Sesquiterpene Lactones from Cotula cinerea with Antibiotic Activity against Clinical Isolates of Enterococcus faecalis
Abstract
1. Introduction
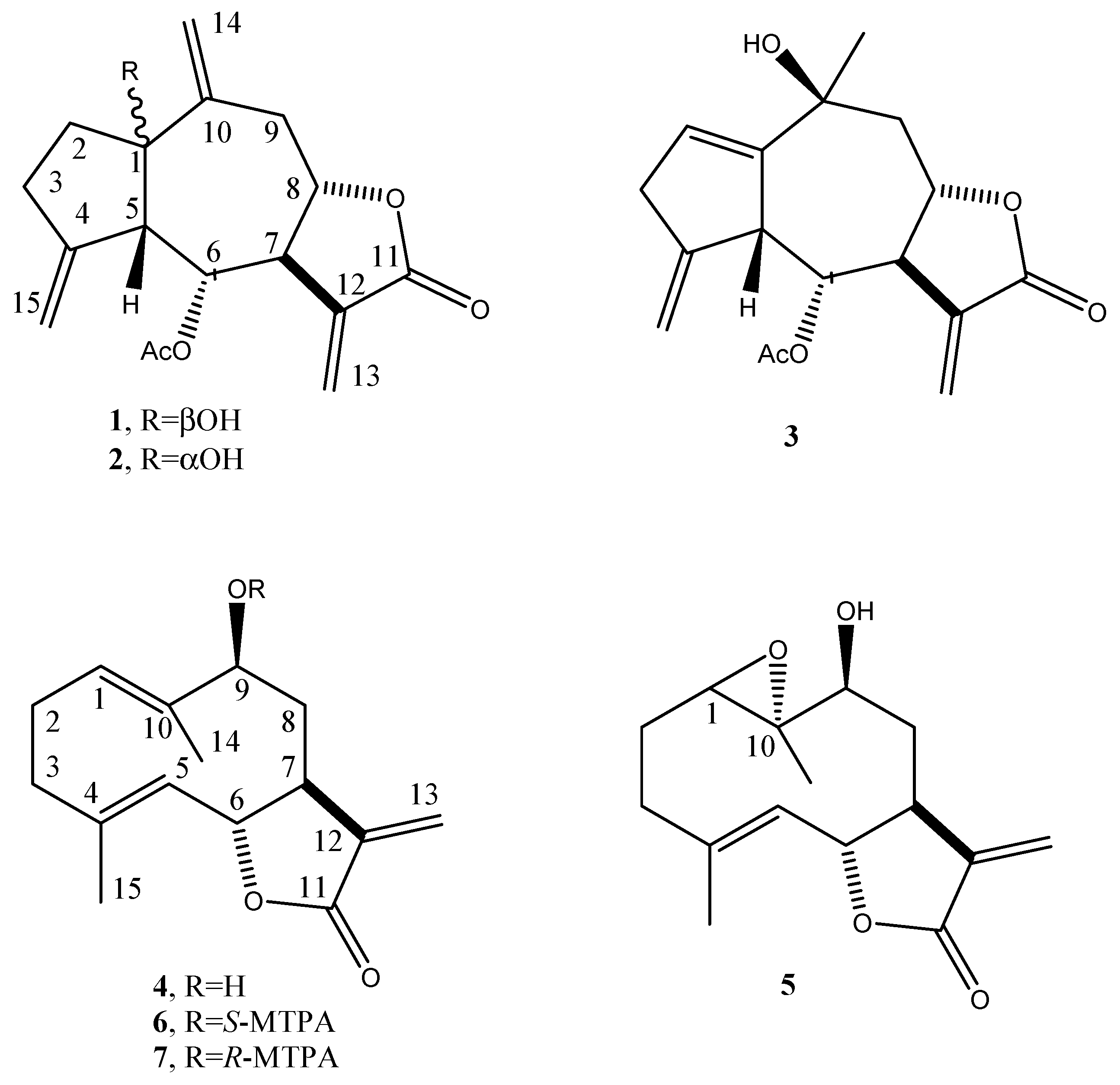
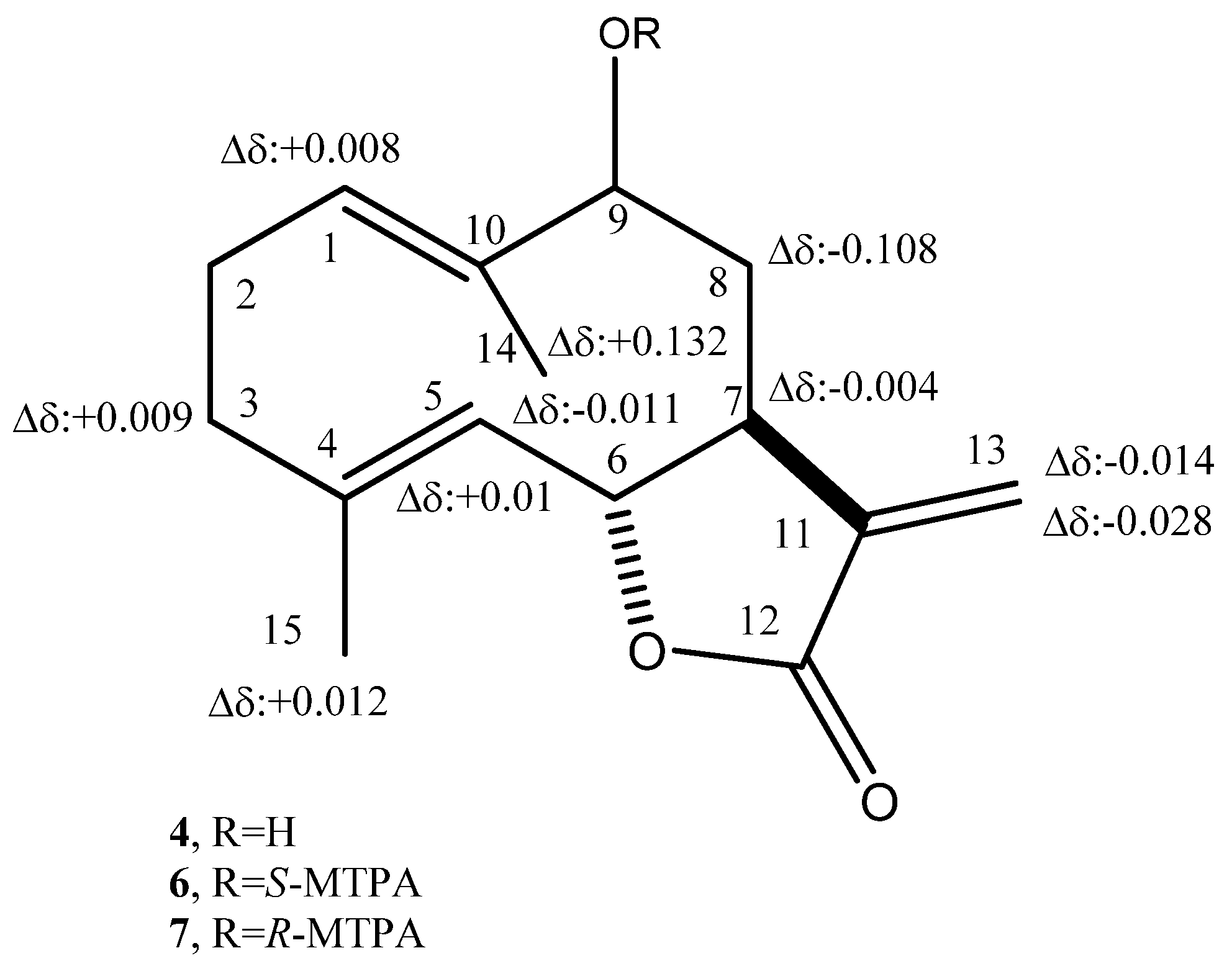
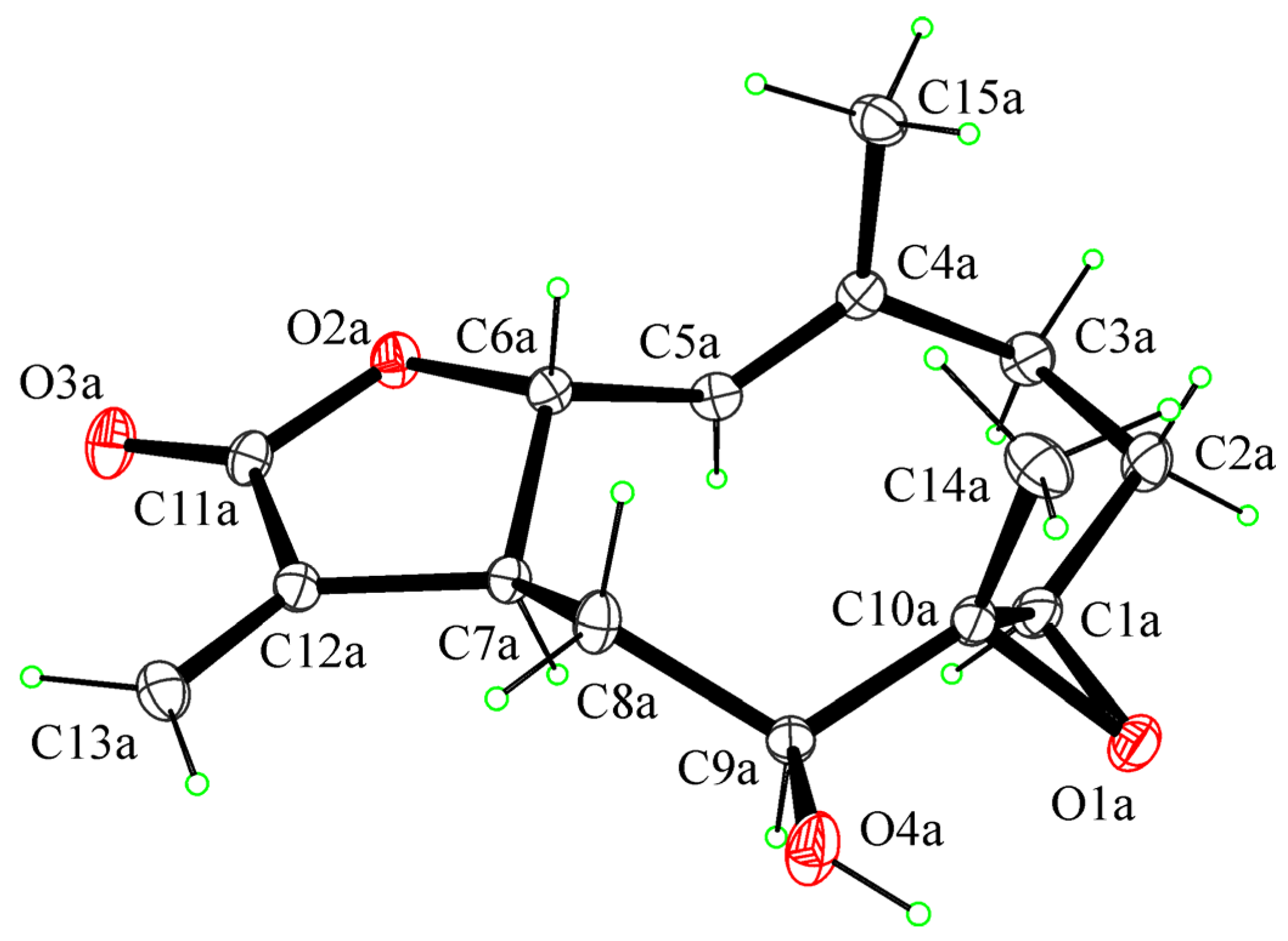
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Isolation of Plant Metabolites
3.4. Crystallographic Data of 1,10-Epoxyhaagenolide (5)
3.5. Test Bacterial Strains and Culture Conditions
3.6. Antimicrobial Assays
3.7. Biofilm Formation Inhibition Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 16, 303–336. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, T.I.; Mandalakis, M.; Krigas, N.; Vézignol, T.; Lazari, D.; Katharios, P.; Dailianis, T.; Antonopoulou, E. Comparative evaluation of essential oils from medicinal-aromatic plants of Greece: Chemical composition, antioxidant capacity and antimicrobial activity against bacterial fish pathogens. Molecules 2019, 25, 148. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Roscetto, E.; Cimmino, A.; Catania, M.R.; Surico, G.; Evidente, A. Farnesane-type sesquiterpenoids with antibiotic activity from Chiliadenus Lopadusanus. Antibiotics 2021, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Antimicrobial Resistance No Time to Wait: Securing the Future from Drug-Resistant Infections. Report to the Secretary-General of the United Nations. Available online: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_final_report_EN.pdf?ua=1 (accessed on 8 April 2020).
- Ciofu, O.; Mandsberg, L.F.; Wang, H.; Høiby, N. Phenotypes selected during chronic lung infection in cystic fibrosis patients: Implications for the treatment of Pseudomonas aeruginosa biofilm infections. FEMS Immunol. Med. Microbiol. 2012, 65, 215–225. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Prada, I.; Micó-Muñoz, P.; Giner-Lluesma, T.; Micó-Martínez, P.; Collado-Castellano, N.; Manzano-Saiz, A. Influence of microbiology on endodontic failure. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, e364–e372. [Google Scholar] [CrossRef]
- Balato, G.; Roscetto, E.; Vollaro, A.; Galasso, O.; Gasparini, G.; Ascione, T.; Catania, M.R.; Mariconda, M. Bacterial biofilm formation is variably inhibited by different formulations of antibiotic-loaded bone cement in vitro. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef]
- Van Gestel, J.; Vlamakis, H.; Kolter, R. Division of labor in biofilms: The ecology of cell differentiation. Microbiol Spectr. 2015, 3, 67–97. [Google Scholar] [CrossRef]
- Osbourn, A.E.; Lanzotti, V. Plant-Derived Products; Springer: Dordrecht, Germany, 2009; ISBN 978-0-38-785497-7. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products—A Biosynthetic Approach; Wiley and Sons Ltd.: Chicester, UK, 2009; ISBN 978-0-47-074168-9. [Google Scholar]
- Marrone, P.G. Pesticidal natural products—Status and future potential. Pest Man. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Heywood, V.B.; Harborne, J.B.; Turner, B.L. The Biology and Chemistry of the Compositae; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Lloyd, D.G. A revision of the New Zealand, Subantarctic, and South American species of Cotula, section Leptinella. N. Z. J. Bot. 1972, 10, 277–372. [Google Scholar] [CrossRef]
- Markouk, M.; Redwane, A.; Lazrek, H.B.; Jana, M.; Benjama, A. Antibacterial activity of Cotula cinerea extracts. Fitoterapia 1999, 70, 314–316. [Google Scholar] [CrossRef]
- Ghouti, D.; Rached, W.; Abdallah, M.; Pires, T.C.; Calhelha, R.C.; Alves, M.J.; Abdderrahmane, L.H.; Barros, L.; Ferreira, I.C. Phenolic profile and in vitro bioactive potential of Saharan Juniperus phoenicea L. and Cotula cinerea (Del) growing in Algeria. Food Funct. 2018, 9, 4664–4672. [Google Scholar] [CrossRef] [PubMed]
- Amssayef, A.; Eddouks, M. Antihyperglycemic, antihyperlipidemic and antioxidant effects of Cotula cinerea (Del) in normal and streptozotocin-induced diabetic rats. Endocr. Metab. Immune. Disord. Drug Targets 2020, 20, 1504–1513. [Google Scholar] [CrossRef]
- Mahran, G.H.; Salah, M.A.; Ansary, S.M. A study of the flavonoid content of Cotula cinerea Del. Bull. Far. Pbann. (Cairo Univ.) 1976, 14, 237. [Google Scholar]
- Ahmed, A.A.; El-Sayed, N.H.; El-Negoumy, S.I.; Mabry, T.J. Flavonoids of Cotula cinerea. J. Nat. Prod. 1987, 50, 519–520. [Google Scholar] [CrossRef]
- Metwally, M.A.; El-Dahmy, S.; Jakupovic, J.; Bohlmann, F.; Dawidar, A.M.; Metwally, S.A. Glaucolide-like sesquiterpene lactones from Cotula cinerea. Phytochemistry 1985, 25, 255–257. [Google Scholar] [CrossRef]
- Greger, H.; Hofer, O. Sesquiterpene-coumarin ethers and polyacetylenes from Bfrocchia cinerea. Phytochemistry 1985, 24, 85–88. [Google Scholar] [CrossRef]
- Markouk, M.; Bekkouche, K.; Larhsini, M.; Bousaid, M.; Lazrek, H.B.; Jana, M. Evaluation of some Moroccan medicinal plant extracts for larvicidal activity. J. Ethnopharmacol. 2000, 73, 293–297. [Google Scholar] [CrossRef]
- Larhsini, M.; Markouk, M.; Jaouhari, J.T.; Bekkouche, K.; Lazrek, H.B.; Jana, M. The antipyretic activity of some Moroccan medicinal plants. Phytother. Res. 2002, 16, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Salhi, N.; Mohammed Saghir, S.A.; Terzi, V.; Brahmi, I.; Ghedairi, N.; Bissati, S. Antifungal activity of aqueous extracts of some dominant Algerian medicinal plants. BioMed Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hemada, M.M.; El-Darier, S.M. Management of a noxious weed; Melilotus indicus L. via allelopathy of Cotula cinerea. Int. J. Adv. Res. 2015, 3, 553–561. [Google Scholar]
- Bensizerara, D.; Menasria, T.; Melouka, M.; Cheriet, L.; Chenchouni, H. Antimicrobial activity of xerophytic plant (Cotula cinerea Delile) extracts against some pathogenic bacteria and fungi. J. Appl. Biol. Sci. 2013, 6, 266–271. [Google Scholar] [CrossRef][Green Version]
- Guiton, P.S.; Hannan, T.J.; Ford, B.; Caparon, M.G.; Hultgren, S.J. Enterococcus faecalis overcomes foreign body-mediated inflammation to establish urinary tract infections. Infect. Immun. 2013, 81, 329–339. [Google Scholar] [CrossRef]
- O’Driscoll, T.; Crank, C.W. Vancomycin-resistant enterococcal infections: Epidemiology, clinical manifestations, and optimal management. Infect. Drug Resist. 2015, 24, 217–230. [Google Scholar]
- Ch’ng, J.H.; Chong, K.K.L.; Lam, L.N.; Wong, J.J.; Kline, K.A. Biofilm-associated infection by enterococci. Nat. Rev. Microbiol. 2019, 17, 82–94. [Google Scholar] [CrossRef]
- El Helou, O.C.; Berbari, E.F.; Marculescu, C.E.; El Atrouni, W.I.; Razonable, R.R.; Steckelberg, J.M.; Hanssen, A.D.; Osmon, D.R. Outcome of enterococcal prosthetic joint infection: Is combination systemic therapy superior to monotherapy? Clin. Infect. Dis. 2008, 47, 903–909. [Google Scholar] [CrossRef]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Stefánsdóttir, A.; Johansson, D.; Knutson, K.; Lidgren, L.; Robertsson, O. Microbiology of the infected knee arthroplasty: Report from the Swedish Knee Arthroplasty Register on 426 surgically revised cases. Scand. J. Infect. Dis. 2009, 41, 831–840. [Google Scholar] [CrossRef]
- Rasouli, M.R.; Tripathi, M.S.; Kenyon, R.; Wetters, N.; Della Valle, C.J.; Parvizi, J. Low rate of infection control in enterococcal periprosthetic joint infections. Clin. Orthop. Relat. Res. 2012, 470, 2708–2716. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davidson, D.J.; Spratt, D.; Liddle, A.D. Implant materials and prosthetic joint infection: The battle with the biofilm. EFORT Open Rev. 2019, 4, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Ribeiro, M.; De-Jesus-Soares, A.; Zaia, A.A.; Ferraz, C.C.R.; Almeida, J.F.A.; Gomes, B.P.F.A. Quantification of lipoteichoic acid contents and cultivable bacteria at the different phases of the endodontic retreatment. J. Endod. 2016, 42, 552–556. [Google Scholar] [CrossRef]
- Alghamdi, F.; Shakir, M. The influence of Enterococcus faecalis as a dental root canal pathogen on endodontic treatment: A systematic review. Cureus 2020, 12, e7257. [Google Scholar] [CrossRef]
- García-Solache, M.; Ricea, L.B. The enterococcus: A model of adaptability to its environment. Clin. Microbiol. Rev. 2019, 32, e00058-18. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Samaranayake, L.P.; Lolato, A.; Weinstein, T.; Taschieri, S. Analysis of the secondary endodontic lesions focusing on the extraradicular microorganisms: An overview. J. Investig. Clin. Dent. 2014, 5, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Jakupovic, J.; Aal, M.A.; Eid, F.; Bohlmann, F.; El-Dahmy, S.; Sarg, T. Further glaucolides and other sesquiterpene lactones from Brocchia cinerea. Phytochemistry 1988, 27, 2219–2224. [Google Scholar] [CrossRef]
- Bohlmann, F.; Mahanta, P.K.; Jakupovic, J.; Rastogi, R.C.; Natu, A.A. New sesquiterpene lactones from Inula species. Phytochemistry 1978, 17, 1165–1172. [Google Scholar] [CrossRef]
- Bohlmann, F.; Ates, N.; Grenz, M. New germacranolides from Inula heterolepis. Phytochemistry 1982, 21, 1166–1168. [Google Scholar] [CrossRef]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Application of Mosher’s method for absolute configuration assignment to bioactive plants and fungi metabolites. J. Pharm. Biomed. Anal. 2017, 144, 59–89. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical managemen and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Zaborowska, M.; Tillander, J.; Brånemark, R.; Hagberg, L.; Thomsen, P.; Trobos, M. Biofilm formation and antimicrobial susceptibility of staphylococci and enterococci from osteomyelitis associated with percutaneous orthopaedic implants. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2630–2640. [Google Scholar] [CrossRef]
- Sandoe, J.A.; Wysome, J.; West, A.P.; Heritage, J.; Wilcox, M.H. Measurement of ampicillin, vancomycin, linezolid and gentamicin activity against enterococcal biofilms. J. Antimicrob. Chemother. 2006, 57, 767–770. [Google Scholar] [CrossRef]
- Bayston, R.; Ullas, G.; Ashraf, W. Action of linezolid or vancomycin on biofilms in ventriculoperitoneal shunts in vitro. Antimicrob. Agents Chemother. 2012, 56, 2842–2845. [Google Scholar] [CrossRef]
- Minardi, D.; Cirioni, O.; Ghiselli, R.; Silvestri, C.; Mocchegiani, F.; Gabrielli, E.; d’Anzeo, G.; Conti, A.; Orlando, F.; Rimini, M.; et al. Efficacy of tigecycline and rifampin alone and in combination against Enterococcus faecalis biofilm infection in a rat model of ureteral stent. J. Surg. Res. 2012, 176, 1–6. [Google Scholar] [CrossRef]
- Tang, H.J.; Chen, C.C.; Zhang, C.C.; Su, B.A.; Li, C.M.; Weng, T.C.; Chiang, S.R.; Ko, W.C.; Chuang, Y.C. In vitro efficacy of fosfomycin-based combinations against clinical vancomycin-resistant Enterococcus isolates. Diagn. Microbiol. Infect. Dis. 2013, 77, 254–257. [Google Scholar] [CrossRef]
- Oliva, A.; Furustrand Tafin, U.; Maiolo, E.M.; Jeddari, S.; Bétrisey, B.; Trampuz, A. Activities of fosfomycin and rifampin on planktonic and adherent Enterococcus faecalis strains in an experimental foreign-body infection model. Antimicrob. Agents Chemother. 2014, 58, 1284–1293. [Google Scholar] [CrossRef]
- de Sermeño, R.F.; da Silva, L.A.; Herrera, H.; Herrera, H.; Silva, R.A.; Leonardo, M.R. Tissue damage after sodium hypochlorite extrusion during root canal treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, e46–e49. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Abbott, P.V. The properties and applications of chlorhexidine in endodontics. Int. Endod. J. 2009, 42, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Tornero, E.; Senneville, E.; Euba, G.; Petersdorf, S.; Rodriguez-Pardo, D.; Lakatos, B.; Ferrari, M.C.; Pilares, M.; Bahamonde, A.; Trebse, R.; et al. Characteristics of prosthetic joint infections due to Enterococcus sp. and predictors of failure: A multi-national study. Clin. Microbiol. Infect. 2014, 20, 1219–1224. [Google Scholar] [CrossRef]
- Maale, G.E.; Eager, J.J.; Srinivasaraghavan, A.; Mohammadi, D.K.; Kennard, N. The evolution from the two stage to the one stage procedure for biofilm based periprosthetic joint infections (PJI). Biofilm 2020, 2, 100033. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Braun, S. 200 and More Basic NMR Experiments—A Practical Course, 1st ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R.J. SIR97: A new tool for crystal structure determination and refinement. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Escudero-Adan, E.C.; Benet-Buchholz, J.; Ballester, P. The use of Mo Kα radiation in the assignment of the absolute configuration of light-atom molecules; the importance of high-resolution data. Acta Cryst. 2014, B70, 660–668. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H.D.; Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Cryst. 2013, B69, 249–259. [Google Scholar] [CrossRef]
- Parsons, S. Determination of absolute configuration using X-ray diffraction. Tetrahedron Asymmetry 2017, 28, 1304–1313. [Google Scholar] [CrossRef]
- Spek, J.A.L. Single-crystal structure validation with the program PLATON. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
| Strain | CH2Cl2 Extract % Inhibition |
|---|---|
| S. aureus ATCC 29213 | 49 ± 2.7 |
| E. faecalis ATCC 29212 | 90 ± 1.5 |
| P. aeruginosa ATCC 27853 | n.d. |
| A. baumannii ATCC 747 | n.d. |
| Position | δC c | δH (J in Hz) | HMBC |
|---|---|---|---|
| 1 | 84.6 s | H2-14, H-9B, H-6, H-2B | |
| 2 | 37.4 t | 2.32 ddd (12.0, 8.7, 3.5) 1.88 br dd (12.0, 7.8, 1.5) | |
| 3 | 29.8 t | 2.68 m 2.43 br dd (15.8, 8.7) | H2-15, H-2A |
| 4 | 149.1 s | H-6, H-2B | |
| 5 | 55.4 d | 2.78 m | H2-15, H-6 |
| 6 | 73.2 d | 5.49 dd (5.9, 4.1) | H-8, H-7 |
| 7 | 48.5 d | 3.09 br dd (10.9, 5.9) | H2-13, H-9A, H-6 |
| 8 | 75.6 d | 4.62 dt (10.9, 8.0) | H2-9 |
| 9 | 38.3 t | 3.58 ddt (14.2, 8.0, 1.7) 2.51 dd (14.2, 8.0) | |
| 10 | 145.6 | H2-9 | |
| 11 | 169.5 | H2-13 | |
| 12 | 137.5 | H-13A, H-6 | |
| 13 | 122.2 | 6.28 d (4.4) 5.95 d (4.4) | |
| 14 | 116.9 | 5.37 br s 5.15 br s | H2-9 |
| 15 | 109.3 | 5.14 br s 4.98 br s | |
| CH3CO | 169.5 | CH3CO, H-6 | |
| CH3CO | 20.9 | 2.02 s |
| Position | δC c | δH (J in Hz) | HMBC |
|---|---|---|---|
| 1 | 84.3 s | H2-14, H-6, H-3A, H-2B | |
| 2 | 37.1 t | 2.16 br dd (12.7, 6.8) 1.84 br dd (12.7, 6.3) | |
| 3 | 31.9 t | 2.47 br d (15.0, 6.8) 1.34 m | |
| 4 | 147.9 s | ||
| 5 | 54.6 d | 2.68 | H-3A, H-2B |
| 6 | 72.3 d | 5.87 dd (7.8, 5.2) | |
| 7 | 51.6 d | 3.73 br dd (10.0, 7.8) | H2-13, H2-9 |
| 8 | 77.8 d | 4.18 td (10.0, 3.8) | H2-9 |
| 9 | 41.3 t | 3.06 (2H) m | H2-14 |
| 10 | 146.0 s | H2-9 | |
| 11 | 169.1 s | H2-13 | |
| 12 | 138.1 s | H-13A, H-6 | |
| 13 | 121.6 t | 6.25 d (4.1) 5.76 d (4.1) | |
| 14 | 113.6 t | 5.17 br s 5.08 br s | H2-9 |
| 15 | 109.0 t | 5.14 br s 4.85 br s | |
| CH3CO | 170.0 s | CH3CO, H-6 | |
| CH3CO | 21.2 q | 2.10 |
| Position | δC c | δH (J in Hz) | HMBC |
|---|---|---|---|
| 1 | 146.9 s | Me-14, H-9, H-6, H-5 | |
| 2 | 137.7 d | 6.04 br s | H-5 |
| 3 | 39.1 t | 3.15 2.88 m d | H2-15 |
| 4 | 148.7 s | H-5 | |
| 5 | 50.7 d | 3.80 br d (6.8) | H2-15 |
| 6 | 75.0 d | 5.65 dd (7.8, 6.8) | |
| 7 | 51.4 d | 2.92 md | H-9A |
| 8 | 74.7 d | 4.59 br t (10.5, 2.3) | H2-9 |
| 9 | 48.4 t | 2.61 dd (13.2, 2.3) 1.80 br t (13.0) | Me-14 |
| 10 | 69.4 s | Me-14, H-9 | |
| 11 | 169.4 s | H2-13 | |
| 12 | 137.7 s | H2-13 | |
| 13 | 122.2 t | 6.25 d (3.3) 5.69 d (3.3) | |
| 14 | 29.4 q | 1.61 br s | |
| 15 | 109.8 t | 5.05 br s 4.96 br s | H-5 |
| CH3CO | 170.1 s | CH3CO, H-6 | |
| CH3CO | 21.3 q | 2.07 |
| Position | δC c | δH (J in Hz) | HMBC |
|---|---|---|---|
| 1 | 129.4 d | 5.12 br d (10.7, 1.2) | H2-3, H-9, Me-14 |
| 2 | 35.6 t | 2.00 m | H2-3, Me-15 |
| 3 | 39.4 t | 2.38 br t (11.7) 2.07 td (11.7, 4.6) | H-5, Me-15 |
| 4 | 141.3 s | H-6, Me-15 | |
| 5 | 127.1 d | 4.65 d (9.9) | H-3A, H-6, Me-15 |
| 6 | 81.3 d | 4.59 dd (9.9) | H2-8 |
| 7 | 47.3 d | 2.69 br td (9.9, 3,5) | H-5, H2-8, H2-13, |
| 8 | 25.5 t | 2.31 br t (13.8) 1.95 ddd (13.8, 10.6, 2.2) | H-6 |
| 9 | 79.7 d | 4.26 dd (10.6, 2.2) | H-1, H2-8, Me-14 |
| 10 | 139.0 s | - | H-2, H2-8 Me-14 |
| 11 | 170.0 s | - | H2-13 |
| 12 | 139.2 s | - | H2-13 |
| 13 | 120.1 t | 6.31 d (3.5) 5.61 d (3.5) | H-7 |
| 14 | 10.9 q | 1.48 s | H-1, H-9 |
| 15 | 17.5 q | 1.75 s | H2-3, H-5 |
| Position | δC c | δH (J in Hz) | HMBC |
|---|---|---|---|
| 1 | 67.1 d | 2.90 dd (11.2, 2.3) | H-9, H-3B |
| 2 | 23.4 t | 2.14 br ddd (13.7, 4.8, 2.3) 1.50 br ddd (13.7, 11.2, 4.8) | H-3A, H-1 |
| 3 | 35.9 t | 2.43 d t (12.5, 4.8) 2.21 m | H-5, Me-15 |
| 4 | 144.6 s | H-6, H-3B, Me-15 | |
| 5 | 123.9 d | 5.21 d (10.2) | H-3A, Me-15 |
| 6 | 80.2 d | 4.62 t (10.2) | H2-8 |
| 7 | 47.2 d | 2.70 br t (9.5) | |
| 8 | 32.9 t | 2.29 br d (15.0, 2.0) 1.79 dt (15.0, 9.5) | H-5, H-6, H2-8, H2-13 |
| 9 | 79.9 d | 3.24 dd (9.5, 2.0) | H2-8, Me-14 |
| 10 | 64.7 s | H2-8 Me-14 | |
| 11 | 170.0 s | H2-13 | |
| 12 | 138.7 s | H-13A | |
| 13 | 120.1 t | 6.31 d (3.5) 5.61 d (3.5) | |
| 14 | 11.8 q | 1.48 s | H-9 |
| 15 | 17.9 q | 1.75 s | H-3A |
| 6 | 7 | |
|---|---|---|
| Position | δH | δH |
| 2 | 2.310 (2H) | 2.296 (2H) |
| 3 | 2.388 1.982 | 2.379 2.058 |
| 5 | 4.678 | 4.668 |
| 6 | 4.551 | 4.562 |
| 7 | 2.753 | 2.757 |
| 8 | 2.090 2.019 | 2.198 2.100 |
| 9 | 5.482 | 5.454 |
| 13 | 6.299 5.482 | 6.313 5.510 |
| 14 | 1.436 | 1.304 |
| 15 | 1.725 | 1.713 |
| OMe | 3.519 | 3.557 |
| Ph | 7.508–7.384 | 7.532–7.367 |
| Strains | Minimum Inhibitory Concentration (µg/mL) | |||||
|---|---|---|---|---|---|---|
| AMP | IM | LIN | TEI | VAN | TIG | |
| EF-91823 | ≤2 | ≤2 | 2 | ≤0.5 | 2 | ≤0.25 |
| EF-91804 | ≤2 | ≤2 | ≤0.5 | ≤0.5 | 1 | ≤0.25 |
| EF-165 | ≤2 | ≤1 | 2 | ≤0.5 | 2 | ≤0.12 |
| EF-91705 | ≤2 | ≤1 | 2 | ≤0.5 | 1 | 0.25 |
| ATTC29212 | ≤2 | ≤1 | 2 | ≤0.5 | 2 | ≤0.12 |
| Strains | Compound 1 | Compound 2 | Compound 3 | Compound 4 | Compound 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| µg/mL | %GI | µg/mL | %GI | µg/mL | %GI | µg/mL | %GI | µg/mL | %GI | |
| EF-91804 | 300 | nd | 150 | 78 ± 1.5 | 150 | 80 ± 1.1 | 300 | 77 ± 1.5 | 300 | 82 ± 0.9 |
| EF-91823 | 300 | nd | 300 | 50 ± 1.9 | 300 | 51 ± 0.9 | 300 | 49 ± 1.1 | 300 | 50 ± 1.6 |
| EF-165 | 300 | nd | 300 | nd | 300 | 90 ± 0.7 | 300 | 80 ± 3 | 300 | 81 ± 3.3 |
| EF-91705 | 300 | nd | 300 | 49 ± 2.2 | 300 | 50 ± 2.3 | 300 | 55 ± 2.6 | 150 | 80 ± 0.8 |
| ATCC29212 | 300 | nd | 300 | 80 ± 1.1 | 300 | 79 ± 1.8 | 300 | 70 ± 2.1 | 300 | 79 ± 1.5 |
| Strains | Compound 1 | Compound 2 | Compound 3 | Compound 4 | Compound 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| µg/mL | %BI | µg/mL | %BI | µg/mL | %BI | µg/mL | %BI | µg/mL | %BI | |
| EF-91804 | 150 | 60 ± 2.8 | 18 | n.d. | 18 | 52 ± 2.4 | 37 | n.d. | 37 | 53 ± 2 |
| EF-91823 | 150 | 50 ± 2.1 | 75 | 49 ± 3.1 | 75 | 55 ± 1.9 | 75 | 52 ± 3.1 | 75 | 73 ± 1.1 |
| EF-165 | 150 | 52 ± 1.7 | 150 | 50 ± 2.4 | 37 | 62 ± 1.1 | 37 | 65 ± 1.8 | 37 | 50 ± 1.2 |
| EF-91705 | 150 | 55 ± 3.3 | 75 | 50 ± 3.6 | 75 | 48 ± 2.2 | 75 | 50 ± 2.2 | 37 | 56 ± 3.4 |
| ATCC29212 | 150 | 61 ± 4.1 | 18 | n.d. | 18 | n.d. | 37 | n.d. | 37 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimmino, A.; Roscetto, E.; Masi, M.; Tuzi, A.; Radjai, I.; Gahdab, C.; Paolillo, R.; Guarino, A.; Catania, M.R.; Evidente, A. Sesquiterpene Lactones from Cotula cinerea with Antibiotic Activity against Clinical Isolates of Enterococcus faecalis. Antibiotics 2021, 10, 819. https://doi.org/10.3390/antibiotics10070819
Cimmino A, Roscetto E, Masi M, Tuzi A, Radjai I, Gahdab C, Paolillo R, Guarino A, Catania MR, Evidente A. Sesquiterpene Lactones from Cotula cinerea with Antibiotic Activity against Clinical Isolates of Enterococcus faecalis. Antibiotics. 2021; 10(7):819. https://doi.org/10.3390/antibiotics10070819
Chicago/Turabian StyleCimmino, Alessio, Emanuela Roscetto, Marco Masi, Angela Tuzi, Imene Radjai, Chakali Gahdab, Rossella Paolillo, Amedeo Guarino, Maria Rosaria Catania, and Antonio Evidente. 2021. "Sesquiterpene Lactones from Cotula cinerea with Antibiotic Activity against Clinical Isolates of Enterococcus faecalis" Antibiotics 10, no. 7: 819. https://doi.org/10.3390/antibiotics10070819
APA StyleCimmino, A., Roscetto, E., Masi, M., Tuzi, A., Radjai, I., Gahdab, C., Paolillo, R., Guarino, A., Catania, M. R., & Evidente, A. (2021). Sesquiterpene Lactones from Cotula cinerea with Antibiotic Activity against Clinical Isolates of Enterococcus faecalis. Antibiotics, 10(7), 819. https://doi.org/10.3390/antibiotics10070819










