Abstract
Intensive soil tillage and the resulting erosion constitutes one of the main problems in olive growing. Palliative practices such as implementing vegetable covers are encouraged. Recently, the method of adding inert green roofs to the soil, either alone or in combination with adventitious vegetation, has stood out. Assessing this agronomic measure is our main objective. This research was conducted in 2022 in the Jaén province (southern Spain), wherein olive groves with different managements were selected: (i) conventional, free from vegetation, (ii) ecologically managed olive grove containing a cover of adventitious vegetation (VC), and (iii) ecologically managed olive grove in which the remains of pruning are added to the adventitious cover (VC-MIX). Vegetation inventories and beneficial insect monitoring were performed using a combined device with chromatic and olfactory trapping. The olive moth (Prays oleae) and lacewing predators were selected as the indicator species. Both the beneficial insect diversity and relative plant abundance were higher in the VC-MIX, where the highest rates of predation by lacewings were found (88%). In turn, these parameters in the VC olive surpassed those of the conventional olive grove. The factors involved in the notable differences between the three management types are discussed.
1. Introduction
Olive groves are one of the main woody crops in Spain, with approximately 2.75 million hectares being dedicated to this crop [1]. The largest extension (1.6 million hectares) is in the Andalusian region (southern Spain). Most olive oil production occurs in the province of Jaén, with an average annual yield of around half a million tons [2].
In the Mediterranean area, between 40 and 60 species of phytophagous arthropods have been recorded as almost permanently residing in olive groves [3,4,5]. However, there is a more diverse community of beneficial species that are closely associated with this entomofauna, whose predatory or parasitic activity contributes to the natural control of the phytophagous species; therefore, they must be protected, despite the fact that their action is still little known to farmers. Controlling pests in olive growing is based on applying synthetic pesticides, which have generated progressively impoverished animal communities, notably affecting the natural enemy community [6,7]. Other consequences include the appearance of new emerging pests and lineages resistant to conventional insecticides appearing [7,8,9,10,11]. In parallel, traditional intensive tillage practices have had a very negative impact on flora and fauna diversity [12], thus affecting the soil fertility index [13]. Suppressing plant communities has impoverished the communities of predators and parasitoids, which has led to a reduction in entomophagous activity, an increase in pest populations, and a progressive dependence on synthetic pesticides. In addition, traditional growing involves eradicating the herbaceous plant cover, leaving the soil unprotected against rainfall-induced erosion [14,15,16] and causing significant economic damage to farms and in adjacent areas [17]. To mitigate the effects of erosion, plant cover has been implemented, either by allowing spontaneous herbaceous growth or by artificial planting [18]. However, farmers highlight plant cover’s most controversial facets, such as how it competes with crops for water and nutrients, which is especially intense during water-scarce periods (from mid-spring to early autumn), and the short duration of its protective effect [19], thus prompting many farmers to question its beneficial effect. To mitigate this effect, the herbaceous cover is periodically mowed mechanically, and the resulting residue is added in situ, forming a layer that prevents excessive evapotranspiration during the summer and protects the soil from erosion. A similar practice consists of incorporating the remains from the olive grove pruning, which is previously crushed, into adventitious vegetation. These remains are composed of fine branches (usually less than 5 cm in diameter) and leaves [20]. Traditionally, these pruning residues have been burned in situ, which also provides agronomic benefits in the following harvests. However, it has been pointed out that this management involves environmental drawbacks derived from the emission of polluting particles and gases into the atmosphere, which increase the greenhouse effects and affect public health [21,22,23].
Since the effect of these new agronomic practices on beneficial entomofauna has not been assessed yet, the main objective of this study is to provide data in this regard, particularly regarding their repercussions for communities of natural enemies of pests, paying special attention to the predatory efficacy of the olive moth, Prays oleae Bern, by monitoring green lacewings (Neuroptera: Chrysopidae), as their main natural predators [3,24,25].
2. Materials and Methods
2.1. Description of the Study Area
This study was carried out in olive groves in the province of Jaén (Andalusia, southern Spain) during the spring of 2022. The specific area belongs to the olive groves located in the farm “Virgen de los Milagros”, in the municipality of Mancha Real (Figure 1). Most of the olive trees are between 80 and 100 years old and belong to the Picual variety, cultivated under irrigation and planted in a frame of 10 m × 10 m. In this olive grove, there are two large areas (Figure 1) that are characterized by different types of agricultural farming.
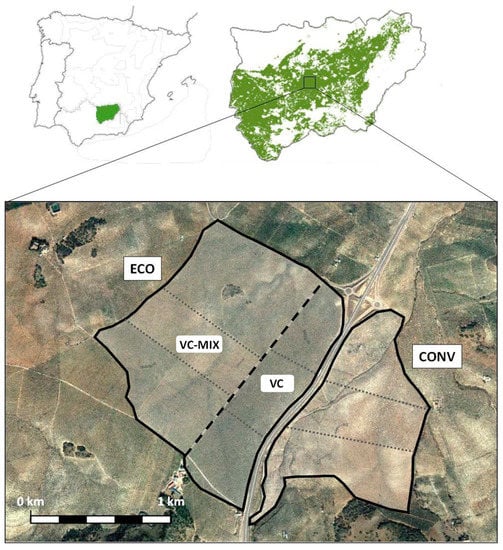
Figure 1.
Location of the study area in the province of Jaén (Southern Spain). Olive grove with conventional management (CONV); olive grove with ecological management (ECO), including two large extensions: Olive grove with mixed cover (VC-MIX) and olive grove with herbaceous cover (VC). By dotted lines, the three blocks in each olive grove are delimited. Source: Own elaboration, using the Google Earth Pro geographic information system.
2.1.1. Olive Groves with Ecological Farming (VC-MIX and VC)
This olive grove has an extension of 238 ha, with the coordinates 37°52′12.55″ N, 3°34′03.33″ W, an altitude of approximately 500 m, and a planting frame of 10 × 10 m. It receives edaphic fertilization through the application, twice a year, of organic and mineral nutrients. In addition, annual foliar fertilization is applied using crystalline urea (nitrogen content 46%), potassium sulfate, and natural amino acids (arginine, glycine, threonine, and proline). In this olive grove, the development of a spontaneous and homogeneous vegetal cover is promoted in a controlled way. The pruning of three olives is carried out by sectors at the end of winter, with all the olive trees being pruned over three or four years.
In this olive grove, two relatively large sub-areas were differentiated (Figure 1) depending on the fate of the pruning residues: (i) in one of these (VC-MIX), with an extension of 163 ha, the finer pruning remains (terminal shoots and leaves) are crushed in situ and the resulting plant material is spread along the central band of the interlines (or streets); (ii) a second subzone (VC) has an extension of 75 ha. In this area, the fine remains are burned.
Regarding pest and disease control, no synthetic chemical products are applied in the organic olive groves, in combination with specific capture devices (McPhail traps, pheromone-baited delta traps, and chromotropic traps). The adventitious vegetation is mechanically controlled by clearing between March and April using a hammer tractor. The resulting plant remains are crushed and spread along the inter lines. To avoid the over-accumulation of plant debris during and reduce the fire risk at the end of spring, a pass is carried out using a tractor equipped with a paddle brush cutter. Its function is to crush the dry remains of the plant cover into very small fragments, reducing the remaining residues to become fragments of a few millimeters, which are scattered on the olive grove’s surface. This results in a protective vegetable layer composed of small elements, the compaction of which makes considerable reduction of its thickness possible.
2.1.2. Olive Grove with Conventional Farming
A 150 ha extension corresponds to the olive trees with conventional farming (Figure 1). In this olive grove, the trees belong to the Picual variety, with a plantation frame of 10 × 10 m. The soil fertilization is performed by applying organic and mineral nutrients twice a year. In addition, the foliar fertilization is carried out annually with crystalline urea (46% nitrogen content), potassium sulfate, and natural amino acids (arginine, glycine, threonine, and proline). To control weeds, intense tillage is carried out by plowing and using the herbicide glyphosate (Roundup UltraPlus© 500mL, Monsanto, St. Louis, MO, USA). For pest control, the most widely used insecticide is Spinetoram-25% (Spintor® 480 SC, Dow AgroSciences, Indianapolis, IN, USA) for controlling the olive moth (Prays oleae) and the olive fly, Bactrocera oleae (Rossi, 1790) (Diptera: Tephritidae). To control the branch borer, Euzophera pinguis (Haworth, 1811) (Lepidoptera: Pyralidae), Chlorpyrifos© 48% (IRAC Group 1B) is applied.
For pathogen control, a fungicide application containing Agrofit ® (Agrocobre 50 colourless, Agroterra) is created as a wettable powder formulation that is 50% copper for use against the olive peacock spot (Spilocaea oleagina = Cycloconium oleaginum).
2.2. Experimental Design
As shown in Figure 1 and Figure 2, in each of the considered olive groves (conventional, VC, and VC-MIX olive groves), three blocks were delimited that correspond to the three series of sample repetitions.
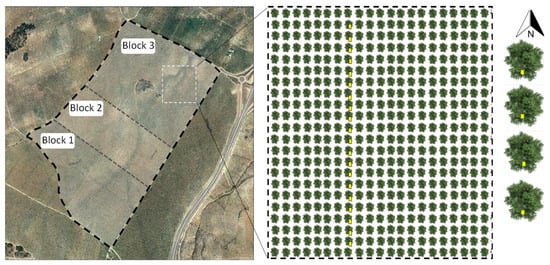
Figure 2.
Aerial view of the VC olive grove, with broken lines indicating the three blocks into which it has been divided. The enlarged box shows the linear arrangement of the chromatic traps along a line of 20 olive trees. On the right, detail of the orientation of the traps. Source: Own elaboration using the Google Earth Pro geographic information system.
2.2.1. Entomological Sampling
Sticky yellow traps: This type of sampling, which is based on how arthropods move toward the traps through chromatic attraction, has shown good results and is appropriate for replication [26,27,28,29].
The traps have been installed in 20 olive trees in each block (Figure 2), so the total number is 60 in each of the three olive groves. These were placed at a height of 1.5 m in the southern sector of each olive tree. Since their placement, the traps have been replaced every 10 days, therefore resulting in the following sampling intervals: 10–20 May; 20–30 May; 30 May–9 June; and 9–19 June.
After their replacement, the traps were temporarily stored in a cold room (4 °C). During each sampling interval, a subsample of six traps from each block was randomly selected, in order to minimize the effects attributable to pseudo-replication.
Olfactory traps: The diversity of lacewings in the study area was sampled through entomological capture nets, which allowed us to obtain the preliminary data on general lacewing species and the particular carnea-complex species [30]. For this study, in addition to the previously described sampling using chromatic stimulation, a second type of traps were used, the McPhail type, which are baited with an aqueous solution of diammonium phosphate (4% w/v). This type of sampling allows for obtaining complementary data, capturing mainly females, unlike the sticky yellow traps that predominantly capture males. The McPhail traps were installed in the three blocks of the different olive groves (conventional, VC, and VC-MIX), which were conveniently away from the yellow traps (at a distance greater than 100 m) and with a minimum distance of at least at 30 m between them in each block. There were two units for each olive grove block; thus, the total number of traps was six for each type of olive grove, with a total of 18 traps. The McPhail traps were installed on 1 June, with renovations being performed at 10-day intervals. For the insect collection, a sieve was used, which allowed captured individuals to be retained. They were subsequently moved, using a brush, into a glass vial containing an aqueous mixture of 70% ethanol. In the laboratory, the captured individuals were taxonomically determined, and the capture numbers for each trap unit were recorded.
2.2.2. Sampling of the Vegetation (VC; VC-MIX Olive Groves)
The study of the herbaceous diversity and abundance in the green roofs was carried out during May 2022. For each of these, five samples were collected. Prior to each sampling, in each of the two olive groves with vegetation cover, the block to be sampled was randomly established. Within each selected block, an olive tree from the central area was chosen at random, and using a flexometer, a 5 m long transect was established, extending in an east–west direction at a distance approximately 3 m from the trunk of the selected olive tree.
To facilitate the data collection, the transect was divided into 5 cm segments, with a total of 20 segments per transect. For each segment, the number of contacts of each plant taxa with the flexometer was recorded, and if there was more than one contact by the same taxon, the total number of contacts was the recorded contacts. Since the flexometer represents an invisible barrier that is perpendicular to the ground, to consider the plants that do not contact the flexometer in their basal area but cross the barrier at a certain height, 25 cm long wooden rods were used. These being nailed to the ground along the margin of the flexometer enabled us to visualize the contacts’ height.
The samples were taxonomically determined in the laboratory in order to obtain the total number of contacts for each of the species.
2.2.3. Estimation of Oophagous Predation (VC, VC-MIX, and CONV Olive Groves)
Among the pests that affect the olive tree, the olive moth, Prays oleae, was selected. In this species, the critical stage in terms of the economic damage is the beginning of the carpophagous generation, i.e., egg stage [31]. Since green lacewings (Neuroptera: Chrysopidae) are their main oophagous predators, they were our focus. Periodic fruit sampling was carried out from the beginning of the phenological olive stage of fruiting (stage G, May, 20–25) at 10-day intervals. For this process, on each sampling date and in each of the blocks of the three olive groves, an olive tree was chosen at random, and correspondingly, to a minimum of 100 fruits were collected. Three olive trees from each of the three blocks for each type of management were sampled at each sampling interval; thus, the total number of olive trees sampled was nine on each sampling date. Since the female of P. oleae preferentially oviposits in the calyx of the olive, the fruits were retained using scissors, cutting into the upper part of the olives’ peduncles. Subsequently, fruits were placed into plastic containers and kept temporarily stored in a cold room (t < 4 °C). The olives were observed using a stereomicroscope (Leica, M205C, Wetzlar, Germany), noting the number of P. oleae eggs on each of them and differentiating between live, predated, and hatched eggs [3,32]. Once all the fruits were examined, the following parameters were calculated for each olive grove on each sampling date:
- Population index: The total number of eggs/100 fruits. This density value reflects the relative density of the ovideponent females in the cultivated area.
- Potential attack: The number of fruits with any type of eggs (live, predated, or hatched) × 100/total number of fruits observed. This represents a value equivalent to the fruit drop that P. oleae would cause in the case of the total absence of oophagous predation activity.
- Hatching rate: The number of hatched eggs × 100/(sum of live and hatched eggs).
- Predation rate: The number of predated eggs/(sum of live eggs + predated + hatched). This allows for assessment of the predatory activity using an index of the activity of predatory eggs.
- Final attack: The number of fruits that contain at least one hatched egg × 100/(number of fruits observed). Given that the loss of fruit caused by P. oleae is exclusively due to the larvae emerging inside the attacked fruits once their larval development is complete, only hatched eggs were considered for its calculation. Therefore, the latter may have survived the predatory action of the natural enemies. For calculating this value, therefore, the live eggs were not accounted for, since these are likely to hatch or be predated. The entomophagous action allows for discarding a proportion of the population of P. oleae eggs; however, in the opposite case, their hatching results in establishing the larva in the endocarp of the fruit and its subsequent fall. The final attack describes the magnitude of this drop, providing a realistic estimation of the impact of P. oleae on the harvest.
- Fruit recovery: The number of fruits in which all the eggs have been predated × 100/(number of fruits that have contained eggs). This parameter indicates predation’s real effectiveness, since it corresponds to the percentage of fruits in which all the eggs have been predated. Once all the P. oleae eggs were eliminated, fruits were considered recovered, so, for practical purposes, the recovery percentage is a parameter that indicates the oophagous predation’s real effectiveness through the lacewings. Importantly, this value does not always correspond to the predation value, except in the cases in which the number of fruits attacked present infestation densities equal to 1 egg/fruit.
2.2.4. Statistical Analysis
For the statistical analysis, the Statgraphics Centurion XVIII package was used. The normality of the distributions was checked using the Shapiro–Wilk and Kolmogorov–Smirnov (K-S, with Liliefors correction) tests. Levene’s test was applied to verify the homogeneity of the variables. Once the normal distributions were assumed, the Student’s t-test was applied to determine any significant differences between samples. To determine the significant differences between the olive groves, an analysis of variance (ANOVA) was performed, followed by a Tukey’s HSD test to determine the statistical significance.
3. Results
3.1. Diversity and Relative Abundance of Plant Species in Vegetation Cover (VC and VC-MIX)
The vegetation samplings showed a total of 19 herbaceous species in the vegetation of the organic olive groves (Table 1), with 16 and 15 species corresponding to VC and VC-MIX olive groves, respectively, of which 12 species were present in both olive groves. Among the common species, three species presented significantly higher population densities in the VC olive grove, and four species had higher values in the VC-MIX. In general, the number of specimens recorded were higher in the VC-MIX olive grove, in which 1422 specimens were collected, while in the VC olive grove, the number of specimens was considerably lower, with 620 specimens. The VC-MIX olive grove’s greater plant density is mainly due to Filago fuscescens, Filago pyramidata, Astragalus ramosus, and Malva parviflora (Table 1), of which abundance was significantly higher (Table 1; p < 0.05; p < 0.01; p < 0.001), and to a lesser extent, to Herniaria cinerea, Bromus rubens, and Hordeum murinum. In contrast, in the VC olive grove, only the Diplotaxis virgata and Trigonella monspeliaca species showed significantly higher abundance values (Table 1; p < 0.01), but they had relatively low abundance values.

Table 1.
List of species inventoried in VC and VC-mix olive groves, and frequency indices in each sampling (S1–S5). The percentages of bare soil in both olive groves are indicated below.
Plantago lagopus was among the VC-MIX olive grove’s exclusive species, being present in samples from 3 to 5 (Table 1), while Stellaria pallida and Anagallis foemina were only accidentally found. In contrast, the species exclusive to the VC olive grove were recorded with much more regularity during the entire sampling period (Diplotaxis virgata, Erodium malacoides, and Crucianella patula).
3.2. Diversity and Relative Abundance of Beneficial Insects (Chromatic Sticky Traps)
A total of 16 parasitoid (Table 2) and nine predator species (Table 3) were caught by the chromatic traps. Among the three compared managements, the greatest diversity corresponded to the VC-MIX olive grove, where all the species were found. In the VC olive grove, the 13 parasitoid species and six predators were also found, while the lowest diversity occurred in the conventional olive grove, where ten parasitoid species and five predators were captured (Table 4).
Regarding the parasitoids, the species belonged to the superfamilies Chalcidoidea (families Pteromalidae, Eupelmidae, Eulophidae and Encyrtidae), Chrysidoidea (family Bethylidae) and Ichneumonoidea (families Icneumonidae and Braconidae). In a decreasing order of relative abundance, the most frequent were Ageniaspis fuscicollis and Chelonus elaeaphilus, which are parasitoids specifically associated with the olive moth P. oleae [33,34], whose larva develops in olive moth larvae, and adults emerge a few days later than their host [3], reaching very high rates of parasitism [35].

Table 2.
Parasitoid species captured in the sticky yellow traps, pest species they are associated with, and first bibliographical reference.
Table 2.
Parasitoid species captured in the sticky yellow traps, pest species they are associated with, and first bibliographical reference.
| Parasitoids spp. | Olive Pest Host | Host Association | |
|---|---|---|---|
| Cephalonomia cursor Westwood, 1881 (Hym.: Bethylidae) |  | Phloeotribus scarabaeoides | [36] Russo, 1938 |
| Apanteles xanthostigma (Haliday, 1834) (Hym.: Braconidae) |  | Prays oleae | [33] Campos, 1981 |
| Psyttalia concolor (Szepligeti, 1910) (Hym.: Braconidae) |  | Bactrocera oleae | [35] Kapatos, 1977 |
| Chelonus elaeaphilus Silvestri, 1908 (Hym.: Braconidae) |  | Prays oleae | [33] Campos, 1981 |
| Ageniaspis fuscicollis (Dalman, 1820) (Hym.: Encyrtidae) |  | Prays oleae | [33] Campos, 1981 |
| Diversinervus elegans Silvestri, 1915 (Hym.: Encyrtidae) |  | Saissetia oleae | [37] Panis, 1977 |
| Leptomastidea abnormis (Girault, 1915) (Hym.: Encyrtidae) |  | Saissetia oleae | [38] Copland, 1983 |
| Scutellista cyanea Motschulsky, 1859 (Hym.: Encyrtidae) |  | Saissetia oleae | [37] Panis, 1977 |
| Chrysocharis gemma (Walker, 1839) (Hym.: Eulophidae) |  | Prays oleae | [39] Noyes, 2019 |
| Pnigalio mediterraneus Ferrierre & Delucchi, 1957 (Hym.: Eulophidae) |  | Bactrocera oleae; Prays oleae | [40] Stavraki, 1970 |
| Tetrastichus cesirae Russo, 1938 (Hym.: Eulophidae) |  | Saissetia oleae; Bactrocera oleae; Liothrips oleae | [3] Arambourg, 1986 |
| Elasmus flabellatus (Fonscolombe, 1832) (Hym.: Eulophidae) |  | Prays oleae | [33] Campos, 1981 |
| Eupelmus urozonus (Dalman, 1820) (Hym.: Eupelmidae) |  | Bactrocera oleae | [33] Campos, 1981 |
| Diadegma semiclausum (Hellén, 1949) (Hym.: Ichneumonidae) |  | Prays oleae | [41] Torres, 2010 |
| Cheiropachus quadrum (Fabricius, 1787) (Hym.: Pteromalidae) |  | Phloeotribus scarabaeoides | [36] Russo, 1938 |
| Cyrtoptyx latipes (Rondani, 1874) (Hym.: Pteromalidae) |  | Bactrocera oleae | [42] Ranaldi and Santoni, 1987 |

Table 3.
Predator species captured in the sticky yellow traps, pest species they are associated with, and first bibliographical reference.
Table 3.
Predator species captured in the sticky yellow traps, pest species they are associated with, and first bibliographical reference.
| Predators spp. | Olive Pest Prey | Prey Association | |
|---|---|---|---|
| Coccinella septempunctata (L., 1758) (Col: Coccinellidae) |  | Saissetia oleae; Lepidosaphes ulmi; Parlatoria oleae; Aspidiotus nerii | [3] Arambourg, 1986 |
| Stethorus punctillum (Weise, 1891) (Col.: Coccinellidae) |  | Saissetia oleae; Lepidosaphes ulmi; Parlatoria oleae; Aspidiotus nerii | [3] Arambourg, 1986 |
| Prolasioptera berlesiana Paoli, 1907 (Dip.: Cecidomyiidae) |  | Bactrocera oleae | [43] Silvestri, 1945 |
| Anthocoris nemoralis (F., 1794) (Hem.: Anthocoridae) |  | Euphyllura olivina; Liothrips oleae; Prays oleae | [3] Arambourg, 1986 |
| Pseudoloxops coccineus (Meyer-Dür, 1843) (Hem.: Miridae) |  | Prays oleae | [44] Paredes et al., 2013 |
| Brachynotocoris ferreri Baena (Hem.: Miridae) |  | Prays oleae | [44] Paredes et al., 2013 |
| Chrysoperla agilis Henry et al., 2003 (Neu.: Chrysopidae) |  | Euphyllura olivina; Prays oleae | [30] Bozsik and González-Ruiz, 2006 |
| Harraphidia laufferi (Navás, 1915) (Rap.: Raphidiidae) |  | Phloeotribus scarabaeoides | [45] Rozas and González-Ruiz, 2017 |
| Aeolothrips intermedius Bagnall, 1934 (Thy.: Aeolothripidae) |  | Liothrips oleae; Aceria oleae; Oxycenus maxwelli | [46] De Liñán, 1998 |

Table 4.
Beneficial species captured in the chromatic traps. Xs indicate their presence in the olive grove.
Table 4.
Beneficial species captured in the chromatic traps. Xs indicate their presence in the olive grove.
| Beneficial Species | VC-MIX | VC | Conventional | |
|---|---|---|---|---|
| Predators | Coccinella septempunctata | X | X | - |
| Stethorus punctillum | X | X | X | |
| Prolasioptera berlesiana | X | - | - | |
| Anthocoris nemoralis | X | X | X | |
| Pseudoloxops coccineus | X | X | X | |
| Brachynotocoris ferreri | X | - | - | |
| Chrysoperla agilis | X | X | X | |
| Harraphidia laufferi | X | - | - | |
| Aeolothrips intermedius | X | X | X | |
| Parasitoids | Cephalonomia cursor | X | X | X |
| Apanteles xanthostigma | X | X | - | |
| Psyttalia concolor | X | X | - | |
| Chelonus elaeaphilus | X | X | X | |
| Ageniaspis fuscicollis | X | X | X | |
| Diversinervus elegans | X | X | - | |
| Leptomastidea abnormis | X | X | X | |
| Scutellista cyanea | X | X | X | |
| Chrysocharis gemma | X | X | X | |
| Pnigalio mediterraneus | X | X | X | |
| Tetrastichus cesirae | X | X | X | |
| Elasmus flabellatus | X | - | - | |
| Eupelmus urozonus | X | - | - | |
| Diadegma semiclausum | X | X | X | |
| Cheiropachus quadrum | X | X | X | |
| Cyrtoptyx latipes | X | - | - |
The other parasitoids caught in the chromatic traps were Elasmus flabellatus, Eupelmus urozonus, and Psyttalia concolor. The first species is associated with the olive moth [35], while the last two are associated with the olive fruit fly [35,36].
Among the predators, the most abundant species was Aeolothrips intermedius (Bagnall, 1934) (Thysanoptera: Aeolothripidae), which is a species that is reportedly associated with the olive thrips, Liothrips oleae (Costa, 1857) (Thysanoptera: Phlaeotripidae) [46], as well as with different phytophagous mites, such as those responsible for acariosis, Aceria oleae (Nalepa, 1900), and Oxycenus maxwelli (Keifer, 1939) (Trombidiformes: Eriophyidae) [3].
Among the heteropterans, Pseudoloxops coccineus, Brachynotocoris ferreri (Myridae), and Anthocoris nemoralis (Anthocoridae) were caught in yellow traps. In general, these species are relatively frequent in the olive groves during spring [47], attacking a variety of species, such as the olive psyllid, Euphyllura olivina (Costa, 1839) (Hemiptera: Psyllidae) [4].
Due to its important ecological role in naturally controlling several pest species, we highlight the presence of green lacewings (Chrysopidae) among the captures. Among the Chrysoperla carnea-complex species, most captured individuals belonged to the Chrysoperla agilis species, whose larvae have been cited as the main predators of the olive moth, P. oleae [3,24,25].
Coccinellids such as Stethorus punctillum (Weise, 1891) and Coccinella septempunctata (Coleoptera: Coccinellidae) have been relatively frequent in color traps. These species are involved in the natural control of scale insects such as Saissetia oleae (Hemiptera: Coccidae), Parlatoria oleae, Lepidosaphes ulmi (Linnaeus, 1758) [3], and Aspidiotus nerii (Bouche, 1833) (Hemiptera: Diaspididae) [4].
In Figure 3, the number of captures of the main beneficial insects are represented in an order of decreasing abundance, indicating the percentage of captures corresponding to each type of olive grove (VC; VC-MIX; and conventional). Concerning their population densities, all beneficial insects showed significantly higher population values in the VC-MIX olive grove (Figure 3, Figure 4 and Figure 5) compared to the VC and conventional olive groves. In the comparison between VC and conventional olive groves, the capture values also presented significant differences in the case of parasitoids (p < 0.05), although this was not fulfilled in the case of predators, whose populations did not statistically differ from each other.
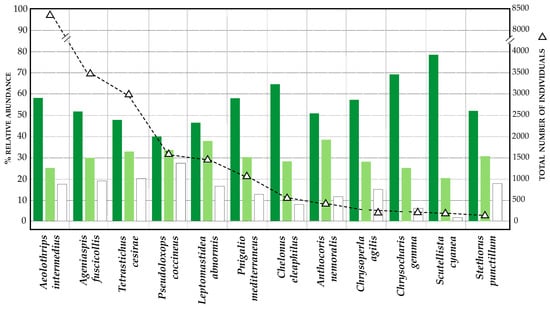
Figure 3.
Total number of individuals captured for the different species of natural enemies (right axis) and capture percentages for each of the three agricultural management (left axis): VC-MIX olive grove (white), VC olive grove (light green), and conventional management (dark green).
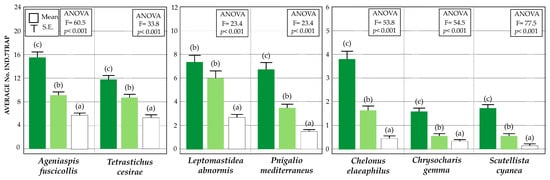
Figure 4.
Statistics (mean and SE) of the relative abundances of main parasitoid species captured in the sticky yellow traps in the three farming managements: Conventional (white), VC olive grove (light green), and VC-MIX olive grove (dark green). Statistically significant differences are indicated by letters (Tukey’s HSD test, p < 0.05).
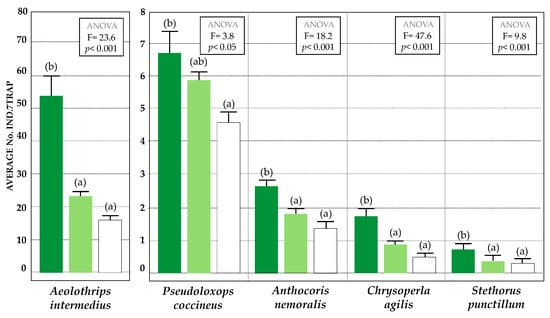
Figure 5.
Statistical values (mean, SE) of the relative abundances of main predator species captured in the sticky yellow traps in the three agricultural managements: Conventional (white), VC olive grove (light green), and VC-MIX olive grove (dark green). Statistically significant differences are indicated by letters (Tukey’s HSD test, p < 0.05).
3.3. Attack Parameters of Olive Moth, P. oleae
3.3.1. Egg Population and Potential Attack
The olive moth egg population on the fruits was significantly lower in the Conventional olive grove throughout the oviposition period of P. oleae (p < 0.05, Figure 6A), where the average value reached 104 eggs/100 fruits. However, the organic olive groves (VC and VC-MIX) presented statistically higher infestation values that were similar to each other, and reached mean values slightly higher than 120 eggs/100 fruits.
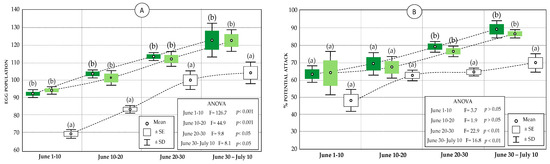
Figure 6.
Statistics (mean, standard error, and standard deviation) of the population of P. oleae eggs in the olive groves subject to different management (A), and potential attack on the fruit (B) in VC-MIX (dark green), VC (light green), and conventional (white). Significant differences within each sampling interval are indicated by letters (Tukey’s HSD test, p < 0.05).
Regarding the potential attack, the values did not initially present significant differences (from 1–20 June, Figure 6B) between the three compared managements; however, as of 20 June, the population values were statistically lower in the conventional olive grove (p < 0.05). According to these data, the potential fall would imply the loss of 70% of the fruits in the conventional olive grove, and around 90% in the organic olive groves.
3.3.2. Evolution of Lacewing Catches (McPhail Traps) and Oophagous Larval Predation
Among the individuals captured in the McPhail traps, a sample of 22% of the captured lacewings were taxonomically determined, resulting in the identification of six species. Most (87.4%) corresponded to Chrysoperla agilis (Henry), followed by 3.4% to Pseudomallada prasinus (Burmeister), 1.5% to Pseudomallada flavifrons (Brauer), 3% to Chrysoperla affinis (Thierry), 3.1% to Chrysoperla lucasina, and 1.6% to Chrysopa viridana (Schneider). In line with previous samplings, Ch. agilis is the dominant species; therefore, within the carnea complex, its impact on the olive moth eggs was considered the greatest [24,25]. The capture values of the adult lacewings in the McPhail traps showed significant differences between the three olive groves in each sampling interval (Figure 7A). The maximum values corresponded to the VC-MIX olive grove, with capture figures of up to 92 individuals/trap. In turn, the capture values of the VC olive grove were significantly higher than those of the traps in the conventional olive grove. Consequently, statistically significant differences were found among them all throughout the study period (p < 0.01; p < 0.001 in Figure 7A).
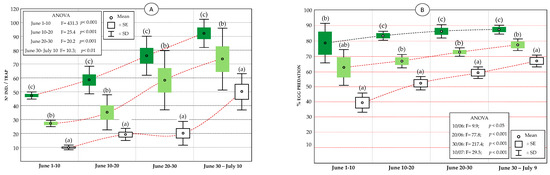
Figure 7.
Statistics (mean, standard error, and standard deviation) of the catch values of lacewings in McPhail traps (A), and evolution of predation percentage (B) in the olive groves subject to different management: VC-MIX (dark green), VC (light green), and conventional (white). Significant differences within each sampling interval are indicated by letters (Tukey’s HSD test, p < 0.05).
Regarding the percentages of oophagous predation, a parallelism is observed when considering the evolution of capturing the adults, with a similar pattern and statistical differences between the three olive groves compared throughout the oviposition period. The values of the oophagous predation caused by lacewing larvae were highest in the VC-MIX olive grove (p < 0.001, Figure 7B), with a final egg predation rate of 88%. Next, in the VC olive grove, a maximum predation rate of 78% was reached, a value that in turn was significantly greater than that of the conventional olive grove, at 67%.
The values of predation by lacewings allow a great reduction of the potential attack on the fruits, decreasing at the end of the oviposition period final attack to values of 23% in the conventional olive grove, 18.5% in the VC olive grove and 12%. in the VC-MIX olive grove (Figure 8A). In relation to the potential attack values (where larval predation is excluded), the final attack values (Figure 8B) represent decreases of 47% in the conventional olive grove, 68.5% in the VC olive grove, and 76% in the VC-MIX olive grove, being therefore in this olive grove the natural control by the lacewings provides the maximum reduction. In close correspondence with these results, the fruit recovery percentages have been 57% in the conventional olive grove, 71.5% in the VC olive grove, and 83% in the VC-MIX olive grove.
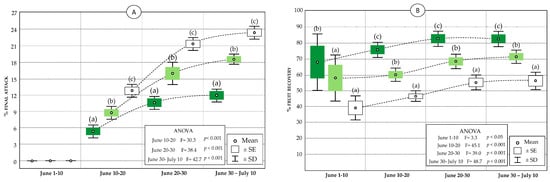
Figure 8.
Statistics (mean, standard error, and standard deviation) of the final attack of P. oleae eggs in the olive groves subject to different management (A), and of fruit recovery (B) in VC-MIX (dark green), VC (light green), and conventional (white). Significant differences within each sampling are indicated by letters (Tukey’s HSD test, p < 0.05).
4. Discussion
Incorporating crop residues into soil provides an important source of nutrients [48] by increasing nitrogen mineralization [49] and positively influencing the soil’s biological, chemical, and physical properties [50]. Several studies highlight that incorporating crop residues into soil improves tilt, reduces erosion processes, prevents nutrient losses by run-off and leaching, and increases microbial biomass [51,52,53]. Therefore, applying agricultural residues (e.g., crop residues and animal manures) to soil has recently become a highly beneficial agricultural practice [2,50,54,55,56,57]. Among its advantages, it provides a longer protective effect against erosion [55] compared to that provided by herbaceous cover [54]. In addition, Taguas and Gómez [56] reported that crushed plants prevent ruderal flora developing, which attenuates the competition of the herbaceous cover with the olive grove for water and nutrients, thus reducing herbicide dependence. The results of this study are consistent with the consulted bibliography [2], in view of the higher abundance of plant species in the olive grove with a mixed plant cover, in which crushed pruning remains were incorporated. At the same time, in this olive grove, the greatest diversity and abundance of beneficial insects were found, in comparison with organic olive groves with only adventitious herbaceous vegetation covers. In any case, this plant cover provides direct benefits to the different dimensions of ecological diversity, since it increases the number of species present in the system, which contributes to establishing more complex relationships between its components. Among the effects provided by the herbaceous vegetation, it is worth considering the different ways they support entomophagous insects:
- -
- Plants provide a settlement for numerous phytophagous species which are potential prey/alternative hosts for numerous entomophagous species, which due to their relatively polyphagous nature, interact with the phytophagous species that are specifically associated with olive trees. Regarding conventional olive growing, in which there is no plant cover, implementing living plant covers creates a settlement for a wide community of species in the cultivation area, which causes the herbaceous plants of the herbaceous cover to be authentic insect reservoirs [12].
- -
- Secondly, herbaceous vegetation provides a food source for the adults of many predatory and parasitoid species [58]. These arthropods require an adequate intake to develop their fecundity, reach their potential longevity [59], and ensure post-diapause survival. The nectar of flowers is especially important for hymenopterous parasitoids [60,61] and predators [62] that are attracted by the release of semiochemical volatiles [63,64]. Several authors [65,66] have reported that Ch. carnea can be caught in traps baited with phenylacetaldehyde, which is a common constituent of a flower scent and is of great practical interest [64,65]. Thus, maintaining biodiversity is essential for achieving greater stability and can be used as an indicator of the system’s resistance to change [67,68].
Additionally, the greater edaphic structural complexity derived from adding crushed pruning remains to the soil contributes to the significant increase in arthropod diversity by stimulating microorganism activity in the soil and serving as food and shelter for beneficial fauna [68]. Chrysoperla spp. overwinter as diapausing adults [69], which in the south of Spain occurs from October to February. However, the intensity and severity of the agricultural practices that occur from early autumn to mid-spring cause the olive trees to be hardly favorable for the winter survival of lacewings. This is because of the harvest, even though the current trend is to bring it forward until early autumn [70]; the harvest involves manually beating the branches or using powerful mechanical vibrators with rods on the branches to cause the fruit to fall [71]. After harvesting, olive pruning is performed (from December to April), whose purpose is to regenerate the tree, removing unnecessary branches [20]; this practice is quite detrimental to the survival of lacewings overwintering in the canopy of olive trees. Thus, ensuring survival through winter largely depends on the population that spends the season in more stable and ecologically favorable refuge areas, such as the plant remains in the marginal areas adjacent to the crops [31], as is the most frequent scenario in conventional farming. For olive groves with ecological farming, the conditions provide a substantial improvement, given the presence of degraded plant remains in them (as is the case in VC farming) resulting from the adventitious vegetation, which, in view of the results of this study, would provide a slightly higher protection to wintering populations in relation to conventional farming. Given the limitations derived from agricultural/forestry practices, an innovative and adequate measure is based on using artificial winter shelters that consist of wooden boxes filled with crushed vegetable matter. These artificial shelters have proven to be a successful practice in integrated pest management, favoring the establishment of adult lacewings [30,72,73,74,75]. As a result, augmentation of the natural lacewing population is stimulated by saving the hibernating adults from the winter coldness and precipitation, and provides an anticipation of the spring occurrence of adults and eggs [30].
The accumulation of plant residues in the soil allows for much more adequate conditions for the winter survival of numerous species of natural enemies of olive pests [34,76,77,78]. Most likely, the accumulation of crushed plant matter in the crop soil resulting from pruning is beneficial, and is similar to that provided by deploying a net of artificial hibernation shelters. This is consistent with the higher post-winter populations of beneficial insects in the VC-MIX olive grove in relation to the VC olive grove, and, more notably, to those of the conventional olive grove. Thus, for the farmer, applying this agronomic practice would result in successive events that are highly beneficial in terms of reducing dependence on synthetic pesticides and improving the evenness of natural enemies, as well as their entomophagous effectiveness.
The implementation of beds of crushed plant material reportedly neither negatively affects crops nor poses a potential danger of a phytosanitary order to them, except for crops close to areas affected by verticillium wilt, the disease caused by Verticillium dahliae [78]. In this regard, Lopez Escudero and Mercado-Blanco reported that the dispersal of infective propagules (microsclerotia) among olive groves may be favored by cultural practices that are inadequate for controlling this disease, such as grinding plant debris from affected trees. Other negative aspects are the workload required by this practice, the high rate of CO2 emissions, and the significant increase in the risk of fire.
5. Conclusions
This research has allowed us to verify the effect of implementing a mixed plant cover in olive groves, consisting of adding the fine residues from olive tree pruning to the adventitious vegetation cover. Compared to an organically managed olive grove provided with a simple plant cover, this practice stimulates the community’s productivity of adventitious herbaceous cover. A clearly stimulating effect on the diversity and abundance of beneficial insects, whose activity is essentially natural pest control, was noted. On the olive moth, as the target pest selected for this study, this practice allows for a notable increase in the effectiveness of the main natural enemies, confirming predation rates of approximately 90%, which in terms of crop loss result in averages of 12% per tree. Comparatively, in organic crops provided with simple plant cover, the average crop losses due to this pest were 19%, while in traditional crops free of any type of plant cover, the crop losses were 23% of the harvest.
Author Contributions
Conceptualization, R.G.-R. and J.A.G.-G.; methodology, R.G.-R., J.A.G.-G. and A.G.-F.; software, J.A.G.-G., A.M.S.-S. and M.M.-R.; validation, R.G.-R., M.d.P.C. and A.G.-F.; formal analysis, R.G.-R. and J.A.G.-G.; investigation, R.G.-R., J.A.G.-G., M.M.-R. and M.S.-P.; resources, J.C.-H.; data curation, J.A.G.-G. and M.S.-P.; writing—original draft preparation, R.G.-R.; writing—review and editing, R.G.-R., M.d.P.C., A.M.S.-S. and A.G.-F.; visualization, M.d.P.C. and A.M.S.-S.; supervision, R.G.-R.; project administration, A.R.-L.; funding acquisition, A.R.-L. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the “Fondo Europeo de Desarrollo Regional (FEDER) y a la Consejería de Transformación Económica, Industria, Conocimiento y Universidades de la Junta de Andalucía, dentro del Programa Operativo FEDER 2014–2020”, for its financial support for the project “Avance multidisciplinar en la gestión y conocimiento de las cubiertas de restos de poda en olivar a escala árbol, parcela y explotación” (US-1380979). M.S-P.’s work was conducted on the basis of a contract (INV_03_2022_I-057) funded by this research project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are highly grateful for the support provided by András Bozsik in the taxonomic determination of the Chrysopidae.
Conflicts of Interest
The authors declare no conflict of interest.
References
- MAGRAMA. Ministerio de Agricultura, Alimentación y Medio Ambiente. Available online: https://www.mapa.gob.es/estadistica/pags/anuario/2012/AE_2012_13_12_01_01.pdf (accessed on 15 February 2023).
- García-Fuentes, A.; Lendínez, M.L.; Salazar, C. Pérdida de diversidad vegetal en los olivares del Alto Valle del Guadalquivir: Alternativas agroecológicas. In La Cultura del Olivo, Ecología, Economía, Sociedad; Anta, J.L., Palacios, J., Guerrero, F., Eds.; Universidad de Jaén: Jaén, Spain, 2005; pp. 300–430. ISBN 84-8439-250-3. [Google Scholar]
- Arambourg, Y. Traité D’entomologie Oléicole; Conseil oléicole International Publishing: Madrid, Spain, 1986; p. 360. ISBN 8439875835/9788439875833. [Google Scholar]
- Andrés-Cantero, F. Enfermedades y Plagas del Olivo, 1st ed.; Riquelme y Vargas Ediciones, SL: Jaén, Spain, 1997; p. 646. ISBN 8486216311/9788486216313. [Google Scholar]
- Gómez-Guzmán, J.A.; Sainz-Pérez, M.; González-Ruiz, R. Monitoring and inference of behavioral resistance in beneficial insects to insecticides in two pest control systems: IPM and organic. Agronomy 2022, 12, 538. [Google Scholar] [CrossRef]
- Vives, J.M. Control de plagas de insectos. Problemas y alternativas. In Insecticidas Biorracionales; Bellés, J., Ed.; Consejo Superior de Investigaciones Científicas; Colección Nuevas Tendencias: Madrid, Spain, 1988; pp. 3–13. [Google Scholar]
- Carrero, J.M. Lucha Integrada Contra Las Plagas Agrícolas y Forestales; Mundi-Prensa Ediciones: Madrid, Spain, 1996; p. 272. ISBN 8471146398/9788471146397. [Google Scholar]
- Gómez-Guzmán, J.A.; González-Ruiz, R. Side effects of insecticides on beneficial insects: A practical tool to identify organic agroecosystems. World J. Agric. Sci. 2019, 4, 1–15. [Google Scholar] [CrossRef]
- Gómez-Guzmán, J.A.; González-Ruiz, R. Determination of the sampling size for the reliable identification of organic crops by inducing sublethal effects in beneficial insects. In Proceedings of the IOP Conference Series: Earth and Enviromental Science, Prague, Czech Republic, 7–11 September 2020. [Google Scholar] [CrossRef]
- Gómez-Guzmán, J.A.; Sainz-Pérez, M.; González-Ruiz, R. Induction of sublethal effects for the characterization of olive groves under different pest management systems. Rev. Bras. Frutic. 2021, 43, 1–15. [Google Scholar] [CrossRef]
- Civantos-Ruiz, M.; Gómez-Guzmán, J.A.; Sainz-Pérez, M.; González-Ruiz, R. Application of accumulated heat units in the control of Prays oleae (Bern.) (Lep., Praydidae). Rev. Bras. Frutic. 2022, 44, e-804. [Google Scholar] [CrossRef]
- Hodkinson, I.D.; Hughes, M.K. Fitofagia en los Insectos, 2nd ed.; Oikos-Tau, S.A., Ed.; Ediciones: Barcelona, Spain, 1993; p. 104. ISBN 978-84-281-0803-4. [Google Scholar]
- Chauvin, R. The World of an Insect; Translated from the French by Harold Oldroyd; Weidenfeld and Nicolson: London, UK, 1967; p. 256. [Google Scholar]
- Cerdà, A.; Giménez-Morera, A.; Bodí, M.B.; Burguet, M.; García-López, J.; Jovani, C.; Segura, M. Pérdida de suelo y agua bajo cubierta de Quercus coccifera en la sierra de enguera. Valencia. C & G 2010, 24, 13–23. [Google Scholar]
- Rodríguez-Lizana, A.; Pereira, M.J.; Ribeiro, M.C.; Soares, A.; Márquez-García, F.; Ramos, A.; Gil-Ribes, J. Assessing Local Uncertainty of Soil Protection in an Olive Grove Area with Pruning Residues Cover: A Geostatistical Cosimulation Approach. Land Degrad. Dev. 2017, 28, 2086–2097. [Google Scholar] [CrossRef]
- Vanwalleghem, T.; Gómez, J.A.; Amate, J.I.; De Molina, M.G.; Vanderlinden, K.; Guzmán, G.; Laguna, A.; Giráldez, J.V. Impact of historical land use and soil management change on soil erosion and agricultural sustainability during the anthropocene. Anthropocene 2017, 17, 13–29. [Google Scholar] [CrossRef]
- Zema, D.A.; Labate, A.; Martino, D.; Zimbone, S.M. Comparing different infiltration methods of the HEC-HMS model: The case study of the mésima torrent (southern italy). Land Degrad. Dev. 2017, 28, 294–308. [Google Scholar] [CrossRef]
- Casado, G.G.; Pulido, L.F. Manejo de la Cubierta Vegetal en el Olivar Ecológico en Andalucía: Siembra de Leguminosas Entre Calles; Dirección General de Agricultura Ecológica; Consejería de Agricultura y Pesca: Junta de Andalucía, Spain, 2007; p. 78. [Google Scholar]
- Alcántara, C.; Pujadas, A.; Saavedra, M. Management of cruciferous cover crops by mowing for soil and water conservation in southern Spain. Agric. Water Manag. 2011, 98, 1071–1080. [Google Scholar] [CrossRef]
- García-Martín, J.F.; Cuevas, M.; Feng, C.H.; Álvarez-Mateos, P.; Torres, M.; Sánchez, S. Energetic valorisation of olive biomass: Olive-tree pruning, olive stones and pomaces. Processes 2020, 8, 511. [Google Scholar] [CrossRef]
- Iranzo, M.; Canizares, J.V.; Roca-Perez, L.; Sainz-Pardo, I.; Mormeneo, S.; Boluda, R. Characteristics of rice straw and sewage sludge as composting materials in Valencia (Spain). Bioresour. Technol. 2004, 95, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Ribo, M.; Albiach, R.; Pomares, F.; Canet, R. Alternativas de gestión de la paja de arroz en la Albufera de Valencia. Vida Rural 2017, 430, 56–60. [Google Scholar]
- Lull, C.; Bautista, I.; Segarra, R.; Monzó, J.M.; Seguí, J.V.; Lidón, A. Efecto de una cubierta de paja de arroz sobre la respiración del suelo. In Proceedings of the IX Simposio Nacional Sobre Control de la Degradación y Recuperación de Suelos, Elche, Spain, 27–29 May 2021. [Google Scholar]
- Campos, M.; Ramos, P. Some relationships between the number of Prays oleae eggs laid on olive fruits and their predation by Chrysoperla carnea. In Integrated Pesticide Control Olive-groves. In Proceedings of the CEC/FAO/IOBC International Joint Meeting, Pisa, Italy, 3–6 April 1985. [Google Scholar]
- Bozsik, A.; González-Ruiz, R.; Lara, B.H. Distribution of the Chysoperla carnea complex in southern Spain (Neuroptera: Chrysopidae). An. Univ. Oradea Fasc. Protectia Mediu. 2009, 14, 60–65. [Google Scholar]
- Trdan, S.; Andjus, L.; Raspudić, E.; Kač, M. Distribution of Aeolothrips intermedius Bagnall (Thysanoptera: Aeolothripidae) and its potential prey Thysanoptera species on different cultivated host plants. J. Pest. Sci. 2005, 78, 217–226. [Google Scholar] [CrossRef]
- González-Ruiz, R.; Gómez-Guzmán, J.A. Agricultural Management Greatly Affects the Beneficial Entomofauna of the Olive Groves. Am. J. Biomed. Sci. Res. 2019, 1, 144–151. [Google Scholar] [CrossRef]
- Ebeling, W.; Wagner, R.; Reierson, D.A. Influence of repellency on the efficacy of blatticides. I. Learned modification of behavior of the German cockroach. J. Econ. Entomol. 1966, 59, 1374–1388. [Google Scholar] [CrossRef]
- Ail-Catzim, C.E.; Cerna-Chávez, E.; Landeros-Flores, J.; Ochoa-Fuentes, Y.; García-López, A.M.; González, R.E. Efecto de Insecticidas Sobre la Mortalidad y Depredación de Chrysoperla carnea (Neuroptera: Chrysopidae). Southwest. Entomol. 2015, 40, 565–574. [Google Scholar] [CrossRef]
- Bozsik, A.; González-Ruiz, R. First data on the sibling species of the common green lacewings in Spain (Neuroptera: Chrysopidae): (The taxonomic status of the most important cryptic species of Chrysoperla carnea complex in Spain). In Proceedings of the 4th International Plant Protection Symposium at Debrecen University and 11th Trans-Tisza Plant Protection Forum, Debrecen, Hungary, 18–19 October 2006. [Google Scholar]
- González Ruiz, R.; Al-Asaad, S.; Bozsik, A. Influencia de las masas forestales en la diversidad y abundancia de los crisópidos (Neur. Chrysopidae) del olivar. Cuad. Soc. Esp. Cienc. For. 2008, 26, 33–38. [Google Scholar]
- Ramos, P.; Ramos, J.M. Veinte años de observaciones sobre la depredación oófaga en Prays oleae Bern. Granada (España), 1970–1989. Boletín Sanid. Veg. Plagas 1990, 16, 119–127. [Google Scholar]
- Campos, M. Contribución al estudio de la entomocenosis de Prays oleae Bern. (Lep. Hyponomeutidae) en Granada (España). Acta Oecol. 1981, 2, 27–35. [Google Scholar]
- Varela, J.L.; González, R. Estudio sobre la entomofauna de un olivar en la provincia de Granada, durante el periodo de vuelo de la generación antófaga de Prays oleae (Lep. Yponomeutidae). Phytoma 1999, 111, 42–55. [Google Scholar]
- Kapatos, E.; Fletcher, B.S.; Pappas, S.; Laudeho, Y. Release of Opius concolor and O. concolor var. siculus [Hym.: Braconidae] against the spring generation of Dacus oleae [Dipt.: Trypetidae] on Corfu. Entomophaga 1977, 22, 265–270. [Google Scholar] [CrossRef]
- Russo, G. VI Contributo alla conoscenza dei coleotteri Scolitidi: Phloeotribus scarabaeoides (Bern.) Fauv. II. Biografia, simbionti, danni e lotta. Boll. R. Lab. Entomol. Agric. Portici 1938, 2, 3–420. [Google Scholar]
- Panis, A. Contribución al conocimiento de la biología de la «cochinilla negra de los agrios» (Saissetia oleae Olivier). Bol. Serv. Plagas 1977, 3, 199–205. [Google Scholar]
- Copland, J.S. Restricciones de temperatura en el control de cochinillas y cochinillas. Bol. SROP 1983, 6, 142–145. [Google Scholar]
- Noyes, J.S. Universal Chalcidoidea Database; World Wide Web Electronic Publication, 2019. Available online: https://www.nhm.ac.uk/our-science/data/chalcidoids/ (accessed on 15 February 2023).
- Stavraki, H. Contribution a l’inventaire du complexe parasitaire de quelques insectes noisibles a l’olivier en Grèce. Entomophaga 1970, 15, 225–231. [Google Scholar] [CrossRef]
- Torres, M.R. Parasitoides de plagas identificados en la provincia de Jaén (España). Bol. Soc. Entomol. Aragonesa 2010, 46, 597–601. [Google Scholar]
- Ranaldi, F.Y.; Santoni, M. Los parasitoides de la mosca del olivo Dacus oleae (Gmel). Inf. Fitopatol. 1987, 37, 15–18. [Google Scholar]
- Silvestri, F. Contribution à la biologie de la petite Cécidomyie des olives (Prolasioptera Berlesiana Paoli) en Italie. Monit. Int. De La Prot. Des Plantes 1945, 19, 73–76. [Google Scholar]
- Paredes, D.; Cayuela, L.; Campos, M. Synergistic effects of ground cover and adjacent vegetation on natural enemies of olive insect pests. Agric. Ecosyst. Environ. 2013, 173, 72–80. [Google Scholar] [CrossRef]
- Rozas, L.; González-Ruiz, R. Primeros datos sobre la influencia de las cubiertas vegetales en la presencia de Raphidioptera (Insecta: Neuropteroidea) en olivares de Jaén. In Proceedings of the XVI Scientific-Technical Symposium on Olive Oil, Fundación del Olivar Jaén, Spain, 8–10 May 2013. [Google Scholar]
- Liñan de, C. Entomología Agroforestal: Insectos y Ácaros que Dañan Montes, Cultivos y Jardines; Ediciones Agrotécnicas, S.L.: Madrid, Spain, 1998; p. 1309. ISBN 9788487480546. [Google Scholar]
- Álvarez, H.A.; Jiménez-Muñoz, R.; Morente, M.; Campos, M.; Ruano, F. La presencia de cobertura vegetal en olivares ecológicos afecta a la interacción de los enemigos naturales contra Prays oleae, favoreciendo una depredación eficaz de los huevos. Agric. Ecosistemas Y Medio Ambiente 2021, 315, 107441. [Google Scholar]
- Azam, F. Comparative effects of organic and inorganic nitrogen sources applied to a flooded soil on rice yield and availability of N. Plant Soil 1990, 125, 255–262. [Google Scholar] [CrossRef]
- Singh, H. Nitrogen mineralization, microbial biomass and crop yield affected by the placement of wheat residues and fertilizers in a semi-arid tropical soil with minimum tillage. J. Appl. Ecol. 1995, 32, 588–595. [Google Scholar] [CrossRef]
- Rodríguez-Lizana, A.; Espejo-Pérez, A.J.; González-Fernández, P.; Ordóñez-Fernández, R. Los residuos de poda como alternativa al laboreo tradicional para reducir la erosión y la dispersión de contaminantes en los olivares. Contam. Del Agua El Aire Y El Suelo 2008, 193, 165–173. [Google Scholar]
- Burkert, A.; Bationo, A.; Dossa, K. Mechanisms of cereal growth induced by residue mulch increases in West Africa. Soil Sci. Soc. Am. J. 2000, 64, 346–358. [Google Scholar] [CrossRef]
- Shah, Z.; Shah, S.H.; Peoples, M.B.; Schwenke, G.D.; Herridge, D.F. Crop residue and fertiliser N effects on nitrogen fixation and yields of legume–cereal rotations and soil organic fertility. Field Crops Res. 2003, 83, 1–11. [Google Scholar] [CrossRef]
- Shafi, M.; Bakht, J.; Jan, M.T.; Shah, Z. Soil C and N dynamics and maize (Zea may L.) yield as affected by cropping systems and residue management in North-western Pakistan. Soil Tillage Res. 2007, 94, 520–529. [Google Scholar] [CrossRef]
- Calatrava, J.; Franco, J.A. Using pruning residues as mulch: Analysis of its adoption and process of diffusion in southern spain olive orchards. J. Environ. Manag. 2011, 92, 620–629. [Google Scholar] [CrossRef]
- Repullo, M.A.; Carbonell, R.; Hidalgo, J.; Rodríguez-Lizana, A.; Ordóñez, R. Using olive pruning residues to cover soil and improve fertility. Soil Tillage Res. 2012, 124, 36–46. [Google Scholar] [CrossRef]
- Taguas, E.V.; Gómez, J.A. Vulnerabilidad de los olivares bajo la actual normativa de la PAC (Política Agraria Común) sobre la erosión del suelo: Un caso de estudio en el Sur de España. Política De Uso De La Tierra 2015, 42, 683–694. [Google Scholar]
- Medina, J.; Monreal, C.; Barea, J.M.; Arriagada, C.; Borie, F.; Cornejo, P. Estabilización de residuos de cultivos y aplicación a suelos agrícolas y degradados: Una revisión. Gestión De Residuos 2015, 42, 41–54. [Google Scholar]
- Chang, Y.F.; Tauber, M.J.; Tauber, C.A. Reproduction and quality of F1 offspring in Chrysoperla carnea: Differential influence of quiescence, artificially-induced diapause, and natural diapause. J. Insect Physiol. 1996, 42, 521–528. [Google Scholar] [CrossRef]
- González-Ruiz, R.; Campos, M. Rearing of Cheiropachus quadrum (Hym.: Pteromalidae) from the olive beetle, Phloeotribus scarabaeoides (Col.: Scolytidae). Potential biological control agent. Redia 1990, 73, 495–505. [Google Scholar]
- Manojlovic, B.; Zabel, A.; Kostic, M.; Stankovic, S. Effect of nutrition of parasites with nectar of melliferous plants on parasitism of the elm bark beetles (col., scolytidae). J. Appl. Entomol. 2000, 124, 155–161. [Google Scholar] [CrossRef]
- Manojlović, B.; Zabel, A.; Stanković, S.; Kostić, M. Ecphylus silesiacus (ratz.) (hymenoptera, braconidae), an important elm bark beetle parasitoid. Agric. For. Entomol. 2000, 2, 63–67. [Google Scholar] [CrossRef]
- Udayagiri, S. Behavioral manipulation of natural enemies: Potential use in insect pest management. Agric. Zool. Rev. 1996, 7, 181–216. [Google Scholar]
- Tóth, M.; Bozsik, A.; Szentkirályi, F.; Letardi, A. Phenylacetaldehyde: A chemical attractant for common green lacewings (Chrysoperla carnea s.l., Neuroptera: Chrysopidae). Eur. J. Entomol. 2006, 103, 267. [Google Scholar] [CrossRef]
- Tóth, M.; Szentkirályi, F.; Vuts, J.; Letardi, A.; Tabilio, M.R.; Jaastad, G.; Knudsen, G.K. Optimization of a phenylacetaldehyde-based attractant for common green lacewings (Chrysoperla carnea sl). J. Chem. Ecol. 2009, 35, 449–458. [Google Scholar] [CrossRef]
- Bruce, T.J.; Cork, A.; Hall, D.R.; Dunkelblum, E. Laboratory and field evaluation of floral odours from African marigold, Tagetes erecta, and sweet pea, Lathyrus odoratus, as kairomones for the cotton bollworm Helicoverpa armigera. IOBC WPRS Bull. 2001, 25, 315–322. [Google Scholar]
- Knudsen, J.T.; Tollsten, L.; Bergström, L.G. Floral scents—A checklist of volatile compounds isolated by headspace techniques. Phytochemistry 1993, 33, 253–280. [Google Scholar] [CrossRef]
- Gliessman, S. La biodiversidad y la estabilidad de los agroecosistemas. In La Práctica de la Agricultura y la Ganadería Ecológicas; Cornejo, J., Ed.; CAAE: Sevilla, Spain, 2001; pp. 69–89. [Google Scholar]
- Foraster-Pulido, L. Las Cubiertas Vegetales en el Rediseño del Olivar para una Transición Agroecológica; Universidad Internacional de Andalucía: Seville, Spain, 2010; p. 104. ISBN 978-84-7993-173-5. Available online: https://core.ac.uk/download/pdf/72018622.pdf (accessed on 17 January 2023).
- Henry, C.S.; Brooks, S.J.; Duelli, P.; Johnson, J.B. A lacewing with the wanderlust: The European song species ‘Maltese’, Chrysoperla agilis, sp. n., of the carnea group of Chrysoperla (Neuroptera: Chrysopidae). Syst. Entomol. 2003, 28, 131–148. [Google Scholar] [CrossRef]
- Parrilla-González, J.A.; Ortega-Alonso, D. Social Innovation in Olive Oil Cooperatives: A Case Study in Southern Spain. Sustainability 2021, 13, 3934. [Google Scholar] [CrossRef]
- Alzoheiry, A.; Ghonimy, M.; El Rahman, E.A.; Abdelwahab, O.; Hassan, A. Improving olive mechanical harvesting using appropriate natural frequency. J. Agric. Eng. 2020, 51, 148–154. [Google Scholar] [CrossRef]
- Weihrauch, F. Overwintering of common green lacewings in hibernation shelters in the Hallertau hop growing area. Bull. Insectol. 2008, 61, 67–71. [Google Scholar]
- McEwen, P.K.; Åkerberg, C.; Bozsik, A.; James, C.J.; Eccleston, L.; Lenartsson, M.; Rossiter, P.; Tuovinen, T. Artificial overwintering chambers for green lacewings: Results of international trials and implications for pest control. J. Appl. Entomol. 2001, 123, 525–527. [Google Scholar] [CrossRef]
- Şengonca, Ç.; Frings, B. Enhancement of the green lacewing Chrysoperla carnea (Stephens) by providing artificial facilities for hibernation. Turk. Entomol. Derg. 1989, 13, 245–250. [Google Scholar]
- Şengonca, Ç.; Henze, M. Conservation and enhancement of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) in the field by providing hibernation shelters. J. Appl. Entomol. 1992, 114, 497–501. [Google Scholar] [CrossRef]
- Varela-Martínez, J.L.; González-Ruiz, R. Bases metodológicas para la evaluación del impacto ocasionado por las aplicaciones insecticidas sobre los enemigos naturales de las plagas del olivo (II). Phytoma 1999, 112, 32–42. [Google Scholar]
- Varela-Martínez, J.L.; González-Ruiz, R. La lucha química contra Prays oleae (lep., Yponomeutidae) y su influencia en los enemigos naturales de las plagas del olivar (y III). Phytoma 2000, 115, 35–47. [Google Scholar]
- López-Escudero, F.J.; Mercado-Blanco, J. Verticillium wilt of olive: A case study to implement an integrated strategy to control a soil-borne pathogen. Plant Soil 2011, 344, 1–50. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).