Abstract
Background: Echinacea species, particularly Echinacea purpurea, Echinacea angustifolia, and Echinacea pallida, are renowned for their immunomodulatory, antibacterial, and antiviral properties. Objectives: This review explores the mechanisms by which echinacea herbal extracts modulate immune responses, focusing on their effects on both innate and adaptive immunity in bacterial and viral infections. Results: Key bioactive compounds, such as alkamides, caffeic acid derivatives, flavonoids, and polysaccharides, contribute to these effects. These compounds enhance immune cell activity, including macrophages and natural killer cells, stimulating cytokine production and phagocytosis. The antibacterial activity of echinacea against respiratory pathogens (Streptococcus pneumoniae, Haemophilus influenzae, Legionella pneumophila) and skin pathogens (Staphylococcus aureus, Propionibacterium acnes) is reviewed, as well as its antiviral efficacy against viruses like herpes simplex, influenza, and rhinovirus. Echinacea’s potential as a complementary treatment alongside conventional antibiotics and antivirals is discussed, particularly in the context of antibiotic resistance and emerging viral threats. Conclusions: Challenges associated with variability in phytochemical content and the need for standardized extraction processes are also addressed. This review provides a comprehensive overview of echinacea’s therapeutic potential and outlines future directions for research, including clinical trials and dosage optimization.
1. Introduction
Herbal and botanical products have been utilized for disease prevention and treatment for thousands of years. Many Native Indian tribes and Asian cultures across the globe are recognized for their significant role in advancing botanical medicine [1]. It is estimated that more than 30,000 herbs and botanicals have been explored for medicinal purposes, though fewer than 300 are actively incorporated into Western medicine today [2]. The genus Echinacea (Asteraceae) includes a small group of hardies, herbaceous perennial species indigenous to parts of North America [3]. Echinacea purpurea, derived from a flowering plant, is a popular herbal medicine. This plant is native to the United States, particularly in regions east of the Rocky Mountains, and the Atlantic drainage area, including the Great Plains, and extends to the central U.S. and nearby areas of Canada [4]. According to the Royal Botanic Kew Gardens’ “Plants of the World Online”, native Echinacea species range from Western Europe to Southeastern Asia [5]. There are at least nine species of echinacea, each potentially varying in medicinal properties. Three species—Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., and Echinacea purpurea (L.) Moench—are commonly used for therapeutic purposes [6]. Some vernacular names for echinacea species include black Sampson, coneflower, pale purple coneflower (E. pallida), purple coneflower (E. purpurea, E. angustifolia), narrow-leaf purple coneflower (E. angustifolia), and Kansas snakeroot (E. angustifolia) [7]. The medicinal parts include the fresh or dried roots and rhizomes of all three species, with E. purpurea also using its fresh or dried flowering tops and fresh-pressed juice [8]. Commercial echinacea products, which may contain one or more of these raw materials from different regions, are available in various forms such as tinctures, tablets, teas, capsules, and parenteral preparations [9]. Echinacea has a long-standing history in medicine for treating diverse conditions, including infections such as syphilis and septic wounds, and has been historically utilized as an “anti-toxin” for snakebites and blood poisoning [10].
Historically, echinacea was regarded as an “anti-infective” agent, commonly used to address bacterial and viral infections, mild septicemia, furunculosis (frequent painful skin nodules), and a range of skin conditions, such as boils, carbuncles, and abscesses [11]. It was also traditionally employed in the treatment of nasopharyngeal catarrh, pyorrhoea (periodontitis), and tonsillitis, as well as a supportive remedy for flu-like symptoms, recurring respiratory tract infections, and urinary tract infections. Externally, it was applied to poorly healing superficial wounds [12]. In recent efforts to substantiate these traditional uses, numerous studies have investigated the effects of standardized E. purpurea formulations on pathogens, inflammatory processes, and gene expression in both infected and healthy human cells and animal models [13]. Today, echinacea is primarily recognized as an over-the-counter herbal remedy for colds and flu and is also used for pain, inflammation, migraines, and other health conditions. However, in some regions, echinacea is regulated as a licensed medicinal product and is even prescribed by doctors, although such prescriptions are not always required [14]. The belief that echinacea should be avoided in autoimmune diseases assumes that boosting any aspect of immune function could be harmful [15]. However, the immune system is highly complex, and substances primarily enhancing phagocytic activity may be safe or beneficial in autoimmune disorders [16,17]. A case study on the long-term use of echinacea in chronic lymphocytic leukemia found no negative effects and underscored its benefits for immune function [18].
Echinacea species, now commonly recognized as immune stimulants, were historically used by North American Indigenous Peoples to treat throat infections, wounds, and pain. In the past, echinacea was also employed in eclectic medicine to treat septic conditions [19]. Ongoing research continues to investigate its pharmacological properties and potential therapeutic applications, including anti-inflammatory, analgesic, anxiolytic, and antimicrobial effects [20]. Echinacea is generally considered safe, with severe side effects being rare. Most users experience few adverse effects, with mild reactions such as gastrointestinal discomfort or skin irritation occurring infrequently [21]. However, like any herbal supplement, there is a potential for allergic reactions, particularly in individuals allergic to members of the Asteraceae family, which includes ragweed, chrysanthemums, marigolds, and daisies [13]. The morphology of the echinacea is depicted in Figure 1.

Figure 1.
Botanical illustration of E. purpurea, depicting the aerial and root structures (Figure drawn by F. Ahmadi).
2. Methodology
Multiple academic databases were utilized to conduct a comprehensive literature search, including Web of Knowledge, Scopus, ScienceDirect, Google Scholar, Web of Science Core Collection by Clarivate Analytics, and ResearchGate. The search focused on publications from 1989 to 2024. Several keywords were employed to obtain a wide range of search results. These keywords encompassed various aspects related to echinacea species, phytochemistry, medicinal plants, echinacea biology, biochemistry, bioactive compounds, antibacterial properties, antivirus, viral infections, immune response, traditional medicine, biological models, and antibacterial mechanisms. In addition to the initial search, the references cited within the obtained publications were collected to ensure a comprehensive literature review. The chosen keywords were combined using operators like “OR” and “AND” to refine the search and obtain more precise results. Including quotation marks around specific terms, such as “echinacea”, ensured accurate retrieval of relevant records. All the keywords were used in all six databases to maximize search coverage and gather a comprehensive collection of literature on the subject. The keywords and topics that are trending in each specific review section were identified. To address the research question and achieve the review objective, a thorough search for peer-reviewed studies was conducted specifically focused on echinacea’s medicinal properties and mechanisms. The search was primarily centered on journal articles, excluding grey literature such as books, book chapters, and conference papers, except in rare cases where they provided valuable insights. The author screened and evaluated titles and abstracts from over 255 articles, employing a rigorous selection process to identify relevant papers. A key criterion for inclusion was the presence of quantitative information within the study. Specifically targeted studies addressed the following topics: (i) echinacea species phytochemistry, physiology, and medicinal properties; and (ii) the mechanisms of echinacea bioactive compounds on bacterial and viral infections. After comparing and analyzing the remaining articles, we categorized them based on relevant keywords. Additionally, the author summarized the conducted research, critically evaluated the content of over 192 studies, extracted essential features, and identified key challenges that warrant further investigation. By following this rigorous methodology, the author aimed to provide a comprehensive review that synthesizes the current state of knowledge, highlights significant findings, and identifies areas that require additional research attention within the context of echinacea phytochemistry and medicinal properties.
3. Phytochemistry
The composition of echinacea varies between species and their different plant parts [12]. It is commonly believed that no single compound or group of compounds is solely responsible for the effects of echinacea [22]. Rather, several classes of compounds—including alkamides, caffeic acid derivatives, polysaccharides, and alkenes (such as polyenes)—are thought to collectively contribute to its activity [23]. Below is a summary of the key constituents found in various echinacea species, compiled from several references, along with the structural formulas of some of these components [24].
3.1. Alkamides
Alkamides are plant-derived, lipophilic compounds characterized by a combination of various amines and aliphatic acids connected via an amine linkage [25]. These amine residues, which can be either aromatic or aliphatic, are produced through the decarboxylation of amino acids [26]. The main alkamides found in different echinacea species are depicted in Figure 2, including the isomeric dodeca-2E, 4E, 8Z, and 10E/Z-tetraenoic acid isobutylamide (8a/8b) (Figure 2). Isobutylamine originates from the amino acid valine and is the most common amine present in alkamides [27]. Other frequent amine residues include piperidine—derived from lysine, pyrrolidine—derived from ornithine, N-2-methylbutyl—whose precursor is isoleucine, and N-phenethyl—derived from phenylalanine [28]. A characteristic feature of echinacea alkamides is the attachment of isobutyl or 2-methylbutyl amines to an unsaturated fatty acid chain, which contains one or more double or triple bonds [29]. However, two alkamides isolated from hydroalcoholic extracts of E. purpurea roots possess a hydroxyl group and a carboxylic acid at the terminal end of the fatty acid chain [30]. Alkamide concentrations vary significantly between the roots, stems, and flowers of E. purpurea, with higher levels of dodeca-2,4-diene-8,10-diyne alkamides in the roots, while dodecatetraene alkamides and nonadeca-2,4-diene-8,10-diynes are more prevalent in the stems [31]. A distribution study found that the root bark and secondary roots of E. angustifolia lacked alkamides. High-quality E. purpurea root material contains up to 6 mg/g of alkamides [32].

Figure 2.
Structure of the main echinacea alkamides (Figure drawn by F. Ahmadi).
3.2. Flavonoids
There are only three documented reports of flavonoids in echinacea species. Previous research [33] identified the main anthocyanin pigments in the flowers of E. purpurea and E. pallida as cyanidin 3-glucoside and cyanidin 3-(6″-malonylglucoside) (Figure 3). Earlier studies [34] also produced anthocyanin-containing callus and suspension cultures from the stem of E. purpurea. From these suspension cultures, three anthocyanins were isolated: cyanidin 3-glucoside and two additional acylated cyanidin glycosides that were not fully characterized [35]. The only other report comes from unpublished data in a doctoral thesis, which noted trace amounts of quercetin and kaempferol glycosides, including the 3-rutinosides (rhamnosyl (1→6) glucosides, 56, 57), in the aerial parts of E. purpurea [36].

Figure 3.
Flavonoids found in echinacea species (Figure drawn by F. Ahmadi).
3.3. Hydrocarbons
Hydrocarbons are key root constituents of E. pallida, with approximately 11 derivatives identified, primarily ketoalkenes and ketoalkynes (polyacetylenes). The major compounds include the ketoalkenes—(Z)-pentadec-8-en-2-one, (8Z,11Z)-pentadeca-8,11-dien-2-one [37], (8Z,11Z,13E)-pentadeca-8,11,13-trien-2-one, and (8Z,11E,13Z)-pentadeca-8,11,13-trien-2-one [38]—and the ketoalkenynes—(8Z,13Z)-pentadeca-8,13-dien-11-yn-2-one, (Z)-tetradeca-8-diene-11,13-diyn-2-one [39], and (Z)-pentadeca-8-ene-11,13-diyn-2-one [40]. Additionally, two notable alkenes, pentadec-1-ene [41] and (Z)-pentadeca-1,8-diene [42], have been detected in the roots of E. angustifolia [43]. In contrast, the only hydrocarbon reported from E. purpurea roots is dodeca-2,4-dien-1-yl isovalerate, which is also found in the roots of E. angustifolia [44]. The absence of polyacetylenes in the roots of E. angustifolia and E. purpurea serves as a distinguishing factor between these species and E. pallida. Research has demonstrated that commercially available E. pallida root preparations are often contaminated with E. angustifolia [45]. This suggests that some early reports of hydrocarbons in E. angustifolia roots may have been due to misidentification or contamination with E. pallida. The hydrocarbons identified in the rhizomes of echinacea species are listed in Table 1.

Table 1.
Hydrocarbons from Rhizomes a of echinacea species [46].
3.4. Polysaccharides
Two immunostimulatory polysaccharides, PS I and PS II, have been isolated from the aerial parts of E. purpurea. PS I is identified as 4-O-methyl-glucuronoarabinoxylan with an average molecular weight of 35,000, while PS II is an acidic arabinorhamnogalactan with a molecular weight of 50,000. Both of these polysaccharides exhibited significant activity in various in vitro and in vivo immunological tests [47]. A crude polysaccharide extract from the roots of E. purpurea has not been fully analyzed, though it seems to have a similar composition to that of the aerial parts [48]. Additionally, from cell cultures of E. purpurea, three homogeneous polysaccharides were obtained: two neutral fucogalactoxyloglucans, with molecular weights of 10,000 and 25,000, and an acidic arabinogalactan, with a molecular weight of 75,000 [49]. The fucogalactoxyloglucan with a molecular weight of 25,000 was found to enhance phagocytosis in both in vitro and in vivo assays, while the arabinogalactan specifically triggered macrophages to secrete tumor necrosis factor (TNF) [50,51]. The acidic arabinorhamnogalactan from E. purpurea is now produced biotechnologically on an industrial scale and is being considered for clinical trials [52]. Previous research classified this polysaccharide as a type II arabinogalactan, characterized by a (1→3)-linked β-D-galactan backbone, which is likely attached to rhamnogalactan and arabinan chains [53,54]. The polysaccharides produced through tissue culture differ structurally from those found in the aerial parts, as they are components of the primary cell walls in cultured cells. As a result, the polysaccharides derived from the aerial parts of E. purpurea show little resemblance to those produced by tissue cultures [55].
3.5. Caffeic Acid Derivatives (CADs)
Another important group of phytochemicals contributing to echinacea’s pharmacological effects is CADs (Figure 4). Unlike alkylamides, which are more limited in their distribution across taxa, phenolic metabolites like CADs are widely distributed among plants. Although echinacea has a unique collection of CADs, these compounds are found across many plant families [56,57]. Two of the most extensively studied CADs in E. purpurea are caftaric acid and chicoric acid, as they represent the dominant polyphenols in this species [58]. In addition to their role in plant defenses, such as deterring herbivores and providing interspecies protection, CADs are associated with echinacea’s immunostimulatory and antioxidant properties [59]. However, oral administration studies indicate that these compounds have poor bioavailability, raising questions about their effectiveness in humans [60]. Chicoric acid is particularly abundant in E. purpurea, making up as much as 20% of total CADs in the roots, while the flowers, stems, and leaves contain approximately 35%, 10%, and 35%, respectively [61]. For natural health products, this distribution suggests that products should prioritize flowers and leaves, which contain higher concentrations of CADs. Some natural health products reflect this, whereas others focus on alkylamides, which are mainly concentrated in the roots and found in smaller amounts in the aerial parts [62].

Figure 4.
A possible biosynthesis pathway for chicoric acid and caffeic acid is derived via the phenylpropanoid pathway in echinacea species. PAL: phenylalanine ammonia-lyase, C4H: cinnamate-4-hydroxylase, C3H: coumarate 3-hydroxylase, 4CL: 4-coumarate-CoA ligase, HCT: shikimate o-hydroxycinnamoyl transferase (Figure drawn by F. Ahmadi).
4. Antibacterial Activities of Echinacea Species
There are numerous reports detailing the traditional use of echinacea species in treating bacterial infections [63]. Three species in particular, E. purpurea, E. angustifolia, and E. pallida, have historically been used as antibacterial remedies. Research [64] has also discussed the application of E. purpurea in Brazil, where a leaf infusion is applied topically to infected areas [65]. Several studies have since been conducted to validate the antibacterial properties traditionally attributed to echinacea species and their preparations [66]. One such study investigated the antibacterial activity of a fermented extract of E. purpurea (5% w/v, fermented with Lactobacillus plantarum) [67]. Using disc diffusion and broth microdilution assays, the extract was tested on a range of bacterial strains, including E. coli, Enterobacter aerogenes, Enterococcus durans, Yersinia enterocolitica, Weissella confusa, Leuconostoc lactis, Propionibacterium jensenii, Lactobacillus sakei, and Bacillus megaterium. The results demonstrated that the fermented extract inhibited the growth of most of the strains tested, with the greatest inhibition seen in B. megaterium and L. lactis, where inhibition halos measured over 3.5 mm and between 2.5–3.5 mm, respectively. No inhibitory effect was observed for L. sakei [68].
A previous study [69] investigated the bactericidal effects of a 65% ethanol extract from freshly harvested aerial parts and roots of E. purpurea at concentrations of 40 and 120 mg of dry mass/mL. The microdilution method, using both light and dark assays, was applied to test the extract against strains responsible for respiratory infections, including L. pneumophila, Streptococcus pyogenes, Mycobacterium smegmatis, and H. influenzae. Results showed that S. pyogenes, H. influenzae, and L. pneumophila were sensitive to the higher concentration of the extract. Another study [70], employing agar diffusion tests, assessed the antibacterial activity of hydroethanol extracts (prepared via conventional and ultrasonic extraction) from the aerial parts of E. purpurea (20 mg/mL). The extracts were tested against standard strains such as E. coli, P. aeruginosa, Bacillus subtilis, and S. aureus. The conventional extraction method produced larger inhibition zones for all strains, with E. coli (11.2 ± 0.2 mm) and B. subtilis (10.9 ± 0.1 mm) showing the most significant inhibition. In another study [71], the antibacterial activity of E. angustifolia extracts was evaluated using solvents of increasing polarity (petroleum ether, methanol, and water) at concentrations of 0.5, 1.5, and 1.5 mg/mL, respectively, against B. subtilis, S. aureus, E. coli, and Staphylococcus epidermidis. The study did not specify the plant parts used for extraction, and the aqueous extracts were less effective than those prepared with other solvents.
Respiratory infections can be caused by various pathogenic bacteria, including Streptococcus pneumoniae, H. influenzae, Moraxella catarrhalis, S. pyogenes, Bordetella pertussis, Mycoplasma pneumoniae, S. aureus, P. aeruginosa, and Burkholderia cepacia [72], among others. In this context, plants from the echinacea genus have shown promising antibacterial activity against microorganisms involved in these infections, as demonstrated by previous studies [73]. Additionally, E. purpurea has been found to inhibit L. pneumophila, the bacterium responsible for pneumonia [74]. However, the results for S. aureus have been inconsistent, possibly due to variations in the echinacea species used, plant parts, extraction methods, and thus their chemical compositions. Earlier research [75] suggested that neither alkylamides nor polysaccharides alone were responsible for the reported bactericidal effects. Furthermore, no clear link was found between bactericidal activity and caffeoyl derivatives [76]. Conversely, other studies [77] have correlated antimicrobial activity with total phenolic and flavonoid content.
Several bacteria, including P. acnes, S. aureus, S. pyogenes, P. aeruginosa, Pasteurella multocida, Capnocytophaga canimorsus, Bartonella spp., Klebsiella rhinoscleromatis, and Vibrio vulnificus, are key pathogens involved in skin infections [78]. Studies have shown that echinacea plants possess active compounds with antimicrobial properties that target these skin pathogens, supporting their traditional use in ethnomedicine for treating skin infections [79]. Additionally, recent research has demonstrated the potential of E. purpurea in addressing throat and mouth conditions (Oto-rhino-laryngological, OTO). One study [80] revealed that extracts from E. angustifolia and its primary alkylamide isomers, dodeca-2E, 4E, 8Z, and 10Z/E-octadecenoic acid (alkylamide 8/9, which is also found in E. purpurea), inhibited the growth of Candida albicans, a leading cause of fungal throat infections [81]. These findings align with ethnobotanical evidence supporting the use of echinacea for OTO-related conditions. Moreover, echinacea has been reported to inhibit microorganisms responsible for respiratory infections, including Legionella pneumophila, Streptococcus pyogenes, and Mycobacterium smegmatis [82]. However, despite being a well-studied respiratory pathogen, Pseudomonas has not been thoroughly investigated concerning echinacea treatments [83].
Purple coneflower (E. purpurea) is known to counteract proinflammatory cytokine stimulation, regardless of the bacterial or viral source of infection [84]. Several studies have examined the effects of E. purpurea on the activation of lipopolysaccharide, an inflammatory mediator typically produced by E. coli, in various human cell cultures and animal models, as well as research involving live bacteria [85]. While these models do not always fully replicate live bacterial infections, they provide useful insights for testing potential anti-inflammatory agents. The findings suggest that E. purpurea acts as a general anti-inflammatory agent, capable of reducing several symptoms associated with respiratory infections [86]. Extracts obtained from the aerial parts of E. purpurea have shown stronger antibacterial and antioxidant properties compared to those derived via ultrasonic extraction methods [87]. Currently, antibiotics and vaccines are the main treatments for bacterial infections, used both therapeutically and prophylactically. However, managing respiratory infections remains a challenge due to the variety of microorganisms involved. In this context, the use of phytopreparations could offer a promising alternative [88]. To better understand the synergistic interactions between echinacea extracts and antibiotics, further research is necessary to elucidate the mechanisms of their antimicrobial effects and identify potential pathways that could be targeted [83].
5. Mechanism of Antibacterial Activity of Polyphenols
Echinacea species exhibit antimicrobial activity through several distinct mechanisms, which together enhance their effectiveness against a wide range of pathogens [89]. A key mechanism is the disruption of microbial cell membranes, primarily due to the hydrophobic compounds like alkamides present in echinacea [90]. These compounds can integrate into the lipid bilayers of microbial membranes, leading to structural instability and increased permeability, ultimately causing cell lysis and death [90]. Another important mechanism is the inhibition of microbial enzymes essential for survival and replication. Compounds such as caffeic acid derivatives inhibit enzymes responsible for processes like cell wall synthesis and nucleic acid production, thereby hindering microbial growth and reproduction [91]. Additionally, echinacea has immunomodulatory effects, stimulating immune cells like macrophages and natural killer cells, enhancing phagocytosis, and increasing cytokine production, which helps coordinate the body’s immune response to infections [92]. Furthermore, echinacea extracts have been shown to prevent biofilm formation—protective layers bacteria form to evade immune responses and resist antibiotics—making bacteria more vulnerable to immune defenses and antimicrobial treatments [93]. The antioxidant properties of echinacea also contribute by neutralizing free radicals generated during infections, reducing oxidative stress, and protecting host tissues from damage, which further supports its antimicrobial action [89]. Together, these multiple mechanisms position echinacea as a versatile antimicrobial agent, capable of combating a variety of pathogens through both direct antimicrobial activity and indirect immune system modulation [90].
5.1. Reactions with Proteins
The antibacterial activity of flavonoids may be attributed to their ability to form complexes with proteins through nonspecific interactions, such as hydrogen bonding and hydrophobic forces, as well as through covalent bond formation [94]. The binding of polyphenols to proteins results in the formation of soluble or insoluble complexes, which affects the functions of both polyphenols and proteins [95]. This interaction can cause certain amino acids in proteins to be blocked or lead to conformational changes, altering the protein’s structure, solubility, hydrophobicity, thermal stability, and isoelectric point [96]. As a result, protein–phenolic complexation can modify their physicochemical and biological properties, influencing the digestibility of food proteins, the activity of digestive enzymes, and nutrient availability [94]. It has been shown that polyphenols, such as condensed tannins, can inhibit several digestive enzymes, including α-glycosidase, α-amylase, lipase, pepsin, trypsin, and chymotrypsin, thereby modulating nutrient availability, and altering microbiota composition [95]. Additionally, polyphenols can bind to crucial bacterial proteins, such as adhesins and enzymes, and transport proteins in the bacterial cell envelope, inactivating them and exerting an antimicrobial effect. However, the complexation of polyphenols with proteins may also impact the bioaccessibility and activity of phenolic compounds [97].
Some flavones have shown activity against E. coli by forming complexes with extracellular and soluble proteins [94]. Flavonoids are also known to influence the activity of bacterial enzymes essential for cell survival, including those involved in synthesizing cell wall components, membrane fatty acids, or adenosine triphosphate (ATP). Fatty acid synthase II (FAS-II) is a critical enzyme in bacterial membrane fatty acid synthesis, catalyzing the elongation of fatty acid chains from 16–24 carbons produced de novo by FAS-I to longer chains of 36–48 carbons, as well as mycolic acids [97]. Flavonoids such as isoliquiritigenin, butein, fisetin, and4′-trihydroxy chalcone have been found to inhibit FAS-II, thus hindering the growth of Mycobacterium smegmatis [98].
5.2. Inhibition of Bacterial DNA Synthesis and Interaction with Nucleic Acids
Flavonoids derived from Elaeagnus glabra were tested for antibacterial effects against Proteus vulgaris and Staphylococcus aureus [99]. The presence of a free 3′, 4′, and 5′-trihydroxy B-ring, along with a free 3-OH group, was found to be necessary for antibacterial activity. In P. vulgaris, DNA synthesis was primarily inhibited by active flavonoids, while in S. aureus, RNA synthesis was inhibited [100]. Robinetin, myricetin, and (−)-epigallocatechin were identified as the most potent inhibitors of DNA synthesis. It is suggested that the B-ring of these flavonoids may interact with nucleic acid bases by forming hydrogen bonds or intercalating, leading to the inhibition of bacterial nucleic acid synthesis. A previous study [101] found that p-coumaric acid exhibited dual bactericidal actions: disrupting the bacterial cell membrane and binding to bacterial genomic DNA, which inhibited essential cellular functions and caused cell death [102]. In S. aureus, membrane depolarization and the inhibition of DNA, RNA, and protein synthesis were observed when treated with flavonoids from Dorstenia species, such as 6,8-diprenyleriodictyol, isobavachalcone, and 4-hydroxylonchocarpin. At higher concentrations, cell lysis occurred.
There is also evidence that flavonoids can inhibit bacterial type II topoisomerases, including DNA gyrase and topoisomerase II (also known as topoisomerase IV) [101]. These enzymes, which regulate DNA topology, are specific to prokaryotes, making them attractive targets for antibacterial drugs. DNA gyrase consists of two key subunits: DNA gyrase subunit A (GyrA), responsible for DNA cleavage and rejoining, and DNA gyrase subunit B (GyrB), which contains the ATP-binding site. Natural compounds like coumarins and cyclothialidines inhibit ATPase activity in DNA gyrase by blocking ATP binding to the GyrB subunit. Research [99] demonstrated that quercetin inhibits the supercoiling activity of bacterial gyrase and induces DNA cleavage, likely through interaction with DNA. Quercetin binds to the 24 kilodalton fragment of GyrB in E. coli with a dissociation constant of 15 µM, inhibiting ATPase activity by competing with ATP for the binding site. Quercetin’s binding site overlaps with the ATP-binding pocket and can be competitively displaced by either ATP or novobiocin [101]. The proposed mechanism suggests that quercetin inhibits gyrases by interacting with either the DNA or the ATP-binding site [101]. Other polyphenols, such as catechins, have also been found to inhibit bacterial DNA gyrase by binding to the ATP site on the GyrB subunit, with epigallocatechin gallate being the most active, followed by epicatechin gallate and epigallocatechin [102]. Flavonoids such as quercetin, apigenin, and 3,3′,4′,6,7-pentahydroxyflavone have also shown inhibitory activity against E. coli DNA gyrase [102].
5.3. Interaction with the Bacterial Cell Wall or Inhibition of Cell Wall Formation
Differences in antimicrobial activity between Gram-negative and Gram-positive bacteria may result from variations in their cell surface structures [103]. The main function of the bacterial cell wall is to provide shape, maintain cell integrity, and serve as an osmotic barrier. Gram-negative bacteria are known to be resistant to many antibacterial agents due to the hydrophilic nature of their outer membrane and the presence of enzymes in the periplasmic space, which can break down external molecules [104]. Additionally, the negatively charged lipopolysaccharide layer in the outer membrane acts as a protective shield against catechins. In contrast, Gram-positive bacteria, which lack an outer membrane, tend to be more vulnerable to the action of phenolic acids. This lack of an outer membrane allows phenolic acids to diffuse more easily through the cell wall and reach the intracellular environment [105]. It is suggested that one potential mechanism behind the antimicrobial action of phenolic acids involves hyperacidification at the plasma membrane interface, caused by the dissociation of these acids. This process disrupts the cell membrane potential, increases membrane permeability, and causes irreversible changes to the sodium-potassium ATPase pump, ultimately leading to cell death [104].
Flavones form complexes with cell wall components, which can inhibit bacterial adhesion and further microbial growth. Flavonoids with a C-7-modified naringenin core have been shown to inhibit bacterial enzymes, such as tyrosyl-tRNA synthetase. These compounds have also demonstrated inhibitory effects on the growth of S. aureus, E. coli, and P. aeruginosa. Baicalein, in particular, was found to be an effective bactericidal agent, and when combined with cefotaxime, it exhibited synergistic effects by inhibiting the expression of extended-spectrum β-lactamase beta-lactamase messenger RNA [105]. Another mechanism contributing to antibacterial activity is the inhibition of bacterial efflux pumps, which enhances the susceptibility of bacteria to existing antibiotics by causing membrane depolarization. Artonin, derived from Morus mesozygia, was effective against S. aureus by blocking efflux mechanisms and inducing membrane depolarization [103]. Furthermore, artonin was able to reverse multidrug resistance, lowering the minimum inhibitory concentrations of antibiotics and increasing their potency.
5.4. Alteration of Cytoplasmic Membrane Function
The bacterial cell membrane is essential for multiple vital functions, including osmoregulation, respiration, transport, biosynthesis, and the cross-linking of peptidoglycan and lipids [106]. Any disruption to its structure or function can result in metabolic failure and cell death, making membrane disruption a key mechanism in the antibacterial action of polyphenols. For example, catechins have been shown to damage bacterial membranes by binding to the lipid bilayer, inhibiting the synthesis of both intracellular and extracellular enzymes [107]. Apigenin was observed to cause dysfunction in fungal membranes, increasing permeability and triggering the release of small intracellular components such as ions and sugars, but not proteins [107]. Epicatechin-3-gallate and caffeic acid were found to target both the cell wall and cytoplasmic membrane of P. aeruginosa, leading to membrane destruction, increased permeability, and the enhanced entry of hydrophobic antibiotics. This process also caused the release of potassium ions and the leakage of nucleotides. Due to their partially lipophilic properties, phenolic acids can penetrate the cell membrane via passive diffusion, increasing permeability, reducing intracellular pH, and causing protein denaturation [108]. A methanol extract from Coriolus versicolor, rich in polyphenols, inhibited cell division in S. aureus by interfering with septum formation and causing the accumulation of peptidoglycan and teichoic acid precursors in the cytoplasm [107]. In Salmonella enteritidis, the extract damaged the cell envelope. Furthermore, at higher concentrations, catechins have been shown to induce the generation of reactive oxygen species, leading to oxidative stress, altered membrane permeability, and subsequent membrane damage.
Flavonoids and flavonol have been shown to destabilize membrane structures by disrupting and disorienting membrane lipids, leading to leakage from vesicles [106]. An inverse relationship was observed between the number of hydroxyl groups in flavonoids and their ability to induce leakage. Previous research [93] suggested that flavonoids lacking hydroxyl groups on their B rings were more effective at inhibiting microbial growth compared to those with –OH groups. On the other hand, previous research found that the presence of hydroxyl groups in the phenyl rings A and B generally did not affect the antibacterial activity level of flavones [107]. A notable increase in activity was observed for hydroxy derivatives of flavones, specifically against S. aureus. Interestingly, in contrast to other studies, the compounds tested showed greater effectiveness against Gram-negative bacteria such as E. coli and P. aeruginosa than against Gram-positive bacteria like Enterococcus faecalis and Staphylococcus aureus [108].
8. Immunomodulatory Activity
The term “immunomodulatory” is increasingly seen as more appropriate than “immunostimulatory” to describe echinacea’s immunological effects, although the latter remains widely used in earlier scientific discussions on the plant [161]. It has been suggested that broadly stimulating the highly complex immune system may not always be beneficial, as some immune responses can be detrimental [162]. Numerous studies, both in vitro and in vivo, have explored the immunological effects of various echinacea preparations, covering different species, plant parts, and extraction methods. Collectively, the findings suggest that echinacea preparations do impact certain immune functions, though there is no consensus on which specific preparations have the strongest effects [163]. Enhanced macrophage function has been observed in response to different echinacea formulations, as demonstrated by in vitro and in vivo studies using methods like the carbon-clearance test and cytokine measurement to assess macrophage activity [164]. In vitro studies on human macrophages found that fresh-pressed juice and dried juice from the aerial parts of E. purpurea stimulated the production of cytokines such as interleukin (IL)-1, IL-10, and tumor necrosis factor (TNF-α) [165].
Other research has reported that purified polysaccharides from E. purpurea triggered macrophages to produce IL-1 [166], while an arabinogalactan polysaccharide, isolated from E. purpurea plant cell cultures, was found to induce the production of TNF-α and interferon-2 in murine macrophages [167]. Polysaccharides from E. purpurea plant cell cultures have also been previously shown to exhibit immunological activity in vitro [168]. In another series of in vitro experiments, E. purpurea promoted macrophage activation, as indicated by TNF-α production, following simulated digestion (incubation with gastric fluid) to replicate the potential effects of oral consumption [169]. Further studies demonstrated that E. purpurea dry root powder (containing 1.5% total polyphenols, calculated as chlorogenic acid) enhanced the resistance of splenic lymphocytes to apoptosis. This effect was seen in splenic lymphocytes from mice that received the echinacea preparation orally at doses of 30 or 100 mg/kg daily for 14 days [170].
In an in vitro study, peripheral blood mononuclear cells from healthy individuals, as well as from patients with chronic fatigue syndrome, were exposed to increasing concentrations of E. purpurea extracts, which resulted in enhanced natural killer cell function [171]. Similarly, in vivo studies have shown that oral administration of E. purpurea root extract increases natural killer cell numbers in normal [172], leukemic [173], and aging mice [174]. A subsequent in-vivo study using a randomized, double-blind design examined the effects of an echinacea supplement (Nature’s Resource; CVS Pharmacy, USA; containing 1.05 g of echinacea aerial parts and 10.5 mg of chicoric acid) in 16 aging male rats [175]. The animals received 50 mg/kg body weight of echinacea (potentially E. purpurea), equivalent to 0.5 mg/kg of chicoric acid, or a placebo administered in peanut butter daily for 8 weeks. During the first two weeks, echinacea-treated rats showed significantly higher mean circulating white blood cell counts compared to the control group (p < 0.05) [176]. IL-2 concentrations were significantly higher in the echinacea group compared to the control for the last five weeks of the study (P<0.05). Additionally, differential white cell counts changed markedly over the 8 weeks: lymphocyte and monocyte levels increased, while neutrophil and eosinophil levels decreased in the echinacea group compared to placebo [177].
No changes in the phagocytic activity of circulating leukocytes, measured by their ability to ingest latex particles, were observed in either group. Other in-vivo studies in rats demonstrated that water-ethanol extracts of E. purpurea roots and aerial parts, containing chicoric acid, polysaccharides, and alkamides, increased macrophage phagocytic activity, with greater concentrations of these components leading to enhanced activity [178]. Moreover, spleen macrophages from echinacea-treated rats displayed increased nitric oxide release when stimulated with lipopolysaccharide. In a similar experiment, alkamides were shown to stimulate alveolar macrophage function in healthy rats [179].
A proprietary preparation combining E. purpurea root extract and licorice root extract [180] has been found to stimulate phagocytosis both in vitro and in vivo, as evidenced by the carbon-clearance test following oral administration in mice [181] (Figure 6). This combination demonstrated a more potent immunostimulatory effect compared to either extract used independently. Another blend, consisting of aqueous ethanol extracts from E. purpurea and E. pallida roots, Baptisia tinctoria root, and Thuja occidentalis herb, was administered orally to mice through their diet or drinking water for 7 days, resulting in an enhanced antibody response to sheep red blood cells [182]. However, despite the substantial research supporting the immunostimulatory properties of echinacea preparations, some recent studies have reported no such effects [183]. For example, no evidence of natural killer cell activity or antibody production was observed in studies where rats were fed various echinacea formulations, including alcoholic extracts of E. purpurea root and alcoholic extracts of the roots of E. angustifolia, E. purpurea, and E. pallida, in their diet [184].
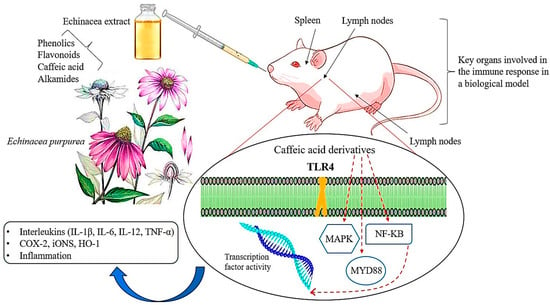
Figure 6.
A schematic representation of the main molecular pathways linked to inflammatory and immunomodulatory activities modulated by echinacea. The solid red line indicates the pathway’s activation, whereas the truncated red line indicates inhibition of the pathway. TLR-4: Toll-like Receptor-4; MyD88: Myeloid Differentiation Primary Response 88; NF-KB: Nuclear Factor kappa B; MAPK: Mitogen-Activated Protein Kinase; COX-2: cyclooxygenase-2; iNOS: inducible Nitric Oxide Synthase; HO-1: Heme Oxygenase-1; IL: Interleukin; TNF: Tumor Necrosis Factor (Figure drawn by F. Ahmadi).
9. Conclusions and Future Perspective on Echinacea Research
Echinacea’s phenolic and flavonoid compounds, including caffeic acid derivatives and alkamides, are known for their antimicrobial properties [185]. Studies have shown that the phytochemicals in E. purpurea significantly reduce inflammatory swelling in mice and rats and lower blood levels of cytokines such as IL-2, IL-6, and TNF-α [186]. These effects are similarly observed with the administration of alkylamides in animal models. With continued research and the establishment of standardized extraction methods, echinacea extract has the potential to become a successful product line in the echinacea industry [187]. Currently, echinacea is predominantly marketed in the US as an immune-boosting supplement [188]. Echinacea alkylamides have been shown to disrupt bacterial cell walls and membranes, with one structural class, the diynoic alkylamides, demonstrating the greatest cell wall disruption [189,190]. The natural variation in phytochemical profiles across echinacea species provides the opportunity to selectively propagate cultivars with unique metabolic profiles for specific purposes, such as producing antimicrobial compounds. Alternatively, echinacea cultivars could be bred to increase the production of caffeic acid derivatives in the flowers, which could be used in dietary supplements. Roots and flowers rich in alkylamides and chicoric acid could be used to produce antibacterial face washes, shampoos, or creams. Although echinacea, like other members of the Asteraceae family, contains some phototoxic polyacetylene compounds, these are unstable and can be easily deactivated by minimal processing [187]. Additionally, the leaves of echinacea, which are rich in vitamin C and phenolic compounds, could be marketed as natural health products. However, the full potential of echinacea’s diverse applications remains largely untapped. The development of new biotechnologies presents significant opportunities for improving echinacea-based products [190,191]. Moving forward, it is important to weigh the benefits and drawbacks of different approaches, such as improving yields, optimizing phytochemical profiles, enhancing propagation efficiency, managing costs, addressing public perception, and ensuring scalability. A combination of tissue culture, chemical treatments, and traditional field cultivation will likely be used to achieve higher production standards and improved phytochemical quality. These advancements will allow the industry to expand the range of echinacea products and meet the growing market demand.
Funding
This research received no external funding or grants.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Ahmadi, F.; Kariman, K.; Mousavi, M.; Rengel, Z. Echinacea: Bioactive compounds and agronomy. Plants 2024, 13, 1235. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhu, H.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Echinacea in hepatopathy: A review of its phytochemistry, pharmacology, and safety. Phytomedicine 2021, 87, 153572. [Google Scholar] [CrossRef] [PubMed]
- Kligler, B. Echinacea. Am. Fam. Physician 2003, 67, 77–80. [Google Scholar] [PubMed]
- Manayi, A.; Vazirian, M.; Saeidnia, S. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharmacogn. Rev. 2015, 9, 63. [Google Scholar]
- Karg, C.A.; Wang, P.; Vollmar, A.M.; Moser, S. Re-opening the stage for Echinacea research-Characterization of phylloxanthobilins as a novel anti-oxidative compound class in Echinacea purpurea. Phytomedicine 2019, 60, 152969. [Google Scholar] [CrossRef] [PubMed]
- Miroshina, T.; Poznyakovskiy, V. Echinacea purpurea as a medicinal plant: Characteristics, use as a biologically active component of feed additives and specialized foods. E3S Web Conf. 2023, 380, 01005. [Google Scholar] [CrossRef]
- Ng, J.Y.; Chiong, J.D.; Liu, M.Y.M.; Pang, K.K. Characteristics of the Echinacea spp. research literature: A bibliometric analysis. Eur. J. Integr. Med. 2023, 57, 102216. [Google Scholar] [CrossRef]
- Hanifah, W.N.; Yunus, A.; Widiyastuti, Y. Morphological, agronomic characteristics, and flavonoid content of Echinacea purpurea at various gamma ray doses. Bulg. J. Agric. Sci. 2024, 30, 451. [Google Scholar]
- Freeman, C.; Spelman, K. A critical evaluation of drug interactions with Echinacea spp. Mol. Nut. Food Res. 2008, 52, 789–798. [Google Scholar] [CrossRef]
- Kakouri, E.; Talebi, M.; Tarantilis, P.A. Echinacea spp.: The cold-fighter herbal remedy? Pharmacol. Res.-Mod. Chin. Med. 2024, 10, 100397. [Google Scholar] [CrossRef]
- Saema, S.; Shaheen, N.; Pandey, V. Immunostimulatory properties of Echinacea purpurea and conservation strategy. In Plants for Immunity and Conservation Strategies; Springer Nature: Singapore, 2023; pp. 153–168. [Google Scholar]
- Petrova, A.; Ognyanov, M.; Petkova, N.; Denev, P. Phytochemical characterization of purple coneflower roots (Echinacea purpurea (L.) Moench.) and their extracts. Molecules 2023, 28, 3956. [Google Scholar] [CrossRef]
- İduğ, T. Herbal food supplements usage awareness of university students: Example of Echinacea and St. John’s Wort. Int. J. Agric. Environ. Food Sci. 2023, 7, 792–797. [Google Scholar] [CrossRef]
- Toselli, F.; Matthias, A.; Gillam, E.M. Echinacea metabolism and drug interactions: The case for standardization of a complementary medicine. Life Sci. 2009, 85, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Jenks, A.; Brinckmann, J. Echinacea purpurea. HerbalGram 2023, 138, 13569. [Google Scholar]
- Shahrajabian, M.H.; Sun, W. Seed biology and pharmacological benefits of fennel, lavender, thyme, and echinacea Species. Seeds 2023, 2, 290–308. [Google Scholar] [CrossRef]
- Kesar, V.; Ahmad, J.; Odin, J.; Kesar, V. Echinacea-induced acute hepatitis transitioning to autoimmune hepatitis. J. Am. Coll. Gastroenterol. ACG 2015, 110, S397. [Google Scholar] [CrossRef]
- Missenda, M.; Morris, D.; Nault, D. Herbal supplement Use for evidence-based indications in US aadults: An analysis of national survey data. J. Integr. Complement. Med. 2023, 29, 584–591. [Google Scholar] [CrossRef]
- Ghutke, T.D.; Parvin, K.; Rashida Banu, A.M.; Bansal, S.; Srivastava, A.; Rout, S.; Ramzan, U. A comprehensive review on the therapeutic properties of medicinal plants. Acta Tradit. Med. 2023, V2i01, 13–18. [Google Scholar]
- Chaughule, R.S.; Barve, R.S. Role of herbal medicines in the treatment of infectious diseases. Vegetos 2024, 37, 41–51. [Google Scholar] [CrossRef]
- Imtiaz, I.; Schloss, J.; Bugarcic, A. Traditional and contemporary herbal medicines in management of cancer: A scoping review. J. Ayurveda Integr. Med. 2024, 15, 100904. [Google Scholar] [CrossRef]
- Ahmadi, F.; Samadi, A.; Sepehr, E.; Rahimi, A.; Shabala, S. Morphological, phytochemical, and essential oil changes induced by different nitrogen supply forms and salinity stress in Echinacea purpurea L. Biocatal. Agric. Biotechnol. 2022, 43, 13932. [Google Scholar] [CrossRef]
- Ahmadi, F.; Samadi, A.; Rahimi, A. Improving morphological properties and phytochemical compounds of Echinacea purpurea (L.) medicinal plant using novel nitrogen slow-release fertilizer under greenhouse conditions. Sci. Rep. 2020, 10, 13842. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Samadi, A.; Sepehr, E.; Rahimi, A.; Shabala, S. Perlite particle size and NO3−/NH4+ ratio affect the growth and chemical composition of purple coneflower (Echinacea purpurea L.) in hydroponics. Ind. Crops Prod. 2021, 162, 113285. [Google Scholar] [CrossRef]
- Micheli, L.; Maggini, V.; Ciampi, C.; Gallo, E.; Bogani, P.; Fani, R.; Firenzuoli, F. Echinacea purpurea against neuropathic pain: Alkamides versus polyphenols efficacy. Phytother. Res. 2023, 37, 1911–1923. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.F.; Fonseca-Rodrigues, D.; Mendanha, D.; Castro, V.I.B.; Pires, R.A.; Reis, R.L.; Pinto-Ribeiro, F. Echinacea purpurea roots extracts. Plant-Deriv. Bioact. Compd. Inflamm. Dis. 2023, 447, 56–63. [Google Scholar]
- AghaAlikhani, M.; Iranpour, A.; Naghdi Badi, H. Changes in agronomical and phytochemical yield of purple coneflower (Echinacea purpurea (L.) moench) under urea and three biofertilizers application. J. Med. Plants 2013, 12, 121–136. [Google Scholar]
- Chopra, H.; Bibi, S.; Gupta, S.K.; Hasan, M.M.; Zeb, M.A.; Khan, M.S.; Yousafi, Q. Alkamides as metabolites in plants. In Strigolactones, Alkamides and Karrikins in Plants; CRC Press: Boca Raton, FL, USA, 2023; pp. 177–193. [Google Scholar]
- Cai, F.; Wang, C. Comprehensive review of the phytochemistry, pharmacology, pharmacokinetics, and toxicology of alkamides (2016–2022). Phytochemistry 2024, 220, 114006. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, D.R.; Kim, H.L.; Choi, B.K.; Kwon, K.B. A randomized, double-blind, placebo-controlled study on immune improvement effects of ethanolic extract of Echinacea purpurea (L.) Moench in Korean adults. Phytother. Res. 2024, 5, 38–45. [Google Scholar] [CrossRef]
- Jose, A.; Karthika, C.; Athira, K.V.; Rahman, M.H.; Sweilam, S.H. Pharmacological potential of plant-derived alkylamides. In Strigolactones, Alkamides and Karrikins in Plants; CRC Press: Boca Raton, FL, USA, 2023; pp. 195–205. [Google Scholar]
- Wang, W.; Jiang, S.; Zhao, Y.; Zhu, G. Echinacoside: A promising active natural products and pharmacological agents. Pharmacol. Res. 2023, 197, 106951. [Google Scholar] [CrossRef]
- Vergun, O.; Svydenko, L.; Shymanska, O.; Hlushchenko, L.; Sedlačková, V.H.; Ivanišová, E.; Brindza, J. Accumulation of total content of polyphenol compounds and antioxidant activity of Echinacea moench species. Agrobiodivers. Improv. Nutr. Health Life Qual. 2024, 8, 63–71. [Google Scholar] [CrossRef]
- Karadağ, A.E.; Baydar, R.; Kırcı, D. Phytochemical quality analysis of commercial preparations containing Echinacea purpurea. Eur. J. Life Sci. 2024, 3, 45–54. [Google Scholar] [CrossRef]
- Choirunnisa, J.P.; Widiyastuti, Y.; Sakya, A.T.; Yunus, A. Morphological characteristics and flavonoid accumulation of Echinacea purpurea cultivated at various salinity. Biodiversitas: J. Biol. Divers. 2021, 22, 156–163. [Google Scholar] [CrossRef]
- Clifford, L.J.; Nair, M.G.; Rana, J.; Dewitt, D.L. Bioactivity of alkamides isolated from Echinacea purpurea (L.) Moench. Phytomedicine 2002, 9, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.; Barros, L.; Dias, M.I.; Finimundy, T.C.; Amaral, J.S.; Alves, M.J.; Ferreira, I.C. Echinacea purpurea (L.) Moench: Chemical characterization and bioactivity of its extracts and fractions. Pharmaceuticals 2020, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, C. Need for additional, specific information in studies with Echinacea. Antimicrob. Agents Chemother. 2001, 45, 369–370. [Google Scholar] [CrossRef]
- Soltanbeigi, A.; Maral, H. Agronomic yield and essential oil properties of purple coneflower (Echinacea purpurea L. Moench) with different nutrient applications. Chil. J. Agric. Anim. Sci. 2022, 38, 164–175. [Google Scholar] [CrossRef]
- Maggini, V.; Bandeira Reidel, R.V.; De Leo, M.; Mengoni, A.; Rosaria Gallo, E.; Miceli, E.; Pistelli, L. Volatile profile of Echinacea purpurea plants after in vitro endophyte infection. Nat. Prod. Res. 2020, 34, 2232–2237. [Google Scholar] [CrossRef]
- Rousseaux, C.G. Herbal remedies. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Academic Press: Cambridge, MA, USA, 2023; pp. 183–303. [Google Scholar]
- Pretorius, T.R.; Charest, C.; Kimpe, L.E.; Blais, J.M. The accumulation of metals, PAHs, and alkyl PAHs in the roots of Echinacea purpurea. PLoS ONE 2018, 13, e0208325. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Phytochemistry of the genus Echinacea. In Echinacea; CRC Press: Boca Raton, FL, USA, 2004; pp. 71–88. [Google Scholar]
- Mirjalili, M.H.; Salehi, P.; Badi, H.N.; Sonboli, A. Volatile constituents of the flowerheads of three Echinacea species cultivated in Iran. Flavour Fragr. J. 2006, 21, 355–358. [Google Scholar] [CrossRef]
- Woelkart, K.; Xu, W.; Pei, Y.; Makriyannis, A.; Picone, R.P.; Bauer, R. The endocannabinoid system as a target for alkamides from Echinacea angustifolia roots. Planta Medica 2005, 71, 701–705. [Google Scholar] [CrossRef]
- Perry, N.B.; Wills, R.B.; Stuart, D.L. Factors affecting echinacea quality: Agronomy and processing. In Echinacea; CRC Press: Boca Raton, FL, USA, 2004; pp. 127–142. [Google Scholar]
- Cozzolino, R.; Malvagna, P.; Spina, E.; Giori, A.; Fuzzati, N.; Anelli, A.; Impallomeni, G. Structural analysis of the polysaccharides from Echinacea angustifolia radix. Carbohydr. Polym. 2006, 65, 263–272. [Google Scholar] [CrossRef]
- Bone, K. Echinacea: What makes it work. Altern. Med. Rev. 1997, 2, 87–93. [Google Scholar]
- Melchart, D.; Clemm, C.; Weber, B.; Draczynski, T.; Worku, F.; Linde, K.; Saller, R. Polysaccharides isolated from Echinacea purpurea herba cell cultures to counteract undesired effects of chemotherapy—A pilot study. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2002, 16, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.T.; Huang, C.C.; Shieh, X.H.; Chen, C.L.; Chen, L.J.; Yu, B.I. Flavonoid, phenol and polysaccharide contents of Echinacea purpurea L. and its immunostimulant capacity in vitro. Int. J. Environ. Sci. Dev. 2010, 1, 5. [Google Scholar] [CrossRef]
- Li, Q.; Yang, F.; Hou, R.; Huang, T.; Hao, Z. Post-screening characterization of an acidic polysaccharide from Echinacea purpurea with potent anti-inflammatory properties in vivo. Food Funct. 2020, 11, 7576–7583. [Google Scholar] [CrossRef]
- Roesler, J.; Steinmüller, C.; Kiderlen, A.; Emmendörffer, A.; Wagner, H.; Lohmann-Matthes, M.L. Application of purified polysaccharides from cell cultures of the plant Echinacea purpurea to mice mediates protection against systemic infections with Listeria monocytogenes and Candida albicans. Int. J. Immunopharmacol. 1991, 13, 27–37. [Google Scholar] [CrossRef]
- Raso, G.M.; Pacilio, M.; Di Carlo, G.; Esposito, E.; Pinto, L.; Meli, R. In-vivo and in-vitro anti-inflammatory effect of Echinacea purpurea and Hypericum perforatum. J. Pharm Pharmacol. 2002, 54, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, Q.; Zhang, Y.; Gao, G.; Shen, P.; Gao, G.; Wu, N. Effects of Echinacea purpurea polysaccharide on IEC-6 cell proliferation. Agric. Sci. Technol. 2014, 15, 1876. [Google Scholar]
- Schöllhorn, C.; Schecklies, E.; Wagner, H. Immunochemical investigations of polysaccharides from Echinacea purpurea cell suspension cultures. Planta Medica 1993, 59, A662–A663. [Google Scholar] [CrossRef]
- Lee, J. Caffeic acid derivatives in dried Lamiaceae and Echinacea purpurea products. J. Funct. Foods 2010, 2, 158–162. [Google Scholar] [CrossRef]
- Kim, H.O.; Durance, T.D.; Scaman, C.H.; Kitts, D.D. Retention of caffeic acid derivatives in dried Echinacea purpurea. J. Agric. Food Chem. 2000, 48, 4182–4186. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.L.; Chiou, S.Y.; Chan, K.C.; Sung, J.M.; Lin, S.D. Caffeic acid derivatives, total phenols, antioxidant and antimutagenic activities of Echinacea purpurea flower extracts. LWT-Food Sci. Technol. 2012, 46, 169–176. [Google Scholar] [CrossRef]
- Wu, C.H.; Murthy, H.N.; Hahn, E.J.; Lee, H.L.; Paek, K.Y. Efficient extraction of caffeic acid derivatives from adventitious roots of Echinacea purpurea. Czech J. Food Sci. 2008, 26, 254–258. [Google Scholar] [CrossRef]
- Erkoyuncu, M.T.; Yorgancilar, M. Optimization of callus cultures at Echinacea purpurea L. for the amount of caffeic acid derivatives. Electron. J. Biotechnol. 2021, 51, 17–27. [Google Scholar] [CrossRef]
- Rady, M.R.; Aboul-Enein, A.M.; Ibrahim, M.M. Active compounds and biological activity of in vitro cultures of some Echinacea purpurea varieties. Bull. Natl. Res. Cent. 2018, 42, 1–12. [Google Scholar] [CrossRef]
- Paek, K.Y.; Murthy, H.N.; Hahn, E.J. Establishment of adventitious root cultures of Echinacea purpurea for the production of caffeic acid derivatives. Protocols for In Vitro Cult. Second. Metab. Anal. Aromat. Med. Plants 2009, 547, 3–16. [Google Scholar]
- Sharifi-Rad, M.; Mnayer, D.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Coutinho, H.D.M.; Sharifi-Rad, J. Echinacea plants as antioxidant and antibacterial agents: From traditional medicine to biotechnological applications. Phytother. Res. 2018, 32, 1653–1663. [Google Scholar] [CrossRef]
- Chiellini, C.; Maida, I.; Maggini, V.; Bosireeman, E.; Mocali, S.; Emiliani, G.; Fani, R. Preliminary data on antibacterial activity of Echinacea purpurea-associated bacterial communities against Burkholderia cepacia complex strains, opportunistic pathogens of Cystic Fibrosis patients. Microbiol. Res. 2017, 196, 34–43. [Google Scholar] [CrossRef]
- Zaushintsena, A.V.; Milentyeva, I.; Babich, O.; Noskova, S.Y.; Kiseleva, T.F.; Popova, D.G.; Lukin, A. Quantitative and qualitative profile of biologically active substances extracted from purple echinacea (Echinacea purpurea L.) growing in the Kemerovo region: Functional foods application. Foods Raw Mater. 2019, 7, 84–92. [Google Scholar] [CrossRef]
- Burlou-Nagy, C.; Bănică, F.; Negrean, R.A.; Jurca, T.; Vicaș, L.G.; Marian, E.; Pallag, A. Determination of the bioactive compounds from Echinacea purpurea (L.) Moench leaves extracts in correlation with the antimicrobial activity and the in vitro wound healing potential. Molecules 2023, 28, 5711. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, H.; Arslanoglu, S.; Edbeib, M.; Kaya, Y.; Marakli, S. Antibacterial activity of Calendula officinalis and Echinacea purpurea extracts against the causal agent of tomatoes’ bacterial canker: Clavibacter michiganensis subsp. michiganensis. Boletín Latinoam. Y Del Caribe De Plantas Med. Y Aromáticas 2021, 20, 496–502. [Google Scholar] [CrossRef]
- Daley, E. A Phytochemical and Antibacterial Analysis of Echinacea purpurea (L.) Moench throughout Seasonal Development. Doctoral Dissertation, Université d’Ottawa/University of Ottawa, Ottawa, ON, Canada, 2019. [Google Scholar]
- Taghizadeh, M.; Jarvandi, S.; Yasa, N. A review of Echinacea. J. Med. Plants. 2002, 1, 13–26. [Google Scholar]
- VAVERKOVÁ, L.B.P.O.Š. Antimicrobial and antimutagenic activities of extracts from different organs of Echinacea angustifolia DC (Asteraceae). J. Food Nutr. Res. 2012, 51, 201–206. [Google Scholar]
- Zazharskyi, V.V.; Davydenko, P.; Kulishenko, O.; Borovik, I.V.; Brygadyrenko, V.V. Antimicrobial activity of 50 plant extracts. Biosyst. Divers. 2019, 27, 163–169. [Google Scholar] [CrossRef]
- Kerem, S.; Özbek, Ö. Antimicrobial activities of some species in Asteraceae and Lamiaceae families from Türkiye. Int. J. Second. Metab. 2024, 11, 277–291. [Google Scholar] [CrossRef]
- Gotti, R.; Pomponio, R.; Bertucci, C.; Cavrini, V. Simultaneous analysis of the lipophilic and hydrophilic markers of Echinacea plant extracts by capillary electrophoresis. J. Separation Sci. 2002, 25, 1079–1086. [Google Scholar] [CrossRef]
- Sharma, S.M.; Anderson, M.; Schoop, S.R.; Hudson, J.B. Bactericidal and anti-inflammatory properties of a standardized Echinacea extract (Echinaforce®): Dual actions against respiratory bacteria. Phytomedicine 2010, 17, 563–568. [Google Scholar] [CrossRef]
- Garzoli, S.; Maggio, F.; Vinciguerra, V.; Rossi, C.; Donadu, M.G.; Serio, A. Chemical Characterization and Antimicrobial Properties of the Hydroalcoholic Solution of Echinacea purpurea (L.) Moench. and Propolis from Northern Italy. Molecules 2023, 28, 1380. [Google Scholar] [CrossRef]
- Rehman, F.; Sudhaker, M.; Roshan, S.; Khan, A. Antibacterial activity of Eachinacia angustfolia. Pharmacogn. J. 2012, 4, 67–70. [Google Scholar] [CrossRef]
- Giles, J.T.; Palat III, C.T.; Chien, S.H.; Chang, Z.G.; Kennedy, D.T. Evaluation of echinacea for treatment of the common cold. Pharmacotherapy: J. Hum. Pharmacol. Drug Ther. 2000, 20, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Currier, N.L.; Miller, S.C. Echinacea purpurea and melatonin augment natural-killer cells in leukemic mice and prolong life span. J. Altern. Complement. Med. 2001, 7, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C. The Echinacea Handbook; Eclectic Medical Publications: Portland, OR, USA, 1989. [Google Scholar]
- Islam, J.; Carter, R. Use of Echinacea in upper respiratory tract infection. South Med. J. 2005, 98, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Jukić, H.; Habeš, S.; Aldžić, A.; Durgo, K.; Kosalec, I. Antioxidant and prooxidant activities of phenolic compounds of the extracts of Echinacea purpurea (L.). Bull. Chem. Technol. Bosnia Herzeg. 2015, 44, 43–52. [Google Scholar]
- Lee, M.; Lin, W.; Yu, B.; Lee, T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian-Australas. J. Anim. Sci. 2017, 30, 299–308. [Google Scholar] [CrossRef]
- Yamada, K.; Hung, P.; Park, T.K.; Park, P.J.; Lim, B.O. A comparison of the immunostimulatory effects of the medicinal herbs Echinacea, Ashwagandha and Brahmi. J. Ethnopharmacol. 2011, 137, 231–235. [Google Scholar] [CrossRef]
- Tan, B.K.; Vanitha, J. Immunomodulatory and antimicrobial effects of some traditional Chinese medicinal herbs: A review. Curr. Med. Chem. 2004, 11, 1423–1430. [Google Scholar] [CrossRef]
- Sabouri, Z.; Barzegar, M.; Sahari, M.; Naghdi Badi, H. Antioxidant and antimicrobial potential of Echinacea purpurea extract and its effect on extension of cake shelf life. J. Med. Plants 2012, 3, 28–40. [Google Scholar]
- Pellati, F.; Benvenuti, S.; Magro, L.; Melegari, M.; Soragni, F. Analysis of phenolic compounds and radical scavenging activity of Echinacea spp. J. Pharm. Biomed. Anal. 2004, 35, 289–301. [Google Scholar] [CrossRef]
- Pellati, F.; Benvenuti, S.; Melegari, M.; Lasseigne, T. Variability in the composition of anti-oxidant compounds in Echinacea species by HPLC. Phytochem. Anal. 2005, 16, 77–85. [Google Scholar] [CrossRef]
- Hudson, J.B. Applications of the phytomedicine Echinacea purpurea (Purple Coneflower) in infectious diseases. BioMed Res. Int. 2012, 2012, 769896. [Google Scholar]
- Cruz, I.; Cheetham, J.J.; Arnason, J.T.; Yack, J.E.; Smith, M.L. Alkamides from Echinacea disrupt the fungal cell wall-membrane complex. Phytomedicine 2014, 21, 435–442. [Google Scholar] [CrossRef]
- Birt, D.F.; Widrlechner, M.P.; LaLone, C.A.; Wu, L.; Bae, J.; Solco, A.K.; Price, J.P. Echinacea in infection. Am. J. Clin. Nutr. 2008, 87, 488S–492S. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.M.; Laba, J.G.; Moore, J.A.; Lee, T.D. Echinacea-induced macrophage activation. Immunopharmacol. Immunotoxicol. 2008, 30, 553–574. [Google Scholar] [CrossRef] [PubMed]
- Tierra, M. Echinacea: An effective alternative to antibiotics. J. Herb. Pharmacother. 2008, 7, 79–89. [Google Scholar] [CrossRef]
- Bałan, B.J.; Sokolnicka, I.; SkopińSka-różEwSka, E.; Skopiński, P. The modulatory influence of some Echinacea-based remedies on antibody production and cellular immunity in mice. Central Eur. J. Immu. 2016, 41, 12–18. [Google Scholar] [CrossRef]
- Classen, B. Characterization of an arabinogalactan-protein from suspension culture of Echinacea purpurea. Plant Cell Tissue Organ Cult. 2007, 88, 267–275. [Google Scholar] [CrossRef]
- Balciunaite, G.; Juodsnukyte, J.; Savickas, A.; Ragazinskiene, O.; Siatkute, L.; Zvirblyte, G.; Savickiene, N. Fractionation and evaluation of proteins in roots of Echinacea purpurea (L.) Moench. Acta Pharm. 2015, 65, 473–479. [Google Scholar] [CrossRef]
- Bossy, A.; Blaschek, W.; Classen, B. Characterization and immunolocalization of arabinogalactan-proteins in roots of Echinacea Purpurea. Planta Medica 2009, 75, 1526–1533. [Google Scholar] [CrossRef]
- Gallo, M.; Ferracane, R.; Naviglio, D. Antioxidant addition to prevent lipid and protein oxidation in chicken meat mixed with supercritical extracts of Echinacea angustifolia. J. Supercrit. Fluids 2012, 72, 198–204. [Google Scholar] [CrossRef]
- Lim, H.S.; Sohn, E.; Kim, Y.J.; Kim, B.Y.; Kim, J.H.; Jeong, S.J. Ethanol Extract of Elaeagnus glabra f. oxyphylla Branches Alleviates the Inflammatory Response Through Suppression of Cyclin D3/Cyclin-Dependent Kinase 11p58 Coupled to Lipopolysaccharide-Activated BV-2 Microglia. Nat. Prod. Commun. 2022, 17. [Google Scholar] [CrossRef]
- Haron, M.H.; Tyler, H.L.; Pugh, N.D.; Moraes, R.M.; Maddox, V.L.; Jackson, C.R.; Pasco, D.S. Activities and prevalence of Proteobacteria members colonizing Echinacea purpurea fully account for macrophage activation exhibited by extracts of this botanical. Planta Medica 2016, 82, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Maggini, V.; Miceli, E.; Fagorzi, C.; Maida, I.; Fondi, M.; Perrin, E.; Fani, R. Antagonism and antibiotic resistance drive a species-specific plant microbiota differentiation in Echinacea spp. FEMS Microbiol. Ecol. 2018, 94, fiy118. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Mir-Rashed, N.; Cruz, I.; Jessulat, M.; Dumontier, M.; Chesnais, C.; Juliana, N.G.; Smith, M.L. Disruption of the fungal cell wall by antifungal Echinacea extracts. Med. Mycol. 2010, 48, 949–958. [Google Scholar] [CrossRef]
- Spelman, K. The extraction, stability, metabolism, and bioactivity of the alkylamides in Echinacea spp. Microbiology 2009, 3, 534–540. [Google Scholar]
- Luettig, B.; Steinmüller, C.; Gifford, G.E.; Wagner, H.; Lohmann-Matthes, M.L. Macrophage activation by the polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea. JNCI J. Natl. Cancer Inst. 1989, 81, 669–675. [Google Scholar] [CrossRef]
- Chicca, A.; Pellati, F.; Adinolfi, B.; Matthias, A.; Massarelli, I.; Benvenuti, S.; Nieri, P. Cytotoxic activity of polyacetylenes and polyenes isolated from roots of Echinacea pallida. Br. J. Pharmacol. 2008, 153, 879–885. [Google Scholar] [CrossRef]
- Sestáková, H.; Turek, B. Effect of Echinacea on cells involved in disease defense. In Echinacea; CRC Press: Boca Raton, FL, USA, 2004; pp. 179–184. [Google Scholar]
- Kindscher, K. (Ed.) Echinacea: Herbal Medicine with a Wild History; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Qu, L.; Chen, Y.; Wang, X.; Scalzo, R.; Davis, J.M. Patterns of variation in alkamides and cichoric acid in roots and aboveground parts of Echinacea purpurea (L.) Moench. HortScience 2005, 40, 1239. [Google Scholar] [CrossRef]
- Senchina, D.S.; Martin, A.E.; Buss, J.E.; Kohut, M.L. Effects of Echinacea extracts on macrophage antiviral activities. Phytother. Res. 2010, 24, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Vimalanathan, S.; Kang, L.; Amiguet, V.T.; Livesey, J.; Arnason, J.T.; Hudson, J. Echinacea purpurea. aerial parts contain multiple antiviral compounds. Pharm. Biol. 2005, 43, 740–745. [Google Scholar] [CrossRef]
- Wu, L.; Bae, J.; Kraus, G.; Wurtele, E.S. Diacetylenic isobutylamides of Echinacea: Synthesis and natural distribution. Phytochemistry 2004, 65, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Signer, J.; Jonsdottir, H.R.; Albrich, W.C.; Strasser, M.; Züst, R.; Ryter, S.; Engler, O.B. In vitro virucidal activity of Echinaforce®, an Echinacea purpurea preparation, against coronaviruses, including common cold coronavirus 229E and SARS-CoV-2. Virol. J. 2020, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mistrikova, I.; Vaverkova, S. Echinacea—Chemical composition, immunostimulatory activities and uses. Thaiszia J. Bot. 2006, 16, 11–26. [Google Scholar]
- Farahani, M. Inhibition of HSV-1 multiplication by five species of medicinal plants. J. Microbiol. Biotechnol. Food Sci. 2013, 3, 69–71. [Google Scholar]
- De Oliveira, J.R.; Antunes, B.S.; do Nascimento, G.O.; Kawall, J.C.D.S.; Oliveira, J.V.B.; Silva, K.G.D.S.; Oliveira, C.R. Antiviral activity of medicinal plant-derived products against SARS-CoV-2. Exp. Biol. Med. 2022, 247, 1797–1809. [Google Scholar] [CrossRef]
- Percaccio, E.; De Angelis, M.; Acquaviva, A.; Nicotra, G.; Ferrante, C.; Mazzanti, G.; Di Sotto, A. ECHOPvir: A Mixture of Echinacea and hop extracts endowed with cytoprotective, immunomodulatory and antiviral properties. Nutrients 2023, 15, 4380. [Google Scholar] [CrossRef] [PubMed]
- Awang, D.V. Immune stimulants and antiviral botanicals: Echinacea and ginseng. Perspectives on new crops and new uses. ASHS Press Alex. 1999, 5, 450–456. [Google Scholar]
- Bergner, P. Antiviral botanicals in herbal medicine. Med. Herbal. 2005, 14, 1–12. [Google Scholar]
- Bruni, R.; Brighenti, V.; Caesar, L.K.; Bertelli, D.; Cech, N.B.; Pellati, F. Analytical methods for the study of bioactive compounds from medicinally used Echinacea species. J. Pharm. Biomed. Anal. 2018, 160, 443–477. [Google Scholar] [CrossRef]
- Aucoin, M.; Cooley, K.; Saunders, P.R.; Carè, J.; Anheyer, D.; Medina, D.N.; Garber, A. The effect of Echinacea spp. on the prevention or treatment of COVID-19 and other respiratory tract infections in humans: A rapid review. Adv. Int. Med. 2020, 7, 203. [Google Scholar] [CrossRef] [PubMed]
- Novika, R.G.; Wahidah, N.J.; Yunus, A.; Sumarno, L.; Ilyas, M.F. Clinical effect of Echinacea purpurea as an antiviral and its effect on reproductive hormones. J. Pharm. Pharmacogn. Res. 2024, 12, 255–263. [Google Scholar] [CrossRef]
- Sun, Y.; Li, C.; Liu, Z.; Zeng, W.; Ahmad, M.J.; Zhang, M.; He, Q. Chinese herbal extracts with antiviral activity: Evaluation, mechanisms, and potential for preventing PRV, PEDV and PRRSV infections. Anim. Dis. 2023, 3, 35. [Google Scholar] [CrossRef]
- Turner, R.B.; Bauer, R.; Woelkart, K.; Hulsey, T.C.; Gangemi, J.D. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N. Engl. J. Med. 2005, 353, 341–348. [Google Scholar] [CrossRef]
- Gakhar, A. Anti-viral phyto medicine: A review. J. Pharmacogn. Phytochem. 2021, 10, 2002–2004. [Google Scholar] [CrossRef]
- Kindscher, K. Ethnobotany of purple coneflower (Echinacea angustifolia, Asteraceae) and other Echinacea species. Econ. Bot. 1989, 43, 498–507. [Google Scholar] [CrossRef]
- Naithani, R.; Mehta, R.G.; Shukla, D.; Chandersekera, S.N.; Moriarty, R.M. Antiviral activity of phytochemicals: A current perspective. In Dietary Components and Immune Function; Humana Press: Totowa, NJ, USA, 2010; pp. 421–468. [Google Scholar]
- Lelešius, R.; Karpovaitė, A.; Mickienė, R.; Drevinskas, T.; Tiso, N.; Ragažinskienė, O.; Šalomskas, A. In vitro antiviral activity of fifteen plant extracts against avian infectious bronchitis virus. BMC Vet. Res. 2019, 15, 1–10. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Shehata, M.; Sadasivam, K.; Delbue, S.; Dolci, M.; Pariani, E.; D’Alessandro, S.; Pleschka, S. Broad antiviral effects of Echinacea purpurea against SARS-CoV-2 variants of concern and potential mechanism of action. Microorganisms 2022, 10, 2145. [Google Scholar] [CrossRef]
- LaLone, C.A.; Hammer, K.D.; Wu, L.; Bae, J.; Leyva, N.; Liu, Y.; Birt, D.F. Echinacea species and alkamides inhibit prostaglandin E2 production in RAW264. 7 mouse macrophage cells. J. Agric. Food Chem. 2007, 55, 7314–7322. [Google Scholar] [CrossRef]
- Foster, S. Herbal Remedies: Echinacea: The Cold and Flu Remedy. Alter Compl Therapies. 1995, 1, 254–257. [Google Scholar] [CrossRef]
- Ogal, M.; Johnston, S.L.; Klein, P.; Schoop, R. Echinacea reduces antibiotic usage in children through respiratory tract infection prevention: A randomized, blinded, controlled clinical trial. Eur. J. Med. Res. 2021, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Brincat, J.P.; Cetin, Y.; Lia, F. Antiviral activity of natural compounds extracted from Mediterranean medicinal plants against SARS-CoV-2. Acad. Biol. 2024, 2, 41–49. [Google Scholar] [CrossRef]
- Nicolussi, S.; Ardjomand-Woelkart, K.; Stange, R.; Gancitano, G.; Klein, P.; Ogal, M. Echinacea as a potential force against coronavirus infections? A mini-review of randomized controlled trials in adults and children. Microorganisms 2022, 10, 211. [Google Scholar] [CrossRef]
- Mohamadi, N.; Sharififar, F.; Rameshk, M.; Khandani, S.K. A Global Perspective on Medicinal Plants and Phytochemicals with Antiviral Potentials in the Respiratory System. Anti-Infect. Agents 2023, 21, 56–78. [Google Scholar] [CrossRef]
- Hassan, S.T.; Masarčíková, R.; Berchová, K. Bioactive natural products with anti-herpes simplex virus properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336. [Google Scholar] [CrossRef]
- Wagner, L.; Cramer, H.; Klose, P.; Lauche, R.; Gass, F.; Dobos, G.; Langhorst, J. Herbal medicine for cough: A systematic review and meta-analysis. Forsch. Komplementärmedizin/Res. Complement. Med. 2015, 22, 359–368. [Google Scholar] [CrossRef]
- Sadeek, A.; Abdallah, E. Medicinal Plants with Antiviral Properties to Tackle Covid-19 Pandemic: A Short-Review. Antivirals 2021, 2, 122–127. [Google Scholar]
- Wu, Y.H.; Chen, Y.; Zhuang, A.Q.; Chen, S.M. Natural Phenolic Acids and Their Derivatives against Human Viral Infections; InTechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Saifulazmi, N.F.; Rohani, E.R.; Harun, S.; Bunawan, H.; Hamezah, H.S.; Nor Muhammad, N.A.; Sarian, M.N. A review with updated perspectives on the antiviral potentials of traditional medicinal plants and their prospects in antiviral therapy. Life 2022, 12, 1287. [Google Scholar] [CrossRef]
- Hudson, J.; Vimalanathan, S.; Kang, L.; Amiguet, V.T.; Livesey, J.; Arnason, J.T. Characterization of antiviral activities in Echinacea. Root preparations. Pharm. Biol. 2005, 43, 790–796. [Google Scholar] [CrossRef]
- Venu, L.N.; Austin, A. Antiviral efficacy of medicinal plants against respiratory viruses: Respiratory Syncytial Virus (RSV) and Coronavirus (CoV)/COVID 19. J. Pharmacol. 2020, 9, 281–290. [Google Scholar] [CrossRef]
- Ghaemi, A.; Soleimanjahi, H.; Farshbaf Moghaddam, M.; Yazdani, N. Evaluation of antiviral activity of aerial part of Echinacea purpurea extract against herpes. Hakim Res. J. 2007, 9, 59–64. [Google Scholar]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic acid and its derivatives: Antimicrobial drugs toward microbial pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, A.; Soleimanjahi, H.; Gill, P.; Arefian, E.; Soudi, S.; Hassan, Z. Echinacea purpurea polysaccharide reduces the latency rate in herpes simplex virus type-1 infections. Intervirology 2009, 52, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.; Vimalanathan, S. Echinacea—A source of potent antivirals for respiratory virus infections. Pharmaceuticals 2011, 4, 1019–1031. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Schoop, R.; Suter, A.; Hudson, J. Prevention of influenza virus-induced bacterial superinfection by standardized Echinacea purpurea, via regulation of surface receptor expression in human bronchial epithelial cells. Virus Res. 2017, 233, 51–59. [Google Scholar] [CrossRef]
- Meeran, M.N.; Javed, H.; Sharma, C.; Goyal, S.N.; Kumar, S.; Jha, N.K.; Ojha, S. Can Echinacea be a potential candidate to target immunity, inflammation, and infection-The trinity of coronavirus disease 2019. Heliyon 2021, 7, 65–72. [Google Scholar]
- Sharma, M.; Anderson, S.A.; Schoop, R.; Hudson, J.B. Induction of multiple pro-inflammatory cytokines by respiratory viruses and reversal by standardized Echinacea, a potent antiviral herbal extract. Antivir. Res. 2009, 83, 165–170. [Google Scholar] [CrossRef]
- Pleschka, S.; Stein, M.; Schoop, R.; Hudson, J.B. Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV). Virol. J. 2009, 6, 1–9. [Google Scholar] [CrossRef]
- Farahani, M. Inhibition of HSV-1 multiplication by aqueous extract of Echinacea purpurea root. J. Mashhad Dent. Sch. 2015, 39, 71–80. [Google Scholar]
- Stevenson, L.M.; Matthias, A.; Banbury, L.; Penman, K.G.; Bone, K.M.; Leach, D.; Lehmann, R.P. Modulation of macrophage immune responses by Echinacea. Molecules 2005, 10, 1279–1285. [Google Scholar] [CrossRef]
- Kembuan, G.; Lie, W.; Tumimomor, A. Potential usage of immune modulating supplements of the Echinacea genus for COVID-19 infection. Int. J. Med. Rev. Case Rep 2020, 4, 2020. [Google Scholar]
- Di Sotto, A.; Vitalone, A.; Di Giacomo, S. Plant-derived nutraceuticals and immune system modulation: An evidence-based overview. Vaccines 2020, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Solco, A.; Wu, L.; Wurtele, E.S.; Kohut, M.L.; Murphy, P.A.; Cunnick, J.E. Echinacea increases arginase activity and has anti-inflammatory properties in RAW 264.7 macrophage cells, indicative of alternative macrophage activation. J. Ethnopharmacol. 2009, 122, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Yuan, Y.; Jiang, L.; Tai, Y.; Yang, X.; Hu, F.; Xie, Z. Anti-inflammatory effects of essential oil in Echinacea purpurea L. Pak. J. Pharm. Sci. 2013, 26, 403–408. [Google Scholar]
- Mohammedsaleh, Z.M.; Aljadani, H.M. Echinacea purpurea root extract modulates diabetes-induced renal dysfunction in rats through hypoglycemic, antioxidant, and anti-inflammatory activities. Med. Sci. 2021, 25, 1033–1043. [Google Scholar]
- Dobrange, E.; Peshev, D.; Loedolff, B.; Van den Ende, W. Fructans as immunomodulatory and antiviral agents: The case of Echinacea. Biomolecules 2019, 9, 615. [Google Scholar] [CrossRef]
- Bajrai, L.H.; El-Kafrawy, S.A.; Hassan, A.M.; Tolah, A.M.; Alnahas, R.S.; Sohrab, S.S.; Azhar, E.I. In vitro screening of anti-viral and virucidal effects against SARS-CoV-2 by Hypericum perforatum and Echinacea. Sci. Rep. 2022, 12, 21723. [Google Scholar] [CrossRef]
- Lim, T.K.; Lim, T.K. Echinacea purpurea. Edible Medicinal and Non-Medicinal Plants. Flowers 2014, 7, 340–371. [Google Scholar]
- Melchart, D.; Linde, K.; Worku, F.; Bauer, R.; Wagner, H. Immunomodulation with Echinacea—A systematic review of controlled clinical trials. Phytomedicine 1994, 1, 245–254. [Google Scholar] [CrossRef]
- Oliveira, B.G.D.; Santos, L.F.F.; Costa, M.C.D.; Bastos, R.W.; Carmo, P.H.F.D.; Santos, D.D.A.; César, I.C. Antimicrobial, and immunomodulatory activities of dried extracts of Echinacea Purpurea. Braz. J. Pharm. Sci. 2022, 58, e21026. [Google Scholar] [CrossRef]
- Senchina, D.S.; McCann, D.A.; Asp, J.M.; Johnson, J.A.; Cunnick, J.E.; Kaiser, M.S.; Kohut, M.L. Changes in immunomodulatory properties of Echinacea spp. root infusions and tinctures stored at 4 C for four days. Clin. Chim. Acta 2005, 355, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Bone, K. Echinacea: Quality, uses, and immunomodulating activity from a phytotherapist’s perspective. In Echinacea; CRC Press: Boca Raton, FL, USA, 2004; pp. 219–230. [Google Scholar]
- Mahmoud, S.H.; Mahmoud, R.M.; Ashoush, I.S.; Attia, M.Y. Immunomodulatory and antioxidant activity of pomegranate juice incorporated with spirulina and echinacea extracts sweetened by stevioside. J. Agric. Vet. Sci. (Qassim Univ.) 2015, 8, 161–174. [Google Scholar]
- Ibrahim, A.; Saleh, R.; Hassan, A.; Amer, M. Immunomodulatory effect of Echinacea purpurea and Curcuma longa in rabbits. Mansoura. Vet. Med. J. 2020, 22, 2. [Google Scholar]
- Raduner, S.; Majewska, A.; Chen, J.Z.; Xie, X.Q.; Hamon, J.; Faller, B.; Gertsch, J. Alkylamides from Echinacea are a new class of cannabinomimetics: Cannabinoid type 2 receptor-dependent and-independent immunomodulatory effects. J. Biol. Chem. 2006, 281, 14192–14206. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, M.; Kim, D.; Oh, D.H.; Prasad, K.S.; Eun, S.; Lee, J. Echinacea purpurea extract enhances natural killer cell activity in vivo by upregulating MHC II and Th1-type CD4+ T cell responses. J. Med. Food 2021, 24, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Matthias, A.; Banbury, L.; Stevenson, L.M.; Bone, K.M.; Leach, D.N.; Lehmann, R.P. Alkylamides from Echinacea modulate induced immune responses in macrophages. Immunol. Investig. 2007, 36, 117–130. [Google Scholar] [CrossRef]
- Coeugniet, E.G.; Elek, E. Immunomodulation with Viscum album and Echinacea purpurea extracts. Oncol. Res. Treat. 1987, 10, 27–33. [Google Scholar] [CrossRef]
- Aarland, R.C.; Bañuelos-Hernández, A.E.; Fragoso-Serrano, M.; Sierra-Palacios, E.D.C.; Díaz de León-Sánchez, F.; Pérez-Flores, L.J.; Mendoza-Espinoza, J.A. Studies on phytochemical, antioxidant, anti-inflammatory, hypoglycaemic and antiproliferative activities of Echinacea purpurea and Echinacea angustifolia extracts. Pharm. Biol. 2017, 55, 649–656. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Perissutti, B.; Grabnar, I.; Farra, R.; Comar, M.; Agostinis, C.; Voinovich, D. Pharmacokinetics and immunomodulatory effect of lipophilic Echinacea extract formulated in softgel capsules. Eur. J. Pharm. Biopharm. 2015, 97, 8–14. [Google Scholar] [CrossRef]
- Classen, B.; Csávás, M.; Borbás, A.; Dingermann, T.; Zündorf, I. Monoclonal antibodies against an arabinogalactan-protein from pressed juice of Echinacea purpurea. Planta Medica 2004, 70, 861–865. [Google Scholar] [CrossRef]
- Zorig, A.; Toko, R.; Sukhbold, E.; Takasugi, M.; Arai, H. Echinacea purpurea water extracts suppress the release of chemical mediators from mast cells. Biosci. Biotechnol. Biochem. 2021, 85, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.C.; Chen, C.H.; Yang, N.S.; Chen, Y.P.; Lo, C.P.; Wang, S.Y.; Shyur, L.F. Comparative metabolomics approach coupled with cell-and gene-based assays for species classification and anti-inflammatory bioactivity validation of Echinacea plants. J. Nutr. Biochem. 2010, 21, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Randolph, R.K.; Gellenbeck, K.; Stonebrook, K.; Brovelli, E.; Qian, Y.; Bankaitis-Davis, D.; Cheronis, J. Regulation of human immune gene expression as influenced by a commercial blended Echinacea product: Preliminary studies. Exp. Biol. Med. 2003, 228, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines. A Guide for Healthcare Professionals, updated CDROM of 2nd ed.; Pharmaceutical Press: London, UK, 2004. [Google Scholar]
- Binns, S.E.; Hudson, J.; Merali, S.; Arnason, J.T. Antiviral activity of characterized extracts from Echinacea spp. (Heliantheae: Asteraceae) against Herpes simplex virus (HSV-1). Planta Med. 2002, 68, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Christakis, D.A.; Lehmann, H.P. Can an herbal preparation of echinacea, propolis, and vitamin C reduce respiratory illnesses in children? Arch. Pediatr. Adolesc. Med. 2004, 158, 222–224. [Google Scholar]
- Cohen, H.A.; Varsano, I.; Kahan, E.; Sarrell, M.; Uziel, Y. Effectiveness of an herbal preparation containing Echinacea, propolis, and vitamin C in preventing respiratory tract infections in children. A randomized, double-blind, placebo-controlled, multicenter study. Arch. Pediatr. Adolesc. Med. 2004, 158, 217–221. [Google Scholar] [CrossRef]
- Grimm, W.; Muller, H.H. A randomized controlled trial of the effect of fluid extract of Echinacea purpurea on the incidence and severity of colds and respiratory infections. Am. J. Med. 1999, 106, 138–143. [Google Scholar] [CrossRef]
- Lee, A.N.; Werth, V.P. Activation of autoimmunity following use of immunostimulatory herbal supplements. Arch. Dermatol 2004, 140, 723–727. [Google Scholar] [CrossRef]
- Matthias, A.; Penman, K.G.; Addison, R.S.; Dickinson, R.G.; Bone, K.M.; Lehmann, R.P. Bioavailability and pharmacokinetics of alkylamides from Echinacea. Int. Congr. Nat. Prod. Res. Phoenix Ariz. 2004, 57, p133. [Google Scholar]
- Wichtl, M. (Ed.) Herbal Drugs and Phytopharmaceuticals. A Handbook for Practice on a Scientific Basis, 3rd ed.; Medpharm Scientific Publishers: Stuttgart, Germany, 2004. [Google Scholar]
- Parsons, J.L.; Cameron, S.I.; Harris, C.S.; Smith, M.L. Echinacea biotechnology: Advances, commercialization, and future considerations. Pharm. Biol. 2018, 56, 485–494. [Google Scholar] [CrossRef]
- Catanzaro, M.; Corsini, E.; Rosini, M.; Racchi, M.; Lanni, C. Immunomodulators inspired by nature: A review on curcumin and echinacea. Molecules 2018, 23, 2778. [Google Scholar] [CrossRef] [PubMed]
- Burlou-Nagy, C.; Bănică, F.; Jurca, T.; Vicaș, L.G.; Marian, E.; Muresan, M.E.; Pallag, A. Echinacea purpurea (L.) Moench: Biological and pharmacological properties. A review. Plants 2022, 11, 1244. [Google Scholar] [CrossRef] [PubMed]
- Justus, W.; Hossain, M.F. Echinacea, The Immune-Boosting Potential of Nature’s Herbal Remedy: A Systematic Review. Am. J. Nat. Med. Facts 2024, 1, 1–4. [Google Scholar]
- Manohar, K.A.; Shahina, N.N.; Sarkar, B.C.; Shukla, G.; Das, S.; Singh, M.; Chakravarty, S. Ethnomedicinal plants: Medicine and nutrition for future generations. In Bioprospecting of Ethnomedicinal Plant Resources; Apple Academic Press: Palm Bay, FL, USA, 2025; pp. 11–34. [Google Scholar]
- McNeil, B.K.; Renaud, D.L.; Steele, M.A.; Keunen, A.J.; DeVries, T.J. Effects of Echinacea purpurea supplementation on markers of immunity, health, intake, and growth of dairy calves. J. Dairy Sci. 2023, 106, 4949–4965. [Google Scholar] [CrossRef] [PubMed]
- Sperber, S.J.; Shah, L.P.; Gilbert, R.D.; Ritchey, T.W.; Monto, A.S. Echinacea purpurea for prevention of experimental rhinovirus colds. Clin. Infect. Dis. 2004, 38, 1367–1371. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).