Further Study of the Polar Group’s Influence on the Antibacterial Activity of the 3-Substituted Quinuclidine Salts with Long Alkyl Chains
Abstract
1. Introduction
2. Results
2.1. Synthesis
2.2. Antibacterial Activity
2.3. Inhibition of the Bacterial Biofilms
2.4. Time-Resolved Growth Analysis
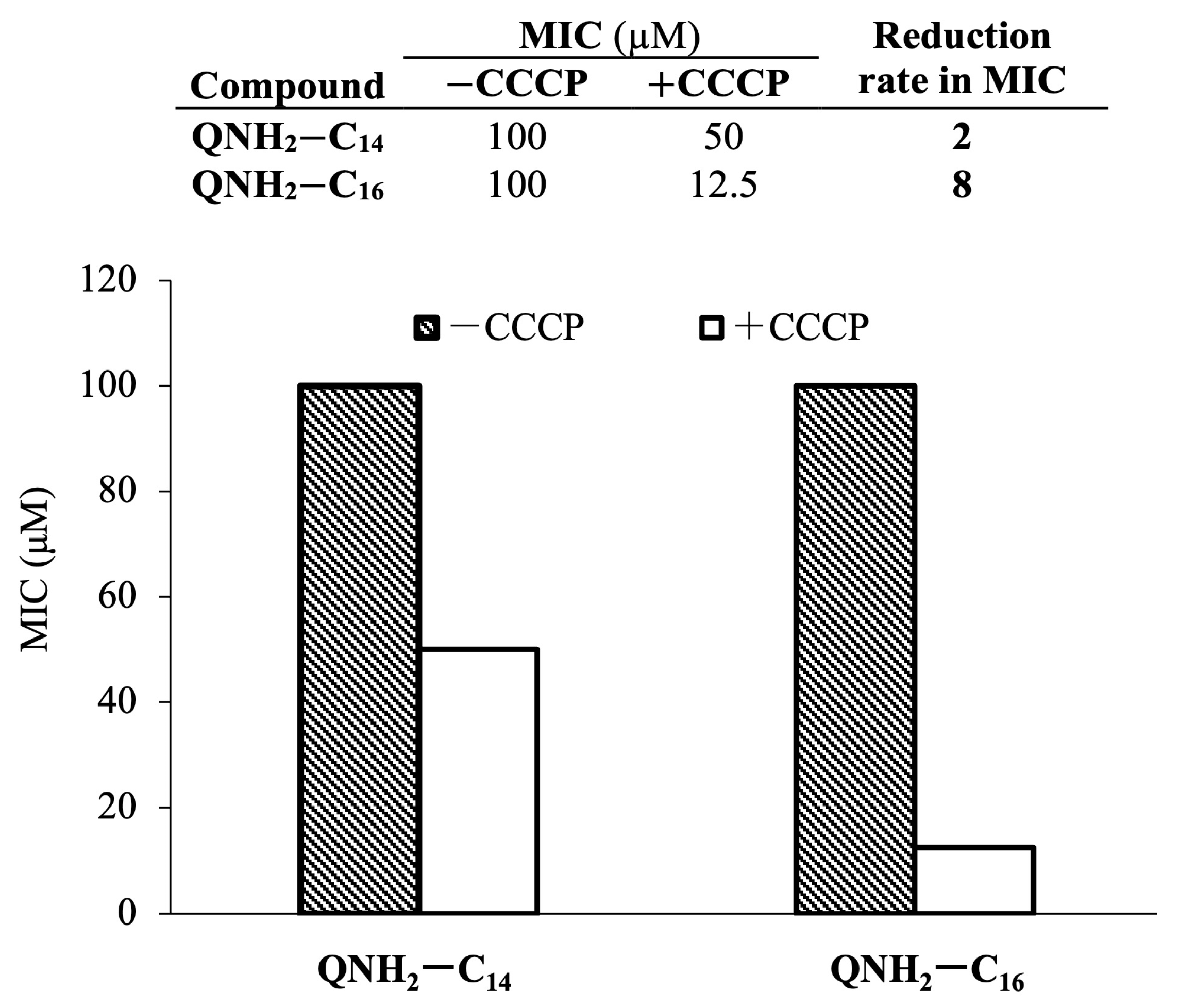
2.5. Potential of Bacterial Resistance Development
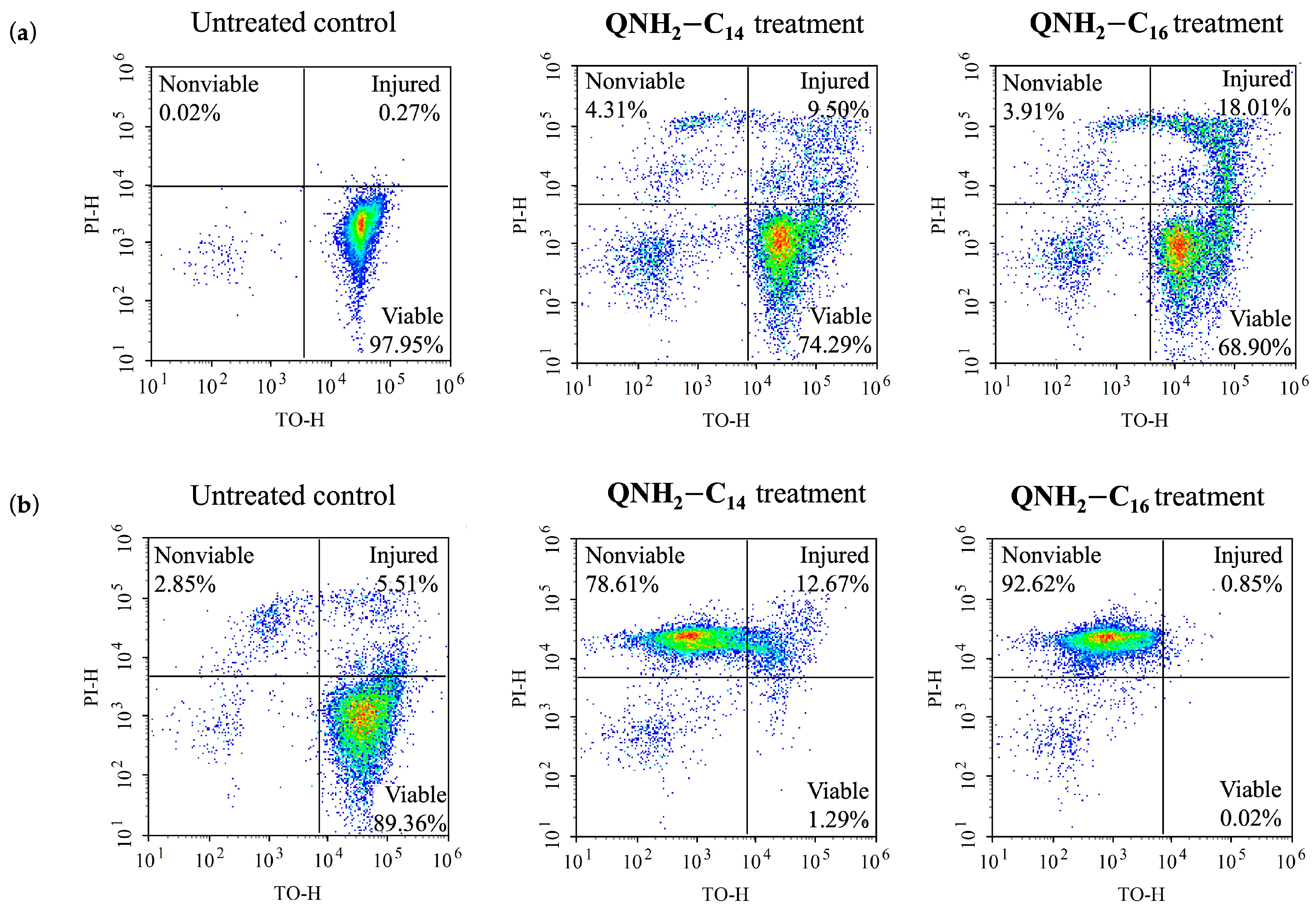
2.6. Cell Viability in Relation to the Antibacterial Treatment Duration
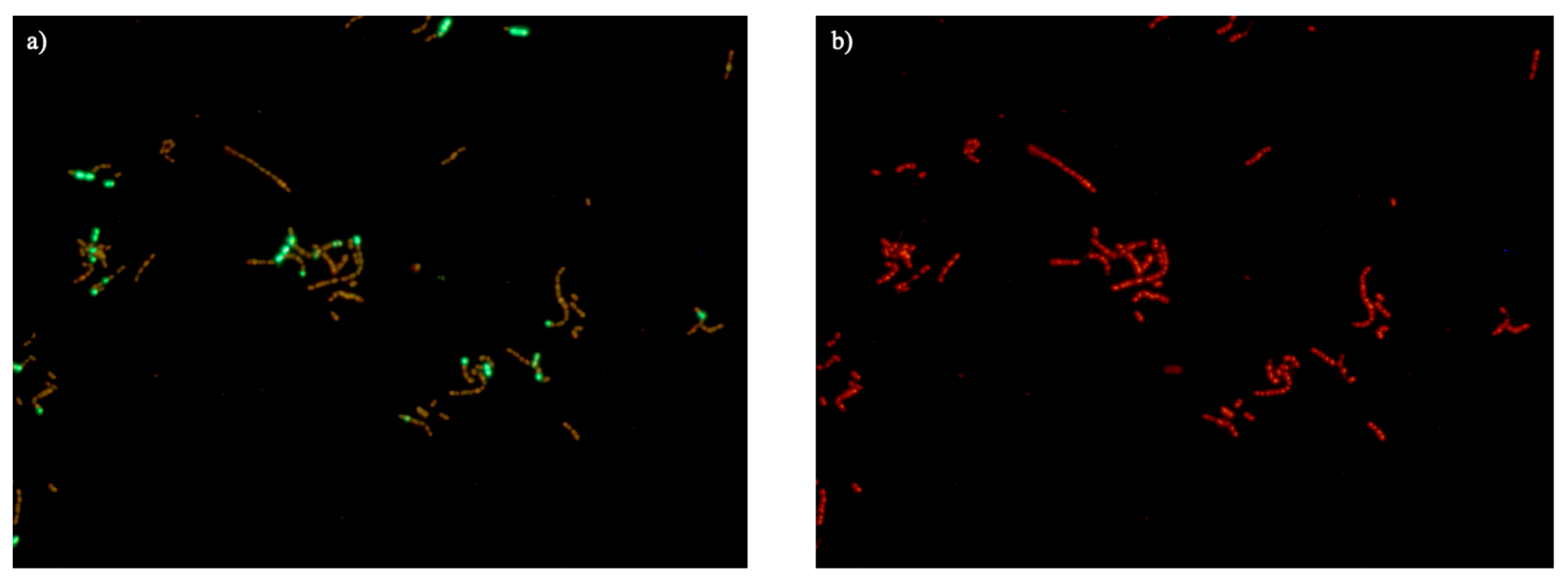
2.7. Disruption of the Bacterial Cell Membrane
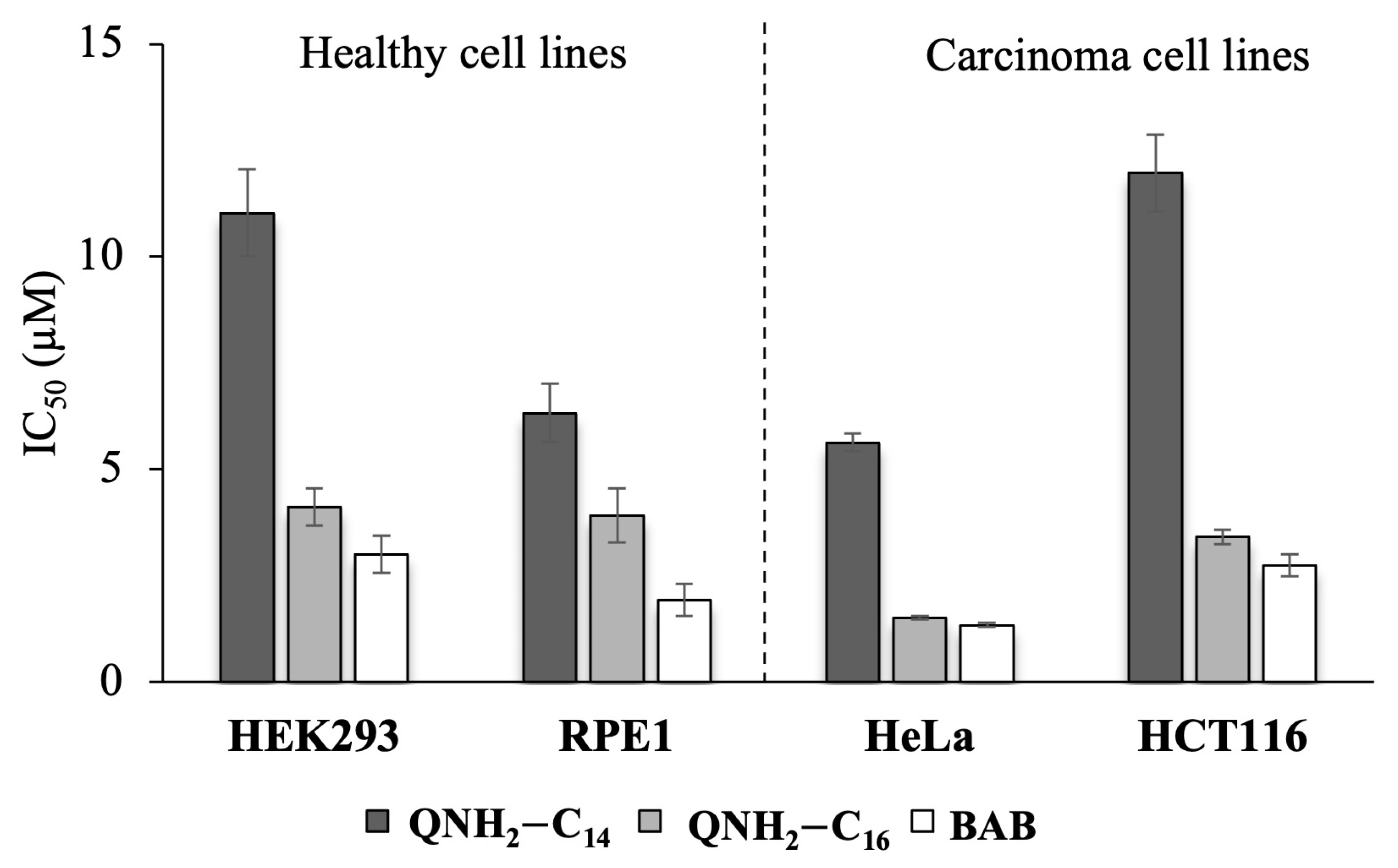
2.8. Cytotoxicity
3. Materials and Methods
3.1. Synthesis
3.1.1. Synthesis of (±)3-Aminoquinuclidine
3.1.2. Synthesis of (±)3-Aminoquinuclidinium Quaternary Compounds
3.2. Broth Microdilution Assay
3.3. Biofilm Inhibition Assay
3.4. Time-Resolved Growth Analysis
3.5. Potential of Bacterial Resistance Development
3.6. Time-Kill Kinetics Assay
3.7. Optical Fluorescence Microscopy Measurements
3.8. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

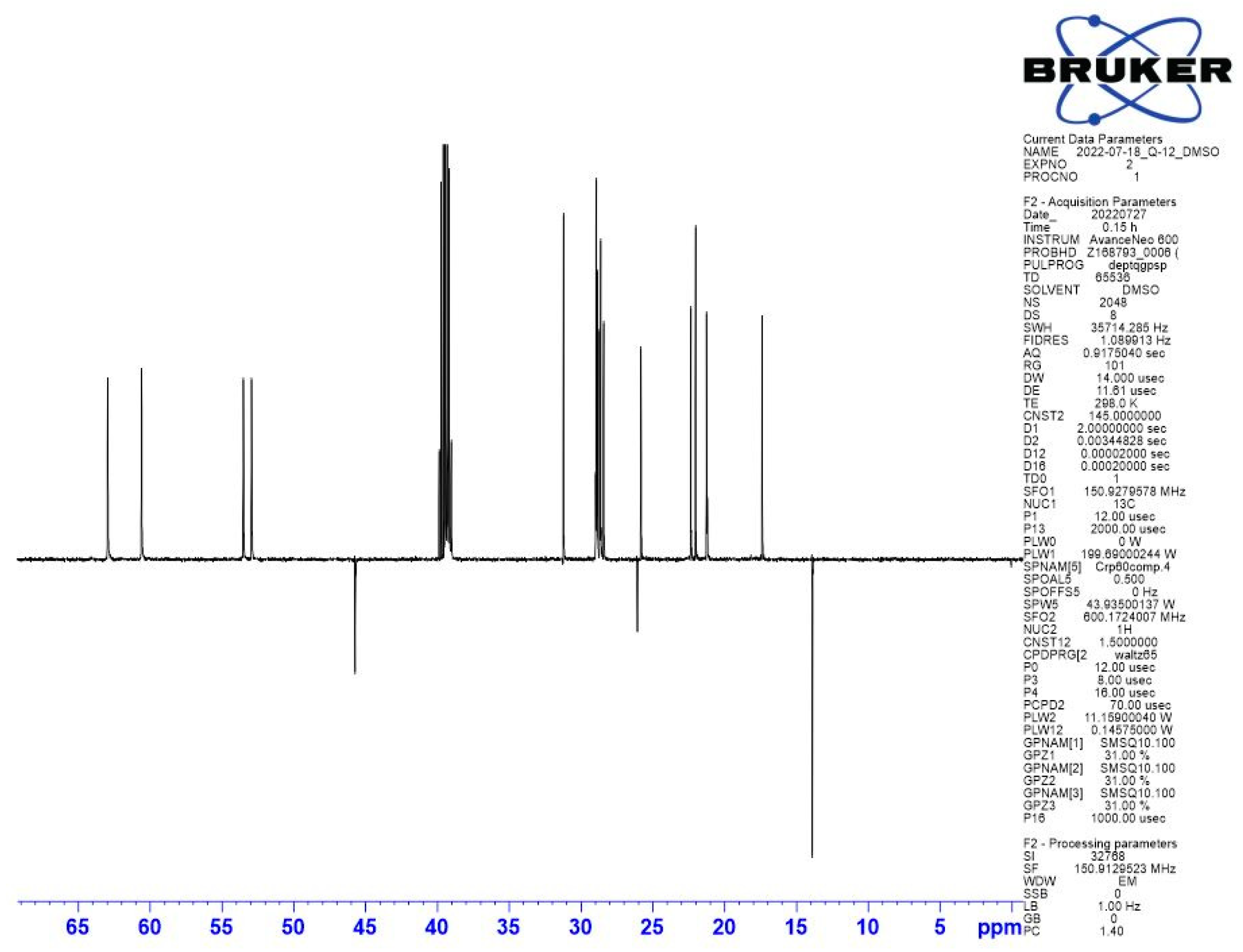
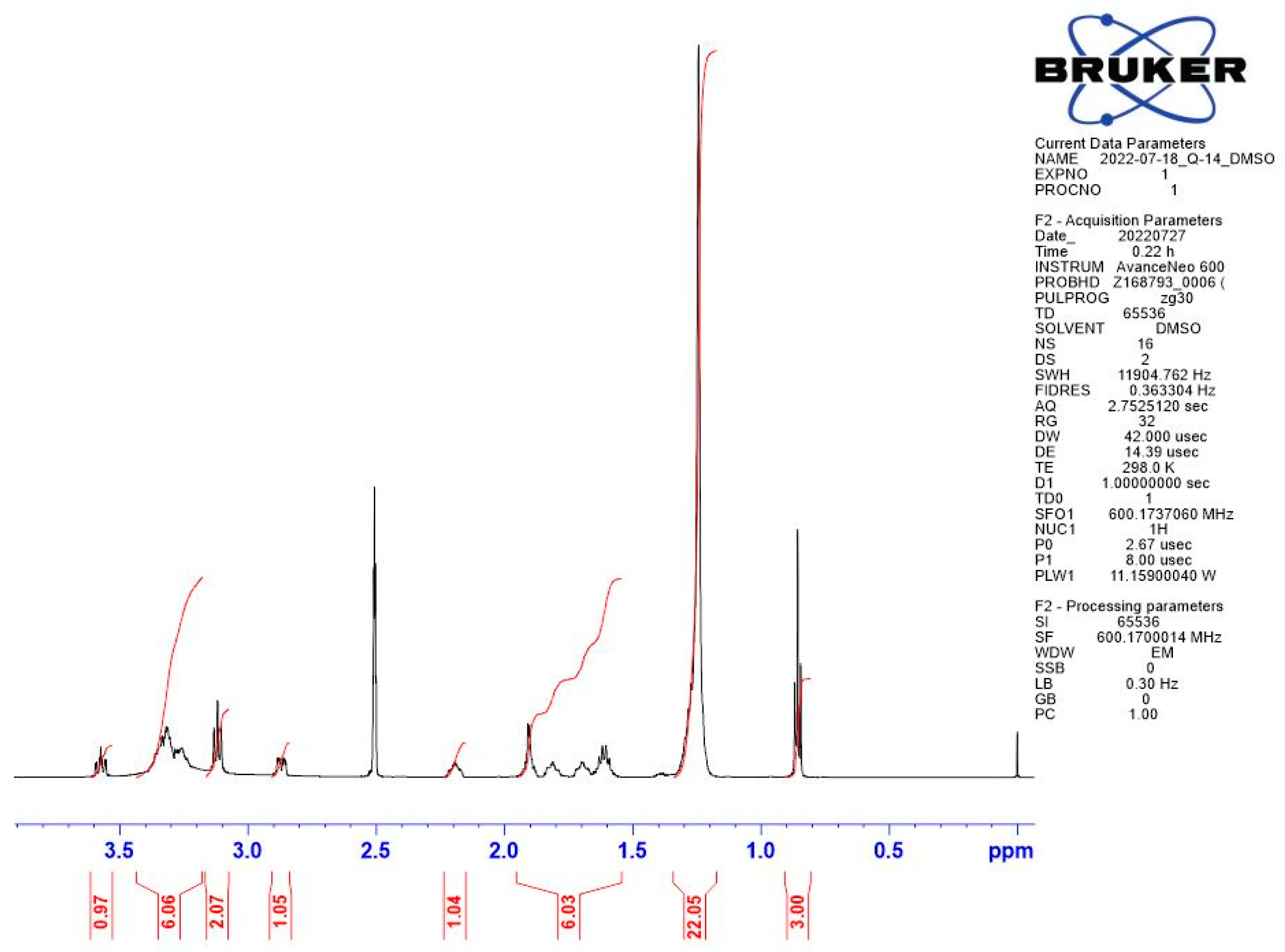

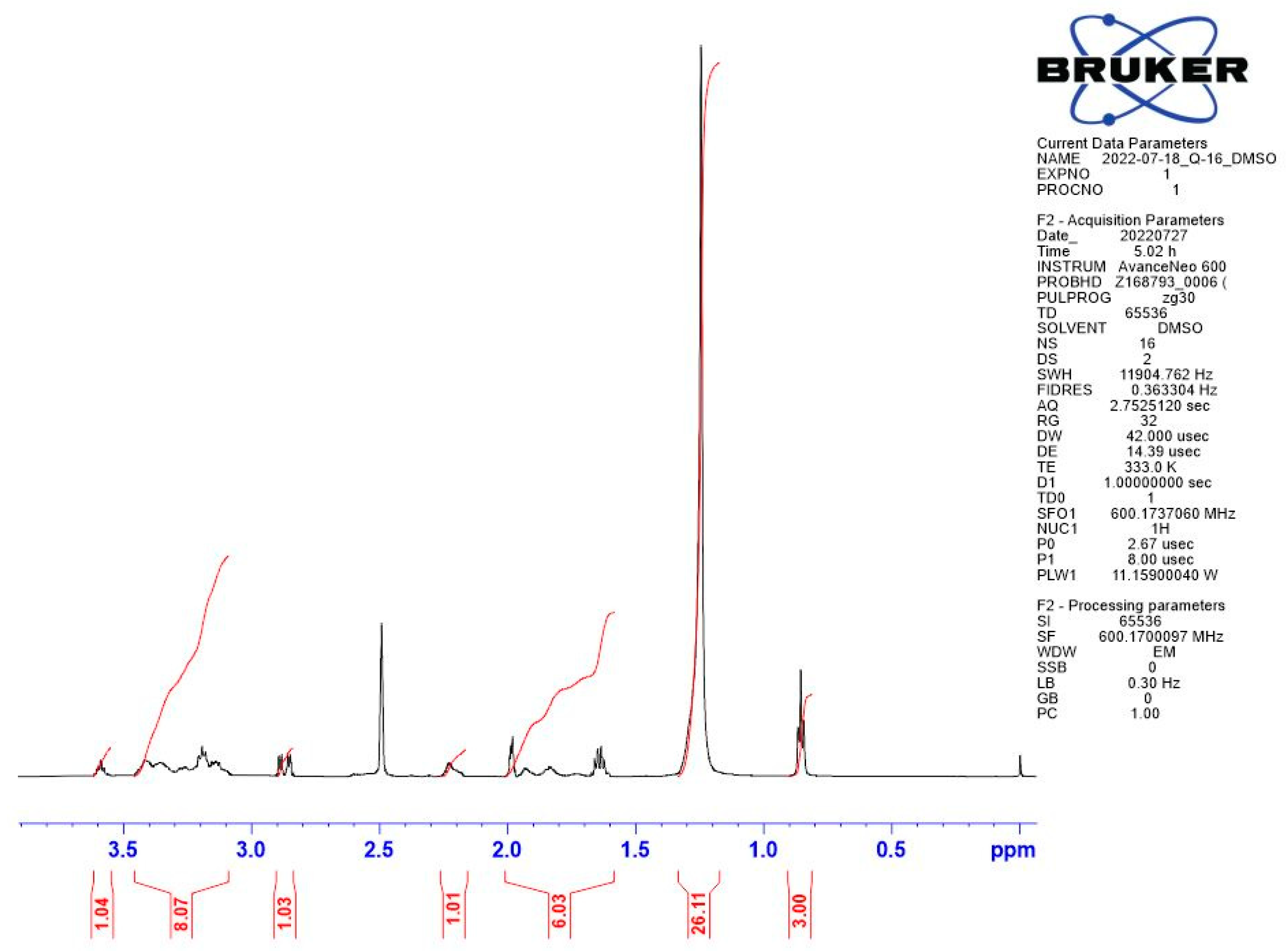

Appendix B
| Species | Strain Origin | (MIC)/µM | |
|---|---|---|---|
| Gram-positive | QNH2 | QAc | |
| Staphylococcus aureus | ATCC 25923 | >100 | >100 |
| Staphylococcus aureus | Clinical/MRSA | >100 | >100 |
| Staphylococcus aureus | ATCC 33591 | >100 | >100 |
| Bacillus cereus | ATCC 14579 | >100 | >100 |
| Listeria monocytogenes | ATCC 7644 | >100 | >100 |
| Enterococcus faecalis | ATCC 29212 | >100 | >100 |
| Gram-negative | |||
| Escherichia coli | ATCC 25922 | >100 | >100 |
| Salmonella enterica | food isolate | >100 | >100 |
| Pseudomonas aeruginosa | ATCC 27853 | >100 | >100 |
Appendix C
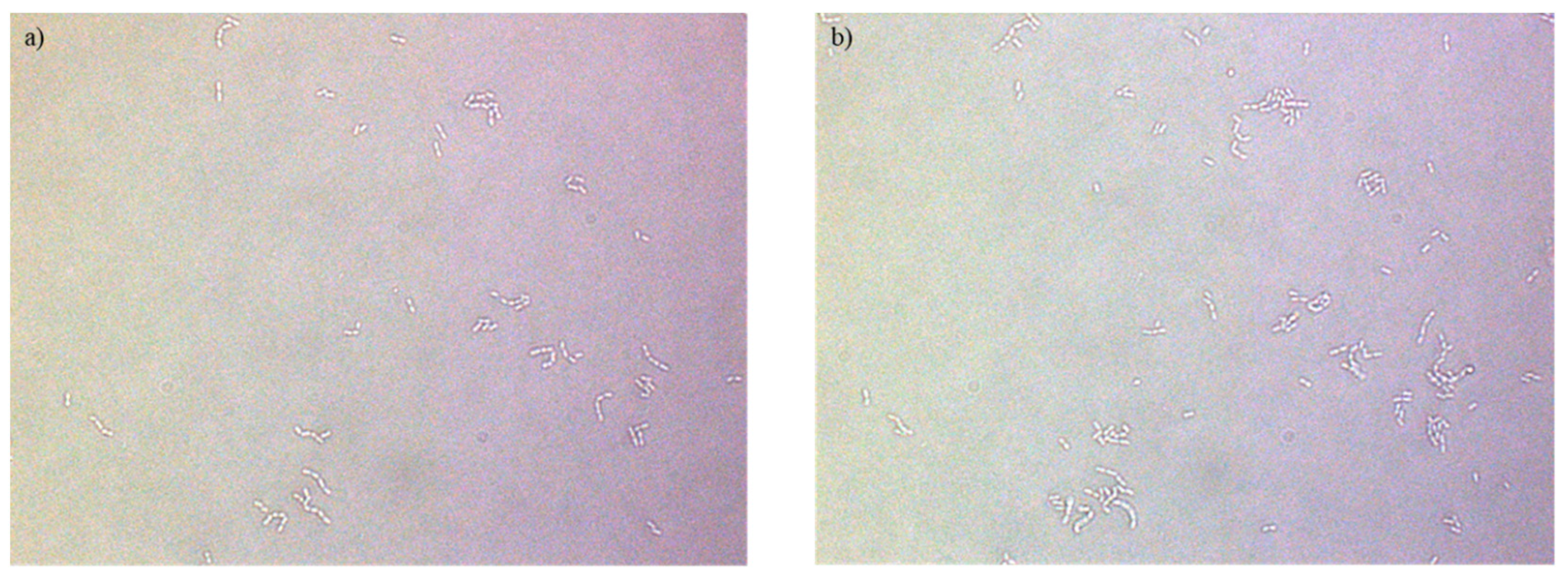
References
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary Ammonium Compounds: An Antimicrobial Mainstay and Platform for Innovation to Address Bacterial Resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef]
- Bureš, F. Quaternary Ammonium Compounds: Simple in Structure, Complex in Application. Top. Curr. Chem. 2019, 377, 14. [Google Scholar] [CrossRef]
- Hora, P.I.; Pati, S.G.; McNamara, P.J.; Arnold, W.A. Increased Use of Quaternary Ammonium Compounds during the SARS-CoV-2 Pandemic and Beyond: Consideration of Environmental Implications. Environ. Sci. Technol. Lett. 2020, 7, 622–631. [Google Scholar] [CrossRef]
- Gerba, C.P. Quaternary Ammonium Biocides: Efficacy in Application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef]
- Guo, X.; Chen, Y.; Wang, L.; Wu, X.; Fan, J.; Li, F.; Zeng, X.; Ge, Y.; Chi, Y.; Cui, L.; et al. In vitro inactivation of SARS-CoV-2 by commonly used disinfection products and methods. Sci. Rep. 2021, 11, 2418. [Google Scholar] [CrossRef]
- Melin, V.E.; Potineni, H.; Hunt, P.; Griswold, J.; Siems, B.; Werre, S.R.; Hrubec, T.C. Exposure to common quaternary ammonium disinfectants decreases fertility in mice. Reprod. Toxicol. 2014, 50, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.A.; Blum, A.; Branyan, J.; Bruton, T.A.; Carignan, C.C.; Cortopassi, G.; Datta, S.; DeWitt, J.; Doherty, A.-C.; Halden, R.U.; et al. Quaternary Ammonium Compounds: A Chemical Class of Emerging Concern. Environ. Sci. Technol. 2023, 57, 7645–7665. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Lu, H.; Zhu, L. Molecular mechanism of antibiotic resistance induced by mono- and twin-chained quaternary ammonium compounds. Sci. Total Environ. 2022, 832, 155090. [Google Scholar] [CrossRef] [PubMed]
- Obłąk, E.; Futoma-Kołoch, B.; Wieczyńska, A. Biological activity of quaternary ammonium salts and resistance of microorganisms to these compounds. World J. Microbiol. Biotechnol. 2021, 37, 22. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.C.; Forman, M.E.; Duggan, S.M.; Minbiole, K.P.C.; Wuest, W.M. Efflux Pumps Might Not Be the Major Drivers of QAC Resistance in Methicillin-Resistant Staphylococcus aureus. Chembiochem 2017, 18, 1573–1577. [Google Scholar] [CrossRef]
- Tischer, M.; Pradel, G.; Ohlsen, K.; Holzgrabe, U. Quaternary Ammonium Salts and Their Antimicrobial Potential: Targets or Nonspecific Interactions? ChemMedChem 2011, 7, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.M.; Schuman, J.T.; Skurray, R.A.; Brown, M.H.; Brennan, R.G.; Schumacher, M.A. QacR−Cation Recognition Is Mediated by a Redundancy of Residues Capable of Charge Neutralization. Biochemistry 2008, 47, 8122–8129. [Google Scholar] [CrossRef]
- IPaulsen, I.T.; Brown, M.H.; Littlejohn, T.G.; Mitchell, B.A.; Skurray, R.A. Multidrug resistance proteins QacA and QacB from Staphylococcus aureus: Membrane topology and identification of residues involved in substrate specificity. Proc. Natl. Acad. Sci. USA 1996, 93, 3630–3635. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Miller, M.C.; Grkovic, S.; Brown, M.H.; Skurray, R.A.; Brennan, R.G. Structural Mechanisms of QacR Induction and Multidrug Recognition. Science 2001, 294, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifa, S.; Jennings, M.C.; Granata, D.; Klein, M.; Wuest, W.M.; Minbiole, K.P.C.; Carnevale, V. Analysis of the Destabilization of Bacterial Membranes by Quaternary Ammonium Compounds: A Combined Experimental and Computational Study. Chembiochem 2019, 21, 1510–1516. [Google Scholar] [CrossRef]
- Black, J.W.; Jennings, M.C.; Azarewicz, J.; Paniak, T.J.; Grenier, M.C.; Wuest, W.M.; Minbiole, K.P. TMEDA-derived biscationic amphiphiles: An economical preparation of potent antibacterial agents. Bioorg. Med. Chem. Lett. 2014, 24, 99–102. [Google Scholar] [CrossRef]
- Grenier, M.C.; Davis, R.W.; Wilson-Henjum, K.L.; LaDow, J.E.; Black, J.W.; Caran, K.L.; Seifert, K.; Minbiole, K.P. The antibacterial activity of 4,4′-bipyridinium amphiphiles with conventional, bicephalic and gemini architectures. Bioorg. Med. Chem. Lett. 2012, 22, 4055–4058. [Google Scholar] [CrossRef]
- Carden, R.G.; Sommers, K.J.; Schrank, C.L.; Leitgeb, A.J.; Feliciano, J.A.; Wuest, W.M.; Minbiole, K.P.C. Advancements in the Development of Non-Nitrogen-Based Amphiphilic Antiseptics to Overcome Pathogenic Bacterial Resistance. ChemMedChem 2020, 15, 1974–1984. [Google Scholar] [CrossRef]
- Sommers, K.J.; Michaud, M.E.; Hogue, C.E.; Scharnow, A.M.; Amoo, L.E.; Petersen, A.A.; Carden, R.G.; Minbiole, K.P.C.; Wuest, W.M. Quaternary Phosphonium Compounds: An Examination of Non-Nitrogenous Cationic Amphiphiles That Evade Disinfectant Resistance. ACS Infect. Dis. 2022, 8, 387–397. [Google Scholar] [CrossRef]
- Feliciano, J.A.; Leitgeb, A.J.; Schrank, C.L.; Allen, R.A.; Minbiole, K.P.; Wuest, W.M.; Carden, R.G. Trivalent sulfonium compounds (TSCs): Tetrahydrothiophene-based amphiphiles exhibit similar antimicrobial activity to analogous ammonium-based amphiphiles. Bioorg. Med. Chem. Lett. 2021, 37, 127809. [Google Scholar] [CrossRef]
- Brayton, S.R.; Toles, Z.E.A.; Sanchez, C.A.; Michaud, M.E.; Thierer, L.M.; Keller, T.M.; Risener, C.J.; Quave, C.L.; Wuest, W.M.; Minbiole, K.P.C. Soft QPCs: Biscationic Quaternary Phosphonium Compounds as Soft Antimicrobial Agents. ACS Infect. Dis. 2023, 9, 943–951. [Google Scholar] [CrossRef]
- Thorsteinsson, T.; Másson, M.; Kristinsson, K.G.; Hjálmarsdóttir, M.A.; Hilmarsson, H.; Loftsson, T. Soft Antimicrobial Agents: Synthesis and Activity of Labile Environmentally Friendly Long Chain Quaternary Ammonium Compounds. J. Med. Chem. 2003, 46, 4173–4181. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.A.; Jennings, M.C.; Mitchell, M.A.; Al-Khalifa, S.E.; Wuest, W.M.; Minbiole, K.P. Ester- and amide-containing multiQACs: Exploring multicationic soft antimicrobial agents. Bioorg. Med. Chem. Lett. 2017, 27, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Crnčević, D.; Krce, L.; Cvitković, M.; Brkljača, Z.; Sabljić, A.; Vuko, E.; Primožič, I.; Odžak, R.; Šprung, M. New Membrane Active Antibacterial and Antiviral Amphiphiles Derived from Heterocyclic Backbone of Pyridinium-4-Aldoxime. Pharmaceuticals 2022, 15, 775. [Google Scholar] [CrossRef] [PubMed]
- Odžak, R.; Tomić, S. 3-Amidoquinuclidine derivatives: Synthesis of compounds and inhibition of butyrylcholinesterase. Bioorganic Chem. 2006, 34, 90–98. [Google Scholar] [CrossRef]
- Bazina, L.; Maravić, A.; Krce, L.; Soldo, B.; Odžak, R.; Popović, V.B.; Aviani, I.; Primožič, I.; Šprung, M. Discovery of novel quaternary ammonium compounds based on quinuclidine-3-ol as new potential antimicrobial candidates. Eur. J. Med. Chem. 2018, 163, 626–635. [Google Scholar] [CrossRef]
- Odžak, R.; Crnčević, D.; Sabljić, A.; Primožič, I.; Šprung, M. Synthesis and Biological Evaluation of 3-Amidoquinuclidine Quaternary Ammonium Compounds as New Soft Antibacterial Agents. Pharmaceuticals 2023, 16, 187. [Google Scholar] [CrossRef]
- Kontos, R.C.; Schallenhammer, S.A.; Bentley, B.S.; Morrison, K.R.; Feliciano, J.A.; Tasca, J.A.; Kaplan, A.R.; Bezpalko, M.W.; Kassel, W.S.; Wuest, W.M.; et al. An Investigation into Rigidity–Activity Relationships in BisQAC Amphiphilic Antiseptics. Chemmedchem 2018, 14, 83–87. [Google Scholar] [CrossRef]
- Leitgeb, A.J.; Feliciano, J.A.; Sanchez, H.A.; Allen, R.A.; Morrison, K.R.; Sommers, K.J.; Carden, R.G.; Wuest, W.M.; Minbiole, K.P.C. Further Investigations into Rigidity-Activity Relationships in BisQAC Amphiphilic Antiseptics. ChemMedChem 2020, 15, 667–670. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2857177/pdf/cshperspect-PRK-a000414.pdf (accessed on 21 March 2023). [CrossRef]
- Mahoney, A.R.; Safaee, M.M.; Wuest, W.M.; Furst, A.L. The silent pandemic: Emergent antibiotic resistances following the global response to SARS-CoV-2. iScience 2021, 24, 102304. [Google Scholar] [CrossRef]
- Mereghetti, L.; Quentin, R.; Der Mee, N.M.-V.; Audurier, A. Low Sensitivity of Listeria monocytogenes to Quaternary Ammonium Compounds. Appl. Environ. Microbiol. 2000, 66, 5083–5086. [Google Scholar] [CrossRef]
- Jiang, X.; Ren, S.; Geng, Y.; Yu, T.; Li, Y.; Liu, L.; Liu, G.; Wang, H.; Shi, L. The sug operon involves in resistance to quaternary ammonium compounds in Listeria monocytogenes EGD-e. Appl. Microbiol. Biotechnol. 2020, 104, 7093–7104. [Google Scholar] [CrossRef]
- Bridier, A.; Briandet, R.; Thomas, V.; Dubois-Brissonnet, F. Resistance of bacterial biofilms to disinfectants: A review. Biofouling 2011, 27, 1017–1032. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.H.; Jennings, M.C.; Wuest, W.M. Draining the moat: Disrupting bacterial biofilms with natural products. Tetrahedron 2014, 70, 6373–6383. [Google Scholar] [CrossRef]
- Kula, N.; Lamch, Ł.; Futoma-Kołoch, B.; Wilk, K.A.; Obłąk, E. The effectiveness of newly synthesized quaternary ammonium salts differing in chain length and type of counterion against priority human pathogens. Sci. Rep. 2022, 12, 21799. [Google Scholar] [CrossRef]
- Theophel, K.; Schacht, V.J.; Schlüter, M.; Schnell, S.; Stingu, C.-S.; Schaumann, R.; Bunge, M. The importance of growth kinetic analysis in determining bacterial susceptibility against antibiotics and silver nanoparticles. Front. Microbiol. 2014, 5, 544. [Google Scholar] [CrossRef] [PubMed]
- Pankey, G.A.; Sabath, L.D. Clinical Relevance of Bacteriostatic versus Bactericidal Mechanisms of Action in the Treatment of Gram-Positive Bacterial Infections. Clin. Infect. Dis. 2004, 38, 864–870. Available online: https://academic.oup.com/cid/article/38/6/864/320723 (accessed on 12 February 2023). [CrossRef] [PubMed]
- Salton, M.R.J. Lytic Agents, Cell Permeability, and Monolayer Penetrability. J. Gen. Physiol. 1968, 52, 227–252. [Google Scholar] [CrossRef]
- Boulos, L.; Prevost, M.; Barbeau, B.; Coallier, J.; Desjardins, R. LIVE/DEAD BacLightE: Application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J. Microbiol. Methods 1999, 37, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cui, F.; Zeng, G.-M.; Jiang, M.; Yang, Z.-Z.; Yu, Z.-G.; Zhu, M.-Y.; Shen, L.-Q. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015, 518–519, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Kwasny, S.M.; Opperman, T.J. Static Biofilm Cultures of Gram-Positive Pathogens Grown in a Microtiter Format Used for Anti-Biofilm Drug Discovery. Curr. Protoc. Pharmacol. 2010, 50, 13A.8.1–13A.8.23. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Carbonel, A.; Mondragón, B.; López-Chegne, N.; Peña-Tuesta, I.; Huayan-Dávila, G.; Blitchtein, D.; Carrillo-Ng, H.; Silva-Caso, W.; Aguilar-Luis, M.A.; del Valle-Mendoza, J. The effect of the efflux pump inhibitor Carbonyl Cyanide m-Chlorophenylhydrazone (CCCP) on the susceptibility to imipenem and cefepime in clinical strains of Acinetobacter baumannii. PLoS ONE 2021, 16, e0259915. [Google Scholar] [CrossRef]




| MIC (µM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Compound | |||||||||
| Gram-positive | QNH2-C12 | QNH2-C14 | QNH2-C16 | QAc-C12 * | QAc-C14 * | QAc-C16 * | BAB | CPC | ||
| Strain Origin | Mr = 375.4 | Mr = 403.5 | Mr = 431.6 | Mr = 417.5 | Mr = 445.5 | Mr = 473.6 | Mr = 384.4 | Mr = 340 | ||
| Staphylococcus aureus | ATCC 25923 | 100 | 25 | 25 | >125 | >125 | >125 | 10.00 | 4.00 | |
| Staphylococcus aureus | Clinical/MRSA | >100 | >100 | >100 | >125 | >125 | >125 | 25.00 | 8.00 | |
| Staphylococcus aureus | ATCC 33591 | >100 | 100 | 100 | >125 | >125 | >125 | 25.00 | 6.00 | |
| Bacillus cereus | ATCC 14579 | 100 | 25 | 12.5 | >125 | >125 | >125 | 25.00 | 16.00 | |
| Listeria monocytogenes | ATCC 7644 | 100 | 50 | 12.5 | >125 | 63 | 8 | 10.00 | 8.00 | |
| Enterococcus faecalis | ATCC 29212 | 100 | 100 | 50 | >125 | 63 | 31 | 15.00 | 8.00 | |
| Gram-negative | ||||||||||
| Escherichia coli | ATCC 25922 | >100 | 100 | 100 | >125 | >125 | >125 | 63.00 | 16.00 | |
| Salmonella enterica | food isolate | >100 | >100 | >100 | >125 | >125 | >125 | 50.00 | 63.00 | |
| Pseudomonas aeruginosa | ATCC 27853 | >100 | >100 | >100 | >125 | >125 | >125 | >125 | 250 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odžak, R.; Crnčević, D.; Sabljić, A.; Krce, L.; Paladin, A.; Primožič, I.; Šprung, M. Further Study of the Polar Group’s Influence on the Antibacterial Activity of the 3-Substituted Quinuclidine Salts with Long Alkyl Chains. Antibiotics 2023, 12, 1231. https://doi.org/10.3390/antibiotics12081231
Odžak R, Crnčević D, Sabljić A, Krce L, Paladin A, Primožič I, Šprung M. Further Study of the Polar Group’s Influence on the Antibacterial Activity of the 3-Substituted Quinuclidine Salts with Long Alkyl Chains. Antibiotics. 2023; 12(8):1231. https://doi.org/10.3390/antibiotics12081231
Chicago/Turabian StyleOdžak, Renata, Doris Crnčević, Antonio Sabljić, Lucija Krce, Antonela Paladin, Ines Primožič, and Matilda Šprung. 2023. "Further Study of the Polar Group’s Influence on the Antibacterial Activity of the 3-Substituted Quinuclidine Salts with Long Alkyl Chains" Antibiotics 12, no. 8: 1231. https://doi.org/10.3390/antibiotics12081231
APA StyleOdžak, R., Crnčević, D., Sabljić, A., Krce, L., Paladin, A., Primožič, I., & Šprung, M. (2023). Further Study of the Polar Group’s Influence on the Antibacterial Activity of the 3-Substituted Quinuclidine Salts with Long Alkyl Chains. Antibiotics, 12(8), 1231. https://doi.org/10.3390/antibiotics12081231






