Advancing Stress-Resilient Rice: Mechanisms, Genes, and Breeding Strategies
Abstract
1. Introduction
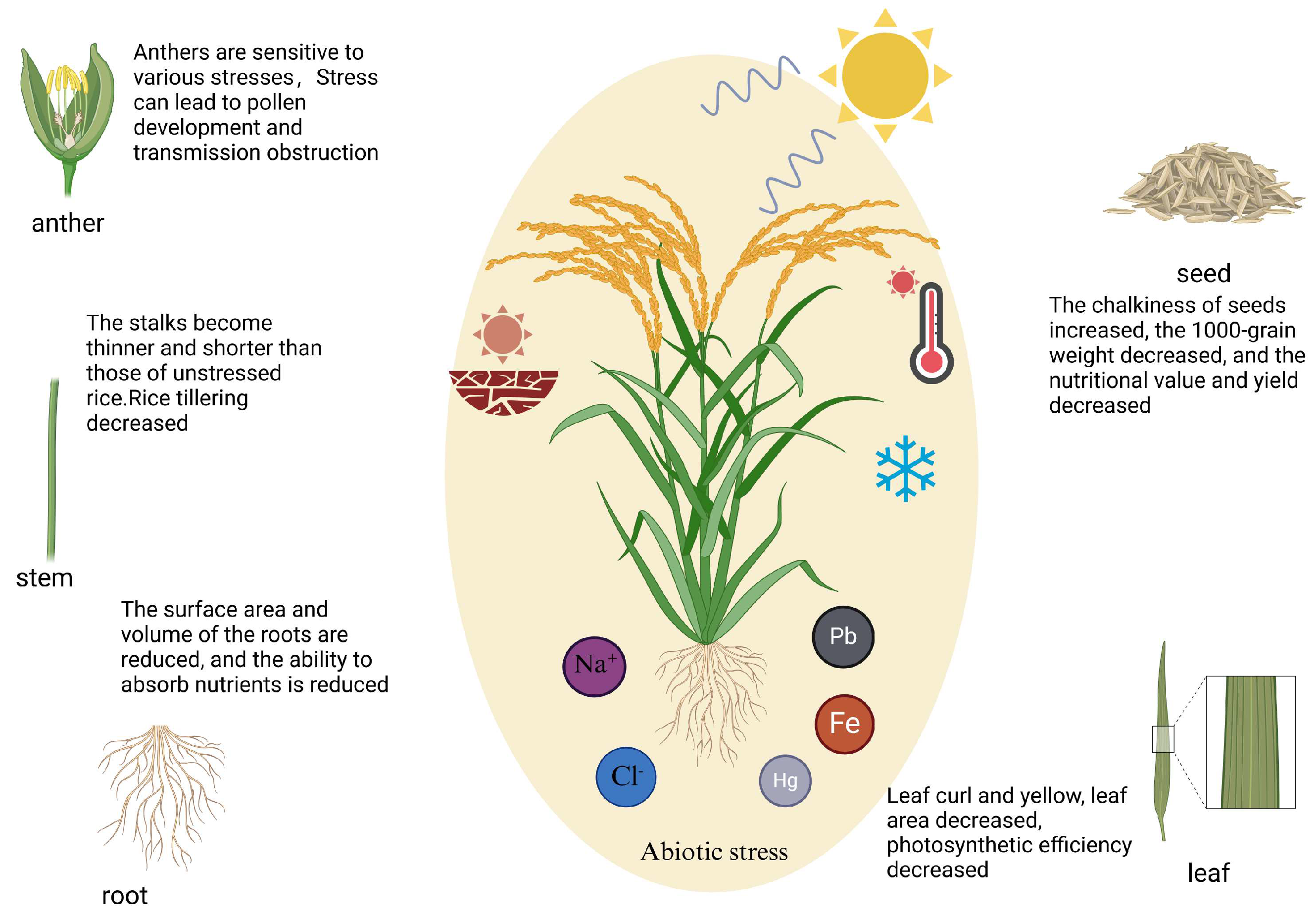
2. Research on the Effects of Abiotic Stress on Rice and Functional Genes
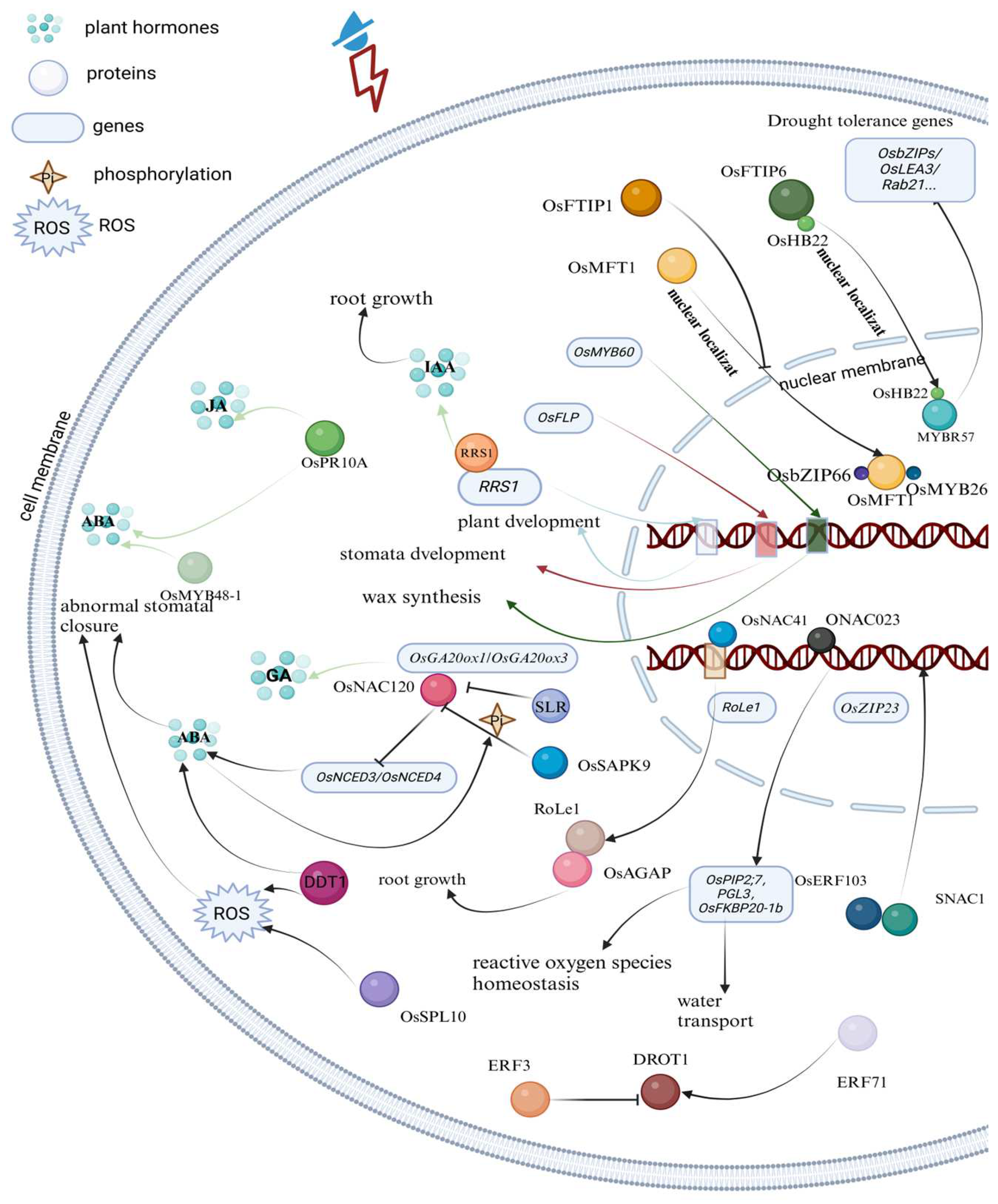
2.1. Drought Stress
2.2. Salt Stress
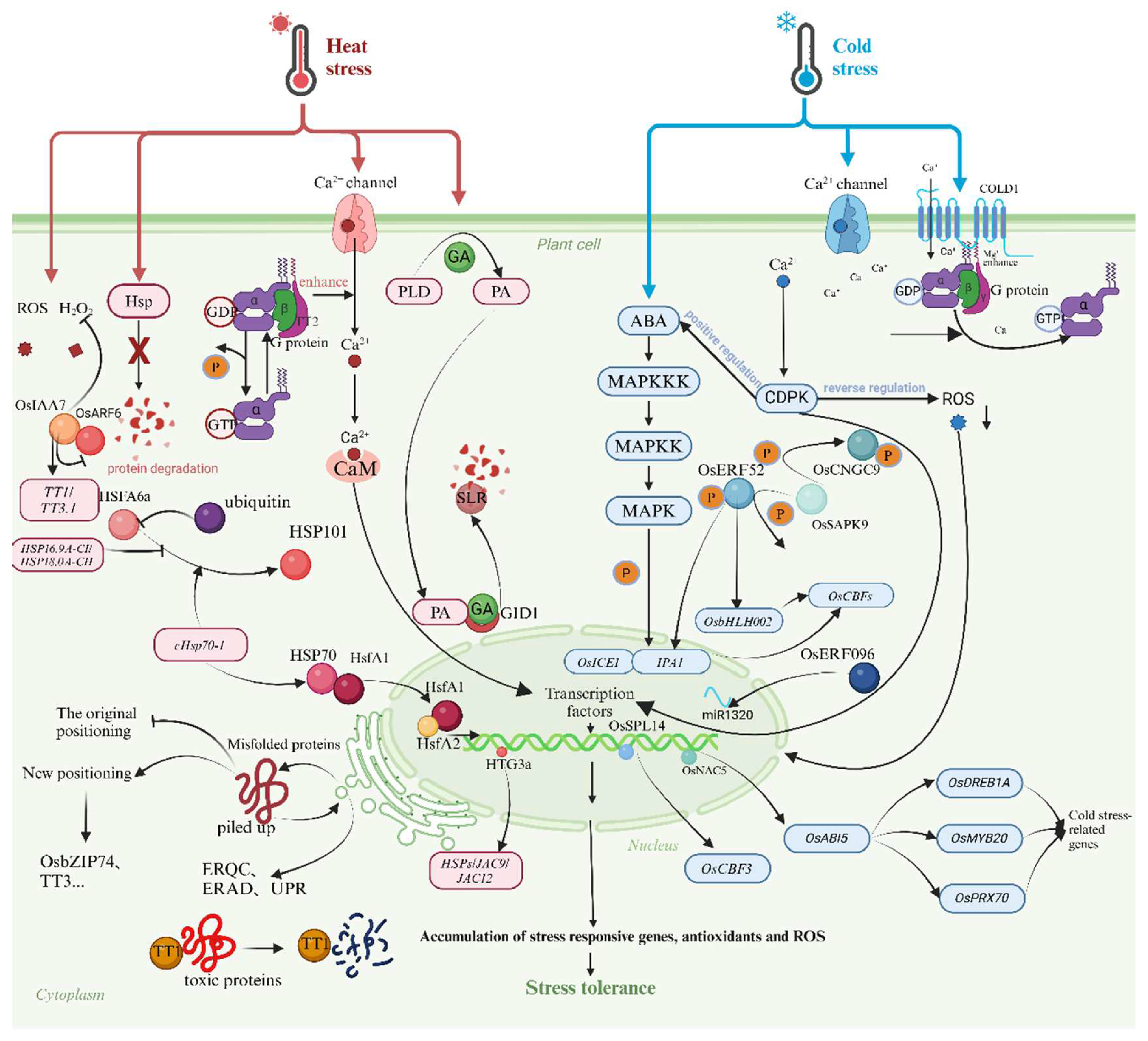
2.3. High-Temperature Stress
2.4. Low Temperature Stress
2.5. UV-B Stress
| Gene | Expression Site | Subcellular Localization | Function | References |
|---|---|---|---|---|
| OsUVR8a | Leaves and leaf sheaths | Nucleus | Enhances UV-B stress tolerance | [130,134] |
| OsUVR8b | Leaves and leaf sheathst | Nucleus | Enhances UV-B stress tolerance | [130,134] |
| OsUGT706C2 | Leaves | Plasma membrane and nucleus | Enhances UV-B stress tolerance | [138] |
| OsMYB44 | Leaves | Nucleus | Enhances UV-B stress tolerance | [139] |
| OsMYB110 | Roots, leaves, and leaf sheaths | Nucleus | Enhances UV-B stress tolerance | [139] |
| OsRLCK160 | Roots, leaves, veins, leaf sheaths, stems, and panicles | Cell membrane and nucleus | Enhances UV-B stress tolerance | [140] |
| OsbZIP48 | Roots, leaf sheaths, stems, and panicles | Nucleus | Enhances UV-B stress tolerance | [140] |
2.6. Heavy Metal Stress
| Gene | Expression Site | Subcellular Localization | Function | References |
|---|---|---|---|---|
| OsHMA3 | Roots | Vacuolar membrane | Transports Cd | [163] |
| OsLCT2 | Leaves, roots | Endoplasmic reticulum | Transports Cd | [164] |
| OsZIP1 | Roots | Plasma membrane and endoplasmic reticulum | Transports Zn, Cu, and Cd | [165] |
| OsZIP5 | Roots | Plasma membrane | Transports Zn and Cd | [166] |
| OsZIP7 | Roots | Plasma membrane | Transports Cd | [169] |
| OsZIP9 | Roots | Plasma membrane | Transports Zn and Cd | [166] |
| OsMT1e | Roots | Nucleus | Increases Cd tolerance | [167] |
| OsIRT1 | Roots | Plasma membrane | Transports Fe and Cd | [168] |
| OsIRT2 | Roots | Plasma membrane | Transports Fe and Cd | [169] |
| OsNramp1 | Leaves, roots | Plasma membrane | Transports Cd | [170] |
| OsNramp2 | Leaves, roots | Vacuolar membrane | Transports Cd | [171] |
| OsNramp5 | Roots | Plasma membrane | Absorbs Cd and Mn | [172] |
3. Mechanisms of Rice Responses to Abiotic Stress
3.1. Physiological and Biochemical Responses
3.2. Molecular Mechanisms
3.2.1. Transcription Factors
3.2.2. Hormone Signaling Pathways
3.2.3. Non-Coding RNAs and Epigenetic Regulation
4. Applications of Abiotic Stress Research in Rice Breeding
4.1. Traditional Breeding Techniques
4.2. Molecular Breeding-Related Technologies
4.3. Gene Editing Techniques
4.4. Integrated Breeding Approaches
5. Challenges and Future Prospects
5.1. Challenges
5.2. Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruan, B.; Shang, L.; Zhang, B.; Hu, J.; Wang, Y.; Lin, H.; Zhang, A.; Liu, C.; Peng, Y.; Zhu, L.; et al. Natural variation in the promoter of TGW2 determines grain width and weight in rice. New Phytol. 2020, 227, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yu, X.; Zhang, C.; Hou, L.; Wu, N.; Zhang, W.; Wang, Y.; Yao, B.; Delaplace, P.; Tian, J. Harnessing microbial interactions with rice: Strategies for abiotic stress alleviation in the face of environmental challenges and climate change. Sci. Total Environ. 2024, 912, 168847. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, G.; Cui, Z.; Kong, X.; Yu, X.; Gui, R.; Han, Y.; Li, Z.; Lang, H.; Hua, Y.; et al. Regain flood adaptation in rice through a 14-3-3 protein OsGF14h. Nat. Commun. 2022, 13, 5664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hu, Y.; Pan, X.; Cao, R.; Hu, Q.; Fu, R.; Risalat, H.; Shang, B. Effects of increased ozone on rice panicle morphology. iScience 2023, 26, 106471. [Google Scholar] [CrossRef]
- Salehin, M. Emerging roles of auxin in plant abiotic stress tolerance. Physiol. Plant 2024, 176, 6. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, F.; Xie, P.; Sun, S.; Qiao, X.; Tang, S.; Chen, C.; Yang, S.; Mei, C.; Yang, D.; et al. A Gγ protein regulates alkaline sensitivity in crops. Science 2023, 24, 379. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant Growth-Promoting Bacteria: Biological Tools for the Mitigation of Salinity Stress in Plants. Front. Microbiol. 2020, 7, 111216. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Ogaya, R.; Fernández-Martínez, M.; Pugh, T.A.M.; Peñuelas, J. Increasing climatic sensitivity of global grassland vegetation biomass and species diversity corre-lates with water availability. New Phytol. 2021, 230, 1761–1771. [Google Scholar] [CrossRef]
- Dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Dolferus, R.; Ji, X.; Richards, R.A. Abiotic stress and control of grain number in cereals. Plant Sci. 2011, 181, 331–341. [Google Scholar] [CrossRef]
- Chirivì, D.; Betti, C. Molecular Links between Flowering and Abiotic Stress Response: A Focus on Poaceae. Plants 2023, 12, 331. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Basu, S.; Kumar, G. A systematic review on the implications of concurrent heat and drought stress in modulating floral development in plants. Plant Sci. 2024, 349, 112248. [Google Scholar]
- Sahebi, M.; Hanafi, M.M.; Rafii, M.Y.; Mahmud, T.M.M.; Azizi, P.; Osman, M.; Abiri, R.; Taheri, S.; Kalhori, N.; Shabanimofrad, M.; et al. Improvement of Drought Tolerance in Rice (O. sativa L.): Genetics, Genomic Tools, and the WRKY Gene Family. BioMed Res. Int. 2018, 2018, 3158474. [Google Scholar]
- Zargar, S.M.; Mir, R.A.; Ebinezer, L.B.; Masi, A.; Hami, A.; Manzoor, M.; Salgotra, R.K.; Sofi, N.R.; Mushtaq, R.; Rohila, J.S.; et al. Physiological and Multi-Omics Approaches for Explaining Drought Stress Tolerance and Supporting Sustainable Production of Rice. Front. Plant Sci. 2021, 12, 803603. [Google Scholar] [CrossRef] [PubMed]
- Geng, A.; Lian, W.; Wang, Y.; Liu, M.; Zhang, Y.; Wang, X.; Chen, G. Molecular Mechanisms and Regulatory Pathways Underlying Drought Stress Response in Rice. Int. J. Mol. Sci. 2024, 25, 1185. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar]
- Li, Q.; Zhu, P.; Yu, X.; Xu, J.; Liu, G. Physiological and Molecular Mechanisms of Rice Tolerance to Salt and Drought Stress: Advances and Future Directions. Int. J. Mol. Sci. 2024, 25, 9404. [Google Scholar] [CrossRef]
- Sun, Y.; Lai, Y.; Wang, Q.; Song, Q.; Jin, L.; Zeng, X.; Feng, Y.; Lu, X. Combination of Water-Saving Irrigation and Nitrogen Fertilization Regulates Greenhouse Gas Emissions and Increases Rice Yields in High-Cold Regions, Northeast China. Int. J. Environ. Res. Public Health 2022, 19, 16506. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Bao, X.; Zhi, Y.; Wu, Q.; Guo, Y.; Yin, X.; Zeng, L.; Li, J.; Zhang, J.; He, W.; et al. Overexpression of a MYB Family Gene, OsMYB6, Increases Drought and Salinity Stress Tolerance in Transgenic Rice. Front. Plant Sci. 2019, 10, 168. [Google Scholar] [CrossRef]
- Du, D.; Zhang, C.; Xing, Y.; Lu, X.; Cai, L.; Yun, H.; Zhang, Q.; Zhang, Y.; Chen, X.; Liu, M.; et al. The CC-NB-LRR OsRLR1 mediates rice disease resistance through interaction with OsWRKY19. Plant Biotechnol. J. 2021, 19, 1052–1064. [Google Scholar]
- Raza, A.; Charagh, S.; Karikari, B.; Sharif, R.; Yadav, V.; Mubarik, M.S.; Habib, M.; Zhuang, Y.; Zhang, C.; Chen, H.; et al. miRNAs for crop improvement. Plant Physiol. Biochem. 2023, 201, 107857. [Google Scholar]
- Qu, X.; Zou, J.; Wang, J.; Yang, K.; Wang, X.; Le, J. A Rice R2R3-Type MYB Transcription Factor OsFLP Positively Regulates Drought Stress Response via OsNAC. Int. J. Mol. Sci. 2022, 23, 5873. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, J.; Zhang, L.; Qi, H.; Yang, L.; Wang, H.; Wang, J.; Wang, Y.; Du, H.; Tao, Z.; et al. Nuclear translocation of OsMFT1 that is impeded by OsFTIP1 promotes drought tolerance in rice. Mol. Plant 2021, 14, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tang, M.; Wang, M.; Yu, Y.; Ruan, B. Advances in Understanding Drought Stress Responses in Rice: Molecular Mechanisms of ABA Signaling and Breeding Prospects. Genes. Genet. Syst. 2024, 15, 1529. [Google Scholar]
- Xie, Z.; Jin, L.; Sun, Y.; Zhan, C.; Tang, S.; Qin, T.; Liu, N.; Huang, J. OsNAC120 balances plant growth and drought tolerance by integrating GA and ABA signaling in rice. Plant Commun. 2024, 5, 100782. [Google Scholar]
- Han, S.; Wang, Y.; Li, Y.; Zhu, R.; Gu, Y.; Li, J.; Guo, H.; Ye, W.; Nabi, H.G.; Yang, T.; et al. The OsNAC41-RoLe1-OsAGAP module promotes root development and drought resistance in upland rice. Mol. Plant 2024, 17, 1573–1593. [Google Scholar]
- Yang, L.; Xu, L.; Guo, J.; Li, A.; Qi, H.; Wang, J.; Song, S. SNAC1-OsERF103-OsSDG705 module mediates drought response in rice. New Phytol. 2024, 241, 2480–2494. [Google Scholar] [CrossRef]
- Chang, Y.; Fang, Y.; Liu, J.; Ye, T.; Li, X.; Tu, H.; Ye, Y.; Wang, Y.; Xiong, L. Stress-induced nuclear translocation of ONAC023 improves drought and heat tolerance through multiple processes in rice. Nat. Commun. 2024, 15, 5877. [Google Scholar]
- Li, Y.; Han, S.; Sun, X.; Khan, N.U.; Zhong, Q.; Zhang, Z.; Zhang, H.; Ming, F.; Li, Z.; Li, J. Variations in OsSPL10 confer drought tolerance by directly regulating OsNAC2 expression and ROS production in rice. J. Integr. Plant Biol. 2023, 65, 918–933. [Google Scholar]
- Ruan, B.; Jiang, Y.; Ma, Y.; Zhou, M.; Chen, F.; Zhang, Y.; Yu, Y.; Wu, L. Characterization of the ddt1 Mutant in Rice and Its Impact on Plant Height Reduction and Water Use Efficiency. Int. J. Mol. Sci. 2024, 25, 7629. [Google Scholar] [CrossRef]
- Hopmans, J.W.; Qureshi, A.S.; Kisekka, I.; Munns, R.; Grattan, S.R.; Rengasamy, P.; Ben-Gal, A.; Assouline, S.; Javaux, M.; Minhas, P.S.; et al. Chapter One-Critical knowledge gaps and research priorities in global soil salinity. Adv. Agron. 2021, 169, 1–191. [Google Scholar]
- Hamani, A.K.M.; Chen, J.; Soothar, M.K.; Wang, G.; Shen, X.; Gao, Y.; Qiu, R. Application of Exogenous Protectants Mitigates Salt-Induced Na+ Toxicity and Sustains Cotton (Gossypium hirsutum L.) Seedling Growth: Comparison of Glycine Betaine and Salicylic Acid. Plants 2021, 10, 380. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liu, C.; Liu, L.; Tan, Y.; Sheng, X.; Yu, D.; Sun, Z.; Sun, X.; Chen, J.; Yuan, D.; et al. Effect of salinity stress on rice yield and grain quality: A meta-analysis. Eur. J. Agron. 2023, 144, 126765. [Google Scholar]
- Shen, L.; Fan, W.; Li, N.; Wu, Q.; Chen, D.; Luan, J.; Zhang, G.; Tian, Q.; Jing, W.; Zhang, Q.; et al. Rice potassium transporter OsHAK18 mediates phloem K+ loading and redistribution. Plant J. 2023, 116, 201–216. [Google Scholar] [PubMed]
- Qin, H.; Wang, J.; Chen, X.; Wang, F.; Peng, P.; Zhou, Y.; Miao, Y.; Zhang, Y.; Gao, Y.; Qi, Y.; et al. Rice OsDOF15 contributes to ethylene-inhibited primary root elongation under salt stress. New Phytol. 2019, 223, 798–813. [Google Scholar]
- Wang, H.Q.; Zhao, X.Y.; Xuan, W.; Wang, P.; Zhao, F.J. Rice roots avoid asymmetric heavy metal and salinity stress via an RBOH-ROS-auxin signaling cascade. Mol. Plant 2023, 16, 1678–1694. [Google Scholar]
- Razzaq, A.; Ali, A.; Safdar, L.B.; Zafar, M.M.; Rui, Y.; Shakeel, A.; Shaukat, A.; Ashraf, M.; Gong, W.; Yuan, Y. Salt stress induces physiochemical alterations in rice grain composition and quality. J. Food Sci. 2020, 85, 14–20. [Google Scholar]
- Zhao, J.; Meng, X.; Zhang, Z.; Wang, M.; Nie, F.; Liu, Q. OsLPR5 Encoding Ferroxidase Positively Regulates the Tolerance to Salt Stress in Rice. Int. J. Mol. Sci. 2023, 24, 8115. [Google Scholar] [CrossRef]
- Liang, X.; Li, J.; Yang, Y.; Jiang, C.; Guo, Y. Designing salt stress-resilient crops: Current progress and future challenges. J. Integr. Plant Biol. 2024, 66, 303–329. [Google Scholar]
- Singh, R.K.; Kota, S.; Flowers, T.J. Salt tolerance in rice: Seedling and reproductive stage QTL mapping come of age. Theor. Appl. Genet. 2021, 134, 3495–3533. [Google Scholar] [CrossRef]
- Qin, H.; Li, Y.; Huang, R. Advances and Challenges in the Breeding of Salt-Tolerant Rice. Int. J. Mol. Sci. 2020, 21, 8385. [Google Scholar] [CrossRef]
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.; Srivastava, S. Morphological, Physiological and Molecular Markers for Salt-Stressed Plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Ponce, K.S.; Meng, L.; Guo, L.; Leng, Y.; Ye, G. Advances in Sensing, Response and Regulation Mechanism of Salt Tolerance in Rice. Int. J. Mol. Sci. 2021, 22, 2254. [Google Scholar] [CrossRef]
- Yu, H.; Teng, Z.; Liu, B.; Lv, J.; Chen, Y.; Qin, Z.; Peng, Y.; Meng, S.; He, Y.; Duan, M.; et al. Transcription factor OsMYB30 increases trehalose content to inhibit α-amylase and seed germination at low temperature. Plant Physiol. 2024, 194, 1815–1833. [Google Scholar] [CrossRef] [PubMed]
- Arabia, S.; Shah, M.N.A.; Sami, A.A.; Ghosh, A.; Islam, T. Identification and expression profiling of proline metabolizing genes in Arabidopsis thaliana and Oryza sativa to reveal their stress-specific transcript alteration. Physiol. Mol. Biol. Plants 2021, 27, 1469–1485. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Huang, R. The phytohormonal regulation of Na+/K+ and reactive oxygen species homeostasis in rice salt response. Mol. Breed. 2020, 40, 47. [Google Scholar]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Nadarajah, K.; Kumar, I.S. Drought Response in Rice: The miRNA Story. Int. J. Mol. Sci. 2019, 20, 3766. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Xie, T.; Shen, J.; Liang, T.; Yin, L.; Liu, K.; He, Y.; Chen, M.; Tang, H.; Chen, S.; et al. Potassium transporter OsHAK9 regulates seed germination under salt stress by preventing gibberellin degradation through mediating OsGA2ox7 in rice. J. Integr. Plant Biol. 2024, 66, 731–748. [Google Scholar] [CrossRef]
- Pachamuthu, K.; Hari Sundar, V.; Narjala, A.; Singh, R.R.; Das, S.; Avik Pal, H.C.Y.; Shivaprasad, P.V. Nitrate-dependent regulation of miR444-OsMADS27 signalling cascade controls root development in rice. J. Exp. Bot. 2022, 73, 3511–3530. [Google Scholar] [CrossRef]
- Li, X.M.; Chao, D.Y.; Wu, Y.; Huang, X.; Chen, K.; Cui, L.G.; Su, L.; Ye, W.W.; Chen, H.; Chen, H.C.; et al. Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat. Genet. 2015, 47, 827–833. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Zhai, Y.; Wen, Z.; Liu, J.; Xi, C.; Zhao, H.; Wang, Y.; Han, S. OsOSCA1.1 Mediates Hyperosmolality and Salt Stress Sensing in Oryza sativa. Biology 2022, 11, 678. [Google Scholar] [CrossRef]
- Xie, Q.; Zhou, Y.; Jiang, X. Structure, Function, and Regulation of the Plasma Membrane Na+/H+ Antiporter Salt Overly Sensitive 1 in Plants. Front. Plant Sci. 2022, 13, 866265. [Google Scholar]
- Kumar, G.; Basu, S.; Singla-Pareek, S.L.; Pareek, A. Unraveling the contribution of OsSOS2 in conferring salinity and drought tolerance in a high-yielding rice. Physiol. Plant 2022, 174, e13638. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, L.; Han, X.; He, G.; Fan, W.; Li, Y.; Yang, S.; Zhang, Z.; Yang, Y.; Jin, W.; et al. Phosphatidic acid-regulated SOS2 controls sodium and potassium homeostasis in Arabidopsis under salt stress. EMBO J. 2023, 42, e112401. [Google Scholar] [CrossRef]
- Li, J.; Zhou, H.; Zhang, Y.; Li, Z.; Yang, Y.; Guo, Y. The GSK3-like Kinase BIN2 Is a Molecular Switch between the Salt Stress Response and Growth Recovery in Arabidopsis thaliana. Dev. Cell 2020, 55, 367–380.e6. [Google Scholar] [CrossRef]
- Cai, Y.S.; Cai, J.L.; Lee, J.T.; Li, Y.M.; Balladona, F.K.; Sukma, D.; Chan, M.T. Arabidopsis AtMSRB5 functions as a salt-stress protector for both Arabidopsis and rice. Front. Plant Sci. 2023, 14, 1072173. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, C.; Xuan, W.; An, H.; Tian, Y.; Wang, B.; Chi, W.; Chen, G.; Ge, Y.; Li, J.; et al. Genome-wide association studies identify OsWRKY53 as a key regulator of salt tolerance in rice. Nat. Commun. 2023, 14, 3550. [Google Scholar] [CrossRef]
- Gao, R.; Jia, Y.; Xu, X.; Fu, P.; Zhou, J.; Yang, G. Structural insights into the Oryza sativa cation transporters HKTs in salt tolerance. J. Integr. Plant Biol. 2024, 66, 700–708. [Google Scholar]
- Alam, M.M.; Tanaka, T.; Nakamura, H.; Ichikawa, H.; Kobayashi, K.; Yaeno, T.; Yamaoka, N.; Shimomoto, K.; Takayama, K.; Nishina, H.; et al. Overexpression of a rice heme activator protein gene (OsHAP2E) confers resistance to pathogens, salinity and drought, and increases photosynthesis and tiller number. Plant Biotechnol. J. 2015, 13, 85–96. [Google Scholar]
- Ly, L.K.; Ho, T.M.; Bui, T.P.; Nguyen, L.T.; Phan, Q.; Le, N.T.; Khuat, L.T.M.; Le, L.H.; Chu, H.H.; Pham, N.B.; et al. CRISPR/Cas9 targeted mutations of OsDSG1 gene enhanced salt tolerance in rice. Funct. Integr. Genom. 2024, 24, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, J.; Chen, Y.; Huang, J.; Liang, W. Genome-Wide Identification of MKK Gene Family and Response to Hormone and Abiotic Stress in Rice. Plants 2024, 13, 2922. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, X.K.; Liu, X.; Yang, X.Q.; Li, Y.J.; Hou, B.K. Rice glycosyltransferase gene UGT2 functions in salt stress tolerance under the regulation of bZIP23 transcription factor. Plant Cell Rep. 2023, 42, 17–28. [Google Scholar] [PubMed]
- Molla, K.A. Molecular switch to regulate salt tolerance in rice. Plant Cell 2023, 35, 3396–3397. [Google Scholar]
- Park, S.I.; Kim, J.J.; Shin, S.Y.; Kim, Y.S.; Yoon, H.S. ASR Enhances Environmental Stress Tolerance and Improves Grain Yield by Modulating Stomatal Closure in Rice. Front. Plant Sci. 2019, 10, 1752. [Google Scholar]
- Zhang, Q.; Liu, Y.; Jiang, Y.; Li, A.; Cheng, B.; Wu, J. OsASR6 Enhances Salt Stress Tolerance in Rice. Int. J. Mol. Sci. 2022, 23, 9340. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Z.; Wang, Y.; Wang, J.; Xiao, M.; Liu, H.; Quan, R.; Zhang, H.; Huang, R.; Zhu, L.; et al. Cellulose synthase-like protein OsCSLD4 plays an important role in the response of rice to salt stress by mediating abscisic acid biosynthesis to regulate osmotic stress tolerance. Plant Biotechnol. J. 2022, 20, 468–484. [Google Scholar]
- Oda, S.; Kaneko, F.; Yano, K.; Fujioka, T.; Masuko, H.; Park, J.I.; Kikuchi, S.; Hamada, K.; Endo, M.; Nagano, K.; et al. Morphological and gene expression analysis under cool temperature conditions in rice anther development. Genes. Genet. Syst. 2010, 85, 107–120. [Google Scholar]
- Shi, W.; Li, X.; Schmidt, R.C.; Struik, P.C.; Yin, X.; Jagadish, S.V.K. Pollen germination and in vivo fertilization in response to high-temperature during flowering in hybrid and inbred rice. Plant Cell Environ. 2018, 41, 1287–1297. [Google Scholar] [PubMed]
- Coast, O.; Murdoch, A.J.; Ellis, R.H.; Hay, F.R.; Jagadish, K.S. Resilience of rice (Oryza spp.) pollen germination and tube growth to temperature stress. Plant Cell Environ. 2016, 39, 26–37. [Google Scholar]
- Guo, H.; Zeng, Y.; Li, J.; Ma, X.; Zhang, Z.; Lou, Q.; Li, J.; Gu, Y.; Zhang, H.; Li, J.; et al. Differentiation, evolution and utilization of natural alleles for cold adaptability at the reproductive stage in rice. Plant Biotechnol. J. 2020, 18, 2491–2503. [Google Scholar]
- Yan, H.; Zhang, B.; Zhang, Y.; Chen, X.; Xiong, H.; Matsui, T.; Tian, X. High Temperature Induced Glume Closure Resulted in Lower Fertility in Hybrid Rice Seed Production. Front. Plant Sci. 2016, 7, 1960. [Google Scholar]
- Yang, J.; Zhang, Y.Z.; He, H.H.; Li, Y.C.; Chen, X.R.; Bian, J.M.; Jin, G.H.; Li, X.X.; Huang, S.E. Current status and research advances of high-temperature hazards in rice. J. Appl. Ecol. 2020, 31, 2817–2830. [Google Scholar]
- Riaz, A.; Thomas, J.; Ali, H.H.; Zaheer, M.S.; Ahmad, N.; Pereira, A. High night temperature stress on rice (Oryza sativa)-insights from phenomics to physiology. A review. Funct. Plant Biol. 2024, 51, FP24057. [Google Scholar]
- Ren, H.; Bao, J.; Gao, Z.; Sun, D.; Zheng, S.; Bai, J. How rice adapts to high temperatures. Front. Plant Sci. 2023, 14, 1137923. [Google Scholar]
- Xing, Y.H.; Lu, H.; Zhu, X.; Deng, Y.; Xie, Y.; Luo, Q.; Yu, J. How Rice Responds to Temperature Changes and Defeats Heat Stress. Rice 2024, 17, 73. [Google Scholar]
- Wang, Y.; Chen, F.; Chen, Y.; Ren, K.; Zhao, D.; Li, K.; Li, H.; Wan, X.; Peng, M.; Xiang, Z.; et al. Identification and analysis of drought-responsive F-box genes in upland rice and involvement of OsFBX148 in ABA response and ROS accumulation. BMC Plant Biol. 2024, 24, 1120. [Google Scholar]
- Rezaul, I.M.; Baohua, F.; Tingting, C.; Weimeng, F.; Caixia, Z.; Longxing, T.; Guanfu, F. Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiol. Plant 2019, 165, 644–663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, J.F.; Kan, Y.; Shan, J.X.; Ye, W.W.; Dong, N.Q.; Guo, T.; Xiang, Y.H.; Yang, Y.B.; Li, Y.C.; et al. A genetic module at one locus in rice protects chloroplasts to enhance thermotolerance. Science 2022, 376, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Mu, X.R.; Zhang, H.; Gao, J.; Shan, J.X.; Ye, W.W.; Lin, H.X. TT2 controls rice thermotolerance through SCT1-dependent alteration of wax biosynthesis. Nat. Plants 2022, 8, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Yao, P.; Yang, J.; Hou, J.; Xiao, H.; Wu, Y.; Tu, D.; Ma, X.; Zhao, Y.; Li, L. OsIAA7 enhances heat stress tolerance by inhibiting the activity of OsARF6 in rice. Int. J. Biol. Macromol. 2024, 288, 138746. [Google Scholar] [CrossRef]
- Wang, D.; Pei, K.; Fu, Y.; Sun, Z.; Li, S.; Liu, H.; Tang, K.; Han, B.; Tao, Y. Genome-wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa). Gene 2007, 394, 13–24. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Ou, S.; Wang, R.; Wang, Y.; Chu, C.; Yao, S. Natural variations of SLG1 confer high-temperature tolerance in indica rice. Nat. Commun. 2020, 11, 5441. [Google Scholar] [CrossRef]
- Andrási, N.; Pettkó-Szandtner, A.; Szabados, L. Diversity of plant heat shock factors: Regulation, interactions, and functions. J. Exp. Bot. 2021, 72, 1558–1575. [Google Scholar] [CrossRef]
- Lavania, D.; Dhingra, A.; Grover, A. Analysis of transactivation potential of rice (Oryza sativa L.) heat shock factors. Planta 2018, 247, 1267–1276. [Google Scholar] [CrossRef]
- Li, J.; Cao, Y.; Zhang, J.; Zhu, C.; Tang, G.; Yan, J. The miR165/166-PHABULOSA module promotes thermotolerance by transcriptionally and posttranslationally regulating HSFA1. Plant Cell 2023, 35, 2952–2971. [Google Scholar] [CrossRef]
- Singh, G.; Sarkar, N.K.; Grover, A. Hsp70, sHsps and ubiquitin proteins modulate HsfA6a-mediated Hsp101 transcript expression in rice (Oryza sativa L.). Physiol. Plant 2021, 173, 2055–2067. [Google Scholar] [CrossRef]
- Zhu, T.; Herrfurth, C.; Xin, M.; Savchenko, T.; Feussner, I.; Goossens, A.; De Smet, I. Warm temperature triggers JOX and ST2A-mediated jasmonate catabolism to promote plant growth. Nat. Commun. 2021, 12, 4804. [Google Scholar] [PubMed]
- Chang, L.; Liu, Z.; Ying, X.; Kalandarov, B.; Ergashev, M.; Tong, X.; Zhang, J.; Jin, J.; Ying, J. Molecular Basis of Lipid Metabolism in Oryza sativa L. Plants 2024, 13, 3263. [Google Scholar] [CrossRef]
- Liu, J.; Sun, X.; Xu, F.; Zhang, Y.; Zhang, Q.; Miao, R.; Zhang, J.; Liang, J.; Xu, W. Suppression of OsMDHAR4 enhances heat tolerance by mediating H2O2-induced stomatal closure in rice plants. Rice 2018, 11, 38. [Google Scholar] [PubMed]
- Vinarao, R.; Proud, C.; Snell, P.; Fukai, S.; Mitchell, J. Genomic Regions and Floral Traits Contributing to Low Temperature Tolerance at Young Microspore Stage in a Rice (Oryza sativa L.) Recombinant Inbred Line Population of Sherpa/IRAT109. Front. Plant Sci. 2022, 13, 873677. [Google Scholar]
- Shrestha, S.; Mahat, J.; Shrestha, J.; Madhav, K.C.; Paudel, K. Influence of high-temperature stress on rice growth and development. A review. Heliyon 2022, 8, e12651. [Google Scholar]
- Fahad, S.; Hussain, S.; Saud, S.; Tanveer, M.; Bajwa, A.A.; Hassan, S.; Shah, A.N.; Ullah, A.; Wu, C.; Khan, F.A.; et al. A biochar application protects rice pollen from high-temperature stress. Plant Physiol. Biochem. 2015, 96, 281–287. [Google Scholar] [PubMed]
- Wang, G.; Li, X.; Shen, W.; Li, M.W.; Huang, M.; Zhang, J.; Li, H. The chromatin accessibility landscape of pistils and anthers in rice. Plant Physiol. 2022, 190, 2797–2811. [Google Scholar]
- Huang, B.; Fan, Y.; Cui, L.; Li, C.; Guo, C. Cold Stress Response Mechanisms in Anther Development. Int. J. Mol. Sci. 2022, 24, 30. [Google Scholar] [CrossRef]
- Al Mamun, E.; Cantrill, L.C.; Overall, R.L.; Sutton, B.G. Mechanism of low-temperature-induced pollen failure in rice. Cell Biol. Int. 2010, 34, 469–476. [Google Scholar]
- Yamamori, K.; Ogasawara, K.; Ishiguro, S.; Koide, Y.; Takamure, I.; Fujino, K.; Sato, Y.; Kishima, Y. Revision of the relationship between anther morphology and pollen sterility by cold stress at the booting stage in rice. Ann. Bot. 2021, 128, 559–575. [Google Scholar]
- Costa Silva Neta, I.; Vilela De Resende Von Pinho, É.; De Abreu, V.M.; Rezende Vilela, D.; Santos, M.C.; Oliveira Dos Santos, H.; Diniz Cabral Ferreira, R.A.; Garcia Von Pinho, R.; Coelho De Castro Vasconcellos, R. Gene expression and genetic control to cold tolerance during maize seed germination. BMC Plant Biol. 2020, 20, 188. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Hsu, Y.T. Biochemical responses of rice roots to cold stress. Bot. Stud. 2019, 60, 14. [Google Scholar] [CrossRef]
- Wei, X.; Liu, S.; Sun, C.; Xie, G.; Wang, L. Convergence and Divergence: Signal Perception and Transduction Mechanisms of Cold Stress in Arabidopsis and Rice. Plants 2021, 10, 1864. [Google Scholar] [CrossRef]
- Venzhik, Y.; Deryabin, A.; Moshkov, I. Adaptive strategy of plant cells during chilling: Aspect of ultrastructural reorganization. Plant Sci. 2023, 332, 111722. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, J.; Liu, Y.; Gong, X.; Xu, J.; Lin, D.; Dong, Y. The Rice Pentatricopeptide Repeat Gene TCD10 is Needed for Chloroplast Development under Cold Stress. Rice 2016, 9, 67. [Google Scholar] [CrossRef]
- González-Schain, N.; Roig-Villanova, I.; Kater, M.M. Early cold stress responses in post-meiotic anthers from tolerant and sensitive rice cultivars. Rice 2019, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.F.; Zhang, S.H.; Zhao, J.; Huang, Z.H.; Zhang, G.Q.; Liu, B. Meta-analysis of QTLs underlying cold tolerance in rice (Oryza sativa L.). Mol. Plant Breed. 2015, 13, 1–15. [Google Scholar]
- Lu, X.; Zhou, Y.; Fan, F.; Peng, J.; Zhang, J. Coordination of light, circadian clock with temperature: The potential mechanisms regulating chilling tolerance in rice. J. Integr. Plant Biol. 2020, 62, 737–760. [Google Scholar] [CrossRef]
- Li, N.; Miao, J.; Li, Y.; Ji, F.; Yang, M.; Dai, K.; Zhou, Z.; Hu, D.; Guo, H.; Fang, H.; et al. Comparative transcriptome analysis and meta-QTLs mapping reveal the regulatory mechanism of cold tolerance in rice at the budding stage. Heliyon 2024, 10, e37933. [Google Scholar] [CrossRef]
- Solis, C.A.; Yong, M.T.; Venkataraman, G.; Milham, P.; Zhou, M.; Shabala, L.; Holford, P.; Shabala, S.; Chen, Z.H. Sodium sequestration confers salinity tolerance in an ancestral wild rice. Physiol. Plant 2021, 172, 1594–1608. [Google Scholar] [CrossRef]
- Nasiri, N.; Shokri, E.; Nematzadeh, G.A. Aeluropus littoralis NaCl-induced vacuolar H+-ATPase subunit c: Molecular cloning and expression analysis. Genetika 2012, 48, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; He, M.; Zhang, C.; Liu, Y.; Li, X.; Wang, Z.; Jin, X.; Sui, J.; Zhou, W.; et al. OsMAPK6 positively regulates rice cold tolerance at seedling stage via phosphorylating and stabilizing OsICE1 and OsIPA1. Theor. Appl. Genet. 2023, 137, 10. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Li, H.; Ding, Y.; Shi, Y.; Song, C.; Gong, Z.; Yang, S. BRASSINOSTEROID-INSENSITIVE2 Negatively Regulates the Stability of Transcription Factor ICE1 in Response to Cold Stress in Arabidopsis. Plant Cell 2019, 31, 2682–2696. [Google Scholar]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Wang, B.; Yang, S.; Guo, Y.; Gong, Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [PubMed]
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 2022, 27, 922–935. [Google Scholar]
- Jia, M.; Meng, X.; Song, X.; Zhang, D.; Kou, L.; Zhang, J.; Jing, Y.; Liu, G.; Liu, H.; Huang, X.; et al. Chilling-induced phosphorylation of IPA1 by OsSAPK6 activates chilling tolerance responses in rice. Sci. Rep. 2022, 8, 71. [Google Scholar]
- Sun, M.; Shen, Y.; Chen, Y.; Wang, Y.; Cai, X.; Yang, J.; Jia, B.; Dong, W.; Chen, X.; Sun, X. Osa-miR1320 targets the ERF transcription factor OsERF096 to regulate cold tolerance via JA-mediated signaling. Plant Physiol. 2022, 189, 2500–2516. [Google Scholar]
- Xu, L.; Yang, L.; Li, A.; Guo, J.; Wang, H.; Qi, H.; Li, M.; Yang, P.; Song, S. An AP2/ERF transcription factor confers chilling tolerance in rice. Sci. Adv. 2024, 10, eado4788. [Google Scholar]
- Wang, J.; Ren, Y.; Liu, X.; Luo, S.; Zhang, X.; Liu, X.; Lin, Q.; Zhu, S.; Wan, H.; Yang, Y.; et al. Transcriptional activation and phosphorylation of OsCNGC9 confer enhanced chilling tolerance in rice. Mol. Plant 2021, 14, 315–329. [Google Scholar]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef]
- Frohnmeyer, H.; Staiger, D. Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 2003, 133, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, K.K.; Agrawal, S.B. Effect of elevated ultraviolet-B on four tropical soybean cultivars: Quantitative and qualitative aspects with special emphasis on gas exchange, chlorophyll fluorescence, biomass and yield. Acta Physiol. Plant 2015, 37, 31. [Google Scholar] [CrossRef]
- Dotto, M.; Casati, P. Developmental reprogramming by UV-B radiation in plants. Plant Sci. 2017, 264, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Kataria, S.; Jajoo, A.; Guruprasad, K.N. Impact of increasing Ultraviolet-B (UV-B) radiation on photosynthetic processes. J. Photochem. Photobiol. B 2014, 137, 55–66. [Google Scholar] [CrossRef]
- Kibria, M.G.; Hossain, M.; Murata, Y.; Hoque, M.A. Antioxidant Defense Mechanisms of Salinity Tolerance in Rice Genotypes. Rice Sci. 2017, 24, 155–162. [Google Scholar] [CrossRef]
- Escobar-Bravo, R.; Klinkhamer, P.G.; Leiss, K.A. Interactive Effects of UV-B Light with Abiotic Factors on Plant Growth and Chemistry, and Their Consequences for Defense against Arthropod Herbivores. Front. Plant Sci. 2017, 8, 278. [Google Scholar] [CrossRef]
- Nascimento, L.B.; Moreira Ndos, S.; Leal-Costa, M.V.; Costa, S.S.; Tavares, E.S. Induction of wound-periderm-like tissue in Kalanchoe pinnata (Lam.) Pers. (Crassulaceae) leaves as a defence response to high UV-B radiation levels. Ann. Bot. 2015, 116, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Willick, I.R.; Lahlali, R.; Vijayan, P.; Muir, D.; Karunakaran, C.; Tanino, K.K. Wheat flag leaf epicuticular wax morphology and composition in response to moderate drought stress are revealed by SEM, FTIR-ATR and synchrotron X-ray spectroscopy. Physiol. Plant 2018, 162, 316–332. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Jin, L.Q.; Li, Y.T.; Tikkanen, M.; Li, Q.M.; Ai, X.Z.; Gao, H.Y. Ultraviolet-B Radiation (UV-B) Relieves Chilling-Light-Induced PSI Photoinhibition And Accelerates The Recovery Of CO2 Assimilation In Cucumber (Cucumis sativus L.) Leaves. Sci. Rep. 2016, 6, 34455. [Google Scholar] [CrossRef]
- Idris, M.; Seo, N.; Jiang, L.; Kiyota, S.; Hidema, J.; Iino, M. UV-B signalling in rice: Response identification, gene expression profiling and mutant isolation. Plant Cell Environ. 2021, 44, 1468–1485. [Google Scholar] [CrossRef]
- Thomas, D.T.; Puthur, J.T. Amplification of abiotic stress tolerance potential in rice seedlings with a low dose of UV-B seed priming. Funct. Plant Biol. 2019, 46, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Challabathula, D.; Puthur, J.T. UV-B Priming of Oryza sativa Seeds Augments the Innate Tolerance Potential in a Tolerant Variety more Effectively Toward NaCl and PEG Stressors. J. Plant Growth Regul. 2021, 40, 1166–1180. [Google Scholar]
- Dhanya Thomas, T.T.; Dinakar, C.; Puthur, J.T. Effect of UV-B priming on the abiotic stress tolerance of stress-sensitive rice seedlings: Priming imprints and cross-tolerance. Plant. Physiol. Biochem. 2020, 147, 21–30. [Google Scholar] [PubMed]
- Hu, S.; Chen, Y.; Qian, C.; Ren, H.; Liang, X.; Tao, W.; Chen, Y.; Wang, J.; Dong, Y.; Han, J.; et al. Nuclear accumulation of rice UV-B photoreceptors is UV-B- and OsCOP1-independent for UV-B responses. Nat. Commun. 2024, 15, 6396. [Google Scholar] [CrossRef]
- Faseela, P.; Puthur, J.T. Intraspecific variation in sensitivity of high yielding rice varieties towards UV-B radiation. Physiol. Mol. Biol. Plants 2019, 25, 727–740. [Google Scholar] [CrossRef]
- Shahzad, R.; Ewas, M.; Harlina, P.W.; Khan, S.U.; Zhenyuan, P.; Nie, X.; Nishawy, E. β-Sitosterol differentially regulates key metabolites for growth improvement and stress tolerance in rice plants during prolonged UV-B stress. J. Genet. Eng. Biotechnol. 2021, 19, 79. [Google Scholar]
- Zedek, F.; Veselý, P.; Tichý, L.; Elliott, T.L.; Garbolino, E.; De Ruffray, P.; Bureš, P. Holocentric plants are more competitive under higher UV-B doses. New Phytol. 2022, 233, 15–21. [Google Scholar]
- Zhang, F.; Guo, H.; Huang, J.; Yang, C.; Li, Y.; Wang, X.; Qu, L.; Liu, X.; Luo, J. A UV-B-responsive glycosyltransferase, OsUGT706C2, modulates flavonoid metabolism in rice. Sci. China Life Sci. 2020, 63, 1037–1052. [Google Scholar]
- Zhang, F.; Yang, C.; Guo, H.; Li, Y.; Shen, S.; Zhou, Q.; Li, C.; Wang, C.; Zhai, T.; Qu, L.; et al. Dissecting the genetic basis of UV-B responsive metabolites in rice. Genome Biol. 2024, 25, 234. [Google Scholar]
- Zhang, F.; Huang, J.; Guo, H.; Yang, C.; Li, Y.; Shen, S.; Zhan, C.; Qu, L.; Liu, X.; Wang, S.; et al. OsRLCK160 contributes to flavonoid accumulation and UV-B tolerance by regulating OsbZIP48 in rice. Sci. China Life Sci. 2022, 65, 1380–1394. [Google Scholar]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Wang, Y.; Wang, W.; Gu, C.; Huang, S.; Yuan, H.; Dhankher, O.P. Quantitative proteomic analysis reveals complex regulatory and metabolic response of Iris lactea Pall. var. chinensis to cadmium toxicity. J. Hazard. Mater. 2020, 400, 123165. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Chu, S.; You, Y.; Chi, Y.; Wang, R.; Yang, X.; Hayat, K.; Zhang, D.; Zhou, P. Comparative cytology combined with transcriptomic and metabolomic analyses of Solanum nigrum L. in response to Cd toxicity. J. Hazard. Mater. 2022, 423, 127168. [Google Scholar] [PubMed]
- Wei, H.Y.; Li, Y.; Yan, J.; Peng, S.Y.; Wei, S.J.; Yin, Y.; Li, K.T.; Cheng, X. Root cell wall remodeling: A way for exopolysaccharides to mitigate cadmium toxicity in rice seedling. J. Hazard. Mater. 2023, 443, 130186. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, J.; Song, K.; Sun, Y.; Qin, Q.; Xue, Y. Transcriptome analysis of rice (Oryza sativa L.) shoots responsive to cadmium stress. Sci. Rep. 2019, 9, 10177. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Rolly, N.K.; Al Azzawi, T.N.I.; Imran, M.; Mun, B.-G.; Lee, I.-J.; Yun, B.-W. Lead (Pb)-Induced Oxidative Stress Alters the Morphological and Physio-Biochemical Properties of Rice (Oryza sativa L.). Agronomy 2021, 11, 409. [Google Scholar] [CrossRef]
- Cong, W.; Li, N.; Miao, Y.; Huang, Y.; Zhao, W.; Kang, Y.; Zhang, B.; Wang, J.; Zhang, J.; Lv, Y.; et al. DNA hypomethylation-associated transcriptional rewiring enables resistance to heavy metal mercury (Hg) stress in rice. J. Hazard. Mater. 2024, 461, 132649. [Google Scholar]
- Mao, Q.; Tang, L.; Ji, W.; Rennenberg, H.; Hu, B.; Ma, M. Elevated CO2 and soil mercury stress affect photosynthetic characteristics and mercury accumulation of rice. Ecotoxicol. Environ. Saf. 2021, 208, 111605. [Google Scholar]
- Murugaiyan, V.; Zeibig, F.; Anumalla, M.; Siddiq, S.A.; Frei, M.; Murugaiyan, J.; Ali, J. Arsenic Stress Responses and Accumulation in Rice. In Rice Improvement; Ali, J., Wani, S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 281–313. [Google Scholar]
- Raghuvanshi, R.; Raut, V.V.; Pandey, M.; Jeyakumar, S.; Verulkar, S.; Suprasanna, P.; Srivastava, A.K. Arsenic and cadmium induced macronutrient deficiencies trigger contrasting gene expression changes in rice. Environ. Pollut. 2022, 300, 118923. [Google Scholar] [CrossRef]
- Senoura, T.; Sakashita, E.; Kobayashi, T.; Takahashi, M.; Aung, M.S.; Masuda, H.; Nakanishi, H.; Nishizawa, N.K. The iron-chelate transporter OsYSL9 plays a role in iron distribution in developing rice grains. Plant Mol. Biol. 2017, 95, 375–387. [Google Scholar]
- Miyadate, H.; Adachi, S.; Hiraizumi, A.; Tezuka, K.; Nakazawa, N.; Kawamoto, T.; Katou, K.; Kodama, I.; Sakurai, K.; Takahashi, H.; et al. OsHMA3, α P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol. 2011, 189, 190–199. [Google Scholar] [PubMed]
- Sasaki, A.; Yamaji, N.; Ma, J.F. Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J. Exp. Bot. 2014, 65, 6013–6021. [Google Scholar] [PubMed]
- Nevo, Y.; Nelson, N. The NRAMP family of metal-ion transporters. Biochim. Biophys. Acta 2006, 1763, 609–620. [Google Scholar]
- Zvobgo, G.; Lwalabawalwalaba, J.; Sagonda, T.; Mutemachani Mapodzeke, J.; Muhammad, N.; Haider Shamsi, I.; Zhang, G. Phosphate alleviates arsenate toxicity by altering expression of phosphate transporters in the tolerant barley genotypes. Ecotoxicol. Environ. Saf. 2018, 147, 832–839. [Google Scholar] [PubMed]
- Ram, H.; Kaur, A.; Gandass, N.; Singh, S.; Deshmukh, R.; Sonah, H.; Sharma, T.R. Molecular characterization and expression dynamics of MTP genes under various spatio-temporal stages and metal stress conditions in rice. PLoS ONE 2019, 14, e0217360. [Google Scholar]
- Peto, A.; Lehotai, N.; Lozano-Juste, J.; León, J.; Tari, I.; Erdei, L.; Kolbert, Z. Involvement of nitric oxide and auxin in signal transduction of copper-induced morphological responses in Arabidopsis seedlings. Ann. Bot. 2011, 108, 449–457. [Google Scholar]
- Chen, Y.A.; Chi, W.C.; Trinh, N.N.; Huang, L.Y.; Chen, Y.C.; Cheng, K.T.; Huang, T.L.; Lin, C.Y.; Huang, H.J. Transcriptome profiling and physiological studies reveal a major role for aromatic amino acids in mercury stress tolerance in rice seedlings. PLoS ONE 2014, 9, e95163. [Google Scholar]
- Trinh, N.N.; Huang, T.-L.; Chi, W.-C.; Fu, S.-F.; Chen, C.-C.; Huang, H.-J.J.P.P. Chromium stress response effect on signal transduction and expression of signaling genes in rice. Physiol. Plant 2014, 150, 205–224. [Google Scholar]
- Jagodzik, P.; Tajdel-Zielinska, M.; Ciesla, A.; Marczak, M.; Ludwikow, A. Mitogen-Activated Protein Kinase Cascades in Plant Hormone Signaling. Front. Plant Sci. 2018, 9, 1387. [Google Scholar]
- Ding, Y.; Ye, Y.; Jiang, Z.; Wang, Y.; Zhu, C. MicroRNA390 Is Involved in Cadmium Tolerance and Accumulation in Rice. Front. Plant Sci. 2016, 7, 235. [Google Scholar]
- Liu, Q.; Zhang, H. Molecular identification and analysis of arsenite stress-responsive miRNAs in rice. J. Agric. Food Chem. 2012, 60, 6524–6536. [Google Scholar]
- Lu, C.; Zhang, L.; Tang, Z.; Huang, X.Y.; Ma, J.F.; Zhao, F.J. Producing cadmium-free Indica rice by overexpressing OsHMA3. Environ. Int. 2019, 126, 619–626. [Google Scholar] [PubMed]
- Tang, L.; Dong, J.; Tan, L.; Ji, Z.; Li, Y.; Sun, Y.; Chen, C.; Lv, Q.; Mao, B.; Hu, Y.; et al. Overexpression of OsLCT2, a Low-Affinity Cation Transporter Gene, Reduces Cadmium Accumulation in Shoots and Grains of Rice. Rice 2021, 14, 89. [Google Scholar] [CrossRef]
- Liu, X.S.; Feng, S.J.; Zhang, B.Q.; Wang, M.Q.; Cao, H.W.; Rono, J.K.; Chen, X.; Yang, Z.M. OsZIP1 functions as a metal efflux transporter limiting excess zinc, copper and cadmium accumulation in rice. BMC Plant Biol. 2019, 19, 283. [Google Scholar]
- Tan, L.; Qu, M.; Zhu, Y.; Peng, C.; Wang, J.; Gao, D.; Chen, C. ZINC TRANSPORTER5 and ZINC TRANSPORTER9 Function Synergistically in Zinc/Cadmium Uptake. Plant Physiol. 2020, 183, 1235–1249. [Google Scholar] [PubMed]
- Rono, J.K.; Le Wang, L.; Wu, X.C.; Cao, H.W.; Zhao, Y.N.; Khan, I.U.; Yang, Z.M. Identification of a new function of metallothionein-like gene OsMT1e for cadmium detoxification and potential phytoremediation. Chemosphere 2021, 265, 129136. [Google Scholar]
- Tao, J.; Lu, L. Advances in Genes-Encoding Transporters for Cadmium Uptake, Translocation, and Accumulation in Plants. Toxics 2022, 10, 411. [Google Scholar] [CrossRef]
- Tan, L.; Zhu, Y.; Fan, T.; Peng, C.; Wang, J.; Sun, L.; Chen, C. OsZIP7 functions in xylem loading in roots and inter-vascular transfer in nodes to deliver Zn/Cd to grain in rice. Biochem. Biophys. Res. Commun. 2019, 23, 112–118. [Google Scholar]
- Chang, J.D.; Huang, S.; Yamaji, N.; Zhang, W.; Ma, J.F.; Zhao, F.J. OsNRAMP1 transporter contributes to cadmium and manganese uptake in rice. Plant Cell Environ. 2020, 43, 2476–2491. [Google Scholar]
- Chang, J.D.; Xie, Y.; Zhang, H.; Zhang, S.; Zhao, F.J. The vacuolar transporter OsNRAMP2 mediates Fe remobilization during germina-tion and affectsCddistribution to rice grain. Plant Soil. 2022, 476, 79–95. [Google Scholar]
- Chang, J.D.; Huang, S.; Noriyuki, K.; Wang, P.; Chen, J.; Huang, X.Y.; Ma, J.F.; Zhao, F.J. Over expression of the manganese/cadmium transporter OsNRAMP5 reduces cadmium accumulation in rice grain. J. Exp. Bot. 2020, 71, 5705–5715. [Google Scholar] [CrossRef]
- Rajkumari, N.; Chowrasia, S.; Nishad, J.; Ganie, S.A.; Mondal, T.K. Metabolomics-mediated elucidation of rice responses to salt stress. Planta 2023, 258, 111. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Wei, C.; Liu, X.; Jiang, L.; Gao, J. Multifunctional Transcription Factor YABBY6 Regulates Morphogenesis, Drought and Cold Stress Responses in Rice. Rice 2024, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ma, H.; Li, T.; Guo, E.; Zhang, T.; Zhang, X.; Yang, X.; Wang, L.; Jiang, S.; Deng, Y.; et al. Innovative modeling on the effects of low-temperature stress on rice yields. J. Exp. Bot. 2024, 76, erae452. [Google Scholar] [CrossRef] [PubMed]
- Resentini, F.; Orozco-Arroyo, G.; Cucinotta, M.; Mendes, M.A. The impact of heat stress in plant reproduction. Front. Plant Sci. 2023, 14, 1271644. [Google Scholar] [CrossRef]
- Soualiou, S.; Duan, F.; Li, X.; Zhou, W. Crop production under cold stress: An understanding of plant responses, acclimation processes, and management strategies. Plant Physiol. Biochem. 2022, 190, 47–61. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Zolla, L.; Rinalducci, S. Low temperature tolerance in plants: Changes at the protein level. Phytochemistry 2015, 117, 76–89. [Google Scholar] [CrossRef]
- Shelp, B.J.; Aghdam, M.S.; Flaherty, E.J. γ-Aminobutyrate (GABA) Regulated Plant Defense: Mechanisms and Opportunities. Plants 2021, 10, 1939. [Google Scholar] [CrossRef]
- Shim, J.S.; Jeong, H.I.; Bang, S.W.; Jung, S.E.; Kim, G.; Kim, Y.S.; Redillas, M.; Oh, S.J.; Seo, J.S.; Kim, J.K. Drought-induced branched-chain amino acid aminotransferase enhances drought tolerance in rice. Plant Physiol. 2023, 191, 1435–1447. [Google Scholar] [CrossRef]
- Bahuguna, R.N.; Jha, J.; Pal, M.; Shah, D.; Lawas, L.M.; Khetarpal, S.; Jagadish, K.S. Physiological and biochemical characterization of NERICA-L-44: A novel source of heat tolerance at the vegetative and reproductive stages in rice. Physiol. Plant 2015, 154, 543–559. [Google Scholar] [CrossRef]
- Larkindale, J.; Knight, M.R. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [PubMed]
- Xu, M.; Ni, Y.; Tu, Y.; Wang, Y.; Zhang, Z.; Jiao, Y.; Zhang, X. A SCYL2 gene from Oryza sativa is involved in phytosterol accumulation and regulates plant growth and salt stress. Plant Sci. 2024, 343, 112062. [Google Scholar]
- Vashisth, V.; Sharma, G.; Giri, J.; Sharma, A.K.; Tyagi, A.K. Rice A20/AN1 protein, OsSAP10, confers water-deficit stress tolerance via proteasome pathway and positive regulation of ABA signaling in Arabidopsis. Plant Cell Rep. 2024, 43, 215. [Google Scholar]
- Wang, Y.; Wang, J.; Sarwar, R.; Zhang, W.; Geng, R.; Zhu, K.M.; Tan, X.L. Research progress on the physiological response and molecular mechanism of cold response in plants. Front. Plant Sci. 2024, 15, 1334913. [Google Scholar]
- Gusain, S.; Joshi, S.; Joshi, R. Sensing, signalling, and regulatory mechanism of cold-stress tolerance in plants. Plant Physiol. Biochem. 2023, 197, 107646. [Google Scholar]
- Liu, M.; Zhou, Y.; Sun, J.; Mao, F.; Yao, Q.; Li, B.; Wang, Y.; Gao, Y.; Dong, X.; Liao, S.; et al. From the floret to the canopy: High temperature tolerance during flowering. Plant Commun. 2023, 4, 100629. [Google Scholar] [PubMed]
- You, J.; Chan, Z. ROS Regulation During Abiotic Stress Responses in Crop Plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Quiñones, C.O.; Gesto-Borroto, R.; Wilson, R.V.; Hernández-Madrigal, S.V.; Lorence, A. Alternative pathways leading to ascorbate biosynthesis in plants: Lessons from the last 25 years. J. Exp. Bot. 2024, 75, 2644–2663. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Q.; Lu, L.; Gao, H.; Liu, Q.; Liu, Y.; Yang, C.; Peng, C. The role of ascorbic acid in rice leaf senescence and photo-carbon imbalance. Funct. Plant Biol. 2020, 47, 263–278. [Google Scholar]
- Fàbregas, N.; Fernie, A.R. The metabolic response to drought. J. Exp. Bot. 2019, 70, 1077–1085. [Google Scholar]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription Factors Associated with Abiotic and Biotic Stress Tolerance and Their Potential for Crops Improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Blumwald, E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Lee, K.H.; Dong, T.; Jeong, J.C.; Jin, J.B.; Kanno, Y.; Kim, D.H.; Kim, S.Y.; Seo, M.; Bressan, R.A.; et al. A vacuolar β-glucosidase homolog that possesses glucose-conjugated abscisic acid hydrolyzing activity plays an important role in osmotic stress responses in Arabidopsis. Plant Cell 2012, 24, 2184–2199. [Google Scholar] [CrossRef]
- Zhang, Y.; Kilambi, H.V.; Liu, J.; Bar, H.; Lazary, S.; Egbaria, A.; Ripper, D.; Charrier, L.; Belew, Z.M.; Wulff, N.; et al. ABA homeostasis and long-distance translocation are redundantly regulated by ABCG ABA importers. Sci. Adv. 2021, 7, eabf6069. [Google Scholar] [CrossRef]
- Kurowska, M.; Daszkowska-Golec, A. Molecular mechanisms of SNAC1 (Stress-responsive NAC1) in conferring the abiotic stress tolerance. Plant Sci. 2023, 337, 111894. [Google Scholar] [CrossRef] [PubMed]
- Kavi Kishor, P.B.; Tiozon, R.N., Jr.; Fernie, A.R.; Sreenivasulu, N. Abscisic acid and its role in the modulation of plant growth, development, and yield stability. Trends Plant Sci. 2022, 27, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.A.U.; Zakari, S.A.; Zhao, Q.; Zhou, L.; Ye, Y.; Cheng, F. Abiotic Stresses Intervene with ABA Signaling to Induce Destructive Metabolic Pathways Leading to Death: Premature Leaf Senescence in Plants. Int. J. Mol. Sci. 2019, 20, 256. [Google Scholar] [CrossRef]
- Li, X.; Yu, B.; Wu, Q.; Min, Q.; Zeng, R.; Xie, Z.; Huang, J. OsMADS23 phosphorylated by SAPK9 confers drought and salt tolerance by regulating ABA biosynthesis in rice. PLoS Genet. 2021, 17, e1009699. [Google Scholar] [CrossRef]
- Fu, X.; Liu, C.; Li, Y.; Liao, S.; Cheng, H.; Tu, Y.; Zhu, X.; Chen, K.; He, Y.; Wang, G. The coordination of OsbZIP72 and OsMYBS2 with reverse roles regulates the transcription of OsPsbS1 in rice. New Phytol. 2021, 229, 370–387. [Google Scholar] [CrossRef]
- Lim, C.; Kang, K.; Shim, Y.; Yoo, S.C.; Paek, N.C. Inactivating transcription factor OsWRKY5 enhances drought tolerance through abscisic acid signaling pathways. Plant Physiol. 2022, 188, 1900–1916. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A Century of Gibberellin Research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Hua, C.; Shen, L.; Yu, H. New insights into gibberellin signaling in regulating flowering in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, J.; Ohashi, Y.; Takahashi, R.; Nakai, K.; Takahashi, Y. Della degradation by gibberellin promotes flowering via GAF1-TPR-dependent repression of floral repressors in Arabidopsis. Plant Cell 2021, 33, 2258–2272. [Google Scholar] [PubMed]
- Dar, T.A.; Uddin, M.; Khan, M.M.A.; Hakeem, K.R.; Jaleel, H. Jasmonates counter plant stress: A Review. Environ. Exp. Bot. 2015, 115, 49–57. [Google Scholar]
- Awan, S.A.; Khan, I.; Rizwan, M.; Zhang, X.; Brestic, M.; Khan, A.; El-Sheikh, M.A.; Alyemeni, M.N.; Ali, S.; Huang, L. Exogenous abscisic acid and jasmonic acid restrain polyethylene glycol-induced drought by improving the growth and antioxidative enzyme activities in pearl millet. Physiol. Plant 2021, 172, 809–819. [Google Scholar]
- Kiba, T.; Mizutani, K.; Nakahara, A.; Takebayashi, Y.; Kojima, M.; Hobo, T.; Osakabe, Y.; Osakabe, K.; Sakakibara, H. The trans-zeatin-type side-chain modification of cytokinins controls rice growth. Plant Physiol. 2023, 192, 2457–2474. [Google Scholar]
- Perata, P. Ethylene Signaling Controls Fast Oxygen Sensing in Plants. Trends Plant Sci. 2020, 25, 3–6. [Google Scholar]
- Zhao, H.; Yin, C.C.; Ma, B.; Chen, S.Y.; Zhang, J.S. Ethylene signaling in rice and Arabidopsis: New regulators and mechanisms. J. Integr. Plant Biol. 2021, 63, 102–125. [Google Scholar]
- Zhang, M.; Zhao, R.; Huang, K.; Huang, S.; Wang, H.; Wei, Z.; Li, Z.; Bian, M.; Jiang, W.; Wu, T.; et al. The OsWRKY63–OsWRKY76–OsDREB1B module regulates chilling tolerance in rice. Plant J. 2022, 112, 383–398. [Google Scholar]
- Xie, W.; Ding, C.; Hu, H.; Dong, G.; Zhang, G.; Qian, Q.; Ren, D. Molecular Events of Rice AP2/ERF Transcription Factors. Int. J. Mol. Sci. 2022, 23, 12013. [Google Scholar] [CrossRef]
- Wei, S.; Li, X.; Lu, Z.; Zhang, H.; Ye, X.; Zhou, Y.; Li, J.; Yan, Y.; Pei, H.; Duan, F.; et al. A transcriptional regulator that boosts grain yields and shortens the growth duration of rice. Science 2022, 377, eabi8455. [Google Scholar] [PubMed]
- Um, T.Y.; Lee, H.Y.; Lee, S.; Chang, S.H.; Chung, P.J.; Oh, K.B.; Kim, J.K.; Jang, G.; Choi, Y.D. Jasmonate Zim-Domain Protein 9 Interacts With Slender Rice 1 to Mediate the Antagonistic Interaction Between Jasmonic and Gibberellic Acid Signals in Rice. Front. Plant Sci. 2018, 9, 1866. [Google Scholar]
- Jang, G.; Chang, S.H.; Um, T.Y.; Lee, S.; Kim, J.K.; Choi, Y.D. Antagonistic interaction between jasmonic acid and cytokinin in xylem development. Sci. Rep. 2017, 7, 10212. [Google Scholar]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef]
- Wan, J.; Meng, S.; Wang, Q.; Zhao, J.; Qiu, X.; Wang, L.; Li, J.; Lin, Y.; Mu, L.; Dang, K.; et al. Suppression of microRNA168 enhances salt tolerance in rice (Oryza sativa L.). BMC Plant Biol. 2022, 22, 563. [Google Scholar]
- Yang, W.; Chen, Y.; Gao, R.; Chen, Y.; Zhou, Y.; Xie, J.; Zhang, F. MicroRNA2871b of Dongxiang Wild Rice (Oryza rufipogon Griff.) Negatively Regulates Cold and Salt Stress Tolerance in Transgenic Rice Plants. Int. J. Mol. Sci. 2023, 24, 14502. [Google Scholar] [CrossRef]
- Yuan, H.; Cheng, M.; Wang, R.; Wang, Z.; Fan, F.; Wang, W.; Si, F.; Gao, F.; Li, S. miR396b/GRF6 module contributes to salt tolerance in rice. Plant Biotechnol. J. 2024, 22, 2079–2092. [Google Scholar]
- Lan, T.; Zheng, Y.; Su, Z.; Yu, S.; Song, H.; Zheng, X.; Lin, G.; Wu, W. OsSPL10, a SBP-Box Gene, Plays a Dual Role in Salt Tolerance and Trichome Formation in Rice (Oryza sativa L.). G3 2019, 9, 4107–4114. [Google Scholar]
- Shen, Y.; Zhou, G.; Liang, C.; Tian, Z. Omics-based interdisciplinarity is accelerating plant breeding. Curr. Opin. Plant Biol. 2022, 66, 102167. [Google Scholar]
- Du, X.; Xu, W.; Peng, C.; Li, C.; Zhang, Y.; Hu, L. Identification and validation of a novel locus, Qpm-3BL, for adult plant resistance to powdery mildew in wheat using multilocus GWAS. BMC Plant Biol. 2021, 21, 357. [Google Scholar]
- Ma, L.; Qing, C.; Zhang, M.; Zou, C.; Pan, G.; Shen, Y. GWAS with a PCA uncovers candidate genes for accumulations of microelements in maize seedlings. Physiol. Plant 2021, 172, 2170–2180. [Google Scholar] [CrossRef] [PubMed]
- Tekeu, H.; Ngonkeu, E.L.M.; Bélanger, S.; Djocgoué, P.F.; Abed, A.; Torkamaneh, D.; Boyle, B.; Tsimi, P.M.; Tadesse, W.; Jean, M.; et al. GWAS identifies an ortholog of the rice D11 gene as a candidate gene for grain size in an international collection of hexaploid wheat. Sci. Rep. 2021, 11, 19483. [Google Scholar]
- Dai, L.; Li, P.; Li, Q.; Leng, Y.; Zeng, D.; Qian, Q. Integrated Multi-Omics Perspective to Strengthen the Understanding of Salt Tolerance in Rice. Int. J. Mol. Sci. 2022, 23, 5236. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ma, C.; Tai, H.; Qiu, H.; Yang, A. Comparative transcriptome analysis of two rice genotypes differing in their tolerance to saline-alkaline stress. PLoS ONE 2020, 15, e0243112. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Zhang, G.; Jiang, J.; Lu, J.; Zhang, F. Combining Genome-Wide Association Study and Gene-Based Haplotype Analysis to Identify Candidate Genes for Alkali Tolerance at the Germination Stage in Rice. Front. Plant Sci. 2022, 13, 887239. [Google Scholar] [CrossRef]
- Lei, L.; Zheng, H.; Bi, Y.; Yang, L.; Liu, H.; Wang, J.; Sun, J.; Zhao, H.; Li, X.; Li, J.; et al. Identification of a Major QTL and Candidate Gene Analysis of Salt Tolerance at the Bud Burst Stage in Rice (Oryza sativa L.) Using QTL-Seq and RNA-Seq. Rice 2020, 13, 55. [Google Scholar]
- Kong, W.; Zhang, C.; Zhang, S.; Qiang, Y.; Zhang, Y.; Zhong, H.; Li, Y. Uncovering the Novel QTLs and Candidate Genes of SaltTolerance in Rice with Linkage Mapping, RTM-GWAS, and RNA-seq. Rice 2021, 14, 93. [Google Scholar] [CrossRef]
- Babu, N.N.; Krishnan, S.G.; Vinod, K.K.; Krishnamurthy, S.L.; Singh, V.K.; Singh, M.P.; Singh, R.; Ellur, R.K.; Rai, V.; Bollinedi, H.; et al. Marker Aided Incorporation of Saltol, a Major QTL Associated with Seedling Stage Salt Tolerance, into Oryza sativa ‘Pusa Basmati 1121’. Front. Plant Sci. 2017, 8, 41. [Google Scholar]
- Bimpong, I.K.; Manneh, B.; Sock, M.; Diaw, F.; Amoah, N.K.A.; Ismail, A.M.; Gregorio, G.; Singh, R.K.; Wopereis, M. Improving salt tolerance of lowland rice cultivar ‘Rassi’ through marker-aided backcross breeding in West Africa. Plant Sci. 2016, 242, 288–299. [Google Scholar] [CrossRef]
- Krishnamurthy, S.L.; Pundir, P.; Warraich, A.S.; Rathor, S.; Lokeshkumar, B.M.; Singh, N.K.; Sharma, P.C. Introgressed Saltol QTL Lines Improves the Salinity Tolerance in Rice at Seedling Stage. Front. Plant Sci. 2020, 11, 833. [Google Scholar]
- Singh, V.K.; Singh, B.D.; Kumar, A.; Maurya, S.; Krishnan, S.G.; Vinod, K.K.; Singh, M.P.; Ellur, R.K.; Bhowmick, P.K.; Singh, A.K. Marker-Assisted Introgression of Saltol QTL Enhances Seedling Stage Salt Tolerance in the Rice Variety “Pusa Basmati 1”. Int. J. Genom. 2018, 2018, 8319879. [Google Scholar]
- Yadav, A.K.; Kumar, A.; Grover, N.; Ellur, R.K.; Krishnan, S.G.; Bollinedi, H.; Bhowmick, P.K.; Vinod, K.K.; Nagarajan, M.; Krishnamurthy, S.L.; et al. Marker aided introgression of ‘Saltol’, a major QTL for seedling stage salinity tolerance into an elite Basmati rice variety ‘Pusa Basmati 1509’. Sci. Rep. 2020, 10, 13877. [Google Scholar]
- Yuyu, C.; Aike, Z.; Pao, X.; Xiaoxia, W.; Yongrun, C.; Beifang, W.; Yue, Z.; Liaqat, S.; Shihua, C.; Liyong, C.; et al. Effects of GS3 and GL3.1 for Grain Size Editing by CRISPR/Cas9 in Rice. Rice Sci. 2020, 27, 405–413. [Google Scholar]
- Cui, Y.; Hu, X.; Liang, G.; Feng, A.; Wang, F.; Ruan, S.; Dong, G.; Shen, L.; Zhang, B.; Chen, D.; et al. Production of novel beneficial alleles of a rice yield-related QTL by CRISPR/Cas9. Plant Biotechnol. J. 2020, 18, 1987–1989. [Google Scholar]
- Ma, X.; Feng, F.; Zhang, Y.; Elesawi, I.E.; Xu, K.; Li, T.; Mei, H.; Liu, H.; Gao, N.; Chen, C.; et al. A novel rice grain size gene OsSNB was identified by genome-wide association study in natural population. PLoS Genet. 2019, 15, e1008191. [Google Scholar]
- Kuang, Y.; Li, S.; Ren, B.; Yan, F.; Spetz, C.; Li, X.; Zhou, X.; Zhou, H. Base-Editing-Mediated Artificial Evolution of OsALS1 In Planta to Develop Novel Herbicide-Tolerant Rice Germplasms. Mol. Plant 2020, 13, 565–572. [Google Scholar]
- Sheela, H.S.; Vennapusa, A.R.; Melmaiee, K.; Prasad, T.G.; Reddy, C.P. Pyramiding of transcription factor, PgHSF4, and stress-responsive genes of p68, Pg47, and PsAKR1 impart multiple abiotic stress tolerance in rice (Oryza sativa L.). Front. Plant Sci. 2023, 14, 1233248. [Google Scholar]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar]
- Spök, A.; Sprink, T.; Allan, A.C.; Yamaguchi, T.; Dayé, C. Towards social acceptability of genome-edited plants in industrialised countries? Emerging evidence from Europe, United States, Canada, Australia, New Zealand, and Japan. Front. Genome Ed. 2022, 4, 899331. [Google Scholar]
- Shailani, A.; Joshi, R.; Singla-Pareek, S.L.; Pareek, A. Stacking for future: Pyramiding genes to improve drought and salinity tolerance in rice. Physiol. Plant 2021, 172, 1352–1362. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Yao, Y.; Wang, J.; Ruan, B.; Yu, Y. Advancing Stress-Resilient Rice: Mechanisms, Genes, and Breeding Strategies. Agriculture 2025, 15, 721. https://doi.org/10.3390/agriculture15070721
Wang S, Yao Y, Wang J, Ruan B, Yu Y. Advancing Stress-Resilient Rice: Mechanisms, Genes, and Breeding Strategies. Agriculture. 2025; 15(7):721. https://doi.org/10.3390/agriculture15070721
Chicago/Turabian StyleWang, Sining, Yao Yao, Jing Wang, Banpu Ruan, and Yanchun Yu. 2025. "Advancing Stress-Resilient Rice: Mechanisms, Genes, and Breeding Strategies" Agriculture 15, no. 7: 721. https://doi.org/10.3390/agriculture15070721
APA StyleWang, S., Yao, Y., Wang, J., Ruan, B., & Yu, Y. (2025). Advancing Stress-Resilient Rice: Mechanisms, Genes, and Breeding Strategies. Agriculture, 15(7), 721. https://doi.org/10.3390/agriculture15070721






