Influence of Concentration Levels of β-Tricalcium Phosphate on the Physical Properties of a Dental Adhesive
Abstract
:1. Introduction
2. Materials and Methods
2.1. Amalgamation of Nanoparticles with the Dental Adhesive
2.2. Characterization of the Filler Nanoparticles
2.3. Tooth Specimens Preparation and Bonding Protocol
2.4. SBS Testing and Investigation of the Failure Modes
2.5. Adhesive–Dentin Interface Analysis
2.6. FTIR Spectroscopy and DC Investigation
2.7. Statistical Analysis
3. Results
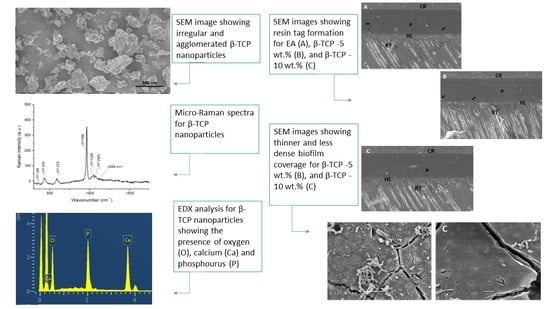
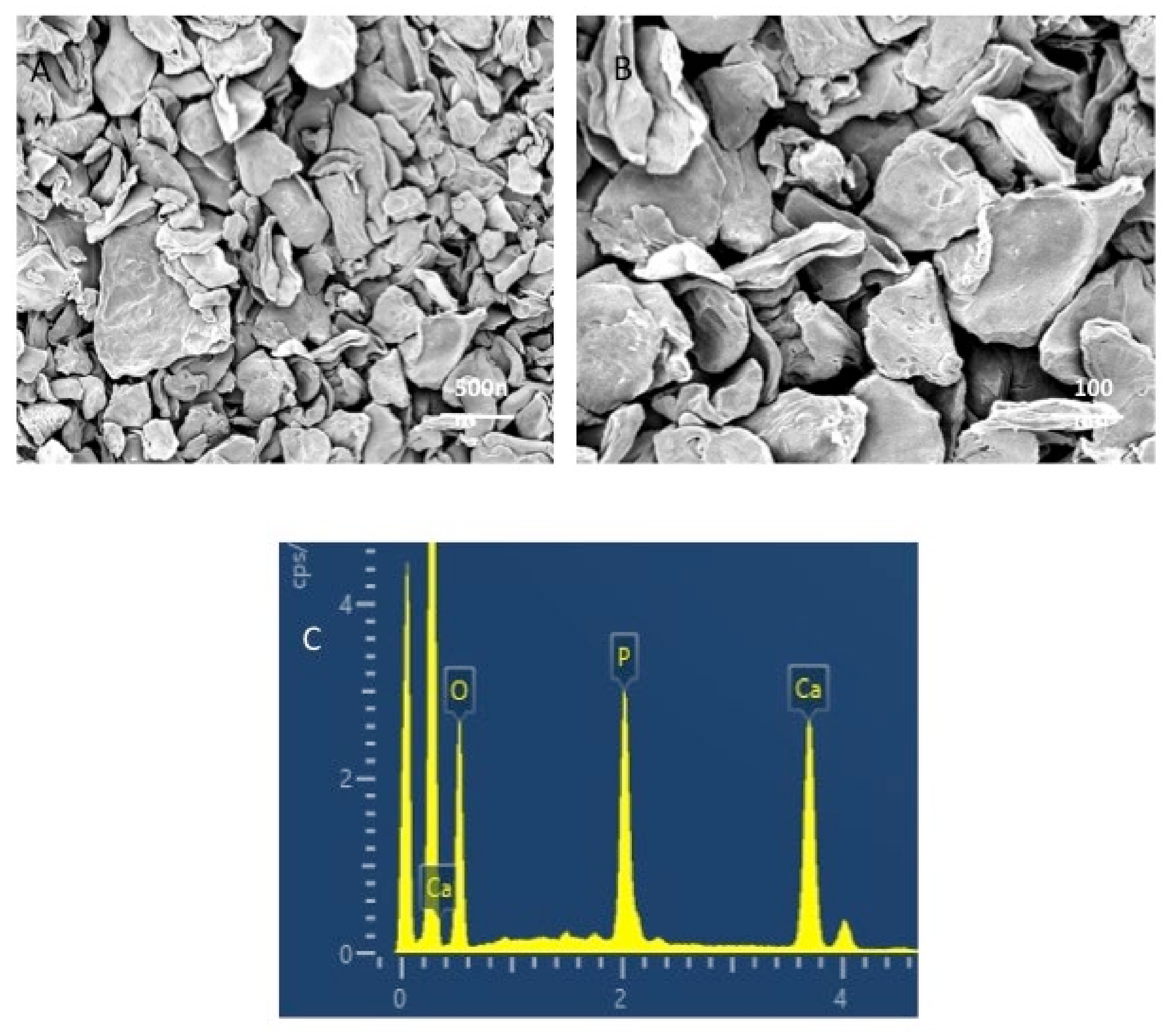
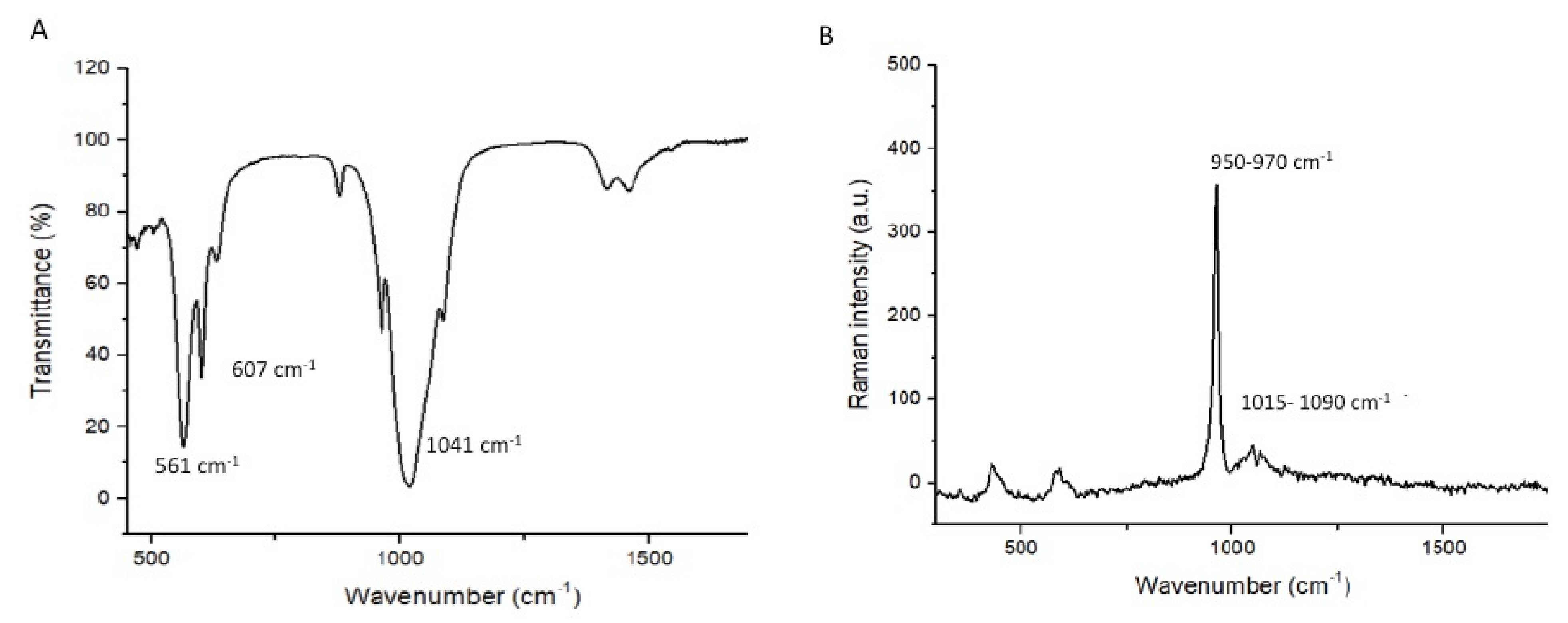
3.1. Outcomes of the Filler Nanoparticle’s Characterization
3.2. Outcomes of the SBS and Failure Mode Analysis
3.3. Outcomes of the Analysis of the Adhesive–Dentin Interface
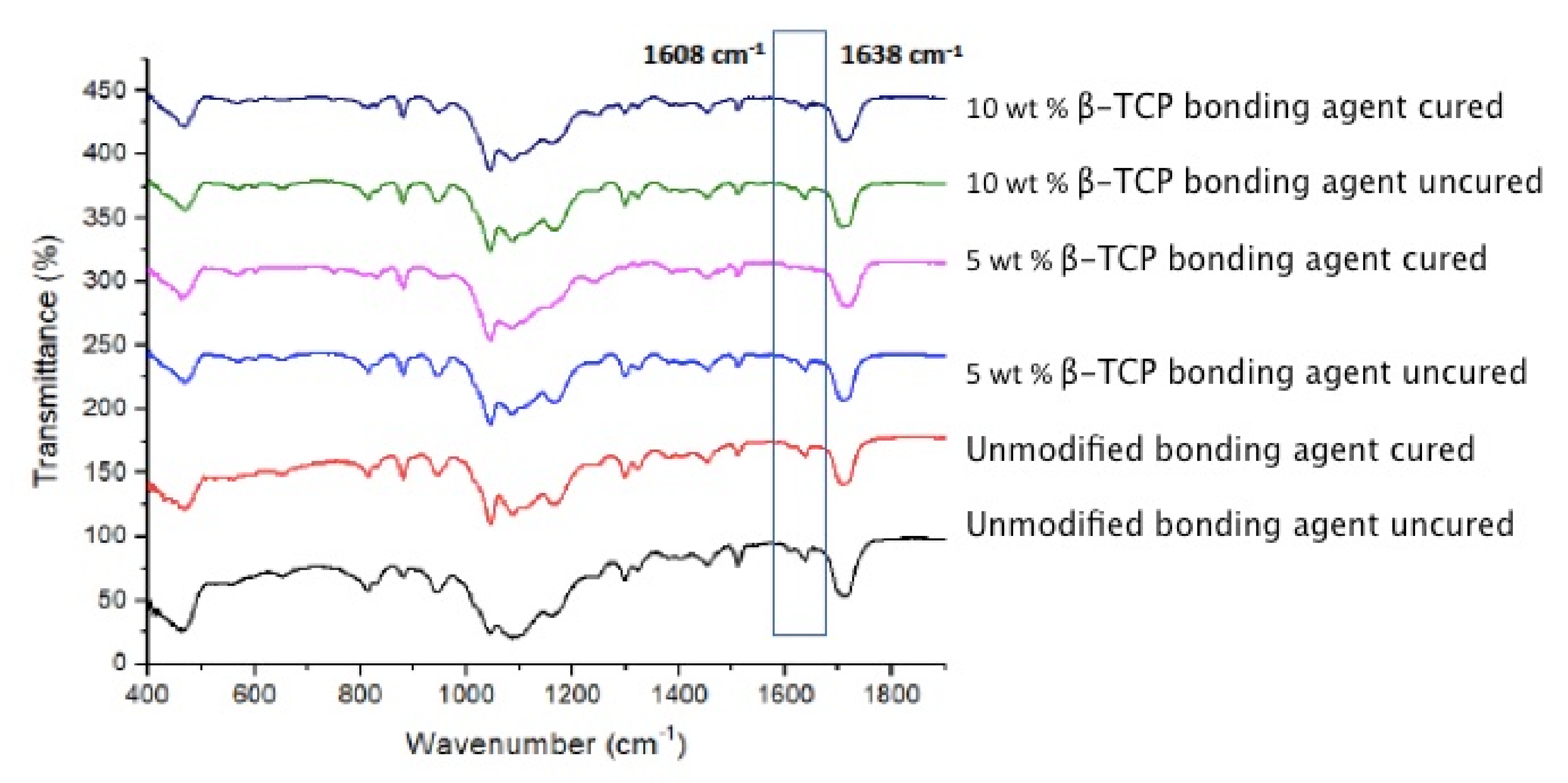
3.4. Outcomes of the FTIR and DC Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perdigao, J. Current perspectives on dental adhesion: (1) Dentin adhesion—Not there yet. Jpn. Dent. Sci. Rev. 2020, 56, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Perdigao, J.; Araujo, E.; Ramos, R.Q.; Gomes, G.; Pizzolotto, L. Adhesive dentistry: Current concepts and clinical considerations. J Esthet. Restor. Dent. 2021, 33, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Siqueira, W.L.; Cvitkovitch, D.G.; Finer, Y. Esterase from a cariogenic bacterium hydrolyzes dental resins. Acta Biomater. 2018, 71, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Perdigao, J. Dentin bonding-variables related to the clinical situation and the substrate treatment. Dent. Mater. 2010, 26, e24–e37. [Google Scholar] [CrossRef] [PubMed]
- Farooq, I.; Ali, S.; Al-Saleh, S.; AlHamdan, E.M.; AlRefeai, M.H.; Abduljabbar, T.; Vohra, F. Synergistic Effect of Bioactive Inorganic Fillers in Enhancing Properties of Dentin Adhesives—A Review. Polymers 2021, 13, 2169. [Google Scholar] [CrossRef]
- Kim, J.S.; Cho, B.H.; Lee, I.B.; Um, C.M.; Lim, B.S.; Oh, M.H.; Chang, C.G.; Son, H.H. Effect of the hydrophilic nanofiller loading on the mechanical properties and the microtensile bond strength of an ethanol-based one-bottle dentin adhesive. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Finer, Y. Biostable, antidegradative and antimicrobial restorative systems based on host-biomaterials and microbial interactions. Dent. Mater. 2019, 35, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, S.; Zhou, X.; Hannig, M.; Rupf, S.; Feng, J.; Peng, X.; Cheng, L. Modifying Adhesive Materials to Improve the Longevity of Resinous Restorations. Int. J. Mol. Sci. 2019, 20, 723. [Google Scholar] [CrossRef] [Green Version]
- Balhaddad, A.A.; Garcia, I.M.; Mokeem, L.; Alsahafi, R.; Collares, F.M.; Sampaio de Melo, M.A. Metal Oxide Nanoparticles and Nanotubes: Ultrasmall Nanostructures to Engineer Antibacterial and Improved Dental Adhesives and Composites. Bioengineering 2021, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Saafan, A.; Zaazou, M.H.; Sallam, M.K.; Mosallam, O.; El Danaf, H.A. Assessment of Photodynamic Therapy and Nanoparticles Effects on Caries Models. Open Access. Maced. J. Med. Sci. 2018, 6, 1289–1295. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Zhang, Y.; Wang, X.; Li, Q.; Xiao, Y.; Li, P.; Wang, L.; Ye, Z.; Xing, X. Novel resin-based dental material with anti-biofilm activity and improved mechanical property by incorporating hydrophilic cationic copolymer functionalized nanodiamond. J. Mater. Sci. Mater. Med. 2018, 29, 162. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, J.; Chai, Z.; Zhang, L.; Xiao, Y.; Fang, M.; Ma, S. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J. Dent. 2009, 37, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Kozmos, M.; Virant, P.; Rojko, F.; Abram, A.; Rudolf, R.; Raspor, P.; Zore, A.; Bohinc, K. Bacterial Adhesion of Streptococcus mutans to Dental Material Surfaces. Molecules 2021, 26, 1152. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Santoni, B.L.G.; Dobelin, N. beta-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Al-Sanabani, J.S.; Madfa, A.A.; Al-Sanabani, F.A. Application of calcium phosphate materials in dentistry. Int. J. Biomater. 2013, 2013, 876132. [Google Scholar] [CrossRef] [Green Version]
- Hamba, H.; Nakamura, K.; Nikaido, T.; Tagami, J.; Muramatsu, T. Remineralization of enamel subsurface lesions using toothpaste containing tricalcium phosphate and fluoride: An in vitro microCT analysis. BMC Oral Health 2020, 20, 292. [Google Scholar] [CrossRef]
- Ji, H.; Huang, Z.; Xia, Z.; Molokeev, M.S.; Chen, M.; Atuchin, V.V.; Fang, M.; Liu, Y.G.; Wu, X. Phase transformation in Ca3 (PO4) 2: Eu2+ via the controlled quenching and increased Eu2+ content: Identification of new cyan-emitting α-Ca3 (PO4) 2: Eu2+ phosphor. J. Am. Ceram. Soc. 2015, 98, 3280–3284. [Google Scholar] [CrossRef]
- Schiroky, P.G.; Garcia, I.M.; Noal, F.C.; Leitune, V.C.B.; de Araújo, F.B.; Collares, F.M. Adhesive system with alpha-tricalcium phosphate addition for mineral deposition on caries-affected dentin. Int. J. Adhes. Adhes. 2021, 105, 102790. [Google Scholar] [CrossRef]
- Kang, K.R.; Piao, Z.G.; Kim, J.S.; Cho, I.A.; Yim, M.J.; Kim, B.H.; Oh, J.S.; Son, J.S.; Kim, C.S.; Kim, D.K.; et al. Synthesis and Characterization of beta-Tricalcium Phosphate Derived from Haliotis sp. Shells. Implant Dent. 2017, 26, 378–387. [Google Scholar] [CrossRef]
- Mangano, C.; Sinjari, B.; Shibli, J.A.; Mangano, F.; Hamisch, S.; Piattelli, A.; Perrotti, V.; Iezzi, G. A human clinical, histological, histomorphometrical, and radiographical study on biphasic HA-Beta-TCP 30/70 in maxillary sinus augmentation. Clin. Implant Dent. Relat. Res. 2015, 17, 610–618. [Google Scholar] [CrossRef]
- AlRefeai, M.H.A.E.M.; Al-Saleh, S.; Alqahtani, A.S.; Al-Rifaiy, M.Q.; Alshiddi, I.F.; Farooq, I.; Vohra, F.; Abduljabbar, T. Application of β-Tricalcium Phosphate in Adhesive Dentin Bonding. Polymers 2021, 13, 2285. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamdan, R.S.; Almutairi, B.; Kattan, H.F.; Alsuwailem, N.A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of Hydroxyapatite Nanospheres in Dentin Adhesive on the Dentin Bond Integrity and Degree of Conversion: A Scanning Electron Microscopy (SEM), Raman, Fourier Transform-Infrared (FTIR), and Microtensile Study. Polymers 2020, 12, 2948. [Google Scholar] [CrossRef] [PubMed]
- AlFawaz, Y.F.; Almutairi, B.; Kattan, H.F.; Zafar, M.S.; Farooq, I.; Naseem, M.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Hydroxyapatite Containing Resin Adhesive Enhanced with Graphene Oxide Nano-Particles-An SEM, EDX, Micro-Raman, and Microtensile Bond Strength Study. Polymers 2020, 12, 2978. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yamaguchi, K.; Tsubota, K.; Takamizawa, T.; Kurokawa, H.; Rikuta, A.; Ando, S.; Miyazaki, M. Effect of metal conditioners on polymerization behavior of bonding agents. J. Oral Sci. 2005, 47, 171–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almutairi, B.; Kattan, H.F.; BinMahfooz, A.M.; Qutub, O.A.; Basunbul, G.; ArRejaie, A.S.; Farooq, I.; Vohra, F.; Abduljabbar, T. Synergistic effect of graphene oxide/calcium phosphate nanofiller in a dentin adhesive on its dentin bond integrity and degree of conversion. A scanning electron microscopy, energy dispersive X-ray spectroscopy, Fourier transform infrared, micro-Raman, and bond strength study. Microsc. Res. Tech. 2021, 84, 2082–2094. [Google Scholar]
- Farooq, I.; Bugshan, A. The role of salivary contents and modern technologies in the remineralization of dental enamel: A narrative review. F1000Res 2020, 9, 171. [Google Scholar]
- Alhussain, A.M.; Alhaddad, A.A.; Ghazwi, M.M.; Farooq, I. Remineralization of artificial carious lesions using a novel fluoride incorporated bioactive glass dentifrice. Dent. Med. Probl. 2018, 55, 379–382. [Google Scholar] [CrossRef] [Green Version]
- Abou Neel, E.A.; Bozec, L.; Perez, R.A.; Kim, H.W.; Knowles, J.C. Nanotechnology in dentistry: Prevention, diagnosis, and therapy. Int. J. Nanomed. 2015, 10, 6371–6394. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Aguilara, C.O.-P.U.; Aguilar-Reyes, E.A.; López-Juárez, R.; Alfonso, I. Characterization of β-tricalcium phosphate powders synthesized by sol–gel and mechanosynthesis. Bol. Soc. Esp. Ceram. Vidr. 2018, 57, 213–220. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef]
- Arbez, B.; Libouban, H. Behavior of macrophage and osteoblast cell lines in contact with the beta-TCP biomaterial (beta-tricalcium phosphate). Morphologie 2017, 101, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Ren, B.; Zhou, X.; Xu, H.H.K.; Wang, S.; Li, M.; Weir, M.D.; Feng, M.; Cheng, L. Novel Dental Adhesive with Biofilm-Regulating and Remineralization Capabilities. Materials 2017, 10, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anchieta, R.B.; Oliveira, F.G.; Sundfeld, R.H.; Rahal, V.; Machado, L.S.; Alexandre, R.S.; Sundefeld, M.L.; Rocha, E.P. Analysis of hybrid layer thickness, resin tag length and their correlation with microtensile bond strength using a total etch adhesive to intact dentin. Acta Odontol. Latinoam. 2011, 24, 272–278. [Google Scholar] [PubMed]
- Vohra, F.; Labban, N.; Al-Hussaini, A.; Al-Jarboua, M.; Zawawi, R.; Alrahlah, A.; Naseem, M. Influence of Er; Cr: YSGG laser on shear bond strength and color stability of lithium disilicate ceramics: An in vitro study. Photobiomodul. Photomed. Laser Surg. 2019, 37, 483–488. [Google Scholar] [CrossRef]
- Alnassar, T.; Vohra, F.; Abualsaud, H.; Al-Thobity, A.M.; Flinton, R. Efficacy of novel cleansing agent for the decontamination of lithium disilicate ceramics: A shear bond strength study. J. Adhes. Sci. Technol. 2017, 17, 202–210. [Google Scholar] [CrossRef]
- Wagner, A.; Belli, R.; Stotzel, C.; Hilpert, A.; Muller, F.A.; Lohbauer, U. Biomimetically- and hydrothermally-grown HAp nanoparticles as reinforcing fillers for dental adhesives. J. Adhes. Dent. 2013, 15, 413–422. [Google Scholar]
- Garcia, I.M.; Leitune, V.C.B.; Samuel, S.M.W.; Collares, F.M. Influence of Different Calcium Phosphates on an Experimental Adhesive Resin. J. Adhes. Dent. 2017, 19, 379–384. [Google Scholar]
- Noorani, T.Y.; Luddin, N.; Rahman, I.A.; Masudi, S.M. In Vitro Cytotoxicity Evaluation of Novel Nano-Hydroxyapatite-Silica Incorporated Glass Ionomer Cement. J. Clin. Diagn. Res. 2017, 11, ZC105–ZC109. [Google Scholar] [CrossRef]
- Alqarawi, F.K.; Alkahtany, M.F.; Almadi, K.H.; Ben Gassem, A.A.; Alshahrani, F.A.; AlRefeai, M.H.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of Different Conditioning Treatments on the Bond Integrity of Root Dentin to rGO Infiltrated Dentin Adhesive. SEM, EDX, FTIR and MicroRaman Study. Polymers 2021, 13, 1555. [Google Scholar] [CrossRef]
- Peumans, M.; Kanumilli, P.; De Munck, J.; Van Landuyt, K.; Lambrechts, P.; Van Meerbeek, B. Clinical effectiveness of contemporary adhesives: A systematic review of current clinical trials. Dent. Mater. 2005, 21, 864–881. [Google Scholar] [CrossRef]
- International Organization for Standardization, ISO/TS 11405. Dentistry—Testing of Adhesion to Tooth Structure, 3rd ed.; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Helvatjoglu-Antoniades, M.; Koliniotou-Kubia, E.; Dionyssopoulos, P. The effect of thermal cycling on the bovine dentine shear bond strength of current adhesive systems. J. Oral Rehabil. 2004, 31, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Alhenaki, A.M.; Attar, E.A.; Alshahrani, A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Filled and Unfilled Resin Adhesive Enhanced with Silica Nanoparticles-An SEM, EDX, Micro-Raman, FTIR and Micro-Tensile Bond Strength Study. Polymers 2021, 13, 1093. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, T.R.; de Oliveira, M.; Arrais, C.A.; Ambrosano, G.M.; Rueggeberg, F.; Giannini, M. The effect of photopolymerization on the degree of conversion, polymerization kinetic, biaxial flexure strength, and modulus of self-adhesive resin cements. J. Prosthet. Dent. 2015, 113, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, M.; Balazsi, R.; Soanca, A.; Roman, A.; Sarosi, C.; Prodan, D.; Vlassa, M.; Cojocaru, I.; Saceleanu, V.; Cristescu, I. Evaluation of the Degree of Conversion, Residual Monomers and Mechanical Properties of Some Light-Cured Dental Resin Composites. Materials 2019, 12, 2109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utneja, S.; Talwar, S.; Nawal, R.R.; Sapra, S.; Mittal, M.; Rajain, A.; Verma, M. Evaluation of remineralization potential and mechanical properties of pit and fissure sealants fortified with nano-hydroxyapatite and nano-amorphous calcium phosphate fillers: An in vitro study. J. Conserv. Dent. 2018, 21, 681–690. [Google Scholar] [CrossRef] [PubMed]


| SBS (MPa) (Mean ± SD) | Failure Mode Analysis (%) | |||||
|---|---|---|---|---|---|---|
| Group (n = 10) | NTC | TC | p-Value | Adhesive | Cohesive | Mixed |
| Unmodified CA | 27.34 ± 3.11 a A | - | <0.01 | 100 | 0 | 0 |
| - | 24.70 ± 3.64 b A | 100 | 0 | 0 | ||
| 5.0 wt.% nano-β-TCP | 32.37 ± 3.10 a B | - | 80 | 0 | 20 | |
| - | 27.75 ± 3.15 b B | 80 | 0 | 20 | ||
| 10.0 wt.% nano- β-TCP | 33.55 ± 3.73 a B | - | 90 | 0 | 10 | |
| - | 30.50 ± 3.25 b B | 80 | 0 | 20 | ||
| Group | Degree of Conversion (Mean ± SD) | Tukey Test (p < 0.01) * |
|---|---|---|
| Unmodified CA | 41.3 ± 4.5 | A |
| 5.0 wt.% β-TCP | 39.4 ± 6.2 | AC |
| 10.0 wt.% β-TCP | 35.5 ± 4.5 | C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qahtani, A.S.; Tulbah, H.I.; Binhasan, M.; Shabib, S.; Al-Aali, K.A.; Alhamdan, M.M.; Abduljabbar, T. Influence of Concentration Levels of β-Tricalcium Phosphate on the Physical Properties of a Dental Adhesive. Nanomaterials 2022, 12, 853. https://doi.org/10.3390/nano12050853
Al-Qahtani AS, Tulbah HI, Binhasan M, Shabib S, Al-Aali KA, Alhamdan MM, Abduljabbar T. Influence of Concentration Levels of β-Tricalcium Phosphate on the Physical Properties of a Dental Adhesive. Nanomaterials. 2022; 12(5):853. https://doi.org/10.3390/nano12050853
Chicago/Turabian StyleAl-Qahtani, Amal S., Huda I. Tulbah, Mashael Binhasan, Sara Shabib, Khulud A. Al-Aali, Mai M. Alhamdan, and Tariq Abduljabbar. 2022. "Influence of Concentration Levels of β-Tricalcium Phosphate on the Physical Properties of a Dental Adhesive" Nanomaterials 12, no. 5: 853. https://doi.org/10.3390/nano12050853