Efficacy of Beauveria bassiana and Mechanical Traps for the Control of Aclees taiwanensis (Coleoptera: Curculionidae) in Fig Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Semi-Field Trials in Entomological Cages
2.2. Field Trials
2.3. Data Analysis
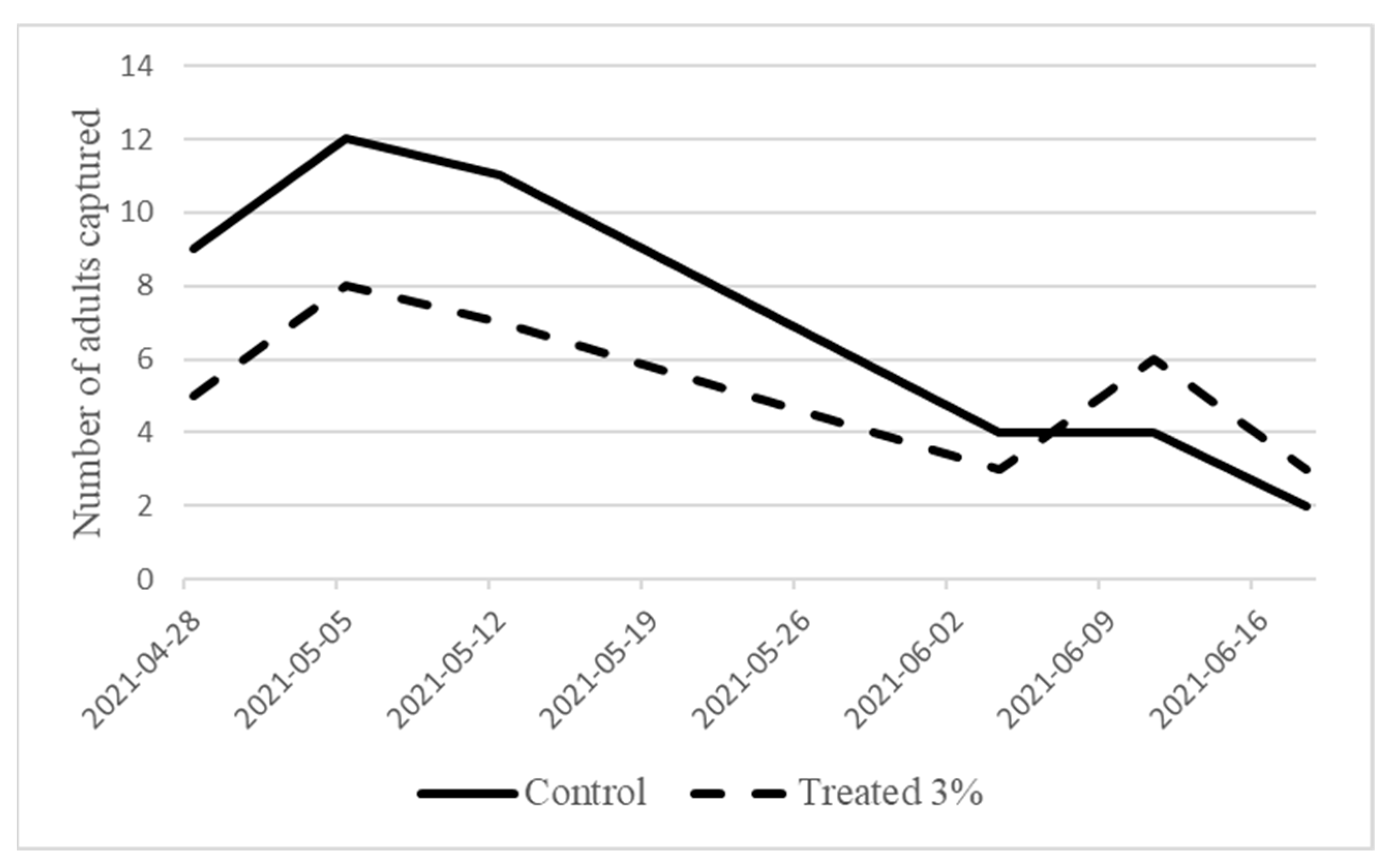
3. Results
3.1. Semi-Field Trials in Entomological Cages
3.2. Field Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sportelli, G.F. Fico, Un’antica Pianta per Una Moderna Industria. Riv. Fruttic. Ortofloric. 2021. Available online: https://rivistafrutticoltura.edagricole.it/ (accessed on 20 October 2023).
- Nuzzo, V.; Gatto, A.; Montanaro, G. Morphological Characterization of Some Local Varieties of Fig (Ficus carica L.) Cultivated in Southern Italy. Sustainability 2022, 14, 15970. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Gozlan, R.E.; Vaissière, A.C.; Assailly, C.; Nuninger, L.; Roiz, D.; Jourdain, F.; Jari’c, I.; Courchamp, F. InvaCost, a public database of the economic costs of biological invasions worldwide. Sci. Data 2020, 7, 277. [Google Scholar] [CrossRef] [PubMed]
- Ciampolini, M.; Regalin, R.; Farnesi, I.; Lorenzi, C. Prime osservazioni sulla bio-etologia di Aclees sp. (Curculionidae, Molytinae) esiziale a Ficus carica L. in Italia. Boll. Zool. Agrar. Bachic. 2007, 39, 51–60. [Google Scholar]
- Benelli, G.; Meregalli, M.; Canale, A. Field Observations on the Mating Behavior of Aclees sp. cf. foveatus Voss (Coleoptera: Curculionidae), an Exotic Pest Noxious to Fig Orchards. J. Insect. Behav. 2014, 27, 419–427. [Google Scholar] [CrossRef]
- Meregalli, M.; Boriani, M.; Taddei, A.; Hsu, C.F.; Tseng, W.Z.; Mouttet, R. A new species of Aclees from Taiwan with notes on other species of the genus. (Coleoptera: Curculionidae: Molytinae). Zootaxa 2020, 4868, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Farina, P.; Mazza, G.; Benvenuti, C.; Cutino, I.; Giannotti, P.; Conti, B.; Bedini, S.; Gargani, E. Biological notes and distribution in Southern Europe of Aclees taiwanensis Kono, 1933 (Coleoptera: Curculionidae): A new pest of the fig tree. Insects 2021, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Malossini, G.; Bernardinelli, I.; Noacco, A. Prima segnalazione del punteruolo nero del fico in Friuli Venezia Giulia. Notiziario ERSA 2021, 3, 42–45. [Google Scholar]
- Crivelli, L. Non si Perda Altro Tempo con il Punteruolo del Fico. Terra e Vita 2022. Available online: https://terraevita.edagricole.it/agrofarmaci-difesa/non-si-perda-altro-tempo-con-il-punteruolo-del-fico/ (accessed on 20 October 2023).
- Lo Cascio, P.; Altadonna, G.; Ponel, P. Diversity and distribution of beetles in a Mediterranean volcanic archipelago: An updated checklist of the Coleoptera of the Aeolian Islands (Sicily, Italy). Biodivers. J. 2022, 13, 531–585. [Google Scholar] [CrossRef]
- Mouttet, R.; Haran, J.; Boriani, M.; Meregalli, M.; Taddei, A.; Panchaud, K.; Vernier, F.; Streito, J.C. Aclees sp. ravageur des Figuiers établi en France métropolitaine (Coleoptera Curculionidae). L’Entomologiste 2020, 76, 65–68. [Google Scholar]
- Hong, K.J.; Park, D.K.; Lee, S.M. First report of the exotic fig weevil, Aclees taiwanensis Kino (Coleoptera: Curculionidae) in Korea. Korean J. Appl. Entomol. 2020, 59, 277–280. [Google Scholar]
- Tani, C.; Conti, B.; Bedini, S. Biological Insights on the Invasive Fig Pest Aclees taiwanensis Kono, 1933 (Coleoptera: Curculionidae). Insects 2023, 14, 223. [Google Scholar] [CrossRef]
- Ciampolini, M.; Farnesi, I.; Scarselli, F.; Lorenzi, C. Contro il curculionide del fico decisiva la lotta agli adulti. Inf. Agrar. 2008, 64, 57. [Google Scholar]
- Gargani, E.; Simoni, S.; Benvenuti, C.; Frosinini, R.; Barzanti, G.P.; Roversi, P.F.; Caselli, A.; Guidotti, M. Aclees cf. sp. foveatus (Coleoptera Curculionidae), an exotic pest of Ficus carica in Italy: A sustainable approach to defence based on aluminosilicate minerals as host plant masking solids. Redia 2018, 101, 201–205. [Google Scholar] [CrossRef]
- Lodolini, E.M.; Nolasco, A.; Cutino, I.; Gargan, E. Use of a commercial organic product to control the black weevil (Aclees sp. cf. foveatus) of the fig tree. Redia 2020, 103, 29–34. [Google Scholar] [CrossRef]
- Gargani, E.; Cutino, I.; Barzanti, G.P.; Benvenuti, C.; Lodolini, E.M.; Nolasco, A.; Caboni, E.; Macchioni, V.; Carbone, K. The black weevil (Aclees sp. cf. foveatus) of the fig tree: Control trials with plant extracts. Acta Hortic. 2021, 1310, 255–260. [Google Scholar] [CrossRef]
- Gargani, E.; Mazza, G.; Benvenuti, C.; Torrini, G.; Strangi, A.; Pennacchio, F.; Roversi, P.F. Biological control of Aclees sp. cf. foveatus and first recovery of an associate Beauveria bassiana strain. Redia 2016, 99, 29–33. [Google Scholar] [CrossRef]
- Gargani, E.; Benvenuti, C. Il Punteruolo Nero del fico: Un’emergenza in Toscana; si Cercano Soluzioni. Riv. Agraria. 2017. Available online: https://www.rivistadiagraria.org/articoli/anno-2017/punteruolo-nero-del-fico-unemergenza-toscana-si-cercano-soluzioni/?print=print (accessed on 20 October 2023).
- Gargani, E.; Barzanti, G.P.; Strangi, A.; Mazza, G.; Benvenuti, C.; Frosinini, R.; Roversi, P.F.; Cutino, I. Aclees sp. cf. foveatus, a real threat to Ficus carica in the Mediterranean area. Acta Hortic. 2021, 1310, 243–250. [Google Scholar] [CrossRef]
- Mwamburi, L.A.; Laing, M.D.; Miller, R.M. Effect of surfactants and temperature on germination and vegetative growth of Beauveria bassiana. Braz. J. Microbiol. 2015, 46, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, C.; Gargani, E.; Mazza, G.; Cutino, I. Utilizzo di Beauveria bassiana per il controllo del punteruolo nero del fico, Aclees taiwanensis. In Proceedings of the Atti Giornate Fitopatologiche, Bologna, Italy, 21–24 June 2022; Volume 1, pp. 125–132. [Google Scholar]
- Zimmerman, G. The ‘Galleria bait method’ for detection of entomopathogenic fungi in soil. J. Appl. Entomol. 1986, 102, 213–215. [Google Scholar] [CrossRef]
- Llacer, E.; Santiago-Alvarez, C.; Jacas, J.A. Could sterile males be used to vector a microbiological control agent? The case of Rhynchophorus ferrugineus and Beauveria bassiana. Bull. Entomol. Res. 2013, 103, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Skinner, M.; Brownbridge, M.; Parker, B.L. Characterization of Beauveria bassiana and Metarhizium anisopliae isolates for management of tarnished plant bug, Lygus lineolaris (Hemiptera:Miridae). J. Invertebr. Pathol. 2003, 82, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.E.; Meyling, N.V.; Luangsa-ard, J.J.; Blackwell, M. 6. Fungal entomopathogens, Environmental Factors Influencing Stability. In Insect Pathology, 2nd ed.; Vega, F.E., Kaya, H.K., Eds.; Elsevier: London, UK, 2012; pp. 188–189. ISBN 978-0-12-384984-7. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Falco, R.; Milella, G. Applicazione di fasce di fibre sintetiche per la cattura massima dell’oziorrinco dell’olivo (Otiorhynchus Cribricollis GYLL.). In Proceedings of the Atti Giornate Fitopatologiche, Perugia, Italia, 16–20 April 2000; Volume 1, pp. 407–412. [Google Scholar]
- Shapiro-Ilan, D.I.; Gardner, W.A.; Cottrell, T.E.; Behle, R.W.; Wood, B.W. Comparison of Application Methods for Suppressing the Pecan Weevil (Coleoptera: Curculionidae) with Beauveria bassiana Under Field Conditions. Environ. Entomol. 2008, 37, 162–171. [Google Scholar] [CrossRef] [PubMed]
- El-Sufty, R.A.; Awash, S.A.; Al-Bgham, S.; Shahdad, A.S.; Al-Bathra, A.H. Pathogenicity of the Fungs Beauveria bassiana (Bals.) Vuill to the Red Palm Weevil, Rhynchophorus ferrugineus (Oilv.) (Col.: Curculionidae) under Laboratory and Field Conditions. EJBPC 2009, 19, 81–85. [Google Scholar]
- Iovinella, I.; Pierattini, E.C.; Bedini, S.; Dani, F.R.; Guarino, S.; Lucchi, A.; Giannotti, P.; Cuzzupoli, G.; Girardi, J.; Conti, B. Semiochemicals for intraspecific communication of the fig weevil Aclees sp. cf. foveatus (Coleoptera:Curculionidae): A first survey. Sci. Rep. 2020, 10, 1092. [Google Scholar] [CrossRef]
- Pereault, R.J.; Whalon, M.E.; Alston, D.G. Field Efficacy of Entomopathogenic Fungi and Nematodes Targeting Caged Last-Instar Plum Curculio (Coleoptera: Curculionidae) in Michigan Cherry and Apple Orchards. Environmen. Entomol. 2009, 38, 1126–1134. [Google Scholar] [CrossRef]
- Tinzaara, T.; Gold, C.S.; Dicke, M.; Van Huis, A.; Nankinga, C.M.; Godfrey, H.; Kagezi, G.H.; Ragama, P.E. The use of aggregation pheromone to enhance dissemination of Beauveria bassiana for the control of the banana weevil in Uganda. Biocontrol Sci. Technol. 2007, 17, 111–124. [Google Scholar] [CrossRef]
- Tinzaara, W.; Emudong, P.; Nankinga, C.; Tushemereirwe, W.; Kagezi, G.; Gold, C.S.; Dicke, M.; Van Huis, A.; Karamura, E. Enhancing dissemination of Beauveria bassiana with host plant base incision trap for the management of the banana weevil Cosmopolites sordidus. Acad. J. 2015, 10, 3878–3884. [Google Scholar] [CrossRef]
- Inglis, G.D.; Goettel, M.S.; Johnson, D.L. Influence of Ultraviolet Light Protectants on Persistence of the Entomopathogenic Fungus, Beauveria bassiana. Biol. Control 1995, 5, 581–590. [Google Scholar] [CrossRef]
- Sabbahi, R.; Merzouki, A.; Guertin, C. Potential Effect of Beauveria Bassiana (Hypocreales: Clavicipitaceae) on Anthonomus Signatus (Coleoptera: Curculionidae) in Strawberries. Biocontrol Sci. Technol. 2009, 19, 729–741. [Google Scholar] [CrossRef]

| Plot | Adults Captured | Total Dead Adults | Adults Killed by B. bassiana |
|---|---|---|---|
| Control | 42 | 10 | 7 |
| Treated | 32 | 14 | 10 |
| Total | 74 | 24 | 17 |
| Spore Concentration | Germination | |
|---|---|---|
| T0 | 1.8 × 107 ± 4.6 × 106 a | 40.33 ± 2.4 a |
| T1 | 8.3 × 106 ± 1.9 × 106 a | 23.33 ± 0.9 b |
| T2 | 7.4 × 106 ± 1.6 × 106 a | 23.33 ± 1.20 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutino, I.; Benvenuti, C.; Mazza, G.; Conti, B.; Marraccini, D.; Gargani, E. Efficacy of Beauveria bassiana and Mechanical Traps for the Control of Aclees taiwanensis (Coleoptera: Curculionidae) in Fig Plants. Agriculture 2023, 13, 2050. https://doi.org/10.3390/agriculture13112050
Cutino I, Benvenuti C, Mazza G, Conti B, Marraccini D, Gargani E. Efficacy of Beauveria bassiana and Mechanical Traps for the Control of Aclees taiwanensis (Coleoptera: Curculionidae) in Fig Plants. Agriculture. 2023; 13(11):2050. https://doi.org/10.3390/agriculture13112050
Chicago/Turabian StyleCutino, Ilaria, Claudia Benvenuti, Giuseppe Mazza, Barbara Conti, Daniele Marraccini, and Elisabetta Gargani. 2023. "Efficacy of Beauveria bassiana and Mechanical Traps for the Control of Aclees taiwanensis (Coleoptera: Curculionidae) in Fig Plants" Agriculture 13, no. 11: 2050. https://doi.org/10.3390/agriculture13112050
APA StyleCutino, I., Benvenuti, C., Mazza, G., Conti, B., Marraccini, D., & Gargani, E. (2023). Efficacy of Beauveria bassiana and Mechanical Traps for the Control of Aclees taiwanensis (Coleoptera: Curculionidae) in Fig Plants. Agriculture, 13(11), 2050. https://doi.org/10.3390/agriculture13112050










