A Review on Microfluidics-Based Impedance Biosensors
Abstract
:1. Introduction
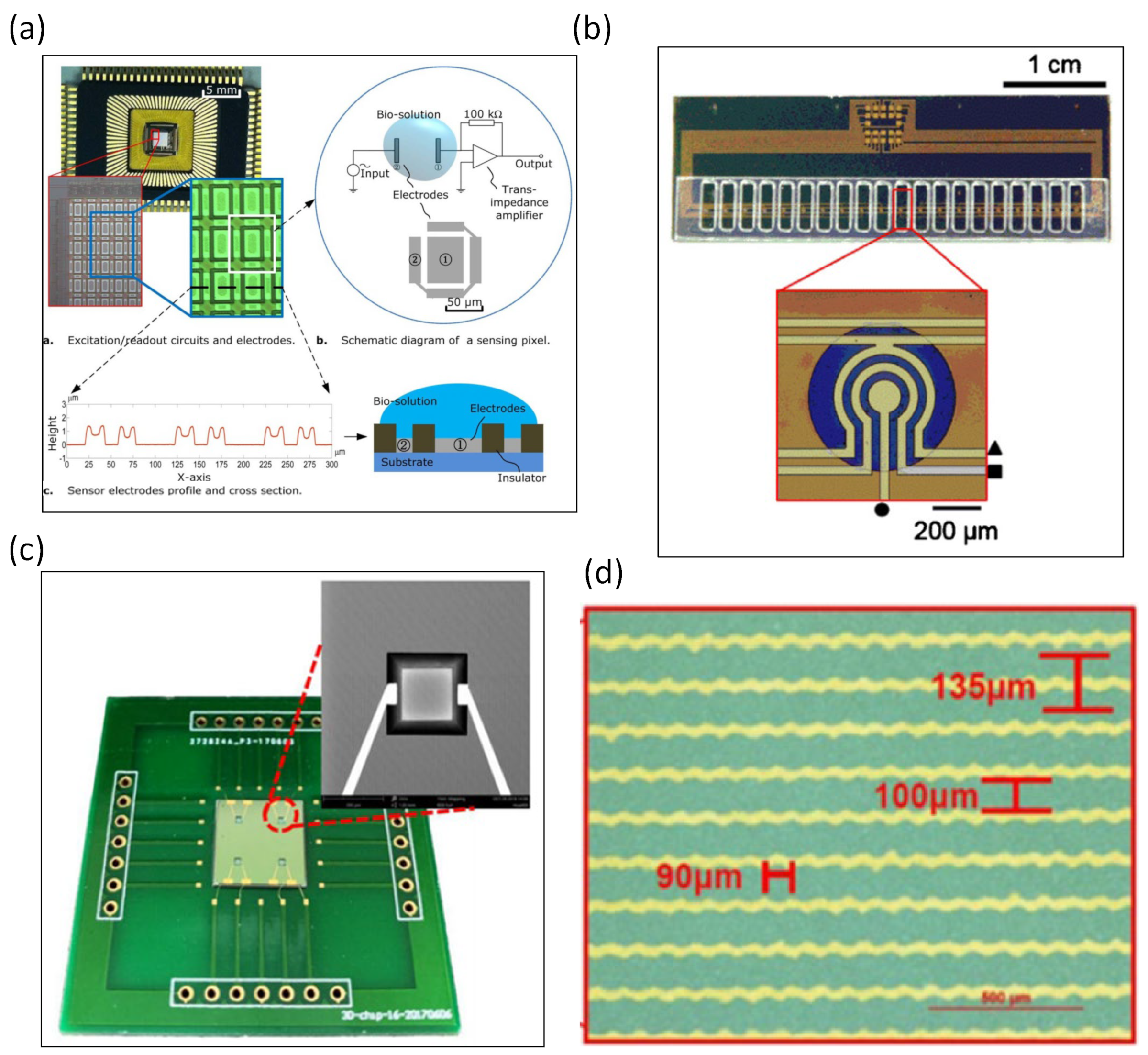
2. Silicon-Based Impedance Biosensors
3. Printed Circuit Board (PCB)-Based Impedance Biosensors
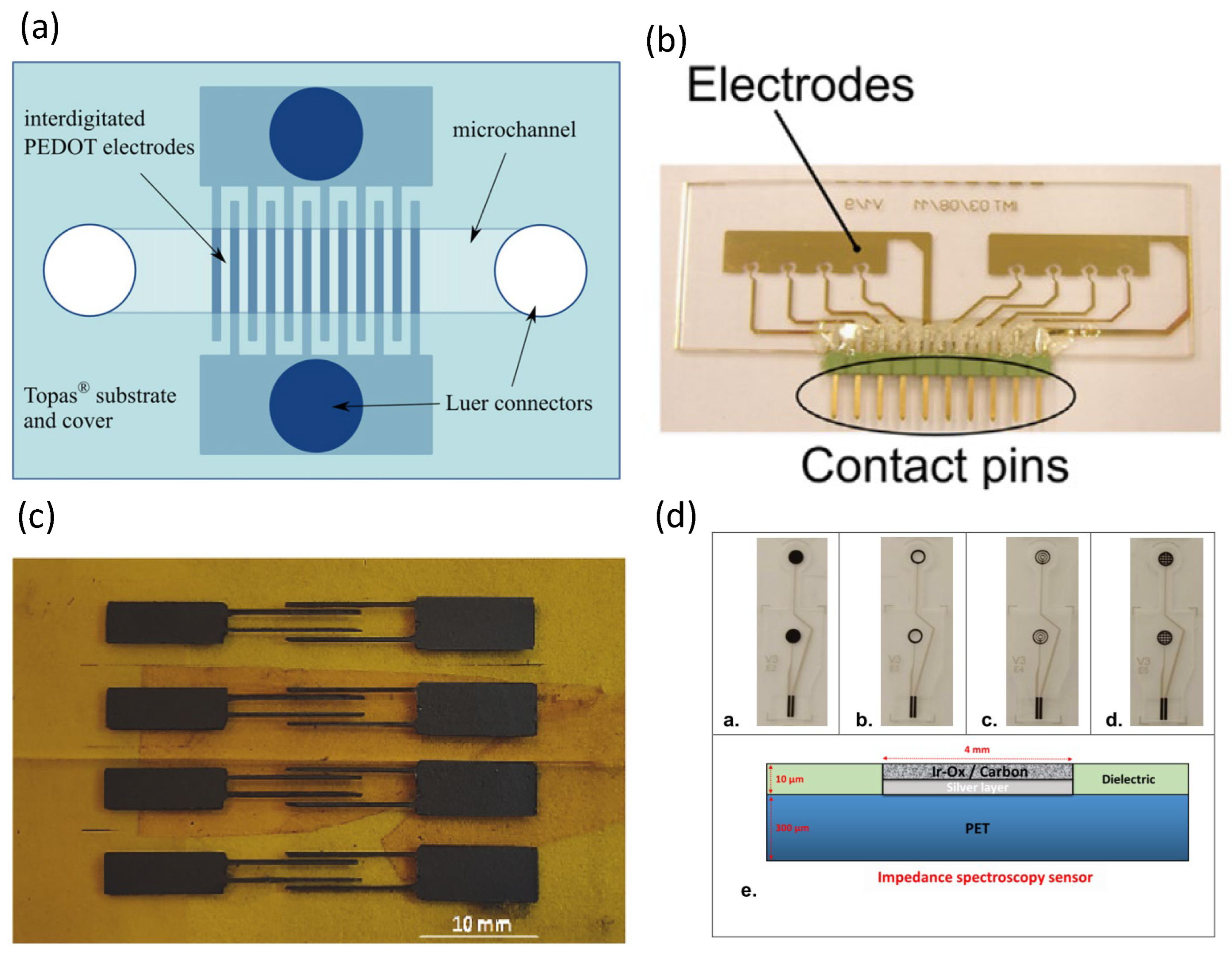
4. Polymer-Based Impedance Biosensors
5. Glass-Based Impedance Biosensors
5.1. Detection of Bacteria
5.2. Detection of Blood Samples
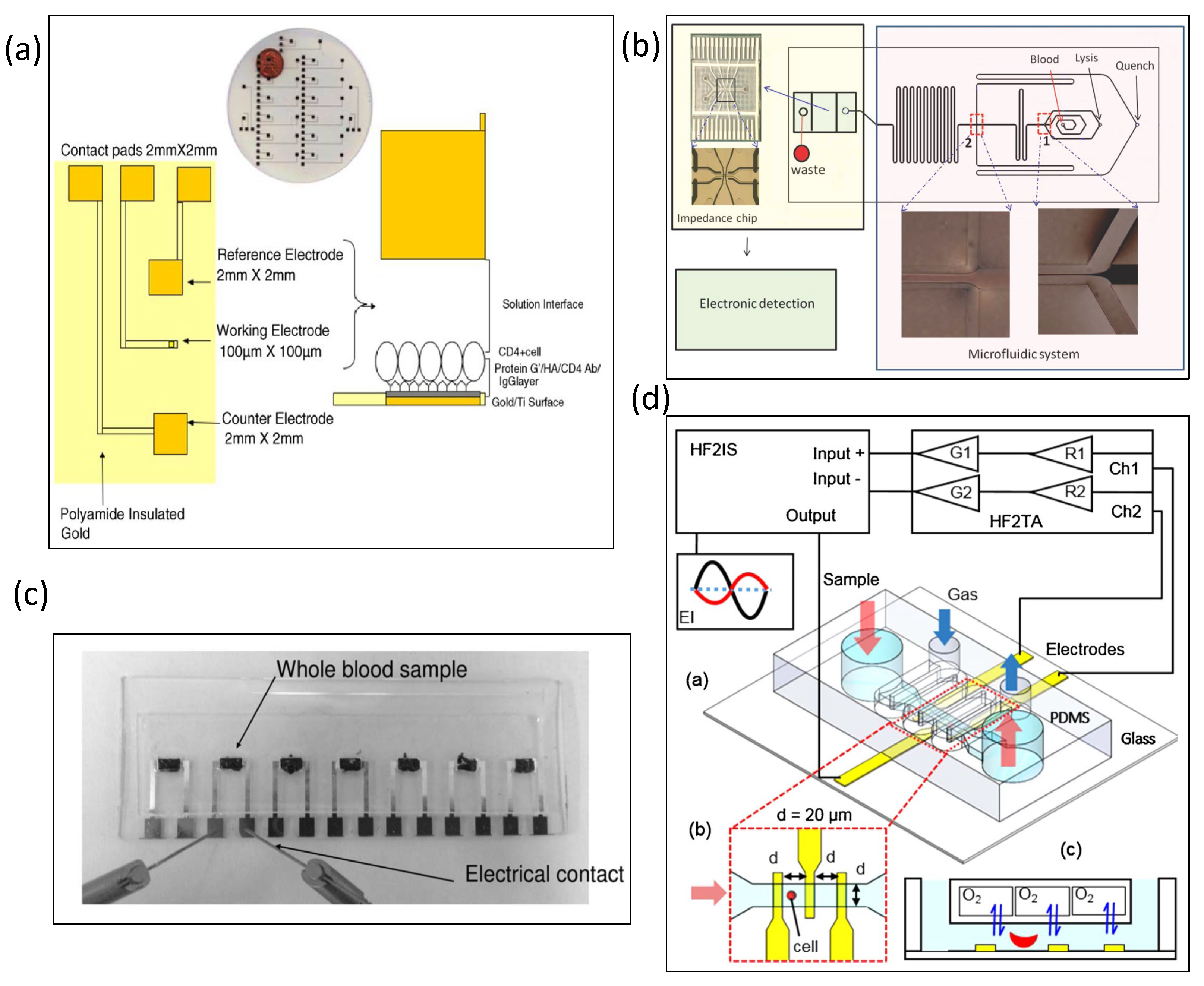
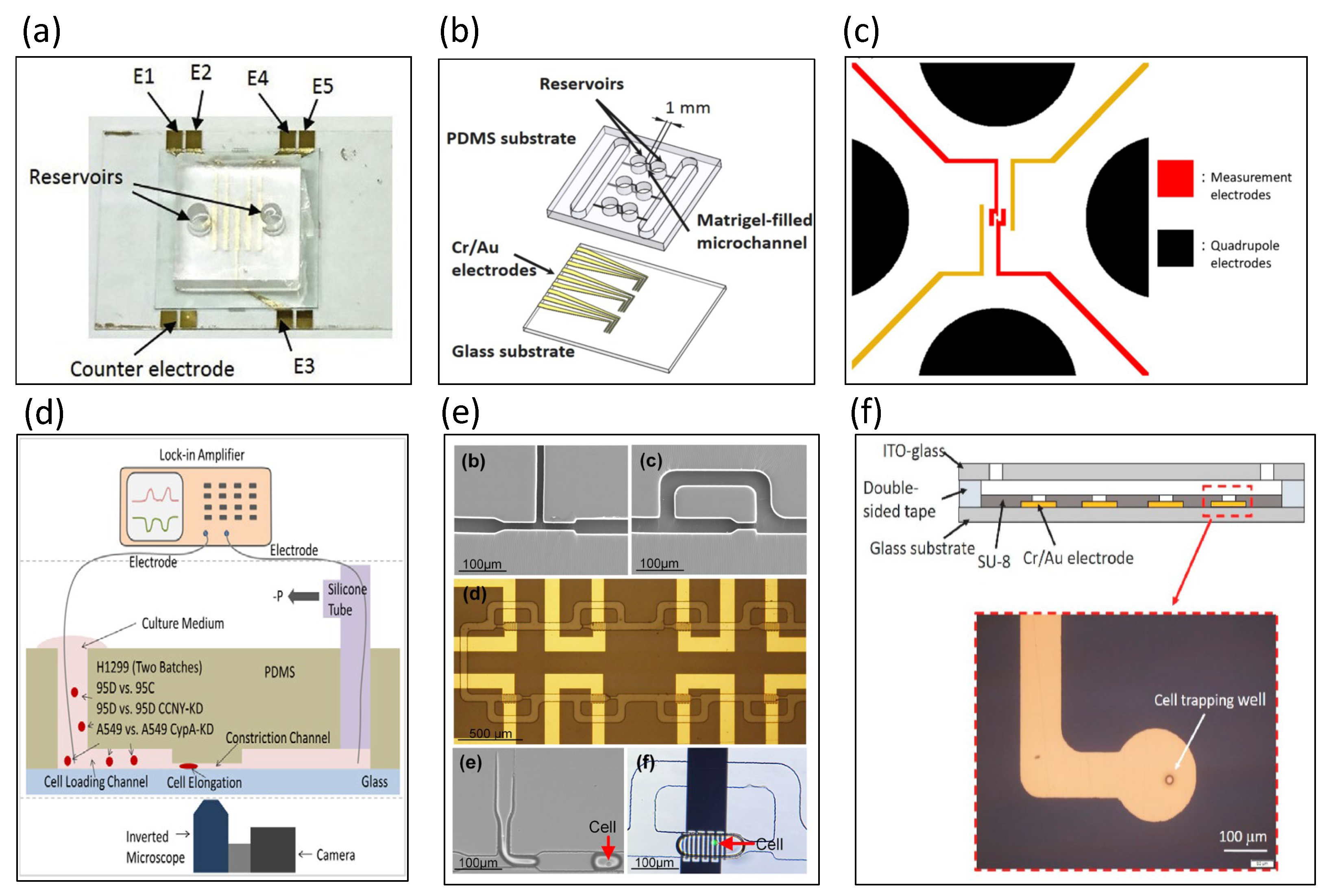
5.3. Static Cell Analyzed by Electrical Impedance Spectroscopy
5.4. Dynamic Cell Analyzed by Microfluidic Impedance Cytometry
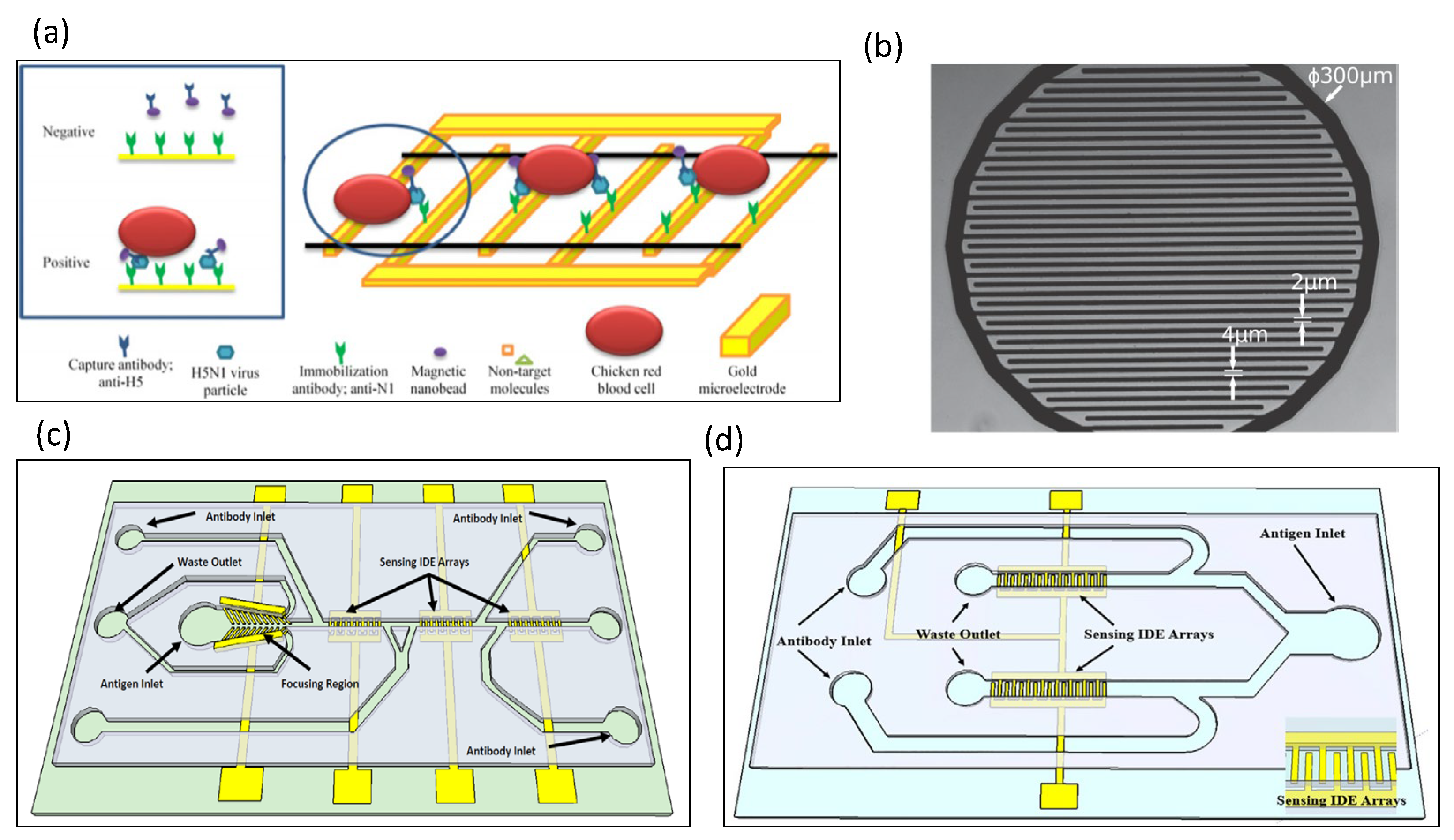
5.5. Detection of Viruses
5.6. Detection of Other Analytes and Chemicals
5.7. Conclusions of the Glass Chips
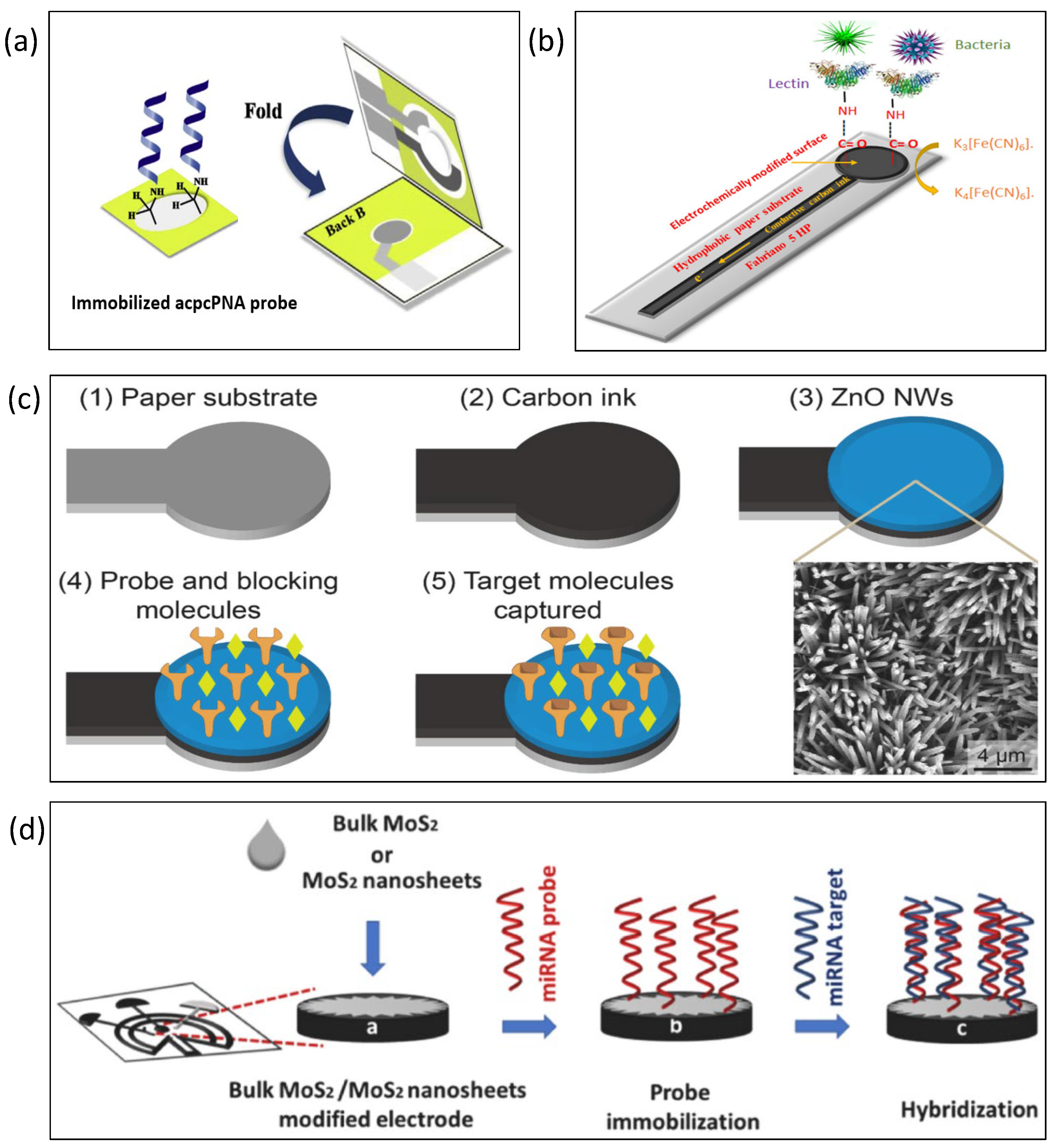
6. Paper-Based Impedance Biosensors
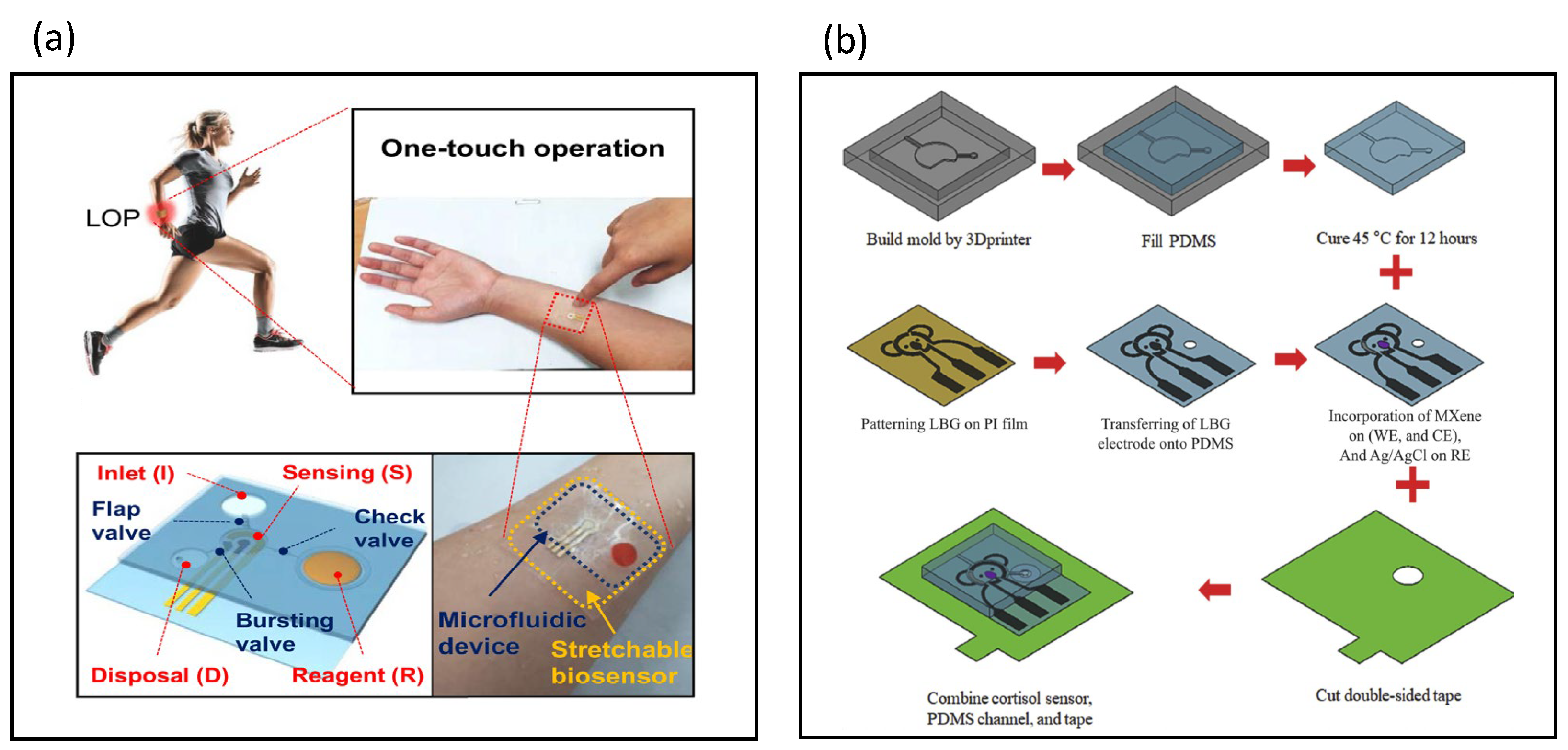
7. Stretchable Biosensors
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.; Song, H.; Ahn, H.; Kim, T.; Jung, J.; Cho, S.K.; Shin, D.M.; Choi, J.R.; Hwang, Y.H.; Kim, K. A Review of Advanced Impedance Biosensors with Microfluidic Chips for Single-Cell Analysis. Biosensors 2021, 11, 412. [Google Scholar] [CrossRef]
- Ayliffe, H.E.; Frazier, A.B.; Rabbitt, R.D. Electric impedance spectroscopy using microchannels with integrated metal electrodes. J. Microelectromech. Syst. 1999, 8, 50–57. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, X.W.; Liu, K.; Lan, T.C.; Wang, Z.X.; Zhu, Z. Recent Advances in Electrical Impedance Sensing Technology for Single-Cell Analysis. Biosensors 2021, 11, 470. [Google Scholar] [CrossRef]
- Lei, K.F. Review on Impedance Detection of Cellular Responses in Micro/Nano Environment. Micromachines 2014, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Giaever, I.; Keese, C.R. Monitoring fibroblast behavior in tissue culture with an applied electric field. Proc. Natl. Acad. Sci. USA 1984, 81, 3761–3764. [Google Scholar] [CrossRef] [Green Version]
- Giaever, I.; Keese, C.R. Use of electric fields to monitor the dynamical aspect of cell behavior in tissue culture. IEEE Trans. Biomed. Eng. 1986, 33, 242–247. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. Micromotion of mammalian-cells measured electrically. Proc. Natl. Acad. Sci. USA 1991, 88, 7896–7900. [Google Scholar] [CrossRef] [Green Version]
- Giaever, I.; Keese, C.R. A morphological biosensor for mammalian-cells. Nature 1993, 366, 591–592. [Google Scholar] [CrossRef]
- Grossi, M.; Riccò, B. Electrical impedance spectroscopy (EIS) for biological analysis and food characterization: A review. J. Sens. Sens. Syst. 2017, 6, 303–325. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Bernabini, C.; Morgan, H. Single-Colloidal Particle Impedance Spectroscopy: Complete Equivalent Circuit Analysis of Polyelectrolyte Microcapsules. Langmuir 2010, 26, 3821–3828. [Google Scholar] [CrossRef]
- Kamentsky, L.A.; Melamed, M.R.; Derman, H. Spectrophotometer: New instrument for ultrarapid cell analysis. Science 1965, 150, 630–631. [Google Scholar] [CrossRef] [PubMed]
- Gawad, S.; Schild, L.; Renaud, P. Micromachined impedance spectroscopy flow cytometer for cell analysis and particle sizing. Lab Chip 2001, 1, 76–82. [Google Scholar] [CrossRef]
- Gawad, S.; Cheung, K.; Seger, U.; Bertsch, A.; Renaud, P. Dielectric spectroscopy in a micromachined flow cytometer: Theoretical and practical considerations. Lab Chip 2004, 4, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Gawad, S.; Renaud, P. Impedance spectroscopy flow cytometry: On-chip label-free cell differentiation. Cytom. Part A 2005, 65A, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.A.; Johnson, T.S.; Britt, W.B. Flow cytometric electronic direct current volume and radiofrequency impedance measurements of single cells and particles. Cytometry 1981, 1, 377–384. [Google Scholar] [CrossRef]
- Sun, T.; Morgan, H. Single-cell microfluidic impedance cytometry: A review. Microfluid. Nanofluid. 2010, 8, 423–443. [Google Scholar] [CrossRef]
- Van Gerwen, P.; Laureyn, W.; Laureys, W.; Huyberechts, G.; De Beeck, M.O.; Baert, K.; Suls, J.; Sansen, W.; Jacobs, P.; Hermans, L.; et al. Nanoscaled interdigitated electrode arrays for biochemical sensors. Sens. Actuator B Chem. 1998, 49, 73–80. [Google Scholar] [CrossRef]
- Gomez, R.; Bashir, R.; Sarikaya, A.; Ladisch, M.R.; Sturgis, J.; Robinson, J.P.; Geng, T.; Bhunia, A.K.; Apple, H.L.; Wereley, S. Microfluidic Biochip for Impedance Spectroscopy of Biological Species. Biomed. Microdevices 2001, 3, 201–209. [Google Scholar] [CrossRef]
- Radke, S.M.; Alocilja, E.C. Design and fabrication of a microimpedance biosensor for bacterial detection. IEEE Sens. J. 2004, 4, 434–440. [Google Scholar] [CrossRef]
- Radke, S.M.; Alocilja, E.C. A microfabricated biosensor for detecting foodborne bioterrorism agents. IEEE Sens. J. 2005, 5, 744–750. [Google Scholar] [CrossRef]
- Hong, J.; Yoon, D.S.; Kim, S.K.; Kim, T.S.; Kim, S.; Pak, E.Y.; No, K. AC frequency characteristics of coplanar impedance sensors as design parameters. Lab Chip 2005, 5, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Kloss, D.; Fischer, M.; Rothermel, A.; Simon, J.C.; Robitzki, A.A. Drug testing on 3D in vitro tissues trapped on a microcavity chip. Lab Chip 2008, 8, 879–884. [Google Scholar] [CrossRef]
- James, C.D.; Reuel, N.; Lee, E.S.; Davalos, R.V.; Mani, S.S.; Carroll-Portillo, A.; Rebeil, R.; Martino, A.; Apblett, C.A. Impedimetric and optical interrogation of single cells in a microfluidic device for real-time viability and chemical response assessment. Biosens. Bioelectron. 2008, 23, 845–851. [Google Scholar] [CrossRef]
- Singh, K.V.; Whited, A.M.; Ragineni, Y.; Barrett, T.W.; King, J.; Solanki, R. 3D nanogap interdigitated electrode array biosensors. Anal. Bioanal. Chem. 2010, 397, 1493–1502. [Google Scholar] [CrossRef]
- Arya, S.K.; Chornokur, G.; Venugopal, M.; Bhansali, S. Antibody functionalized interdigitated mu-electrode (ID mu E) based impedimetric cortisol biosensor. Analyst 2010, 135, 1941–1946. [Google Scholar] [CrossRef]
- Chen, Y.; Wong, C.C.; Pui, T.S.; Nadipalli, R.; Weerasekera, R.; Chandran, J.; Yu, H.; Rahman, A.R.A. CMOS high density electrical impedance biosensor array for tumor cell detection. Sens. Actuator B Chem. 2012, 173, 903–907. [Google Scholar] [CrossRef]
- Ma, H.B.; Wallbank, R.W.R.; Chaji, R.; Li, J.H.; Suzuki, Y.; Jiggins, C.; Nathan, A. An impedance-based integrated biosensor for suspended DNA characterization. Sci. Rep. 2013, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Cunci, L.; Vargas, M.M.; Cunci, R.; Gomez-Moreno, R.; Perez, I.; Baerga-Ortiz, A.; Gonzalez, C.I.; Cabrera, C.R. Real-time detection of telomerase activity in cancer cells using a label-free electrochemical impedimetric biosensing microchip. RSC Adv. 2014, 4, 52357–52365. [Google Scholar] [CrossRef] [Green Version]
- Shin, K.S.; Ji, J.H.; Hwang, K.S.; Jun, S.C.; Kang, J.Y. Sensitivity Enhancement of Bead-based Electrochemical Impedance Spectroscopy (BEIS) biosensor by electric field-focusing in microwells. Biosens. Bioelectron. 2016, 85, 16–24. [Google Scholar] [CrossRef]
- Pursey, J.P.; Chen, Y.; Stulz, E.; Park, M.K.; Kongsuphol, P. Microfluidic electrochemical multiplex detection of bladder cancer DNA markers. Sens. Actuator B Chem. 2017, 251, 34–39. [Google Scholar] [CrossRef]
- Brosel-Oliu, S.; Mergel, O.; Uria, N.; Abramova, N.; van Rijn, P.; Bratov, A. 3D impedimetric sensors as a tool for monitoring bacterial response to antibiotics. Lab Chip 2019, 19, 1436–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.X.; Jiang, D.M.; Gu, C.L.; Qiu, Y.; Wan, H.; Wang, P. 3D microgroove electrical impedance sensing to examine 3D cell cultures for antineoplastic drug assessment. Microsyst. Nanoeng. 2020, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, Y.P.; Mukundan, A.; Chen, W.C.; Wu, M.T.; Hsieh, S.C.; Wang, H.C. Design of a Lab-On-Chip for Cancer Cell Detection through Impedance and Photoelectrochemical Response Analysis. Biosensors 2022, 12, 405. [Google Scholar] [CrossRef] [PubMed]
- Valekunja, R.B.; Kamakoti, V.; Peter, A.; Phadnis, S.; Prasad, S.; Nagaraj, V.J. The detection of papaya ringspot virus coat protein using an electrochemical immunosensor. Anal. Methods 2016, 8, 8534–8541. [Google Scholar] [CrossRef]
- Jin, S.R.; Ye, Z.Z.; Wang, Y.X.; Ying, Y.B. A Novel Impedimetric Microfluidic Analysis System for Transgenic Protein Cry1Ab Detection. Sci. Rep. 2017, 7, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.J.; Ho, C.Y.; Zhou, X.M.; Yen, H.R. Determination of degree of RBC agglutination for blood typing using a small quantity of blood sample in a microfluidic system. Biosens. Bioelectron. 2018, 102, 234–241. [Google Scholar] [CrossRef]
- Fuentes-Velez, S.; Fagoonee, S.; Sanginario, A.; Pizzi, M.; Altruda, F.; Demarchi, D. Electrical Impedance-Based Characterization of Hepatic Tissue with Early-Stage Fibrosis. Biosensors 2022, 12, 116. [Google Scholar] [CrossRef]
- Zou, Z.W.; Kai, J.H.; Rust, M.J.; Han, J.; Ahn, C.H. Functionalized nano interdigitated electrodes arrays on polymer with integrated microfluidics for direct bio-affinity sensing using impedimetric measurement. Sens. Actuator A Phys. 2007, 136, 518–526. [Google Scholar] [CrossRef]
- Sabounchi, P.; Morales, A.M.; Ponce, P.; Lee, L.P.; Simmons, B.A.; Davalos, R.V. Sample concentration and impedance detection on a microfluidic polymer chip. Biomed. Microdevices 2008, 10, 661–670. [Google Scholar] [CrossRef]
- Dapra, J.; Lauridsen, L.H.; Nielsen, A.T.; Rozlosnik, N. Comparative study on aptamers as recognition elements for antibiotics in a label-free all-polymer biosensor. Biosens. Bioelectron. 2013, 43, 315–320. [Google Scholar] [CrossRef]
- Pires, L.; Sachsenheimer, K.; Kleintschek, T.; Waldbaur, A.; Schwartz, T.; Rapp, B.E. Online monitoring of biofilm growth and activity using a combined multi-channel impedimetric and amperometric sensor. Biosens. Bioelectron. 2013, 47, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Rosati, G.; Dapra, J.; Cherre, S.; Rozlosnik, N. Performance Improvement by Layout Designs of Conductive Polymer Microelectrode Based Impedimetric Biosensors. Electroanalysis 2014, 26, 1400–1408. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Tian, Y.L.; Wu, X.S.; Li, C.M.; Yu, L. A one-piece lateral flow impedimetric test strip for label-free clenbuterol detection. Anal. Methods 2015, 7, 4957–4964. [Google Scholar] [CrossRef]
- Sharif, S.; Wang, Y.X.; Ye, Z.Z.; Wang, Z.; Qiu, Q.M.; Ying, S.N.; Ying, Y.B. A novel impedimetric sensor for detecting LAMP amplicons of pathogenic DNA based on magnetic separation. Sens. Actuator B Chem. 2019, 301, 6. [Google Scholar] [CrossRef]
- Lakey, A.; Ali, Z.; Scott, S.M.; Chebil, S.; Korri-Youssoufi, H.; Hunor, S.; Ohlander, A.; Kuphal, M.; Marti, J.S. Impedimetric array in polymer microfluidic cartridge for low cost point-of-care diagnostics. Biosens. Bioelectron. 2019, 129, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.R.; Liu, L.L.; Xu, Y.; Wang, L.; Chen, L.; Yan, S.; Shui, L.L.; Wang, Z.J.; Li, S.B. A highly efficient preconcentration route for rapid and sensitive detection of endotoxin based on an electrochemical biosensor. Analyst 2020, 145, 4204–4211. [Google Scholar] [CrossRef]
- Niaraki, A.; Shirsavar, M.A.; Aykar, S.S.; Taghavimehr, M.; Montazami, R.; Hashemi, N.N. Minute-sensitive real-time monitoring of neural cells through printed graphene microelectrodes. Biosens. Bioelectron. 2022, 210, 8. [Google Scholar] [CrossRef]
- Chmayssem, A.; Tanase, C.E.; Verplanck, N.; Gougis, M.; Mourier, V.; Zebda, A.; Ghaemmaghami, A.M.; Mailley, P. New Microfluidic System for Electrochemical Impedance Spectroscopy Assessment of Cell Culture Performance: Design and Development of New Electrode Material. Biosensors 2022, 12, 452. [Google Scholar] [CrossRef]
- Hantschke, M.; Triantis, I.F. Optimisation of an Electrical Impedance Sensor for Use in Microfluidic Chip Electrophoresis. IEEE Sens. J. 2022, 22, 16–24. [Google Scholar] [CrossRef]
- Ruan, C.M.; Yang, L.J.; Li, Y.B. Immunobiosensor chips for detection of Escherichia coli O157:H7 using electrochemical impedance spectroscopy. Anal. Chem. 2002, 74, 4814–4820. [Google Scholar] [CrossRef]
- Yang, L.J.; Li, Y.B.; Griffis, C.L.; Johnson, M.G. Interdigitated microelectrode (IME) impedance sensor for the detection of viable Salmonella typhimurium. Biosens. Bioelectron. 2004, 19, 1139–1147. [Google Scholar] [CrossRef]
- Yang, L.J.; Li, Y.B.; Erf, G.F. Interdigitated array microelectrode-based electrochemical impedance immunosensor for detection of Escherichia coli O157:H7. Anal. Chem. 2004, 76, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Ruan, C.M.; Kanayeva, D.; Lassiter, K.; Li, Y.B. TiO2 nanowire bundle microelectrode based impedance immunosensor for rapid and sensitive detection of Listeria monocytogenes. Nano Lett. 2008, 8, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Leung, P.H.M.; Liu, Z.B.; Zhang, Y.; Xiao, L.D.; Ye, W.W.; Zhang, X.; Yi, L.; Yang, M. A PDMS microfluidic impedance immunosensor for E. coli O157:H7 and Staphylococcus aureus detection via antibody-immobilized nanoporous membrane. Sens. Actuator B Chem. 2011, 159, 328–335. [Google Scholar] [CrossRef]
- Lum, J.; Wang, R.H.; Lassiter, K.; Srinivasan, B.; Abi-Ghanem, D.; Berghman, L.; Hargis, B.; Tung, S.; Lu, H.G.; Li, Y.B. Rapid detection of avian influenza H5N1 virus using impedance measurement of immuno-reaction coupled with RBC amplification. Biosens. Bioelectron. 2012, 38, 67–73. [Google Scholar] [CrossRef]
- Dastider, S.G.; Barizuddin, S.; Dweik, M.; Almasri, M. A micromachined impedance biosensor for accurate and rapid detection of E. coli O157:H7. RSC Adv. 2013, 3, 26297–26306. [Google Scholar] [CrossRef]
- Couniot, N.; Vanzieleghem, T.; Rasson, J.; Van Overstraeten-Schlogel, N.; Poncelet, O.; Mahillon, J.; Francis, L.A.; Flandre, D. Lytic enzymes as selectivity means for label-free, microfluidic and impedimetric detection of whole-cell bacteria using ALD-Al2O3 passivated microelectrodes. Biosens. Bioelectron. 2015, 67, 154–161. [Google Scholar] [CrossRef]
- Liu, J.Y.; Jasim, I.; Abdullah, A.; Shen, Z.Y.; Zhao, L.; El-Dweik, M.; Zhang, S.P.; Almasri, M. An integrated impedance biosensor platform for detection of pathogens in poultry products. Sci. Rep. 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dastider, S.G.; Abdullah, A.; Jasim, I.; Yuksek, N.S.; Dweik, M.; Almasri, M. Low concentration E. coli O157:H7 bacteria sensing using microfluidic MEMS biosensor. Rev. Sci. Instrum. 2018, 89, 9. [Google Scholar] [CrossRef]
- Liu, J.Y.; Jasim, I.; Shen, Z.Y.; Zhao, L.; Dweik, M.; Zhang, S.P.; Almasri, M. A microfluidic based biosensor for rapid detection of Salmonella in food products. PLoS ONE 2019, 14, e0216873. [Google Scholar] [CrossRef]
- Mishra, N.N.; Retterer, S.; Zieziulewicz, T.J.; Isaacson, M.; Szarowski, D.; Mousseau, D.E.; Lawrence, D.A.; Turner, J.N. On-chip micro-biosensor for the detection of human CD4(+) cells based on AC impedance and optical analysis. Biosens. Bioelectron. 2005, 21, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Kuttel, C.; Nascimento, E.; Demierre, N.; Silva, T.; Braschler, T.; Renaud, P.; Oliva, A.G. Label-free detection of Babesia bovis infected red blood cells using impedance spectroscopy on a microfabricated flow cytometer. Acta Trop. 2007, 102, 63–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, D.; Pettigrew, D.; Reccius, C.H.; Gwyer, J.D.; van Berkel, C.; Holloway, J.; Davies, D.E.; Morgan, H. Leukocyte analysis and differentiation using high speed microfluidic single cell impedance cytometry. Lab Chip 2009, 9, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Han, X.J.; van Berkel, C.; Gwyer, J.; Capretto, L.; Morgan, H. Microfluidic Lysis of Human Blood for Leukocyte Analysis Using Single Cell Impedance Cytometry. Anal. Chem. 2012, 84, 1070–1075. [Google Scholar] [CrossRef]
- Lei, K.F.; Chen, K.H.; Tsui, P.H.; Tsang, N.M. Real-Time Electrical Impedimetric Monitoring of Blood Coagulation Process under Temperature and Hematocrit Variations Conducted in a Microfluidic Chip. PLoS ONE 2013, 8, e76243. [Google Scholar] [CrossRef]
- Song, H.J.; Wang, Y.; Rosano, J.M.; Prabhakarpandian, B.; Garson, C.; Pant, K.; Lai, E. A microfluidic impedance flow cytometer for identification of differentiation state of stem cells. Lab Chip 2013, 13, 2300–2310. [Google Scholar] [CrossRef]
- Du, E.; Ha, S.; Diez-Silva, M.; Dao, M.; Suresh, S.; Chandrakasan, A.P. Electric impedance microflow cytometry for characterization of cell disease states. Lab Chip 2013, 13, 3903–3909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, D.; Hollis, V.; Morgan, H. Microfluidic impedance cytometry of tumour cells in blood. Biomicrofluidics 2014, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Qiang, Y.H.; Alvarez, O.; Du, E. Electrical impedance microflow cytometry with oxygen control for detection of sickle cells. Sens. Actuator B Chem. 2018, 255, 2392–2398. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Yin, T.I.; Reyes, D.; Urban, G.A. Microfluidic Chip with Integrated Electrical Cell-Impedance Sensing for Monitoring Single Cancer Cell Migration in Three-Dimensional Matrixes. Anal. Chem. 2013, 85, 11068–11076. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, X.; Lei, K.F.; Huang, C.H. Quantitative impedimetric monitoring of cell migration under the stimulation of cytokine or anti-cancer drug in a microfluidic chip. Biomicrofluidics 2015, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.H.; Lei, K.F. Impedimetric quantification of migration speed of cancer cells migrating along a Matrigel-filled microchannel. Anal. Chim. Acta 2020, 1121, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Lei, K.F. Quantitative study of tumor angiogenesis in three-dimensional matrigel barrier using electric impedance measurement technique. Sens. Actuator B Chem. 2022, 370, 8. [Google Scholar] [CrossRef]
- Hong, J.L.; Lan, K.C.; Jang, L.S. Electrical characteristics analysis of various cancer cells using a microfluidic device based on single-cell impedance measurement. Sens. Actuator B Chem. 2012, 173, 927–934. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.T.; Chen, D.Y.; Luo, Y.N.; Jiang, M.; Wei, C.; Long, R.; Yue, W.T.; Wang, J.B.; Chen, J. Tumor cell characterization and classification based on cellular specific membrane capacitance and cytoplasm conductivity. Biosens. Bioelectron. 2014, 57, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.H.; Chen, X.; Ge, Y.Q.; Jin, Y.; Jin, Q.H.; Zhao, J.L. Single-cell impedance analysis of osteogenic differentiation by droplet-based microfluidics. Biosens. Bioelectron. 2019, 145, 8. [Google Scholar] [CrossRef]
- Lei, K.F.; Ho, Y.C.; Huang, C.H.; Huang, C.H.; Pai, P.C. Characterization of stem cell-like property in cancer cells based on single-cell impedance measurement in a microfluidic platform. Talanta 2021, 229, 8. [Google Scholar] [CrossRef] [PubMed]
- Bieberich, E.; Guiseppi-Elie, A. Neuronal differentiation and synapse formation of PC12 and embryonic stem cells on interdigitated microelectrode arrays: Contact structures for neuron-to-electrode signal transmission (NEST). Biosens. Bioelectron. 2004, 19, 923–931. [Google Scholar] [CrossRef]
- Jang, L.S.; Wang, M.H. Microfluidic device for cell capture and impedance measurement. Biomed. Microdevices 2007, 9, 737–743. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, H.S.; Frazier, A.B.; Chen, Z.G.; Shin, D.M.; Han, A. Whole-Cell Impedance Analysis for Highly and Poorly Metastatic Cancer Cells. J. Microelectromech. Syst. 2009, 18, 808–817. [Google Scholar] [CrossRef]
- Hildebrandt, C.; Buth, H.; Cho, S.B.; Impidjati; Thielecke, H. Detection of the osteogenic differentiation of mesenchymal stem cells in 2D and 3D cultures by electrochemical impedance spectroscopy. J. Biotechnol. 2010, 148, 83–90. [Google Scholar] [CrossRef]
- Houssin, T.; Folleta, J.; Follet, A.; Dei-Cas, E.; Senez, V. Label-free analysis of water-polluting parasite by electrochemical impedance spectroscopy. Biosens. Bioelectron. 2010, 25, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Dalmay, C.; Cheray, M.; Pothier, A.; Lalloue, F.; Jauberteau, M.O.; Blondy, P. Ultra sensitive biosensor based on impedance spectroscopy at microwave frequencies for cell scale analysis. Sens. Actuator A Phys. 2010, 162, 189–197. [Google Scholar] [CrossRef]
- Bagnaninchi, P.O.; Drummond, N. Real-time label-free monitoring of adipose-derived stem cell differentiation with electric cell-substrate impedance sensing. Proc. Natl. Acad. Sci. USA 2011, 108, 6462–6467. [Google Scholar] [CrossRef] [Green Version]
- Lei, K.F.; Wu, Z.M.; Huang, C.H. Impedimetric quantification of the formation process and the chemosensitivity of cancer cell colonies suspended in 3D environment. Biosens. Bioelectron. 2015, 74, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.C.; Chen, C.H.; Chen, M.K.; Jang, L.S.; Wang, M.H. Single-cell trapping and impedance measurement utilizing dielectrophoresis in a parallel-plate microfluidic device. Sens. Actuator B Chem. 2014, 190, 570–577. [Google Scholar] [CrossRef]
- Lei, K.F.; Kao, C.H.; Tsang, N.M. High throughput and automatic colony formation assay based on impedance measurement technique. Anal. Bioanal. Chem. 2017, 409, 3271–3277. [Google Scholar] [CrossRef]
- Nguyen, N.V.; Jen, C.P. Impedance detection integrated with dielectrophoresis enrichment platform for lung circulating tumor cells in a microfluidic channel. Biosens. Bioelectron. 2018, 121, 10–18. [Google Scholar] [CrossRef]
- Lei, K.F.; Lin, B.Y.; Tsang, N.M. Real-time and label-free impedimetric analysis of the formation and drug testing of tumor spheroids formed via the liquid overlay technique. RSC Adv. 2017, 7, 13939–13946. [Google Scholar] [CrossRef] [Green Version]
- Lei, K.F.; Wu, M.H.; Hsu, C.W.; Chen, Y.D. Real-time and non-invasive impedimetric monitoring of cell proliferation and chemosensitivity in a perfusion 3D cell culture microfluidic chip. Biosens. Bioelectron. 2014, 51, 16–21. [Google Scholar] [CrossRef]
- Sun, T.; Green, N.G.; Gawad, S.; Morgan, H. Analytical electric field and sensitivity analysis for two microfluidic impedance cytometer designs. IET Nanobiotechnol. 2007, 1, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, D.; She, J.K.; Roach, P.L.; Morgan, H. Bead-based immunoassays using a micro-chip flow cytometer. Lab Chip 2007, 7, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Holmes, D.; Gawad, S.; Green, N.G.; Morgan, H. High speed multi-frequency impedance analysis of single particles in a microfluidic cytometer using maximum length sequences. Lab Chip 2007, 7, 1034–1040. [Google Scholar] [CrossRef] [Green Version]
- Kummrow, A.; Theisen, J.; Frankowski, M.; Tuchscheerer, A.; Yildirim, H.; Brattke, K.; Schmidt, M.; Neukammer, J. Microfluidic structures for flow cytometric analysis of hydrodynamically focussed blood cells fabricated by ultraprecision micromachining. Lab Chip 2009, 9, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Spencer, D.; Morgan, H. Positional dependence of particles in microfludic impedance cytometry. Lab Chip 2011, 11, 1234–1239. [Google Scholar] [CrossRef]
- Barat, D.; Spencer, D.; Benazzi, G.; Mowlem, M.C.; Morgan, H. Simultaneous high speed optical and impedance analysis of single particles with a microfluidic cytometer. Lab Chip 2012, 12, 118–126. [Google Scholar] [CrossRef]
- Spencer, D.; Elliott, G.; Morgan, H. A sheath-less combined optical and impedance micro-cytometer. Lab Chip 2014, 14, 3064–3073. [Google Scholar] [CrossRef] [PubMed]
- Haandbaek, N.; Burgel, S.C.; Heer, F.; Hierlemann, A. Characterization of subcellular morphology of single yeast cells using high frequency microfluidic impedance cytometer. Lab Chip 2014, 14, 369–377. [Google Scholar] [CrossRef]
- David, F.; Hebeisen, M.; Schade, G.; Franco-Lara, E.; Di Berardino, M. Viability and membrane potential analysis of Bacillus megaterium cells by impedance flow cytometry. Biotechnol. Bioeng. 2012, 109, 483–492. [Google Scholar] [CrossRef]
- Evander, M.; Ricco, A.J.; Morser, J.; Kovacs, G.T.A.; Leung, L.L.K.; Giovangrandi, L. Microfluidic impedance cytometer for platelet analysis. Lab Chip 2013, 13, 722–729. [Google Scholar] [CrossRef]
- Lin, Z.; Cao, X.; Xie, P.; Liu, M.; Javanmard, M. PicoMolar level detection of protein biomarkers based on electronic sizing of bead aggregates: Theoretical and experimental considerations. Biomed. Microdevices 2015, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Frankowski, M.; Bock, N.; Neukammer, J. Label-free whole blood cell differentiation based on multiple frequency AC impedance and light scattering analysis in a micro flow cytometer. Lab Chip 2016, 16, 2326–2338. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.F.; Cao, X.N.; Lin, Z.T.; Javanmard, M. Top-down fabrication meets bottom-up synthesis for nanoelectronic barcoding of microparticles. Lab Chip 2017, 17, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.S.; Honrado, C.; Spencer, D.; Horton, B.; Bridle, H.L.; Morgan, H. Analysis of Parasitic Protozoa at the Single-cell Level using Microfluidic Impedance Cytometry. Sci. Rep. 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.L.; Tang, D.Z.; Ni, Z.H.; Xiang, N.; Yi, H. Microfluidic Impedance Cytometer with Inertial Focusing and Liquid Electrodes for High-Throughput Cell Counting and Discrimination. Anal. Chem. 2017, 89, 3154–3161. [Google Scholar] [CrossRef]
- Mansor, M.A.; Takeuchi, M.; Nakajima, M.; Hasegawa, Y.; Ahmad, M.R. Electrical Impedance Spectroscopy for Detection of Cells in Suspensions Using Microfluidic Device with Integrated Microneedles. Appl. Sci. 2017, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, K.; Rather, G.M.; Lin, Z.T.; Sui, J.Y.; Xie, P.F.; Le, T.; Bertino, J.R.; Javanmard, M. Toward point-of-care assessment of patient response: A portable tool for rapidly assessing cancer drug efficacy using multifrequency impedance cytometry and supervised machine learning. Microsyst. Nanoeng. 2019, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Sui, J.Y.; Foflonker, F.; Bhattacharya, D.; Javanmard, M. Electrical impedance as an indicator of microalgal cell health. Sci. Rep. 2020, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Mahesh, K.; Varma, M.; Sen, P. Double-peak signal features in microfluidic impedance flow cytometry enable sensitive measurement of cell membrane capacitance. Lab Chip 2020, 20, 4296–4309. [Google Scholar] [CrossRef]
- Petchakup, C.; Hutchinson, P.E.; Tay, H.M.; Leong, S.Y.; Li, K.H.H.; Hou, H.W. Label-free quantitative lymphocyte activation profiling using microfluidic impedance cytometry. Sens. Actuator B Chem. 2021, 339, 9. [Google Scholar] [CrossRef]
- Caselli, F.; Reale, R.; De Ninno, A.; Spencer, D.; Morgan, H.; Bisegna, P. Deciphering impedance cytometry signals with neural networks. Lab Chip 2022, 22, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Wang, Y.; Lassiter, K.; Li, Y.B.; Hargis, B.; Tung, S.; Berghman, L.; Bottje, W. Interdigitated array microelectrode based impedance immunosensor for detection of avian influenza virus H5N1. Talanta 2009, 79, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Lin, J.H.; Lassiter, K.; Srinivasan, B.; Lin, L.; Lu, H.G.; Tung, S.; Hargis, B.; Bottje, W.; Berghman, L.; et al. Evaluation study of a portable impedance biosensor for detection of avian influenza virus. J. Virol. Methods 2011, 178, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, H.; Jahangir, M.; Inci, F.; Wang, S.Q.; Willenbrecht, R.B.M.; Giguel, F.F.; Tsibris, A.M.N.; Kuritzkes, D.R.; Demirci, U. Acute On-Chip HIV Detection Through Label-Free Electrical Sensing of Viral Nano-Lysate. Small 2013, 9, 2553–2563. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Hong, S.; Jang, J. Label-free Detection of Influenza Viruses using a Reduced Graphene Oxide-based Electrochemical Immunosensor Integrated with a Microfluidic Platform. Sci. Rep. 2017, 7, 11. [Google Scholar] [CrossRef] [Green Version]
- Berdat, D.; Rodriguez, A.C.M.; Herrera, F.; Gijs, M.A.M. Label-free detection of DNA with interdigitated micro-electrodes in a fluidic cell. Lab Chip 2008, 8, 302–308. [Google Scholar] [CrossRef]
- Javanmard, M.; Davis, R.W. A microfluidic platform for electrical detection of DNA hybridization. Sens. Actuator B Chem. 2011, 154, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Ben-Yoav, H.; Dykstra, P.H.; Bentley, W.E.; Ghodssi, R. A microfluidic-based electrochemical biochip for label-free diffusion-restricted DNA hybridization analysis. Biosens. Bioelectron. 2012, 38, 114–120. [Google Scholar] [CrossRef]
- Chuang, C.H.; Du, Y.C.; Wu, T.F.; Chen, C.H.; Lee, D.H.; Chen, S.M.; Huang, T.C.; Wu, H.P.; Shaikh, M.O. Immunosensor for the ultrasensitive and quantitative detection of bladder cancer in point of care testing. Biosens. Bioelectron. 2016, 84, 126–132. [Google Scholar] [CrossRef]
- Ondevilla, N.A.P.; Wong, T.W.; Lee, N.Y.; Chang, H.C. An AC electrokinetics-based electrochemical aptasensor for the rapid detection of microRNA-155. Biosens. Bioelectron. 2022, 199, 7. [Google Scholar] [CrossRef]
- Wu, C.C.; Huang, W.C.; Hu, C.C. An ultrasensitive label-free electrochemical impedimetric DNA biosensing chip integrated with a DC-biased AC electroosmotic vortex. Sens. Actuator B Chem. 2015, 209, 61–68. [Google Scholar] [CrossRef]
- Alsabbagh, K.; Hornung, T.; Voigt, A.; Sadir, S.; Rajabi, T.; Lange, K. Microfluidic Impedance Biosensor Chips Using Sensing Layers Based on DNA-Based Self-Assembled Monolayers for Label-Free Detection of Proteins. Biosensors 2021, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Sheen, H.J.; Panigrahi, B.; Kuo, T.R.; Hsu, W.C.; Chung, P.S.; Xie, Q.Z.; Lin, C.Y.; Chang, Y.S.; Lin, C.T.; Fan, Y.J. Electrochemical biosensor with electrokinetics-assisted molecular trapping for enhancing C-reactive protein detection. Biosens. Bioelectron. 2022, 210, 9. [Google Scholar] [CrossRef]
- Chinnadayyala, S.R.; Cho, S. Electrochemical Immunosensor for the Early Detection of Rheumatoid Arthritis Biomarker: Anti-Cyclic Citrullinated Peptide Antibody in Human Serum Based on Avidin-Biotin System. Sensors 2021, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Selvam, S.P.; Chinnadayyala, S.R.; Cho, S. Electrochemical nanobiosensor for early detection of rheumatoid arthritis biomarker: Anti- cyclic citrullinated peptide antibodies based on polyaniline (PANI)/MoS2-modified screen-printed electrode with PANI-Au nanomatrix-based signal amplification. Sens. Actuator B Chem. 2021, 333, 12. [Google Scholar] [CrossRef]
- Lin, C.Y.; Nguyen, U.T.N.; Hsieh, H.Y.; Tahara, H.; Chang, Y.S.; Wang, B.Y.; Gu, B.C.; Dai, Y.H.; Wu, C.C.; Tsai, I.J.; et al. Peptide-based electrochemical sensor with nanogold enhancement for detecting rheumatoid arthritis. Talanta 2022, 236, 9. [Google Scholar] [CrossRef]
- Chiriaco, M.S.; Primiceri, E.; D’Amone, E.; Ionescu, R.E.; Rinaldi, R.; Maruccio, G. EIS microfluidic chips for flow immunoassay and ultrasensitive cholera toxin detection. Lab Chip 2011, 11, 658–663. [Google Scholar] [CrossRef]
- Liu, F.; Nordin, A.N.; Li, F.; Voiculescu, I. A lab-on-chip cell-based biosensor for label-free sensing of water toxicants. Lab Chip 2014, 14, 1270–1280. [Google Scholar] [CrossRef]
- Pradhan, R.; Kalkal, A.; Jindal, S.; Packirisamy, G.; Manhas, S. Four electrode-based impedimetric biosensors for evaluating cytotoxicity of tamoxifen on cervical cancer cells. RSC Adv. 2021, 11, 798–806. [Google Scholar] [CrossRef]
- Guo, Y.M.; Liu, X.F.; Sun, X.; Cao, Y.Y.; Wang, X.Y. A PDMS Microfluidic Impedance Immunosensor for Sensitive Detection Of Pesticide Residues in Vegetable Real Samples. Int. J. Electrochem. Sci. 2015, 10, 4155–4164. [Google Scholar]
- Zeng, L.; Wang, W.Q.; Rogers, F.; Zhang, H.P.; Zhang, X.M.; Yang, D.X. A High Sensitivity Micro Impedance Sensor Based on Magnetic Focusing for Oil Condition Monitoring. IEEE Sens. J. 2020, 20, 3813–3821. [Google Scholar] [CrossRef]
- Lei, K.F.; Huang, C.H.; Tsang, N.M. Impedimetric quantification of cells encapsulated in hydrogel cultured in a paper-based microchamber. Talanta 2016, 147, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Teengam, P.; Siangproh, W.; Tuantranont, A.; Vilaivan, T.; Chailapakul, O.; Henry, C.S. Electrochemical impedance-based DNA sensor using pyrrolidinyl peptide nucleic acids for tuberculosis detection. Anal. Chim. Acta 2018, 1044, 102–109. [Google Scholar] [CrossRef]
- Rengaraj, S.; Cruz-Izquierdo, A.; Scott, J.L.; Di Lorenzo, M. Impedimetric paper-based biosensor for the detection of bacterial contamination in water. Sens. Actuator B Chem. 2018, 265, 50–58. [Google Scholar] [CrossRef]
- Moazeni, M.; Karimzadeh, F.; Kermanpur, A. Peptide modified paper based impedimetric immunoassay with nanocomposite electrodes as a point-of-care testing of Alpha-fetoprotein in human serum. Biosens. Bioelectron. 2018, 117, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.F.; Liu, T.K.; Tsang, N.M. Towards a high throughput impedimetric screening of chemosensitivity of cancer cells suspended in hydrogel and cultured in a paper substrate. Biosens. Bioelectron. 2018, 100, 355–360. [Google Scholar] [CrossRef]
- Congur, G.; Erdem, A. PAMAM dendrimer modified screen printed electrodes for impedimetric detection of miRNA-34a. Microchem. J. 2019, 148, 748–758. [Google Scholar] [CrossRef]
- Vasantham, S.; Alhans, R.; Singhal, C.; Nagabooshanam, S.; Nissar, S.; Basu, T.; Ray, S.C.; Wadhwa, S.; Narang, J.; Mathur, A. Paper based point of care immunosensor for the impedimetric detection of cardiac troponin I biomarker. Biomed. Microdevices 2019, 22, 9. [Google Scholar] [CrossRef]
- Li, X.; Qin, Z.; Fu, H.; Li, T.; Peng, R.; Li, Z.J.; Rini, J.M.; Liu, X.Y. Enhancing the performance of paper-based electrochemical impedance spectroscopy nanobiosensors: An experimental approach. Biosens. Bioelectron. 2021, 177, 8. [Google Scholar] [CrossRef]
- Kare, S.P.O.; Das, D.; Chaudhury, K.; Das, S. Hand-drawn electrode based disposable paper chip for artificial sweat analysis using impedance spectroscopy. Biomed. Microdevices 2021, 23, 12. [Google Scholar] [CrossRef]
- Eksin, E.; Torul, H.; Yarali, E.; Tamer, U.; Papakonstantinou, P.; Erdem, A. Paper-based electrode assemble for impedimetric detection of miRNA. Talanta 2021, 225, 6. [Google Scholar] [CrossRef] [PubMed]
- Karuppiah, S.; Mishra, N.C.; Tsai, W.C.; Liao, W.S.; Chou, C.F. Ultrasensitive and Low-Cost Paper-Based Graphene Oxide Nanobiosensor for Monitoring Water-Borne Bacterial Contamination. ACS Sens. 2021, 6, 3214–3223. [Google Scholar] [CrossRef] [PubMed]
- Yarali, E.; Eksin, E.; Torul, H.; Ganguly, A.; Tamer, U.; Papakonstantinou, P.; Erdem, A. Impedimetric detection of miRNA biomarkers using paper-based electrodes modified with bulk crystals or nanosheets of molybdenum disulfide. Talanta 2022, 241, 10. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Pilloton, R.; Jain, S.; Roy, S.; Khanuja, M.; Mathur, A.; Narang, J. Paper-Based Electrodes Conjugated with Tungsten Disulfide Nanostructure and Aptamer for Impedimetric Detection of Listeria monocytogenes. Biosensors 2022, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Furniturewalla, A.; Chan, M.; Sui, J.; Ahuja, K.; Javanmard, M. Fully integrated wearable impedance cytometry platform on flexible circuit board with online smartphone readout. Microsyst. Nanoeng. 2018, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Guzman, K.; Al-Kharusi, G.; Levingstone, T.; Morrin, A. Robust epidermal tattoo electrode platform for skin physiology monitoring. Anal. Methods 2019, 11, 1460–1468. [Google Scholar] [CrossRef]
- Lee, H.B.; Meeseepong, M.; Trung, T.Q.; Kim, B.Y.; Lee, N.E. A wearable lab-on-a-patch platform with stretchable nanostructured biosensor for non-invasive immunodetection of biomarker in sweat. Biosens. Bioelectron. 2020, 156, 8. [Google Scholar] [CrossRef]
- Huynh, V.L.; Trung, T.Q.; Meeseepong, M.; Lee, H.B.; Nguyen, T.D.; Lee, N.E. Hollow Microfibers of Elastomeric Nanocomposites for Fully Stretchable and Highly Sensitive Microfluidic Immunobiosensor Patch. Adv. Funct. Mater. 2020, 30, 12. [Google Scholar] [CrossRef]
- Nah, J.S.; Barman, S.C.; Abu Zahed, M.; Sharifuzzaman, M.; Yoon, H.; Park, C.; Yoon, S.; Zhang, S.P.; Park, J.Y. A wearable microfluidics-integrated impedimetric immunosensor based on Ti3C2Tx MXene incorporated laser-burned graphene for noninvasive sweat cortisol detection. Sens. Actuator B Chem. 2021, 329, 9. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-S.; Huang, C.-H.; Pai, P.-C.; Seo, J.; Lei, K.F. A Review on Microfluidics-Based Impedance Biosensors. Biosensors 2023, 13, 83. https://doi.org/10.3390/bios13010083
Chen Y-S, Huang C-H, Pai P-C, Seo J, Lei KF. A Review on Microfluidics-Based Impedance Biosensors. Biosensors. 2023; 13(1):83. https://doi.org/10.3390/bios13010083
Chicago/Turabian StyleChen, Yu-Shih, Chun-Hao Huang, Ping-Ching Pai, Jungmok Seo, and Kin Fong Lei. 2023. "A Review on Microfluidics-Based Impedance Biosensors" Biosensors 13, no. 1: 83. https://doi.org/10.3390/bios13010083
APA StyleChen, Y.-S., Huang, C.-H., Pai, P.-C., Seo, J., & Lei, K. F. (2023). A Review on Microfluidics-Based Impedance Biosensors. Biosensors, 13(1), 83. https://doi.org/10.3390/bios13010083




