardiol. 2006, 47, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Niazi, I.; Baker, J., 2nd; Corbisiero, R.; Love, C.; Martin, D.; Sheppard, R.; Worley, S.J.; Varma, N.; Lee, K.; Tomassoni, G. Safety and Efficacy of Multipoint Pacing in Cardiac Resynchronization Therapy: The MultiPoint Pacing Trial. JACC Clin. Electrophysiol. 2017, 3, 1510–1518. [Google Scholar] [CrossRef]
- Leclercq, C.; Burri, H.; Curnis, A.; Delnoy, P.P.; Rinaldi, C.A.; Sperzel, J.; Lee, K.; Calò, L.; Vicentini, A.; Concha, J.F.; et al. Cardiac resynchronization therapy non-responder to responder conversion rate in the more response to cardiac resynchronization therapy with MultiPoint Pacing (MORE-CRTMPP) study: Results from Phase I. Eur. Heart J. 2019, 40, 2979–2987. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.K.; Dilaveris, P.; Chrysohoou, C.; Konstantinou, K.; Magkas, N.; Xydis, P.; Manolakou, P.; Skiadas, I.; Gatzoulis, K.A.; Tousoulis, D.; et al. Multipoint left ventricular pacing effects on hemodynamic parameters and functional status: HUMVEE single-arm clinical trial (NCT03189368). Hell. J. Cardiol. HJC = Hell. Kardiol. Ep. 2022, 63, 8–14. [Google Scholar] [CrossRef]
- Passafaro, F.; Rapacciuolo, A.; Ruocco, A.; Ammirati, G.; Crispo, S.; Pasceri, E.; Santarpia, G.; Mauro, C.; Esposito, G.; Indolfi, C.; et al. COMPArison of Multi-Point Pacing and ConvenTional Cardiac Resynchronization Therapy Through Noninvasive Hemodynamics Measurement: Short- and Long-Term Results of the COMPACT-MPP Study. Am. J. Cardiol. 2024, 215, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay Gaurav, A.; Vijayaraman, P.; Nayak Hemal, M.; Verma, N.; Dandamudi, G.; Sharma Parikshit, S.; Saleem, M.; Mandrola, J.; Genovese, D.; Tung, R.; et al. His Corrective Pacing or Biventricular Pacing for Cardiac Resynchronization in Heart Failure. J. Am. Coll. Cardiol. 2019, 74, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Vinther, M.; Risum, N.; Svendsen Jesper, H.; Møgelvang, R.; Philbert Berit, T. A Randomized Trial of His Pacing Versus Biventricular Pacing in Symptomatic HF Patients With Left Bundle Branch Block (His-Alternative). JACC Clin. Electrophysiol. 2021, 7, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, H.; Hou, X.; Wang, Z.; Zou, F.; Qian, Z.; Wei, Y.; Wang, X.; Zhang, L.; Li, X.; et al. Randomized Trial of Left Bundle Branch vs Biventricular Pacing for Cardiac Resynchronization Therapy. J. Am. Coll. Cardiol. 2022, 80, 1205–1216. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Pokharel, P.; Subzposh, F.A.; Oren, J.W.; Storm, R.H.; Batul, S.A.; Beer, D.A.; Hughes, G.; Leri, G.; Manganiello, M.; et al. His-Purkinje Conduction System Pacing Optimized Trial of Cardiac Resynchronization Therapy vs Biventricular Pacing. JACC Clin. Electrophysiol. 2023, 9, 2628–2638. [Google Scholar] [CrossRef]
- Jastrzębski, M.; Foley, P.; Chandrasekaran, B.; Whinnett, Z.; Vijayaraman, P.; Upadhyay, G.A.; Schaller, R.D.; Gardas, R.; Richardson, T.; Kudlik, D.A.; et al. Multicenter Hemodynamic Assessment of the LOT-CRT Strategy: When Does Combining Left Bundle Branch Pacing and Coronary Venous Pacing Enhance Resynchronization?: Primary Results of the CSPOT Study. Circ. Arrhythmia Electrophysiol. 2024, 17, e013059. [Google Scholar] [CrossRef] [PubMed]
- de Vere, F.; Wijesuriya, N.; Elliott, M.K.; Mehta, V.; Howell, S.; Bishop, M.; Strocchi, M.; Niederer, S.A.; Rinaldi, C.A. Managing arrhythmia in cardiac resynchronisation therapy. Front. Cardiovasc. Med. 2023, 10, 1211560. [Google Scholar] [CrossRef] [PubMed]
- Kies, P.; Bax, J.J.; Molhoek, S.G.; Zeppenfeld, K.; Bootsma, M.; Van Erven, L.; Van der Wall, E.E.; Shalij, M.J. Long-term follow up of CRT: Effects on arrhythmogenesis: 270 Left ventricular remodeling after cardiac resynchronization therapy reduces inducibility of ventricular tachy-arrhythmias. EP Eur. 2005, 7 (Suppl. S1), 65. [Google Scholar]
- Dilaveris, P.; Giannopoulos, G.; Synetos, A.; Aggeli, C.; Raftopoulos, L.; Arsenos, P.; Gatzoulis, K.; Stefanadis, C. Effect of biventricular pacing on ventricular repolarization and functional indices in patients with heart failure: Lack of association with arrhythmic events. EP Eur. 2009, 11, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Lehnart, S.E.; Wehrens, X.H.T. The role of junctophilin proteins in cellular function. Physiol. Rev. 2022, 102, 1211–1261. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, F.R.; MacQuaide, N.; Biesmans, L.; Sipido, K. Dyssynchrony of Ca2+ release from the sarcoplasmic reticulum as subcellular mechanism of cardiac contractile dysfunction. J. Mol. Cell. Cardiol. 2011, 50, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Sapp, J.L.; Parkash, R.; Wells, G.A.; Yetisir, E.; Gardner, M.J.; Healey, J.S.; Thibault, B.; Sterns, L.D.; Birnie, D.; Nery, P.B.; et al. Cardiac Resynchronization Therapy Reduces Ventricular Arrhythmias in Primary but Not Secondary Prophylactic Implantable Cardioverter Defibrillator Patients. Circ. Arrhythmia Electrophysiol. 2017, 10, e004875. [Google Scholar] [CrossRef]
- García-Lunar, I.; Castro-Urda, V.; Toquero-Ramos, J.; Mingo-Santos, S.; Moñivas-Palomero, V.; Daniela Mitroi, C.; Sánchez-García, M.; Pérez-Pereira, E.; Delgado, H.E.; Fernández-Lozano, I. Ventricular Arrhythmias in Super-responders to Cardiac Resynchronization Therapy. Rev. Española De Cardiol. (Engl. Ed.) 2014, 67, 883–889. [Google Scholar] [CrossRef]
- Fang, F.; Yu, C.M. Shall CRT-D be downgraded to CRT-P in super-responders of cardiac resynchronization therapy? Rev. Esp. De Cardiol. (Engl. Ed.) 2014, 67, 875–877. [Google Scholar] [CrossRef]
- Díaz-Infante, E.; Mont, L.; Leal, J.; García-Bolao, I.; Fernández-Lozano, I.; Hernández-Madrid, A.; Pérez-Castellano, N.; Sitges, M.; Pavón-Jiménez, R.; Barba, J.; et al. Predictors of lack of response to resynchronization therapy. Am. J. Cardiol. 2005, 95, 1436–1440. [Google Scholar] [CrossRef]
- Yu, C.M.; Bleeker, G.B.; Fung, J.W.; Schalij, M.J.; Zhang, Q.; van der Wall, E.E.; Chan, Y.S.; Kong, S.L.; Bax, J.J. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation 2005, 112, 1580–1586. [Google Scholar] [CrossRef]
- Varma, N.; Boehmer, J.; Bhargava, K.; Yoo, D.; Leonelli, F.; Costanzo, M.; Saxena, A.; Sun, L.; Gold, M.R.; Singh, J.; et al. Evaluation, Management, and Outcomes of Patients Poorly Responsive to Cardiac Resynchronization Device Therapy. J. Am. Coll. Cardiol. 2019, 74, 2588–2603. [Google Scholar] [CrossRef] [PubMed]
- Wouters, P.C.; Vernooy, K.; Cramer, M.J.; Prinzen, F.W.; Meine, M. Optimizing lead placement for pacing in dyssynchronous heart failure: The patient in the lead. Heart Rhythm 2021, 18, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Sieniewicz, B.J.; Gould, J.; Porter, B.; Sidhu, B.S.; Teall, T.; Webb, J.; Carr-White, G.; Rinaldi, C.A. Understanding non-response to cardiac resynchronisation therapy: Common problems and potential solutions. Heart Fail. Rev. 2019, 24, 41–54. [Google Scholar] [CrossRef]
- Rickard, J.; Michtalik, H.; Sharma, R.; Berger, Z.; Iyoha, E.; Green, A.R.; Haq, N.; Robinson, K.A. Predictors of response to cardiac resynchronization therapy: A systematic review. Int. J. Cardiol. 2016, 225, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Spartalis, M.; Tzatzaki, E.; Spartalis, E.; Damaskos, C.; Athanasiou, A.; Livanis, E.; Voudris, V. The Role of Echocardiography in the Optimization of Cardiac Resynchronization Therapy: Current Evidence and Future Perspectives. Open Cardiovasc. Med. J. 2017, 11, 133–145. [Google Scholar] [CrossRef]
- Chung, E.S.; Leon, A.R.; Tavazzi, L.; Sun, J.P.; Nihoyannopoulos, P.; Merlino, J.; Abraham, W.T.; Ghio, S.; Leclercq, C.; Bax, J.J.; et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008, 117, 2608–2616. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, E.; Alam, M.B.; Schwartzman, D.; Jain, S.; Marek, J.; Gorcsan, J.; Saba, S. Effect of echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy on mortality and risk of defibrillator therapy for ventricular arrhythmias in heart failure patients (from the Speckle Tracking Assisted Resynchronization Therapy for Electrode Region [STARTER] trial). Am. J. Cardiol. 2014, 113, 1518–1522. [Google Scholar]
- van der Bijl, P.; Khidir, M.J.H.; Leung, M.; Yilmaz, D.; Mertens, B.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Reduced left ventricular mechanical dispersion at 6 months follow-up after cardiac resynchronization therapy is associated with superior long-term outcome. Heart Rhythm 2018, 15, 1683–1689. [Google Scholar] [CrossRef]
- Bazoukis, G.; Hui, J.M.H.; Lee, Y.H.A.; Chou, O.H.I.; Sfairopoulos, D.; Vlachos, K.; Saplaouras, A.; Letsas, K.P.; Efremidis, M.; Tse, G.; et al. The role of cardiac magnetic resonance in identifying appropriate candidates for cardiac resynchronization therapy—A systematic review of the literature. Heart Fail Rev. 2022, 27, 2095–2118. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, A.D.; de las Fuentes, L.; Davila-Roman, V.G. Doppler echocardiographic methods for optimization of the atrioventricular delay during cardiac resynchronization therapy. Echocardiography 2008, 25, 1047–1055. [Google Scholar] [CrossRef]
- Auger, D.; Hoke, U.; Bax, J.J.; Boersma, E.; Delgado, V. Effect of atrioventricular and ventriculoventricular delay optimization on clinical and echocardiographic outcomes of patients treated with cardiac resynchronization therapy: A meta-analysis. Am. Heart J. 2013, 166, 20–29. [Google Scholar] [CrossRef]
- Jones, S.; Lumens, J.; Sohaib, S.M.A.; Finegold, J.A.; Kanagaratnam, P.; Tanner, M.; Duncan, E.; Moore, P.; Leyva, F.; Frenneaux, M.; et al. Cardiac resynchronization therapy: Mechanisms of action and scope for further improvement in cardiac function. Europace 2017, 19, 1178–1186. [Google Scholar] [CrossRef]
- Ellenbogen, K.A.; Gold, M.R.; Meyer, T.E.; Fernndez Lozano, I.; Mittal, S.; Waggoner, A.D.; Lemke, B.; Singh, J.P.; Spinale, F.G.; Van Eyk, J.E.; et al. Primary Results From the SmartDelay Determined AV Optimization: A Comparison to Other AV Delay Methods Used in Cardiac Resynchronization Therapy (SMART-AV) Trial. Circulation 2010, 122, 2660–2668. [Google Scholar] [CrossRef]
- Starling, R.C.; Krum, H.; Bril, S.; Tsintzos, S.I.; Rogers, T.; Hudnall, J.H.; Martin, D.O. Impact of a Novel Adaptive Optimization Algorithm on 30-Day Readmissions: Evidence From the Adaptive CRT Trial. JACC Heart Fail. 2015, 3, 565–572. [Google Scholar] [CrossRef]
- Pothineni, N.V.K.; Gondi, S.; Cherian, T.; Kovelamudi, S.; Schaller, R.D.; Lakkireddy, D.; Gopinathannair, R.; Deshmukh, A. Complications of Cardiac Resynchronization Therapy: Comparison of Safety Outcomes from Real-world Studies and Clinical Trials. J. Innov. Card. Rhythm Manag. 2022, 13, 5121–5125. [Google Scholar] [CrossRef]
- Strisciuglio, T.; Ammirati, G.; Pergola, V.; Imparato, L.; Carella, C.; Koci, E.; Chiappetti, R.; Abbate, F.G.; La Fazia, V.M.; Viggiano, A.; et al. Contrast-induced nephropathy after cardiac resynchronization therapy implant impairs the recovery of ejection fraction in responders. ESC Heart Fail. 2019, 6, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.M.; Hoste, E.A.; Braam, B.; Briguori, C.; Kellum, J.A.; McCullough, P.A.; Ronco, C. Cardiorenal syndrome type 3: Pathophysiologic and epidemiologic considerations. Contrib. Nephrol. 2013, 182, 137–157. [Google Scholar]
- Engels, E.B.; Vis, A.; van Rees, B.D.; Marcantoni, L.; Zanon, F.; Vernooy, K.; Prinzen, F.W. Improved acute haemodynamic response to cardiac resynchronization therapy using multipoint pacing cannot solely be explained by better resynchronization. J. Electrocardiol. 2018, 51, S61–S66. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.K.; Xydis, P.; Konstantinou, K.; Magkas, N.; Manolakou, P.; Dilaveris, P.; Chrysohoou, C.; Gatzoulis, K.A.; Tsioufis, C. Multipoint left ventricular pacing as an addition to cardiac resynchronization therapy: A bridge to the holy grail? Am. J. Cardiovasc. Dis. 2021, 11, 429–440. [Google Scholar]
- Sohal, M.; Shetty, A.; Niederer, S.; Lee, A.; Chen, Z.; Jackson, T.; Behar, J.M.; Claridge, S.; Bostock, J.; Hyde, E.; et al. Mechanistic insights into the benefits of multisite pacing in cardiac resynchronization therapy: The importance of electrical substrate and rate of left ventricular activation. Heart Rhythm 2015, 12, 2449–2457. [Google Scholar] [CrossRef]
- Stelzer, J.E.; Patel, J.R.; Walker, J.W.; Moss, R.L. Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation. Circ. Res. 2007, 101, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, M.; Migliore, F.; Badano, L.; Bertaglia, E.; Pedrizzetti, G.; Cavedon, S.; Zorzi, A.; Corrado, D.; Iliceto, S.; Muraru, D. Cardiac resynchronization therapy by multipoint pacing improves response of left ventricular mechanics and fluid dynamics: A three-dimensional and particle image velocimetry echo study. Europace 2017, 19, 1833–1840. [Google Scholar] [CrossRef]
- Bleeker, G.B.; Holman, E.R.; Steendijk, P.; Boersma, E.; van der Wall, E.E.; Schalij, M.J.; Bax, J.J. Cardiac resynchronization therapy in patients with a narrow QRS complex. J. Am. Coll. Cardiol. 2006, 48, 2243–2250. [Google Scholar] [CrossRef]
- Abu Sham’a, R.; Kuperstein, R.; Barsheshet, A.; Bar-Lev, D.; Luria, D.; Gurevitz, O.; Bachar, S.; Eldar, M.; Feinberg, M.; Glikson, M. The effects of anodal stimulation on electrocardiogram, left ventricular dyssynchrony, and acute haemodynamics in patients with biventricular pacemakers. EP Eur. 2011, 13, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Dilaveris, P.; Antoniou, C.K.; Manolakou, P.; Skiadas, I.; Konstantinou, K.; Magkas, N.; Xydis, P.; Chrysohoou, C.; Gatzoulis, K.; Tousoulis, D. Comparison of left ventricular and biventricular pacing: Rationale and clinical implications. Anatol. J. Cardiol. 2019, 22, 132–139. [Google Scholar] [CrossRef]
- Thibault, B.; Mondésert, B.; Cadrin-Tourigny, J.; Dubuc, M.; Macle, L.; Khairy, P. Benefits of Multisite/Multipoint Pacing to Improve Cardiac Resynchronization Therapy Response. Card. Electrophysiol. Clin. 2019, 11, 99–114. [Google Scholar] [CrossRef]
- Parreira, L.; Tsyganov, A.; Artyukhina, E.; Vernooy, K.; Tondo, C.; Adragao, P.; Ascione, C.; Carmo, P.; Carvalho, S.; Egger, M.; et al. Non-invasive three-dimensional electrical activation mapping to predict cardiac resynchronization therapy response: Site of latest left ventricular activation relative to pacing site. Europace 2023, 25, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Ellenbogen, K.A.; Auricchio, A.; Burri, H.; Gold, M.R.; Leclercq, C.; Leyva, F.; Linde, C.; Jastrzebski, M.; Prinzen, F.; Vernooy, K. The evolving state of cardiac resynchronization therapy and conduction system pacing: 25 years of research at EP Europace journal. EP Eur. 2023, 25, euad168. [Google Scholar] [CrossRef] [PubMed]
- Corbisiero, R.; Mathew, A.; Bickert, C.; Muller, D. Multipoint Pacing with Fusion-optimized Cardiac Resynchronization Therapy: Using It All to Narrow QRS Duration. J. Innov. Card. Rhythm Manag. 2021, 12, 4355–4362. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, N.; Jeyaraman, M.; Yadav, S.; Ramasubramanian, S.; Balaji, S. Revolutionizing Healthcare: The Emerging Role of Quantum Computing in Enhancing Medical Technology and Treatment. Cureus 2024, 16, e67486. [Google Scholar] [CrossRef]
- Solenov, D.; Brieler, J.; Scherrer, J.F. The Potential of Quantum Computing and Machine Learning to Advance Clinical Research and Change the Practice of Medicine. Mo. Med. 2018, 115, 463–467. [Google Scholar]
- Koopsen, T.; Gerrits, W.; van Osta, N.; van Loon, T.; Wouters, P.; Prinzen, F.W.; Vernooy, K.; Delhaas, T.; Teske, A.J.; Meine, M.; et al. Virtual pacing of a patient’s digital twin to predict left ventricular reverse remodelling after cardiac resynchronization therapy. EP Eur. 2023, 26, euae009. [Google Scholar] [CrossRef]
- Sel, K.; Osman, D.; Zare, F.; Masoumi Shahrbabak, S.; Brattain, L.; Hahn, J.O.; Pappone, C.; Ćalović, Ž.; Vicedomini, G.; Cuko, A.; et al. Building Digital Twins for Cardiovascular Health: From Principles to Clinical Impact. J. Am. Heart Assoc. 2024, 13, e031981. [Google Scholar] [CrossRef] [PubMed]
- Pappone, C.; Ćalović, Ž.; Vicedomini, G.; Cuko, A.; McSpadden, L.C.; Ryu, K.; Romano, E.; Saviano, M.; Baldi, M.; Pappone, A. Multipoint left ventricular pacing improves acute hemodynamic response assessed with pressure-volume loops in cardiac resynchronization therapy patients. Heart Rhythm. 2014, 11, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, C.A.; Leclercq, C.; Kranig, W.; Kacet, S.; Betts, T.; Bordachar, P.; Gutleben, K.J.; Shetty, A.; Donal, E.; Keel, A.; et al. Improvement in acute contractility and hemodynamics with multipoint pacing via a left ventricular quadripolar pacing lead. J. Interv. Card. Electrophysiol. Int. J. Arrhythm. Pacing 2014, 40, 75–80. [Google Scholar] [CrossRef]
- Thibault, B.; Dubuc, M.; Khairy, P.; Guerra, P.G.; Macle, L.; Rivard, L.; Roy, D.; Talajic, M.; Karst, E.; Ryu, K.; et al. Acute haemodynamic comparison of multisite and biventricular pacing with a quadripolar left ventricular lead. Europace 2013, 15, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Zanon, F.; Baracca, E.; Pastore, G.; Marcantoni, L.; Fraccaro, C.; Lanza, D.; Picariello, C.; Aggio, S.; Roncon, L.; Dell’Avvocata, F.; et al. Multipoint pacing by a left ventricular quadripolar lead improves the acute hemodynamic response to CRT compared with conventional biventricular pacing at any site. Heart Rhythm 2015, 12, 975–981. [Google Scholar] [CrossRef]
- Pappone, C.; Ćalović, Ž.; Vicedomini, G.; Cuko, A.; McSpadden, L.C.; Ryu, K.; Jordan, C.D.; Romano, E.; Baldi, M.; Saviano, M. Improving cardiac resynchronization therapy response with multipoint left ventricular pacing: Twelve-month follow-up study. Heart Rhythm 2015, 12, 1250–1258. [Google Scholar] [CrossRef]
- Leclercq, C.; Burri, H.; Curnis, A.; Delnoy, P.P.; Rinaldi, C.A.; Sperzel, J.; Lee, K.; Cohorn, C.; Thibault, B. Rationale and design of a randomized clinical trial to assess the safety and efficacy of multipoint pacing therapy: MOre REsponse on Cardiac Resynchronization Therapy with MultiPoint Pacing (MORE-CRT MPP-PHASE II). Am. Heart J. 2019, 209, 1–8. [Google Scholar] [CrossRef]
- Mehta, V.S.; Elliott, M.K.; Sidhu, B.S.; Gould, J.; Porter, B.; Niederer, S.; Rinaldi, C.A. Multipoint pacing for cardiac resynchronisation therapy in patients with heart failure: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2021, 32, 2577–2589. [Google Scholar] [CrossRef]
- Leonardo, C.; Ermenegildo, R.; Christof, K.; Amir, J.; Pedro, M.; Pascal, D.; Christelle, M.; Olivier, P.; Andrea, G.; Kwangdeok, L.; et al. Multipoint pacing is associated with improved prognosis and cardiac resynchronization therapy response: MORE-CRT MPP randomized study secondary analyses. Europace 2024, 26, euae259. [Google Scholar] [CrossRef]
- Wisnoskey, B.J.; Varma, N. Left ventricular paced activation in cardiac resynchronization therapy patients with left bundle branch block and relationship to its electrical substrate. Heart Rhythm. O2 2020, 1, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, H.; Fulati, Z.; Liu, Y.; Su, Y.; Shu, X. Left ventricular global longitudinal strain and mechanical dispersion predict response to multipoint pacing for cardiac resynchronization therapy. J. Clin. Ultrasound 2019, 47, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Wijesuriya, N.; Elliott, M.K.; Mehta, V.; De Vere, F.; Strocchi, M.; Behar, J.M.; Niederer, S.A.; Rinaldi, C.A. Pacing interventions in non-responders to cardiac resynchronization therapy. Front. Physiol. 2023, 14, 1054095. [Google Scholar] [CrossRef]
- Archontakis, S.; Sideris, K.; Laina, A.; Arsenos, P.; Paraskevopoulou, D.; Tyrovola, D.; Gatzoulis, K.; Tousoulis, D.; Tsioufis, K.; Sideris, S. His bundle pacing: A promising alternative strategy for anti-bradycardic pacing—Report of a single-center experience. Hell. J. Cardiol. 2022, 64, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.-F.; Yang, L.-C.; Zhao, Y.; Yu, Y.-C.; Liu, B.; Li, Y.-G. Effects of adaptive left bundle branch–optimized cardiac resynchronization therapy: A single centre experience. BMC Cardiovasc. Disord. 2022, 22, 360. [Google Scholar]
- Chen, X.; Ye, Y.; Wang, Z.; Jin, Q.; Qiu, Z.; Wang, J.; Qin, S.; Bai, J.; Wang, W.; Liang, Y.; et al. Cardiac resynchronization therapy via left bundle branch pacing vs. optimized biventricular pacing with adaptive algorithm in heart failure with left bundle branch block: A prospective, multi-centre, observational study. Europace 2022, 24, 807–816. [Google Scholar] [CrossRef]
- Wu, S.; Su, L.; Vijayaraman, P.; Zheng, R.; Cai, M.; Xu, L.; Shi, R.; Huang, Z.; Whinnett, Z.I.; Huang, W. Left Bundle Branch Pacing for Cardiac Resynchronization Therapy: Nonrandomized On-Treatment Comparison With His Bundle Pacing and Biventricular Pacing. Can. J. Cardiol. 2021, 37, 319–328. [Google Scholar] [CrossRef]
- Chen, X.; Jin, Q.; Qiu, Z.; Qian, C.; Liang, Y.; Wang, J.; Qin, S.; Bai, J.; Wang, W.; Chen, H.; et al. Outcomes of Upgrading to LBBP in CRT Nonresponders: A Prospective, Multicenter, Nonrandomized, Case-Control Study. JACC Clin. Electrophysiol. 2024, 10, 108–120. [Google Scholar] [CrossRef]
- Leventopoulos, G.; Travlos, C.K.; Anagnostopoulou, V.; Patrinos, P.; Papageorgiou, A.; Perperis, A.; Gale, C.P.; Gatzoulis, K.A.; Davlouros, P. Clinical Outcomes of Left Bundle Branch Area Pacing Compared with Biventricular Pacing in Patients with Heart Failure Requiring Cardiac Resynchronization Therapy: Systematic Review and Meta-Analysis. RCM 2023, 24, 312. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, S.E.; Ellenbogen, K.A.; Vijayaraman, P.; Chelu, M.G. Clinical outcomes of conduction system pacing versus biventricular pacing for cardiac resynchronization therapy: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2023, 34, 1718–1729. [Google Scholar] [CrossRef]
- Jastrzębski, M.; Moskal, P.; Huybrechts, W.; Curila, K.; Sreekumar, P.; Rademakers, L.M.; Ponnusamy, S.S.; Herweg, B.; Sharma, P.S.; Bednarek, A.; et al. Left bundle branch–optimized cardiac resynchronization therapy (LOT-CRT): Results from an international LBBAP collaborative study group. Heart Rhythm 2022, 19, 13–21. [Google Scholar] [CrossRef]
- Ribes, F.; Perez-Rosello, V.; Gunturiz-Beltran, C.; De La Cruz-Cereceda, S.; Bellver-Navarro, A. QRS narrowing in patients undergoing cardiac resynchronization therapy: LOT-CRT vs. LBBAP. EP Eur. 2024, 26 (Suppl. S1), euae102. [Google Scholar] [CrossRef]
- Boyle, P.M.; Williams, J.C.; Ambrosi, C.M.; Entcheva, E.; Trayanova, N.A. A comprehensive multiscale framework for simulating optogenetics in the heart. Nat. Commun. 2013, 4, 2370. [Google Scholar] [CrossRef]
- Zgierski-Johnston, C.M.; Ayub, S.; Fernández, M.C.; Rog-Zielinska, E.A.; Barz, F.; Paul, O.; Kohl, P.; Ruther, P. Cardiac pacing using transmural multi-LED probes in channelrhodopsin-expressing mouse hearts. Prog. Biophys. Mol. Biol. 2020, 154, 51–61. [Google Scholar] [CrossRef]
| Method | Trial/Study (Year of Publication) | Approaches Compared | Findings |
|---|---|---|---|
| BiVP | PATH-CHF [23] (1999) | Univentricular pacing vs. BiVP | Trends for improvement regarding and 6-min walking distance |
| MUSTIC [24] (2002) | BiVP vs. no pacing (sinus) BiVP vs. Univentricular (patients with Af) | 6 min walking +20% +10% LVEF +5% Mitral regurgitation improved by 45–50% | |
| MIRACLE [25] (2002) | BiVP vs. OMT | 6 min walking +30 mLVEF +4.6% | |
| COMPANION [1] (2004) | BiVP+ICD vs. BiVP vs. OMT (2:2:1 randomization) | Primary endpoint: time to death from/hospitalization for any cause BiVP+ICD 0.8 vs. OMT BiVP 0.81 vs. OMT ICD presence had no impact on the endpoint of death from/hospitalization for heart failure. | |
| MIRACLE-ICD [26] (2003) | BiVP+ICD vs. ICD | BiVP favorably affected quality of life, functional status, and exercise capacity—yet no significant difference in LV function or survival | |
| CONTAK-CD [27] (2003) | BiVP+ICD vs. ICD | 6 min walking +20 m +0.8 mL/kg/min LVEF +2.3% | |
| CARE-HF [2] (2005) | BiVP vs. OMT | Primary endpoint: all-cause death/CV hospitalization HR 0.63 favoring BiVP. Regarding all-cause death specifically HR 0.64 favoring BiVP | |
| HOBIPACE [28] (2006) | BiVP vs. Univentricular pacing (pacing indicated and LV dysfunction) | Favorable effects of BiVP on LV dimensions, ejection fraction, NT-proBNP levels, and functional status | |
| RAFT [4] (2010) | BiVP+ICD vs. ICD | Primary endpoint: all-cause death, CV hospitalization HR 0.75 favoring BiVP. Regarding all-cause death HR 0.75 also favors BiVP group | |
| MPP | IRON-MPP (2017) | Registry MPP vs. BiVP | MPP Absolute LVEF increased +4.4% compared to BiVP. Greater benefit if nonischemic cardiomyopathy or QRS > 150 ms |
| MPP [29] (2017) | MPP noninferiority to BiVP | MPP non-inferior to BiVP Establishment of anatomical separation of MPP dipoles as part of programming | |
| MORE-MPP [30] (2019) | MPP in BiVP non-responders | No significant difference—except in those with >97% pacing (subsequent analysis—see text) | |
| HUMVEE [31] (2022) | Echocardiographically optimized MPP vs. BiVP | Absolute LVEF +4% in MPP Improvement in 6-min walking distance. Significant attrition rate regarding presence of suitable dipoles for MPP may have affected results. | |
| COMPACT-MPP [32] (2024) | MPP vs. BiVP—nonrandomized, method selected based on noninvasive hemodynamic parameter improvement | Improved survival in MPP recipients at 3 years—however, there was no randomization | |
| CS-CRT | HIS-SYNC [33] (2019) | HB-CRT vs. BiVP | Larger QRS duration shortening, trends toward LVEF improvement |
| HIS-ALTERNATIVE [34] (2021) | HB-CRT vs. BiVP | Similar benefits but in the per protocol analysis absolute LVEF +6% in HB-CRT compared to BiVP | |
| LBBP-RESYNC [35] (2022) | LB-CRT vs. BiVP | Absolute LVEF +5.6% in HB-CRT compared to BiVP. Greater NTproBNP reductionSimilar clinical effects | |
| HOT-CRT [36] (2023) | HB/LB-CRT vs. BiVP | At 6 months significantly greater relative increase in LVEF in the CSP-CRT group—12.4% vs. 8%—no higher complication rates | |
| CSPOT [37] (2024) | LBBAP vs. LB-CRT vs. BiVP | Similar hemodynamic effects between LB-CRT and BiVP, superior to those of LBBAP. LB-CRT exhibited the largest QRS duration shortening. LB-CRT superior to LBBAP in those with QRS duration ≥171 ms |
| Feature | BiVP | MPP | CS-CRT |
|---|---|---|---|
| Clearly defined target population | Yes—based on landmark trials | No—a single criterion (electrical dispersion) is currently available for suggesting MPP additional benefit | Indications similar to CRT—there is a trend towards preferential implantation in those with textbook conduction deficits; however, benefits extend to non-specific abnormalities as well |
| Complexity of implantation | Identical | Simpler, especially for LB-CRT | |
| Maintenance of suitable dipole presence | Most often | Attrition rate of up to 20% | Almost always in LB-CRT Most often in HB-CRT |
| Simplicity of programming | Average | Complex | Simple |
| Acute and long-term effects | Both MPP and CP-CRT appear to perform better than BiVP, especially when applied in most suitable cases—i.e., pronounced LV electrical dispersion and LBBB, respectively. H/LOT-CRT has been shown to be superior to conventional BiVP in terms of responder rates and QRS shortening, with 2 meta-analyses suggesting a survival benefit as well. | ||
| Pairing with additional modalities (Multi-fusion, hybrid approaches) | Feasible in all—however, LB-CRT has to be delivered in the bipolar configuration in the presence of a defibrillator, potentially decreasing its efficacy. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoniou, C.-K.; Chrysohoou, C.; Manolakou, P.; Tsiachris, D.; Kordalis, A.; Tsioufis, K.; Gatzoulis, K.A. Multipoint Left Ventricular Pacing as Alternative Approach in Cases of Biventricular Pacing Failure. J. Clin. Med. 2025, 14, 1065. https://doi.org/10.3390/jcm14041065
Antoniou C-K, Chrysohoou C, Manolakou P, Tsiachris D, Kordalis A, Tsioufis K, Gatzoulis KA. Multipoint Left Ventricular Pacing as Alternative Approach in Cases of Biventricular Pacing Failure. Journal of Clinical Medicine. 2025; 14(4):1065. https://doi.org/10.3390/jcm14041065
Chicago/Turabian StyleAntoniou, Christos-Konstantinos, Christina Chrysohoou, Panagiota Manolakou, Dimitrios Tsiachris, Athanasios Kordalis, Konstantinos Tsioufis, and Konstantinos A. Gatzoulis. 2025. "Multipoint Left Ventricular Pacing as Alternative Approach in Cases of Biventricular Pacing Failure" Journal of Clinical Medicine 14, no. 4: 1065. https://doi.org/10.3390/jcm14041065
APA StyleAntoniou, C.-K., Chrysohoou, C., Manolakou, P., Tsiachris, D., Kordalis, A., Tsioufis, K., & Gatzoulis, K. A. (2025). Multipoint Left Ventricular Pacing as Alternative Approach in Cases of Biventricular Pacing Failure. Journal of Clinical Medicine, 14(4), 1065. https://doi.org/10.3390/jcm14041065








