Hemisynthesis and Biological Evaluation of Cinnamylated, Benzylated, and Prenylated Dihydrochalcones from a Common Bio-Sourced Precursor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Synthesis
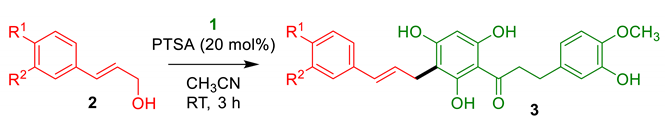
2.1.1. Cinnamylation of DHC 1
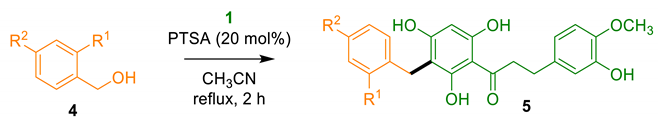
2.1.2. Benzylation of DHC 1
2.1.3. Prenylation of DHC 1
2.2. Biological Evaluation of Alkylated DHCs 3a–e, 5a–d, 7a–b, and 8a
3. Materials and Methods
3.1. General Information
3.1.1. Chemistry
3.1.2. Antibacterial Assays
3.1.3. Cytotoxicity Assays
3.2. Synthesis of Compounds 3a–e
3.2.1. General Procedure for the Cinnamylation of DHC 1
3.2.2. Compound 3a
3.2.3. Compound 3b
3.2.4. Compound 3c
3.2.5. Compound 3d
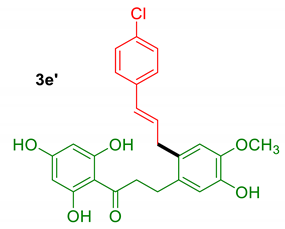
3.2.6. Compound 3e
3.2.7. Compound 3e’
3.3. Synthesis of Compounds 5a–d
3.3.1. General Procedure for the Benzylation of DHC 1
3.3.2. Compound 5a
3.3.3. Compound 5b
3.3.4. Compound 5c
3.3.5. Compound 5d
3.4. Synthesis of Compounds 7a–b and 8a–b
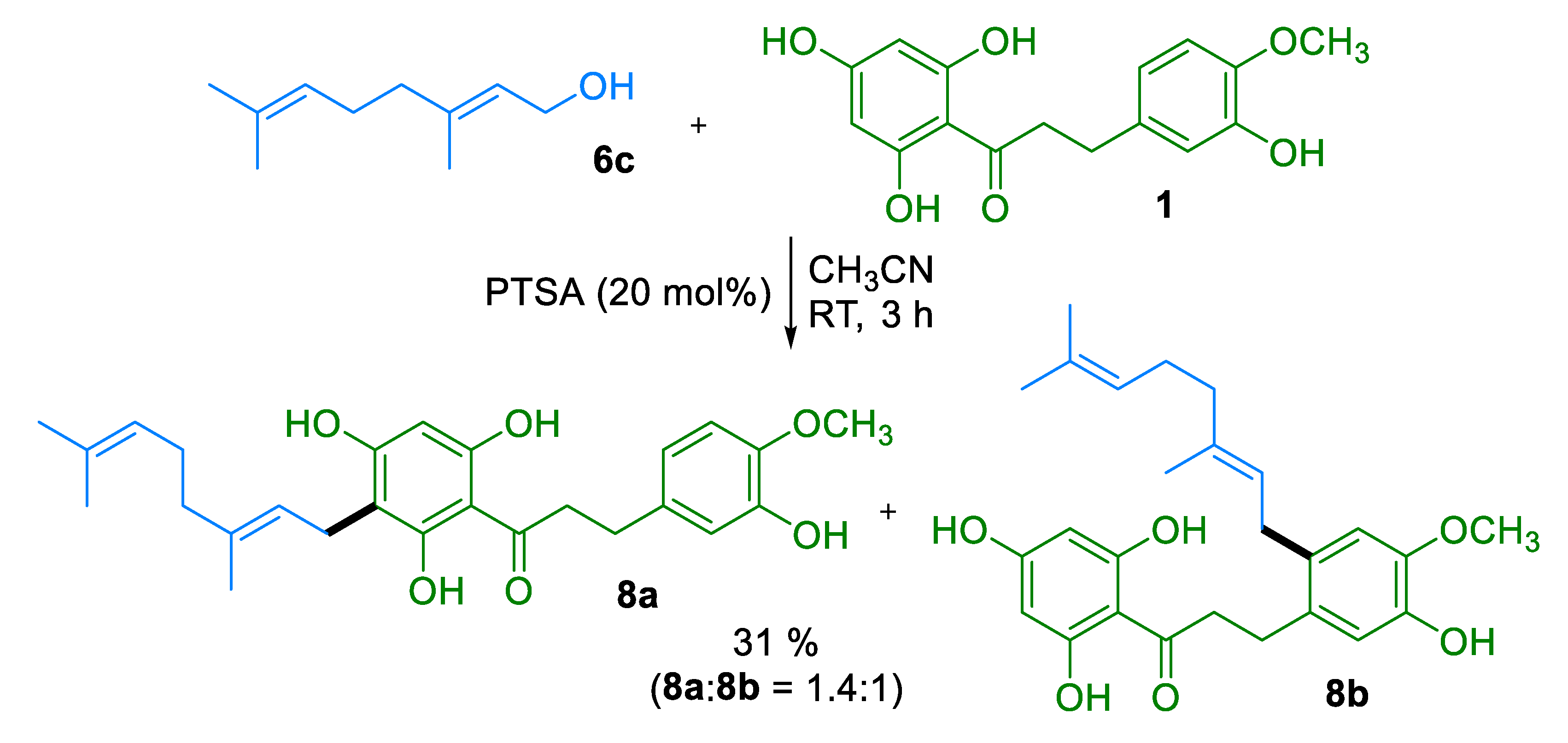
3.4.1. Prenylation of DHC 1
3.4.2. Compound 7a
3.4.3. Compound 7b
3.4.4. Geranylation of DHC 1
3.4.5. Compound 8a
3.4.6. Compound 8b
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibdah, M.; Martens, S.; Gang, D.R. Biosynthetic pathway and metabolic engineering of plant dihydrochalcones. J. Agric. Food Chem. 2018, 66, 2273–2280. [Google Scholar] [CrossRef]
- Stompor, M.; Broda, D.; Bajek-Bil, A. Dihydrochalcones: Methods of acquisition and pharmacological properties—A first systematic review. Molecules 2019, 24, 4468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veitch, N.C.; Grayer, R.J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2011, 28, 1626–1695. [Google Scholar] [CrossRef] [PubMed]
- Snijman, P.W.; Joubert, E.; Ferreira, D.; Li, X.-C.; Ding, Y.; Green, I.R.; Gelderblom, W.C.A. Antioxidant activity of the dihydrochalcones aspalathin and nothofagin and their corresponding flavones in relation to other rooibos (Aspalathus linearis) flavonoids, epigallocatechin gallate, and Trolox. J. Agric. Food Chem. 2009, 57, 6678–6684. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, S.; Legault, J.; Simard, F.; Chiasson, É.; Pichette, A. New antibacterial dihydrochalcone derivatives from buds of Populus balsamifera. Tetrahedron Lett. 2013, 54, 1631–1633. [Google Scholar] [CrossRef]
- Simard, F.; Legault, J.; Lavoie, S.; Pichette, A. Balsacones D-I, dihydrocinnamoyl flavans from Populus balsamifera buds. Phytochemistry 2014, 100, 141–149. [Google Scholar] [CrossRef]
- Simard, F.; Gauthier, C.; Chiasson, É.; Lavoie, S.; Mshvildadze, V.; Legault, J.; Pichette, A. Antibacterial balsacones J–M, hydroxycinnamoylated dihydrochalcones from Populus balsamifera buds. J. Nat. Prod. 2015, 78, 1147–1153. [Google Scholar] [CrossRef]
- Simard, F.; Gauthier, C.; Legault, J.; Lavoie, S.; Mshvildadze, V.; Pichette, A. Structure elucidation of anti-methicillin resistant Staphylococcus aureus (MRSA) flavonoids from balsam poplar buds. Bioorg. Med. Chem. 2016, 24, 4188–4198. [Google Scholar] [CrossRef]
- Muhammad, I.; Waterman, P.G. Chemistry of the Annonaceae, part 18. Benzylated indoles and dihydrochalcones in Uvaria angolensis from Tanzania. J. Nat. Prod. 1985, 48, 571–580. [Google Scholar] [CrossRef]
- Nkunya, M.H.H.; Weenen, H.; Renner, C.; Waibel, R.; Achenbach, H. Benzylated dihydrochalcones from Uvaria leptocladon. Phytochemistry 1993, 32, 1297–1300. [Google Scholar] [CrossRef]
- Prawat, U.; Chairerk, O.; Phupornprasert, U.; Salae, A.-W.; Tuntiwachwuttikul, P. Two New C-benzylated Dihydrochalcone Derivatives from the Leaves of Melodorum siamensis. Planta Med. 2013, 79, 83–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awouafack, M.D.; Kouam, S.F.; Hussain, H.; Ngamga, D.; Tane, P.; Schulz, B.; Green, I.R.; Krohn, K. Antimicrobial Prenylated Dihydrochalcones from Eriosema glomerata. Planta Med. 2008, 74, 50–54. [Google Scholar] [CrossRef]
- Rivière, C. Chapter 7—Dihydrochalcones: Occurrence in the Plant Kingdom, Chemistry and Biological Activities. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 51, pp. 253–281. [Google Scholar] [CrossRef]
- Kozłowska, J.; Potaniec, B.; Żarowska, B.; Anioł, M. Microbial transformations of 4′-methylchalcones as an efficient method of obtaining novel alcohol and dihydrochalcone derivatives with antimicrobial activity. RSC Adv. 2018, 8, 30379–30386. [Google Scholar] [CrossRef] [Green Version]
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, distribution and physiological relevance in plants. Phytochemistry 2010, 71, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, R.M.; Gentili, B. Taste and structure in phenolic glycosides. J. Agric. Food Chem. 1969, 17, 696–700. [Google Scholar] [CrossRef]
- Alsarraf, J.; Bilodeau, J.-F.; Legault, J.; Simard, F.; Pichette, A. Exploring the Biomass-Derived Chemical Space Emerging from Natural Dihydrochalcones through the Single-Step Hemisynthesis of Antibacterial Balsacones. ACS Sustain. Chem. Eng. 2020, 8, 6194–6199. [Google Scholar] [CrossRef]
- Bélanger, A.; Grenier, A.; Simard, F.; Gendreau, I.; Pichette, A.; Legault, J.; Pouliot, R. Dihydrochalcone Derivatives from Populus balsamifera L. Buds for the Treatment of Psoriasis. Int. J. Mol. Sci. 2020, 21, 256. [Google Scholar] [CrossRef] [Green Version]
- Côté, H.; Pichette, A.; Simard, F.; Ouellette, M.-E.; Ripoll, L.; Mihoub, M.; Grimard, D.; Legault, J. Balsacone C, a New Antibiotic Targeting Bacterial Cell Membranes, Inhibits Clinical Isolates of Methicillin-Resistant Staphylococcus aureus (MRSA) Without Inducing Resistance. Front. Microbiol. 2019, 10, 2341. [Google Scholar] [CrossRef] [Green Version]
- Sanz, R.; Martínez, A.; Miguel, D.; Álvarez-Gutiérrez, J.M.; Rodríguez, F. Brønsted Acid-Catalyzed Nucleophilic Substitution of Alcohols. Adv. Synth. Catal. 2006, 348, 1841–1845. [Google Scholar] [CrossRef]
- Bandini, M.; Tragni, M. π-Activated alcohols: An emerging class of alkylating agents for catalytic Friedel–Crafts reactions. Org. Biomol. Chem. 2009, 7, 1501–1507. [Google Scholar] [CrossRef]
- Kumar, R.; Van der Eycken, E.V. Recent approaches for C–C bond formation via direct dehydrative coupling strategies. Chem. Soc. Rev. 2013, 42, 1121–1146. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Hufford, C.D.; Oguntimein, B.O. Dihydrochalcones from Uvaria angolensis. Phytochemistry 1980, 19, 2036–2038. [Google Scholar] [CrossRef]
- Hufford, C.D.; Oguntimein, B.O. New Dihydrochalcones and Flavanones From Uvaria Angolensis. J. Nat. Prod. 1982, 45, 337–342. [Google Scholar] [CrossRef]
- Hufford, C.D.; Oguntimein, B.O.; Shoolery, J.N. Angoluvarin, an antimicrobial dihydrochalcone from Uvaria angolensis. J. Org. Chem. 1987, 52, 5286–5288. [Google Scholar] [CrossRef]
- Dallman, J.; Lansakara, A.; Nguyen, T.; Weeramange, C.; Hulangamuwa, W.; Rafferty, R.J. The winding road of the uvaretin class of natural products: From total synthesis to bioactive agent discovery. MedChemComm 2019, 10, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Terao, J.; Mukai, R. Prenylation modulates the bioavailability and bioaccumulation of dietary flavonoids. Arch. Biochem. Biophys. 2014, 559, 12–16. [Google Scholar] [CrossRef]
- Botta, B.; Vitali, A.; Menendez, P.; Misiti, D.; Delle Monache, G. Prenylated flavonoids: Pharmacology and biotechnology. Curr. Med. Chem. 2005, 12, 717–739. [Google Scholar] [CrossRef]
- Quideau, S.; Ralph, J. Facile large-scale synthesis of coniferyl, sinapyl, and p-coumaryl alcohol. J. Agric. Food Chem. 1992, 40, 1108–1110. [Google Scholar] [CrossRef]
- Malterud, K.E.; Undheim, J.; Erdal, J.E. Synthesis of uvaretin, an antitumour and antimicrobial flavonoid. Tetrahedron Lett. 1985, 26, 4807–4810. [Google Scholar] [CrossRef]
- Sanz, R.; Martínez, A.; Guilarte, V.; Álvarez-Gutiérrez, J.M.; Rodríguez, F. The Ritter Reaction under Truly Catalytic Brønsted Acid Conditions. Eur. J. Org. Chem. 2007, 4642–4645. [Google Scholar] [CrossRef]
- Yu, X.; Li, S.-M. Prenylation of Flavonoids by Using a Dimethylallyltryptophan Synthase, 7-DMATS, from Aspergillus fumigatus. ChemBioChem 2011, 12, 2280–2283. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Porco, J.A. Rapid Access to Polyprenylated Phloroglucinols via Alkylative Dearomatization−Annulation: Total Synthesis of (±)-Clusianone. J. Am. Chem. Soc. 2007, 129, 12682–12683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Ravu, R.R.; Xu, Q.-M.; Ganji, S.; Jacob, M.R.; Khan, S.I.; Yu, B.-Y.; Li, X.-C. Antibacterial Prenylated Acylphloroglucinols from Psorothamnus fremontii. J. Nat. Prod. 2015, 78, 2748–2753. [Google Scholar] [CrossRef]
- Khupse, R.S.; Erhardt, P.W. Total Synthesis of Xanthohumol. J. Nat. Prod. 2007, 70, 1507–1509. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.; Olsson, M.A.; McMillan, D.J.; Cullen, J.K.; Parsons, P.G.; Reddell, P.W.; Ogbourne, S.M. Potent antibacterial prenylated acetophenones from the Australian endemic plant Acronychia crassipetala. Antibiotics 2020, 9, 487. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef] [Green Version]
- Gupte, A.; Buolamwini, J.K. Synthesis and biological evaluation of phloridzin analogs as human concentrative nucleoside transporter 3 (hCNT3) inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 917–921. [Google Scholar] [CrossRef]
- Banfi, E.; Scialino, G.; Monti-Bragadin, C. Development of a microdilution method to evaluate Mycobacterium tuberculosis drug susceptibility. J. Antimicrob. Chemother. 2003, 52, 796–800. [Google Scholar] [CrossRef] [Green Version]
- Rage, R.; Mitchen, J.; Wilding, G. DNA fluorometric assay in 96-well tissue culture plates using Hoechst 33258 after cell lysis by freezing in distilled water. Anal. Biochem. 1990, 191, 31–34. [Google Scholar] [CrossRef]

 | ||
|---|---|---|
| Entry | Alcohol 2 (R 1, R 2) | Product 3 (Yield) 1 |
| 1 | 2a (OH, H) | 3a (69%) |
| 2 | 2b (OCH3, H) | 3b (49%) |
| 3 | 2c (OCH3, OCH3) | 3c (61%) |
| 4 2 | 2d (H, H) | 3d (72%) |
| 5 2 | 2e (Cl, H) | 3e (30%) |
 | ||
|---|---|---|
| Entry | Alcohol 4 (R 1, R 2) | Product 5 (Yield) 1 |
| 1 | 4a (OH, H) | 5a (61%) |
| 2 | 4b (H, OH) | 5b (74%) |
| 3 2 | 4c (H, OCH3) | 5c (36%) |
| 4 | 4d (H, Cl) | 5d (36%) |
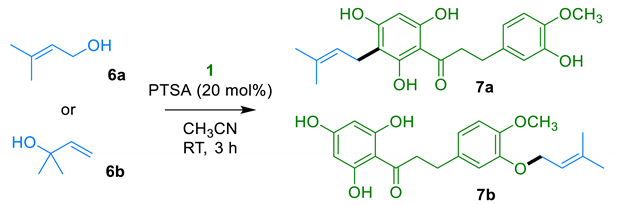 | ||
|---|---|---|
| Entry | Alcohol 6 (Equiv) | Product Ratio 7a:7b 1 (Yield) 2 |
| 1 | 6a (0.25 equiv) | 1:1 (40%) |
| 2 | 6a (0.50 equiv) | 1.3:1 (48%) |
| 3 | 6a (1.0 equiv) | 2.1:1 (46%) |
| 4 | 6b (0.25 equiv) | 1.4:1 (58%) |
| 5 | 6b (0.50 equiv) | 1.4:1 (44%) |
| 6 | 6b (1.0 equiv) | 1.9:1 (26%) |
| Entry | Product | MIC90 (µM) 1 | IC50 (µM) 1,6 | |||
|---|---|---|---|---|---|---|
| E. coli | S. aureus | A-549 | DLD-1 | WS-1 | ||
| 1 | 3a | >100 | 11.6 ± 0.2 | 36 ± 3 | 28 ± 5 | 57 ± 4 |
| 2 | 3b | >100 | 5.7 ± 0.1 | 47 ± 6 | 26 ± 8 | 56 ± 5 |
| 3 | 3c | >100 | 6.0 ± 0.1 | 30 ± 3 | 36 ± 4 | 57 ± 6 |
| 4 | 3d | >100 | 5 ± 0.9 | 43 ± 4 | 35 ± 6 | 58 ± 3 |
| 5 | 3e | >100 | 2.6 ± 0.4 | 44 ± 3 | 44 ± 7 | 59 ± 5 |
| 6 | 5a | >100 | 12 ± 1 | >100 | >100 | >100 |
| 7 | 5b | >100 | 20 ± 1 | 52 ± 9 | 64 ± 5 | 60 ± 5 |
| 8 | 5c | >100 | 4.2 ± 0.5 | 25 ± 2 | 29 ± 3 | 39 ± 4 |
| 9 | 5d | >100 | 2.3 ± 0.1 | 38 ± 2 | 46 ± 3 | 44 ± 4 |
| 10 | 7a | >100 | 80 ± 3 | >100 | >100 | >100 |
| 11 | 7b | >100 | 98 ± 1 | >100 | >100 | >100 |
| 12 | 8a | >100 | 2.5 ± 0.3 | 28 ± 2 | 21 ± 3 | 35 ± 4 |
| 13 | Balsacone A 2 | >100 | 3.3 ± 0.2 | 44 ± 4 | 31 ± 5 | 56 ± 4 |
| 14 | Gentamicin 2 | 0.083 ± 0.007 3 | 0.12 ± 0.01 3 | n. d. 4 | n. d. | n. d. |
| 15 | Etoposide 5 | n. d. | n. d. | 0.56 ± 0.04 | 0.96 ± 0.08 | 10 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardaillou, A.; Alsarraf, J.; Legault, J.; Simard, F.; Pichette, A. Hemisynthesis and Biological Evaluation of Cinnamylated, Benzylated, and Prenylated Dihydrochalcones from a Common Bio-Sourced Precursor. Antibiotics 2021, 10, 620. https://doi.org/10.3390/antibiotics10060620
Ardaillou A, Alsarraf J, Legault J, Simard F, Pichette A. Hemisynthesis and Biological Evaluation of Cinnamylated, Benzylated, and Prenylated Dihydrochalcones from a Common Bio-Sourced Precursor. Antibiotics. 2021; 10(6):620. https://doi.org/10.3390/antibiotics10060620
Chicago/Turabian StyleArdaillou, Anne, Jérôme Alsarraf, Jean Legault, François Simard, and André Pichette. 2021. "Hemisynthesis and Biological Evaluation of Cinnamylated, Benzylated, and Prenylated Dihydrochalcones from a Common Bio-Sourced Precursor" Antibiotics 10, no. 6: 620. https://doi.org/10.3390/antibiotics10060620
APA StyleArdaillou, A., Alsarraf, J., Legault, J., Simard, F., & Pichette, A. (2021). Hemisynthesis and Biological Evaluation of Cinnamylated, Benzylated, and Prenylated Dihydrochalcones from a Common Bio-Sourced Precursor. Antibiotics, 10(6), 620. https://doi.org/10.3390/antibiotics10060620







